Introduction
Plasma membrane-derived microvesicles or microparticles (MPs) are sub-cellular vesicles released upon shear stress, cell activation, injury or apoptosis. They represent factors of the extracellular vesicular compartment1 that also includes smaller (0.03–0.1 μm) multivascular body-derived exosomes and larger (1–5 μm) apoptotic bodies which may contain fragmented DNA1. Exosomes are small export vesicles initially derived from the plasma membrane by an endocytosis-involving internalization of the later. In contrast, MPs are larger than exosomes and are derived directly from the plasma membrane after local cytoskeleton rearrangements and membrane budding2. Despite different generation mechanisms and effects, they have been considered as universal biomarkers of cell activation, injury or apoptosis, in a broad range of physiological and pathological processes.
Typically MPs range in size from 0.1 μm to 1.0 μm. They express surface markers from their parental cells that allow identification of MPs sub-groups according to their origin: from platelets (P-MPs), leukocytes (L-MPs), red blood cells (R-MPs), endothelial cells and other tissue cells. They harbor cell-derived membrane-bound and cytoplasmic proteins (e.g. chaperones) as well as bioactive lipids. However, MPs are not replicas of the maternal cells and plasma membrane, suggesting a level of selectivity in their formation and sorting of cellular proteins to release2,3. In fact, MPs release is a highly controlled process. According to proteomic data, plasma MPs are enriched in Ig μ-chains, J-chains, profilin I and cyclophilin A, suggesting that MPs-bound IgM may provide a mechanism for their clearance4.
As mentioned above, MPs can be formed through several induction pathways, which determine their different molecular profiles and biologic activities. A major aspect in cell biology is communication, which occurs through a direct contact between cells or by means of soluble substances, which react with cells. The fact that MPs released by cells influence other cells is a rather new concept but is a basic mechanism to deliver a message in a highly concentrated manner or to add a missing molecule. MPs exert their effects either via stimulation of target cells by receptor interaction or by direct transfer of their contents which can include membrane proteins and lipids, cytoplasmic components of the parent cell or RNA3,5. By this way, MPs may facilitate cell-to-cell interactions and transfer of signals and receptors between different cell types, inducing thus signalling and response in distant cells. Although not characterized in detail, it may be hypothesized that uptake and removal by cells occurs by similar or identical molecular mechanisms1. The importance of cellular communication via MPs is that they contain highly concentrated signalling components and thus the massive hit of every target cells by MPs’ components is likely to be more effective than the activity of individual components in solution2. As a result, MPs represent nowadays a novel mechanism of intercellular communication mediating inflammation, coagulation and immune responses.
The rate of steady state release of MPs in the blood of healthy individuals is usually low1, however they are found elevated in a variety of pathologies including many thrombotic and inflammatory conditions. While it is not sure if this increase represents a contributor to or a result of the disease deterioration, it identifies MPs as potential biomarkers6,7. On the other hand, L-MPs participate in angiogenesis and as such, they have been suggested as novel therapeutic tools to reset the angiogenic switch in pathologies with altered angiogenesis6.
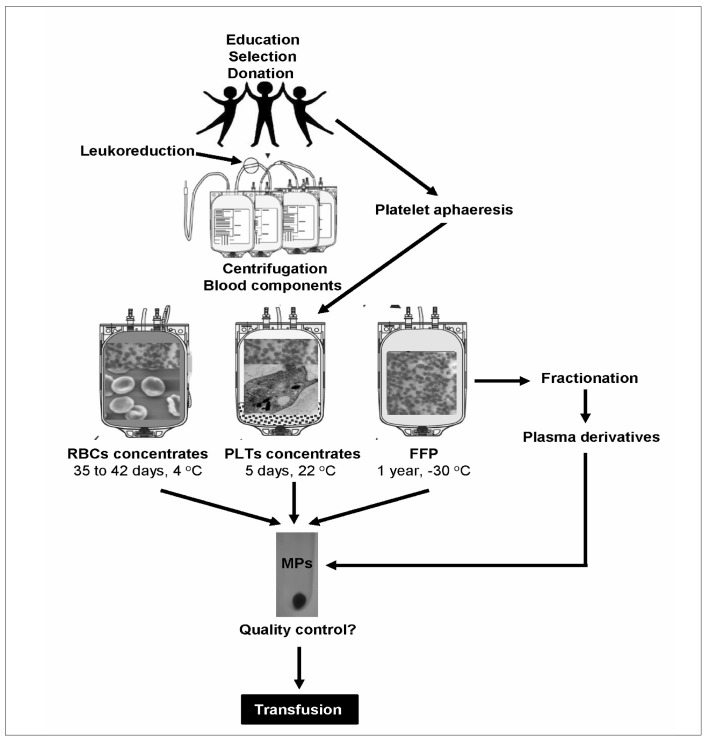
MPs are inherent part of all blood labile products delivered to transfused patients (Figure 1). They are present and accumulate in cellular (red blood cells RBCs, platelets PLTs) concentrates as well as in fresh frozen plasma (FFP) during storage8. A clinically important issue is that MPs may have pro-coagulant activities. It has been demonstrated that P-MPs have from 50 up to 100-times higher pro-coagulant activity than PLTs9. MPs exhibit tissue factor activity, so they may have a role in initiating blood coagulation4. During MPs release, the normal asymmetric distribution of phospholipids between the two leaflets of the plasma membrane is lost, resulting in the exposure of the anionic phospholipid phosphatidylserine (PS). The later contributes to the pro-coagulant property of MPs since it serves for the assembly of coagulation factors into active complexes for thrombin generation. However, some observations also suggest the existence of MPs without PS externalization, like in the case of P-MPs derived from PLTs-poor plasma10 suggesting that they possess other activities aside from pro-coagulant phospholipid one. In recent years, a growing body of literature has demonstrated an increased incidence of adverse clinical outcomes associated with the transfusion of a large number of units or, potentially, with increased storage time of the units. These events include increased risk of infection, renal failure, respiratory failure, multiple organ failure, and death, particularly in physiologically compromised patient populations11,12. Since MPs accumulate in blood labile products during storage, transfusion of more or “older” units will offer to the recipient higher number of MPs. The transfused MPs could increase the risk of adverse reactions, by inducing a hypercoagulable state leading to thromboembolic complications. Inversely, in other situations requiring blood transfusion, a hypercoagulable state may be useful to diminish or even helping to stop the bleeding. Many clinical studies suggest that transfusions might be immunosuppressive, although these observations are not generally accepted13,14. However, a clinical study indicated that transfusions of RBCs might be responsible for a diminished survival in cancer patients15.
Figure 1.
Microparticles (MPs) are inherent part of all blood labile products and are concomitantly delivered by transfusion to recipients. A growing body of literature has demonstrated an increased incidence of adverse clinical outcomes associated with the transfusion of a large number of units or, potentially, with increased storage time of the units. Since MPs accumulate in blood labile products during storage, transfusion of more or “older” units will offer to the recipient higher number of MPs. The transfused MPs have the potential to increase the risk of adverse reactions, by inducing a hypercoagulable state leading to thromboembolic complications. Inversely, in other situations requiring blood transfusion, a hypercoagulable state may be useful to diminish or even helping to stop the bleeding. In both cases, MPs accumulation, cellular origin and composition might be factors of blood labile products quality control.
This review outlines the current knowledge on MPs present in blood labile products used for transfusion: their formation, the effect of storage and their probable activities towards both the “storage lesion” progression as well as the post-transfusion effects. Although the current clinical studies offer only indirect manifestations of MPs activity in the recipients, a lot of data suggest that MPs cotransfused with stored cells and plasma may exert various favorable or adverse effects pre- and post-transfusion, but are definitely not bystanders in any step of this clinically important process.
Methodological approaches
In the past few years, the number of research works on blood MPs has dramatically increased, reflecting the interest on their emerging roles and activities in various diseases as well as in transfusion medicine. The most common approaches to investigate MPs are flow cytometry, coagulation assays (thrombin time, activated partial thromboplastin time etc.), proteomics and ELISA-based solid-phase capture assays8,16. They all aim to gain insights on the biological mechanisms involved in MPs generation and function. Most investigators have designated to characterize blood MPs using flow cytometry and some technical protocols have been reviewed in a recent forum17. Flow cytometry allows the detection, enumeration and assessment of cell origin of MPs after labeling with various markers (cell-specific and annexin V). A severe limitation of the method is that it fails to detect MPs smaller than 200 nm. Microscopy approaches, including transmission electron microscopy, represent the gold standard for the determination of both MPs size and structure18. Moreover, MPs composition and protein topology can be resolved by immune-electron microscopy approaches18–20. Proteomic techniques have lightened the variation in MPs proteome as a function of their origin, time of storage or stimulus that triggers their generation4,21,22. In addition to analyzing MPs antigens, several investigators have assessed the coagulant function of blood MPs using functional assays23–25. In capture-based assays a probe, antibody or annexin V, specifically binds a subset of MPs from plasma. Captured MPs can then be quantified/characterised by using a second probe for example peroxidase conjugated antibody26 or by measurement of the concentration of negatively charged phospholipids (e.g. their pro-coagulant activity)27. Enumeration of total MPs population and information on the size distribution of MPs is unable with captured based assays. There is furthermore interference by soluble antigens. On the opposite, a clear advantage of capture based assays is that they allow measurement of MPs directly in plasma.
Unfortunately, MPs methodologies are poorly standardized and therefore the choice of MPs assay protocol will significantly influence the results obtained8. The last meeting of the Scientific and Standardization Committee of Vascular Biology has focused on MPs. The session was divided into two sections: the Educational session, devoted to MPs function, generation and role in disease, and the business session, focused on MPs determination and standardization (standardized strategies, functional assays and novel methodologies, available at: http://isth.org/default/index.cfm/ssc1/2011-ssc-subcommittee-minutes/2011-vascular-biology-minutes/). The potential causes of variability in MPs measurement and analysis are several and are mostly arisen by the fact that blood cells are highly sensitive to environmental factors and respond by releasing MPs. Pre-analytical variables are a major source of variability and potential artifacts in MPs analysis. Uncontrolled pre-analytical parameters may lead to false interpretation of results. The bulk of new data simultaneously raise questions regarding the roles of MPs, the answers to which depend on forthcoming analytical improvements in the standardization of methods used28. MPs analysis by proteomics and microscopy need PLTs poor plasma to reduce the plasma protein content and to increase the MPs concentration. Multiple washing steps during high-speed centrifugation isolation will certainly cause some loss of MPs in the pellet. In proteomics and other methodologies it is important to work with purified, isolated MPs but unfortunately, there are no standard isolation protocols. Most groups apply centrifugation conditions from 18,000 x g to 250,000 x g4. The problem is that during centrifugation of cells like PLTs some MPs may be selectively depleted, while forced filtration of MPs holds the risk of fragmentation into smaller vesicles1. In any case, PLTs should be pelleted shortly after collection and before freezing stage. Blood collection conditions (e.g. diameter of the needle used for venipuncture and duration of placement of the tourniquet), time before processing, plasma preparation methods, working and storage duration and temperature, the type of anticoagulants used, number/time and other parameters of centrifugation and washing steps needed to yield platelet-free plasma, freeze-thaw cycles, needle-to-analysis time and analysis protocols, cell and cellular fragments contamination, labeling of MPs, fresh vs. frozen plasma, all of them might artificially affect the MPs analytical approaches and must be carefully standardized before any assessment of MPs biological and clinical effect1,28,29. To mention some more, in stored RBCs concentrates the MPs count varies not only with storage time but also with temperature, MPs dilution buffer, vortexing and agitation28. PLTs release MPs in response to shear stress30 and storage31. Furthermore, freeze-thaw cycles of PLTs-reach plasma results in increased number of PS-positive MPs10. Despite difficulties encountered in analyzing MPs and disparities of results obtained with a wide range of methods, MPs generation processes and effects are nowadays better understood.
MPs in stored RBCs concentrates
Stored RBCs undergo a series of time-dependent-yet early enough recognizable-physiological, structural and biochemical alterations, which are only reversible to some extent32. In the RBC storage lesion context, depletion of ATP, pH acidification, physiologically important disturbances in energy metabolism and S-nitrosohaemoglobin content, rheological properties (shape, deformability, aggregability, intracellular viscosity), oxidation stress and finally, in cellular aging process have been widely characterized32–35. Altered membrane surface and cytoskeleton contribute to the RBCs damage and clearance36,37. RBCs lose their membrane stability leading to haemolysis and MPs formation. Although the clinical importance of the RBCs storage lesion is poorly understood, some of the irreversible deteriorations of the stored RBCs, like haemolysis, potassium release and MPs accumulation, are associated with reduced post-transfusion survival/efficacy and increased risk of adverse reactions in the recipients38,39.
The exact mechanism of R-MPs release has not been elucidated; however it is very likely that there may be multiple mechanisms that initiate MPs release40,41. These mechanisms are tightly controlled and associated with different types of cell stimulation42. MPs formation from RBCs in vivo occurs throughout the RBCs lifespan as a part of the normal physiological aging process, but is accelerated in the second half, if a functional spleen is present43. As a result, there is a loss of the 30 and 20% of the RBCs volume and surface, respectively, at the end of their lifespan. MPs are rapidly removed in vivo by the reticuloendothelial system44. RBCs microvesiculation is triggered by different types of stimuli such as shear stress, complement attack, oxidative stress, calcium influx and pro-apoptotic stimulations45. The composition of R-MPs may vary according to the stimulus, or between in vitro and in vivo conditions, and differs from their parental RBCs by nearly complete absence of cytoskeleton-linked molecules, decrease of membrane proteins content, presence of proteins involved in cell metabolism and most importantly, exposure of removal signals45. It should be noted that many stimuli can be additive or even synergistic.
The storage-associated progressive transformation of discocytes to echinocytes and finally to spherical and degenerative shaped cells, is closely associated with membrane loss in the form of MPs46. R-MPs formation represents a continuous process of stored RBCs membrane remodeling, which occurs early during blood banking36,47. Firstly reported in stored RBCs units by Rumsby and colleagues in 197748, the vast majority of MPs collected from the supernatant of RBCs units originate from RBCs and contain haemoglobin (Hb)18. Since their number gradually increases with storage time8,18, the older units contain significantly higher number of MPs compared to the fresh ones. The level of vesiculation in RBCs concentrates may vary not only with the length of storage but also according to the product and the storage solution: lower R-MPs accumulation has been found under RBCs storage in additive solutions that manage effectively the oxidative stress19 as well as after pre-storage leukoreduction of whole blood or packed RBCs units49. Notably, R-MPs accumulation varies also importantly from donor to donor8.
The identity of the storage parameters that slow or promote MPs generation is still elusive. According to theoretical models, cytoskeletal defects induced by low ATP levels can account for discoid RBCs transformation to echinocytes and release of membrane MPs as well as for further loss of cellular ATP50. ATP depletion, RBCs aging, degradation of the spectrin-bilayer anchoring through the band 3-ankyrin complex or calcium loading result in cytoskeleton rigidity and compression forces to the attached fluid membrane that may lead to buckling and MPs formation40,51. In support, in vitro, both echinocytosis and microvesiculation are promoted by intracellular calcium elevation and ATP depletion but during routine storage this correlation is poor52. The composition of R-MPs in RBCs concentrates is similar to those generated in vivo, except for the increased levels of stomatin, making it likely that a raft-based process is responsible for RBCs microvesiculation at low temperatures47. Considering that the cytoskeleton is essential for membrane stability in RBCs, MPs formation has been further associated with the storage-induced local deformation of the spectrin network, tyrosine phosphorylation of band 3 and unstable adhesion of the cytoskeleton to the membrane of stored RBCs that favors budding40,41,44,47,53. Storage reduces the formation of the spectrin-actin-protein 4.1 complex54 and furthermore shifts the redox potential toward oxidative stress37,52, leading to oxidation and proteolysis of several cytoskeleton proteins19. The effect of cytoskeleton protein oxidation damage to the formation of cytoskeleton-depleted8 R-MPs has been previously reported in stored RBCs55.
In fact, R-MPs collected from units of stored RBCs are devoid of most of the RBCs integral membrane proteins or cytoskeletal components, with the exception of actin and band 3, found in aggregated or degraded forms18,21,44,47. The significant difference observed in membrane composition between RBCs and R-MPs outline that MPs are generated by specific processes40, which allow sorting of lipids, as well as of membrane and cytoplasmic proteins21. Numerous components that are predominantly seen in senescent RBCs like C5b-9 complement attack complex, removal signals like PS, IgGs and band 3 neoantigen, as well as oxidized/damaged material, including Hb, methaemoglobin, and activated caspases have been detected in their surface and cytosol respectively18,19,21,45,56. R-MPs-mediated extrusion of those non functional or potentially harmful agents and death signalling mediators from RBCs apparently contributes to cellular homeostasis by preventing the premature removal of viable RBCs57. At the same time, the release of MPs leads to loss of critical surface area56. This depletion contributes to echinocytosis, increased osmotic fragility and cellular deformability defects that threaten post-transfusion viability of stored RBCs39,58,59. According to the absence of a removal mechanism inside the stored RBCs units, R-MPs become more heterogeneous over time, exhibiting a gradual increase in their size and in their content of proteasome components, as well as a decrease in PS exposure18,21,47,57,60, suggesting that MPs structure or nature of MPs formation may vary with storage time.
Although the “loading” of R-MPs with toxic cellular components contributes to the survival of parent cells, it simultaneously renders MPs highly pro-inflammatory and pro-thrombotic21, increasing thus the risk of adverse post-transfusion reactions. As a result, the degree of vesiculation and irreversible transformation of stored RBCs have been used as measures of storage quality61. Proteome information on R-MPs components involved in coagulation is little. However, phospholipid scramblase 1, plasminogen precursor, fibrinogen beta chain precursor, complement component C9 precursor and beta-2-glycoprotein 1, have all been detected in R-MPs collected from the supernatant of stored RBCs21. Notably, MPs pro-coagulant proteins were not found on stored RBCs membrane, suggesting that they are either of plasmatic origin or enriched on MPs microdomains21. In the context of procoagulant property of MPs, it has been proposed that R-MPs may work synergistically with P-MPs in mediating some transfusion-related thrombotic complications62. In support, recent studies reported that the pro-coagulant state in sickle cell disease is partially explained by the factor XI-dependent procoagulant properties of circulating R-MPs63.
There are indications suggesting that stored R-MPs can enhance the inflammatory response observed in patients who receive RBCs of prolonged storage. Indeed, the concentration of PS-positive MPs increases steadily in packed RBCs over time in storage18,64, in particular from the younger cells65 and may modify PLTs-leukocytes, PLTs-endothelial cell and RBCs-leukocytes interactions in transfused patients62. Interestingly, the ability of stored RBCs supernatant to prime neutrophils is present on MPs66. It has been recently reported that MPs collected from older units of stored RBCs contribute to neutrophil priming and/or activation in a murine model of blood banking and haemorrhagic shock67. R-MPs express the Duffy antigen receptor for chemokines, which has been shown to shuttle chemokines to active receptors68. Other studies have also showed that R-MPs interact with platelets to increase inflammatory chemokine bioavailability in vitro64. It is therefore plausible to suggest that RBC-derived MPs can bind to platelets in tissue beds under stress and release chemokines, which then prime neutrophils and exacerbate the inflammatory response. Tung and colleagues have proposed P-MPs and R-MPs of prolonged storage PLTs or RBCs supernatants respectively, as potential mediators of TRALI in a sheep model69. Regarding R-MPs, their high component in complement and IgGs18,21 might also activate neutrophils via neutrophil Fc receptors, which is consistent with MPs proinflammatory potential. In light of studies showing that the majority of CD40L in blood is MPs-bound, it is very important the finding that the soluble CD40L which accumulates during PLTs and RBCs storage has the capacity to activate adherent polymorphonuclear leukocytes causing endothelial damage and possibly TRALI in predisposed patients70.
Furthermore, R-MPs produced during storage of RBCs express their parent cell’s blood group antigens, including the Rhesus antigens8,44,71 and could therefore potentially be immunogenic if present in large numbers. Interestingly, another study demonstrated the immunosuppressive effect of R-MPs in vitro72. Phagocytosis of R-MPs by macrophages inhibits their activation and decreases interleukin-8 and tumor necrosis factor alpha secretion. Consequently, if R-MPs bear the capacity to down-regulate the innate immune system, producing a long lasting anti-inflammatory signal72, MPs transfused with RBCs units may account for some of the immunosuppressive effects of the transfusions. The property of MPs to down-regulate the activity of macrophages may be relevant for those that travel to the spleen. Whether a related down-regulation on specific immune cells might occur in vivo is speculative; however, considering controversy about the immunosuppressive activity of blood transfusion, investigations in this field are welcomed.
In another pathway, MPs release leads to increased consumption of nitric oxide, an important signalling modulator of blood flow73 and deleterious effects such as susceptibility to PLTs activation, inflammation, poor control of blood flow and ROS generation45. Importantly, the effects of MPs on nitric oxide bioavailability could be more severe than those of cell-free Hb as R-MPs are not cleared by haptoglobin74.
Finally, R-MPs could transfer neutral molecules or removal signals to other cells modifying their phenotype. In transfused patients presenting with paroxysmal nocturnal haemoglobinuria, transfer of glycosylphosphatidylinositol-anchored proteins, like CD55 and CD59, from R-MPs to deficient cells after RBCs transfusion has been observed75. Notably, R-MPs may also transfer PS to the surface of nucleated cells and falsely “mark” them as apoptotic76. So, the role of R-MPs in transfusion medicine is dual: on one hand, they improve the survival of transfused RBCs in recipients by allowing the elimination of toxic molecules and removal signals; on the other hand they might enhance the deleterious events of microcirculation impairment and immunosuppression in some patients45.
Although R-MPs are the predominant species in RBCs concentrates, P-MPs and L-MPs are also generated in stored non-leukoreduced packed RBCs. Neutrophils, monocytes/macrophages, and lymphocytes are all candidates for MPs release. They have a role in maintaining or disrupting vascular homeostasis. When they carry tissue factor or coagulation inhibitors, they participate in haemostasis and pathological thrombosis. Both pro-inflammatory and anti-inflammatory processes can be affected by L-MPs, thus ensuring an appropriate inflammatory response. L-MPs also play a dual role in the endothelium by either improving the endothelial function or inducing an endothelial dysfunction. L-MPs modify the endothelial function and promote the recruitment of inflammatory cells in the vascular wall, necessary processes for the progression of the atherosclerotic lesion6. Leukocyte-derived MPs may play a role in the inflammatory process possibly as part of signalling pathways of cellular crosstalk involving soluble cytokines and mediators of direct cell-cell contact77.
The time course of generation of R-MPs, P-MPs and L-MPs vary considerably in the units of non-leukoreduced stored RBCs. The degree of neutrophil activation caused by the RBCs supernatant has been found correlated to the P-MPs levels, while the thrombin generation to the P-MPs and R-MPs levels, suggesting that multiple subpopulations of MPs in the non-leukoreduced units of packed RBCs may variably contribute to the pro-inflammatory and pro-coagulant potential of labile blood product as a function of storage time62.
As mentioned above, the non-leukoreduced units of packed RBCs contain more MPs compared to the leukoreduced ones49. Activation of PLTs during the storage leads to PLTs-leukocytes interaction, leukocytes apoptosis and MPs-associated increase in the pro-coagulant activity of the supernatant78. Leukoreduction however, does not eliminate MPs present in plasma; neither totally suppresses the membrane vesiculation of stored RBCs. As a result, leukoreduction can only mitigate the putative MPs-associated adverse effects of transfusions.
MPs in stored PLTs concentrates
Platelets transfusions are life-saving medical procedures for patients undergoing major surgery, massive bleeding, for patients having not fully functional PLTs and for those suffering from diseases such as cancer or thrombocytopenia. PLTs concentrates still present one of the major challenges to the blood bank, owing to the scarce availability deriving from the short shelf life of PLTs concentrates under standard blood bank conditions. The usual process to collect PLTs is platelet-aphaeresis but it is possible to produce PLTs concentrates by differential centrifugation of whole blood at room temperature. PLTs concentrates have a short period of life (5 days or 7 days after virus inactivation) and are quite susceptible to environmental changes. They are stored between 20 °C to 24 °C with constant agitation. There are two main reasons that PLTs shelf life is limited to a small number of days. The first one is the risk for bacterial contamination, a risk that can be mitigated by bacterial testing of the product or by treatment of PLTs with pathogen inactivation processes79. Although the quality of PLTs concentrates is -to some extent-compromised by the currently applied pathogen reduction technologies80, there is hope that the storage limitation imposed by bacterial risk will one day being gone. The second reason is that over the storage period, PLTs begin to show evidence of a loss of quality that raises concerns about the efficacy of a transfusion that would be performed with such a product.
Collectively, the loss of PLTs quality over storage is known as the platelet storage lesion, or the platelet storage deficit81,82. PLTs storage lesions include activation, proteolysis and changes in morphology, membrane glycoproteins and surface receptor expression83. The normal platelet discoid shape is found to be lost after 5 to 7 days of storage at 22 °C. At this storage time, mainly spherical or fragmented PLTs remain. During PLTs storage, granule release and PLTs activation occurs, as indicated by the accumulation of β-thromboglobulin and platelet factor 4 in the storage medium, and the increase in surface levels of P-selectin, respectively. There is a significantly storage-dependant decrease of platelet aggregation response to a number of agonists used alone, such as adenosine diphosphate, epinephrine, collagen and arachidonic acid84. The reduction of glycoproteins, particular GPIb (the subunit of the GPIb-IX-V complex responsible for the von Willebrand factor interaction) on the platelet surface during storage, is most likely due to proteolysis85. Recent studies86 suggested that protein kinases might be involved in the development of platelet storage lesion and provide a potential target for inhibition in order to reduce it. Furthermore, PLTs proteomic studies have documented several protein changes occurring over storage, that are suggestive of a renewal process regarding proteins involved in cytoskeleton integrity or signalling pathways87,88.
PLTs undergo processes resembling apoptosis, like PS exposure, pro-caspase 3 processing and MPs release89. P-MPs release is induced in vitro by various stimuli, including epinephrine, adenosine diphosphate, thrombin, collagen and calcium42. It has been suggested that there may be more than one mechanism for P-MPs formation90,91. Parameters governing P-MPs release are among others shear stress and biological factors like cytoskeletal reorganization, plasma membrane blebbing and PS exposure. However, PS exposure is not always followed by MPs release, since intracellular calcium threshold required for MPs formation to occur is higher than for PS exposure. Activation of μ-calpain, that is involved in cytoskeleton reorganization and regulation of phosphatases activities, very likely plays a crucial role in P-MPs generation42. There is also a hypothesis that MPs release after pro-apoptotic stimulation is the cell’s early defense mechanism preceding the point of no return in apoptosis. Caspases and calpain might participate in membrane vesiculation through the ceramide pathway that is a marker of cellular stress. Furthermore, ceramide promotes and stabilizes membrane microdomains such as lipid rafts, which commit specific interactions with the cytoskeleton, regulating thus membrane curvature. It has been shown that cytochalasin D, an actin depolymerizer, inhibits MPs release from activated PLTs92. Whole membrane remodeling and lateral organization, changes in calcium homeostasis and disturbances in membrane ion fluxes93, are other factors. From clinical aspect, it is very important to establish or exclude a correlation between in vitro PLTs activation and MPs accumulation with potential adverse effects in vitro and in vivo, in particular with the increase in anti-coagulant and pro-coagulant activity of stored PLTs89.
PLTs-derived MPs are the most abundant MPs in the human circulation. In common to the subtypes of MPs, they expose PS and tissue factor, they promote coagulation and are considered as PLTs activation markers. MPs derived from activated PLTs contain membrane surface proteins (including GPIIIa, GPIIb and P-selectin) as well as enzymes and chemokines (including CXCL4, −7 and −5)22. Elevated numbers of P-MPs have been reported in several diseases associated with arterial thrombosis, like cardiovascular and rheumatic diseases94 and diabetes type II. Their high thrombogenic potential renders P-MPs critical for the vascular diseases. The clinical relevance of P-MPs in supporting coagulation was documented in leukemic and thrombocytopenic patients. Despite having low PLTs counts, these patients do not bleed probably because of the high levels of circulating P-MPs. Apart from their role in coagulation P-MPs are also involved in the transfer of bioactive molecules as well as in cell activation, inflammatory processes and immunomodulation.
Preparations of PLTs that are stored under blood bank conditions and used for transfusion purposes, appear to be enriched in MPs with high coagulant activity90. In fact, during the standard storage of PLTs concentrates there is an obvious increase in P-MPs. The release of MPs is further augmented under storage of PLTs at 4°C95. Storage-dependent MPs generation is probably the result of PLTs activation that is amplified by interaction of PLTs with the bag wall31. In contrast to MPs studies in aphaeresis PLTs concentrates96 or concentrates prepared from whole blood31, in some studies, levels of PS-positive MPs and P-MPs were not found increased during storage for 1–5 days. However, in that case there was a small increase in the fraction of MPs derived from degranulated PLTs97. In similarity to the R-MPs collected after prolonged storage of RBCs19 P-MPs collected from PLTs concentrates supernatant after 5–7 days of storage contain functionally active caspase 3 and can induce apoptosis in human macrophages98. Furthermore, P-MPs can bind and activate neutrophils in vitro via P-selectin-PSGL-1 interaction99. As mentioned above, soluble CD40L released from stored PLTs concentrates has been proposed as a potential mediator for TRALI70. The contribution of shear stress to MPs release was documented in a study reporting that storage of PLTs concentrates on a 6-rpm elliptical rotator resulted in a significantly higher MP release as compared to 2-rpm circular tumbler rotator83. Interestingly, there are reports showing that pre-storage leukoreduction induces significantly higher release of P-MPs after storage as compared with unfiltered PLTs100. Apheresis PLTs concentrates contain MPs from resting PLTs, activated PLTs and endothelial cells that are enriched compared to the blood of donors97. MPs of endothelial origin in PLTs concentrates can arise either from circulating endothelial cells or circulating MPs in the donor. The half time of P-MPs in patients plasma is about 5–6 hours after transfusion of aphaeresis PLTs concentrates101.
The clinical relevance of P-MPs in transfusion products is a subject for further investigation in different (thrombocytopenic) patient groups receiving PLTs concentrates. Dependent on the cause of thrombocytopenia, P-MPs might be helpful in restoring haemostasis and prevention of overt bleeding in patients after surgery or liver biopsy (in case of severe liver cirrhosis). Unfortunately, there is no single laboratory test that can accurately predict the behaviour of PLTs when transfused. However, contemporary scientific approaches to the platelets storage lesion may identify new targets for the development of quality control assays. Several laboratories have recently begun to apply proteomic approaches to the study of platelet storage102.
An illustration of the influences of MPs on coagulation in vivo103,104 is provided by Scott syndrome. Scott syndrome is a rare inherited haemorrhagic disorder linked to the lack of scramblase activity. Scramblase is an enzyme responsible for the transportation of phospholipids randomly across the cell membrane. In the case of deficient scramblase, when PLTs are activated, phospholipid surfaces are not translocated to the outer leaflet and there is neither PS exposure nor MPs release. Thus, there is no catalytic surface for interacting coagulation factors usually provided by PLTs and P-MPs. As a consequence, coagulation is extremely affected. Even if it is not possible to draw clear conclusions about the role of MPs in adverse effects of transfusion, a lot of additional data is being compiled that is expected to elucidate important aspects of transfusion medicine.
MPs and the haemostatic potential of plasma
It is less difficult to store plasma, because it is free of blood cells. Two common processes are used to obtain plasma for transfusion: centrifugation of whole blood (and freezing within 8 hours for fresh frozen plasma [FFP] or 24 hours for frozen plasma [FP] after collection) and plasmaphaeresis. FFP role and administration is still a debate subject in transfusion medicine. A major indication for FFP transfusion is to improve haemostasis in patients with acquired multiple coagulation factor deficiencies105. Furthermore, severe bleeding after injury requires transfusion of blood products, including FFP. Hemorrhage remains a major cause of potentially preventable death and transfused trauma patients are frequently coagulopathic. It is likely that the ability of FFP to generate thrombin and form a clot is the key to its ability to improve haemostasis in vivo. Most clinical uses of FFP, currently recommended by practice guidelines, are not supported by evidence of effectiveness from randomized clinical trials, particularly for the prophylactic use of FFP106. Since in many settings the clinical effectiveness of FFP has not been demonstrated, it is difficult to predict the in vitro characteristics of FFP that might influence its therapeutic efficacy.
Pro-coagulant MPs are not only pathogenic markers of thrombotic disorders and vascular damage but also efficient effectors in the haemostatic response107. The haemostatic potential of FFP, is probably the average result of several parameters including coagulation factor activity, presence or absence of inhibitors and the pro-coagulant activity of MPs. Quantitative or qualitative declines of these plasma components may result in reduced plasma haemostatic potential. Coagulation factors, calcium ions and pro-coagulant negatively charged phospholipid surfaces are the primary components involved in the assembly of coagulation complexes, such as tenase or prothrombinase, and coagulation activation. MPs of FFP are considered essential for clot formation and haemostasis as they expose PS that provide binding sites for activated clotting factors facilitating thus complex association108. Flow cytometry showed that the most numerous MPs in plasma prepared after an overnight hold of whole blood at 4 °C were derived from RBCs and most of them bind annexin V. It has been found that FFP MPs have minimal effect on the prothrombin time and fibrinogen, von Willebrand factor antigen, factor VIII and anti-thrombin III levels, but they exhibit a major effect on endogenous thrombin potential108, possibly contributing to the therapeutic benefit of FFP infusion. In other studies, P-MPs levels have been found higher than those of R-MPs in FFP109.
The number and cellular origin of MPs in FFP is different compared to that observed in peripheral blood108. R-MPs or P-MPs accumulation in FFP depends on whether leukodepletion was performed at whole blood or following separation to plasma110. In the leukodepleted preparations of FFP, filtration decreases the number of P-MPs but increases the number of R-MPs in the resultant product110. Longer blood storage before plasma separation and freezing contributes to P-MPs generation and thus to functional haemostasis109,111. Plasma units prepared after an overnight hold of whole blood at 4 °C prior to processing contained significantly increased levels of MPs in comparison with plasma produced within 8 hours of blood donation111. This was coincidental to stronger and more rapid clot formation as assessed by thrombelastography. Furthermore, this finding is in line with reports of increased P-MPs in PLTs concentrates during storage. On the other hand, in both preparations P-MPs count of the thawed plasma has been found significantly decreased after 5 days of storage at 1–6 °C, in association with an analogous decline in haemostatic potential and clot forming ability109, contributing thus to lower haemostatic potential of thawed product.
Cryoprecipitate is prepared by controlled thawing of frozen plasma to precipitate high molecular weight proteins, which include factor VIII, von Willebrand factor and fibrinogen. The precipitated proteins are separated by centrifugation, re-suspended in a small volume of plasma and stored frozen at −20 °C. Cryoprecipitate is not available in most western European countries but it is still used in the US and UK. FFP and cryoprecipitate contain a significant number of PLTs. When PLTs concentrates are prepared, the resulting supernatant plasma contains more PLTs than if the plasma was harvested directly from whole blood. The concentration of P-MPs in FFP after thawing is proportional to the number of PLTs originally present. It has been shown that P-MPs are highly concentrated by cryoprecipitation. High levels of P-MPs in cryoprecipitate are considered to contribute to its therapeutic effects in bleeding patients112.
The potential clinical relevance of MPs in stored plasma used for massively transfused patients remains to be evaluated. The clinical impact of the MPs within FFP is unknown as few studies have been performed in order to establish their in vivo biological activity. In vivo, the ability of MPs to support and localize haemostasis to the site of injury is not fully known, although in a FVIII-deficient mouse model it has been shown that infusion of leukocyte derived MPs generated in vitro temporarily reversed abnormal haemostasis113. To definitively establish the relative merits of infusing MPs of different cellular origins into patients would require prospective clinical studies. However, both the level and cellular origin of the MPs could give an indication as to any potential biological activity when transfused42.
Conclusions
The important issue raised by the presence of MPs in blood labile products is to determine the factors contributing to their formation as well as their exact involvement in recipient’s reactions to transfusion. To answer them, intensive research on MPs biology, standardization of analytical and pre-analytical approaches as well as large clinical studies focusing on the precise effects (if any) of MPs in blood recipients, are needed. Microparticles are potential candidates for intracellular communication and are important mediators of inflammation and regulation of immune responses. The mechanistic basis of MPs biological effects, the knowledge of how MPs are involved in the coagulation cascade, in the inflammation and in the immunomodulation is critical in order to decipher the complexity of the hypercoagulable states and the effect of transfusion in routine clinical practice. The in vivo correlates and the clinical risks vs. benefits associated with transfusion of aged blood components need to be evaluated. At this point, no data exist as to whether these in vitro findings change clinical outcomes, for better or worse. This is an active area of investigation, with competing studies suggesting a risk and others suggesting that storage duration risk is confounded by the number of units transfused and the associated severity of illness. Although the attention of blood transfusion services was drawn to other important issues such as emerging infectious risks, reducing the costs and transfusion reactions documented in haemovigilance systems, there is nothing more important to our patients than making sure that the transfusions we provide are stored by ways that do not impair their principal functions. Improvement of knowledge on MPs such as their formation, levels in blood and blood labile products as well as MPs proteome under various conditions may help to get the very best from transfusions.
Footnotes
Contributions
Anastasios Kriebardis and Marianna Antonelou contributed equally to this review article.
The Authors declare no conflicts of interest.
References
- 1.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Maio A. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2011;16(3):235–49. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl P, Fricke A, Sander L, et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–44. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin M, Drwal G, Bourgeois T, et al. Distinct proteome features of plasma microparticles. Proteomics. 2005;5:1940–52. doi: 10.1002/pmic.200401057. [DOI] [PubMed] [Google Scholar]
- 5.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 6.Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res. 2012;110:356–69. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 7.Baron M, Boulanger CM, Staels B, Tailleux A. Cell-derived microparticles in atherosclerosis: biomarkers and targets for pharmacological modulation? J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin O, Crettaz D, Canellini G, et al. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang. 2008;95:288–97. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 9.Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–34. [PubMed] [Google Scholar]
- 10.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 11.Vandromme MJ, McGwin G, Jr, Marques MB, et al. Transfusion and pneumonia in the trauma intensive care unit: an examination of the temporal relationship. J Trauma. 2009;67:97–101. doi: 10.1097/TA.0b013e3181a5a8f9. [DOI] [PubMed] [Google Scholar]
- 12.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 13.Vamvakas EC. Possible mechanisms of allogeneic blood transfusion-associated postoperative infection. Transfus Med Rev. 2002;16:144–60. doi: 10.1053/tmrv.2002.31463. [DOI] [PubMed] [Google Scholar]
- 14.Vamvakas EC. White-blood-cell-containing allogeneic blood transfusion, postoperative infection and mortality: a meta-analysis of observational ‘before-and-after’ studies. Vox Sang. 2004;86:111–9. doi: 10.1111/j.0042-9007.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- 15.Jensen LS, Puho E, Pedersen L, et al. Long-term survival after colorectal surgery associated with buffy-coat-poor and leucocyte-depleted blood transfusion: a follow-up study. Lancet. 2005;365:681–2. doi: 10.1016/S0140-6736(05)17949-5. [DOI] [PubMed] [Google Scholar]
- 16.Rubin O, Crettaz D, Tissot JD, Lion N. Microparticles in stored red blood cells: submicron clotting bombs? Blood Transfus. 2010;8(Suppl 3):s31–8. doi: 10.2450/2010.006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jy W, Horstman LL, Jimenez JJ, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–51. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 18.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–53. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 19.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 20.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Apolipoprotein J/clusterin in human erythrocytes is involved in the molecular process of defected material disposal during vesiculation. PLoS One. 2011;6:e26033. doi: 10.1371/journal.pone.0026033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 22.Garcia BA, Smalley DM, Cho H, et al. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–21. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- 23.Joop K, Berckmans RJ, Nieuwland R, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001;85:810–20. [PubMed] [Google Scholar]
- 24.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 25.Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–53. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 26.Ueba T, Haze T, Sugiyama M, et al. Level, distribution and correlates of platelet-derived microparticles in healthy individuals with special reference to the metabolic syndrome. Thromb Haemost. 2008;100:280–5. [PubMed] [Google Scholar]
- 27.Ay C, Freyssinet JM, Sailer T, et al. Circulating procoagulant microparticles in patients with venous thromboembolism. Thromb Res. 2009;123:724–6. doi: 10.1016/j.thromres.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Rubin O, Crettaz D, Tissot JD, Lion N. Pre-analytical and methodological challenges in red blood cell microparticle proteomics. Talanta. 2010;82:1–8. doi: 10.1016/j.talanta.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Ayers L, Kohler M, Harrison P, et al. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011;127:370–7. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88:3456–64. [PubMed] [Google Scholar]
- 31.Bode AP, Orton SM, Frye MJ, Udis BJ. Vesiculation of platelets during in vitro aging. Blood. 1991;77:887–95. [PubMed] [Google Scholar]
- 32.Lion N, Crettaz D, Rubin O, Tissot JD. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73:374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Antonelou MH, Kriebardis AG, Papassideri IS. Aging and death signalling in mature red cells: from basic science to transfusion practice. Blood Transfus. 2010;8(Suppl 3):s39–47. doi: 10.2450/2010.007S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012 doi: 10.1016/j.prot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Storage-dependent remodeling of the red blood cell membrane is associated with increased immunoglobulin G binding, lipid raft rearrangement, and caspase activation. Transfusion. 2007;47:1212–20. doi: 10.1111/j.1537-2995.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 37.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. Progressive oxidation of cytoskeletal proteins and accumulation of denatured hemoglobin in stored red cells. J Cell Mol Med. 2007;11:148–55. doi: 10.1111/j.1582-4934.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 39.Card RT, Mohandas N, Mollison PL. Relationship of post-transfusion viability to deformability of stored red cells. Br J Haematol. 1983;53:237–40. doi: 10.1111/j.1365-2141.1983.tb02016.x. [DOI] [PubMed] [Google Scholar]
- 40.Gov N, Cluitmans J, Sens P, Bosman GJCGM. Cytoskeletal Control of Red Blood Cell Shape: Theory and Practice of Vesicle Formation. In: Leitmannova Liu A, Iglic A, editors. Advances in Planar Lipid Bilayers and Liposomes. Vol. 10. Oxford: Elsevier; 2009. pp. 95–119. [Google Scholar]
- 41.Ferru E, Giger K, Pantaleo A, et al. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117:5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–51. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 44.Greenwalt TJ. The how and why of exocytic vesicles. Transfusion. 2006;46:143–52. doi: 10.1111/j.1537-2995.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 45.Tissot JD, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol. 2010;17:571–7. doi: 10.1097/moh.0b013e32833ec217. [DOI] [PubMed] [Google Scholar]
- 46.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Salzer U, Zhu R, Luten M, et al. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48:451–62. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 48.Rumsby MG, Trotter J, Allan D, Michell RH. Recovery of membrane micro-vesicles from human erythrocytes stored for transfusion: a mechanism for the erythrocyte discocyte-to-spherocyte shape transformation. Biochem Soc Trans. 1977;5:126–8. doi: 10.1042/bst0050126. [DOI] [PubMed] [Google Scholar]
- 49.Greenwalt TJ, Zehner Sostok C, Dumaswala UJ. Studies in red blood cell preservation. 1. Effect of the other formed elements. Vox Sang. 1990;58:85–9. doi: 10.1111/j.1423-0410.1990.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 50.Gov NS, Safran SA. Red blood cell membrane fluctuations and shape controlled by ATP-induced cytoskeletal defects. Biophys J. 2005;88:1859–74. doi: 10.1529/biophysj.104.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sens P, Gov N. Force balance and membrane shedding at the red-blood-cell surface. Phys Rev Lett. 2007;98:018102. doi: 10.1103/PhysRevLett.98.018102. [DOI] [PubMed] [Google Scholar]
- 52.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfus Med Rev. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- 53.Pantaleo A, Ferru E, Carta F, et al. Irreversible AE1 tyrosine phosphorylation leads to membrane vesiculation in G6PD deficient red cells. PLoS One. 2011;6:e15847. doi: 10.1371/journal.pone.0015847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfe LC, Byrne AM, Lux SE. Molecular defect in the membrane skeleton of blood bank-stored red cells. Abnormal spectrin-protein 4.1-actin complex formation. J Clin Invest. 1986;78:1681–6. doi: 10.1172/JCI112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner GM, Chiu DT, Qju JH, et al. Spectrin oxidation correlates with membrane vesiculation in stored RBCs. Blood. 1987;69:1777–81. [PubMed] [Google Scholar]
- 56.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–47. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 57.Willekens FL, Werre JM, Groenen-Dopp YA, et al. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–56. doi: 10.1111/j.1365-2141.2008.07055.x. [DOI] [PubMed] [Google Scholar]
- 58.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 59.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 60.Bosman GJ, Lasonder E, Groenen-Dopp YA, et al. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Dumaswala UJ, Dumaswala RU, Levin DS, Greenwalt TJ. Improved red blood cell preservation correlates with decreased loss of bands 3, 4.1, acetylcholinestrase, and lipids in microvesicles. Blood. 1996;87:1612–6. [PubMed] [Google Scholar]
- 62.Jy W, Ricci M, Shariatmadar S, et al. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion. 2011;51:886–93. doi: 10.1111/j.1537-2995.2011.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Beers EJ, Schaap MC, Berckmans RJ, et al. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–9. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong Z, Cavaretta J, Qu L, et al. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610–21. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greenwalt TJ, Dumaswala UJ. Effect of red cell age on vesiculation in vitro. Br J Haematol. 1988;68:465–7. doi: 10.1111/j.1365-2141.1988.tb04237.x. [DOI] [PubMed] [Google Scholar]
- 66.Cardo LJ, Wilder D, Salata J. Neutrophil priming, caused by cell membranes and microvesicles in packed red blood cell units, is abrogated by leukocyte depletion at collection. Transfus Apher Sci. 2008;38:117–25. doi: 10.1016/j.transci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Belizaire RM, Prakash PS, Richter JR, et al. Microparticles from Stored Red Blood Cells Activate Neutrophils and Cause Lung Injury after Hemorrhage and Resuscitation. J Am Coll Surg. 2012 doi: 10.1016/j.jamcollsurg.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schnabel RB, Baumert J, Barbalic M, et al. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood. 2010;115:5289–99. doi: 10.1182/blood-2009-05-221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tung JP, Fung YL, Nataatmadja M, et al. A novel in vivo ovine model of transfusion-related acute lung injury (TRALI) Vox Sang. 2011;100:219–30. doi: 10.1111/j.1423-0410.2010.01381.x. [DOI] [PubMed] [Google Scholar]
- 70.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oreskovic RT, Dumaswala UJ, Greenwalt TJ. Expression of blood group antigens on red cell microvesicles. Transfusion. 1992;32:848–9. doi: 10.1046/j.1537-2995.1992.32993110758.x. [DOI] [PubMed] [Google Scholar]
- 72.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84:1316–25. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 73.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sloand EM, Mainwaring L, Keyvanfar K, et al. Transfer of glycosylphosphatidylinositol-anchored proteins to deficient cells after erythrocyte transfusion in paroxysmal nocturnal hemoglobinuria. Blood. 2004;104:3782–8. doi: 10.1182/blood-2004-02-0645. [DOI] [PubMed] [Google Scholar]
- 76.Liu R, Klich I, Ratajczak J, et al. Erythrocyte-derived microvesicles may transfer phosphatidylserine to the surface of nucleated cells and falsely ‘mark’ them as apoptotic. Eur J Haematol. 2009;83:220–9. doi: 10.1111/j.1600-0609.2009.01271.x. [DOI] [PubMed] [Google Scholar]
- 77.Distler JH, Huber LC, Gay S, et al. Microparticles as mediators of cellular cross-talk in inflammatory disease. Autoimmunity. 2006;39:683–90. doi: 10.1080/08916930601061538. [DOI] [PubMed] [Google Scholar]
- 78.Keating FK, Butenas S, Fung MK, Schneider DJ. Platelet-white blood cell (WBC) interaction, WBC apoptosis, and procoagulant activity in stored red blood cells. Transfusion. 2011;51:1086–95. doi: 10.1111/j.1537-2995.2010.02950.x. [DOI] [PubMed] [Google Scholar]
- 79.Blajchman MA, Beckers EA, Dickmeiss E, et al. Bacterial detection of platelets: current problems and possible resolutions. Transfus Med Rev. 2005;19:259–72. doi: 10.1016/j.tmrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Schubert P, Culibrk B, Coupland D, et al. Riboflavin and ultraviolet light treatment potentiates vasodilator-stimulated phosphoprotein Ser-239 phosphorylation in platelet concentrates during storage. Transfusion. 2012;52:397–408. doi: 10.1111/j.1537-2995.2011.03287.x. [DOI] [PubMed] [Google Scholar]
- 81.Murphy S, Gardner FH. Platelet storage at 22 degrees C; metabolic, morphologic, and functional studies. J Clin Invest. 1971;50:370–7. doi: 10.1172/JCI106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;41:105–13. doi: 10.1016/j.transci.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 83.George JN, Pickett EB, Heinz R. Platelet membrane glycoprotein changes during the preparation and storage of platelet concentrates. Transfusion. 1988;28:123–6. doi: 10.1046/j.1537-2995.1988.28288179014.x. [DOI] [PubMed] [Google Scholar]
- 84.DiMinno G, Silver MJ, Murphy S. Stored human platelets retain full aggregation potential in response to pairs of aggregating agents. Blood. 1982;59:563–8. [PubMed] [Google Scholar]
- 85.Ribeiro A, Swann JC, Berndt MC. Alterations of the levels of glycoproteins Ib-IX and IIb-IIIa in platelets stored at 22 degrees C. Thromb Res. 1992;66:619–27. doi: 10.1016/0049-3848(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 86.Canault M, Duerschmied D, Brill A, et al. p38 mitogen-activated protein kinase activation during platelet storage: consequences for platelet recovery and hemostatic function in vivo. Blood. 2010;115:1835–42. doi: 10.1182/blood-2009-03-211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thiele T, Steil L, Gebhard S, et al. Profiling of alterations in platelet proteins during storage of platelet concentrates. Transfusion. 2007;47:1221–33. doi: 10.1111/j.1537-2995.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- 88.Thon JN, Schubert P, Devine DV. Platelet storage lesion: a new understanding from a proteomic perspective. Transfus Med Rev. 2008;22:268–79. doi: 10.1016/j.tmrv.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Seghatchian J, Krailadsiri P. Current position on preparation and quality of leucodepleted platelet concentrates for clinical use. Transfus Sci. 2000;22:85–8. doi: 10.1016/s0955-3886(00)00025-4. [DOI] [PubMed] [Google Scholar]
- 90.Curvers J, van Pampus EC, Feijge MA, et al. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion. 2004;44:49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- 91.Fox JE, Austin CD, Reynolds CC, Steffen PK. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J Biol Chem. 1991;266:13289–95. [PubMed] [Google Scholar]
- 92.Yano Y, Kambayashi J, Shiba E, et al. The role of protein phosphorylation and cytoskeletal reorganization in microparticle formation from the platelet plasma membrane. Biochem J. 1994;299:303–8. doi: 10.1042/bj2990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morel O, Morel N, Jesel L, et al. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin Immunopathol. 2011;33:469–86. doi: 10.1007/s00281-010-0239-3. [DOI] [PubMed] [Google Scholar]
- 94.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–9. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 95.Bode AP, Knupp CL. Effect of cold storage on platelet glycoprotein Ib and vesiculation. Transfusion. 1994;34:690–6. doi: 10.1046/j.1537-2995.1994.34894353465.x. [DOI] [PubMed] [Google Scholar]
- 96.Krailadsiri P, Seghatchian J. Are all leucodepleted platelet concentrates equivalent? Comparison of Cobe LRS Turbo, Haemonetics MCS+ LD, and filtered pooled buffy-coat-derived platelets. Vox Sang. 2000;78:171–5. doi: 10.1159/000031176. [DOI] [PubMed] [Google Scholar]
- 97.Rank A, Nieuwland R, Liebhardt S, et al. Apheresis platelet concentrates contain platelet-derived and endothelial cell-derived microparticles. Vox Sang. 2011;100:179–86. doi: 10.1111/j.1423-0410.2010.01385.x. [DOI] [PubMed] [Google Scholar]
- 98.Boing AN, Hau CM, Sturk A, Nieuwland R. Platelet microparticles contain active caspase 3. Platelets. 2008;19:96–103. doi: 10.1080/09537100701777295. [DOI] [PubMed] [Google Scholar]
- 99.Jy W, Mao WW, Horstman L, et al. Platelet microparticles bind, activate and aggregate neutrophils in vitro. Blood Cells Mol Dis. 1995;21:217–31. doi: 10.1006/bcmd.1995.0025. discussion 231a. [DOI] [PubMed] [Google Scholar]
- 100.Devine DV, Bradley AJ, Maurer E, et al. Effects of prestorage white cell reduction on platelet aggregate formation and the activation state of platelets and plasma enzyme systems. Transfusion. 1999;39:724–34. doi: 10.1046/j.1537-2995.1999.39070724.x. [DOI] [PubMed] [Google Scholar]
- 101.Rank A, Nieuwland R, Crispin A, et al. Clearance of platelet microparticles in vivo. Platelets. 2011;22:111–6. doi: 10.3109/09537104.2010.520373. [DOI] [PubMed] [Google Scholar]
- 102.Schubert P, Devine DV. Proteomics meets blood banking: identification of protein targets for the improvement of platelet quality. J Proteomics. 2010;73:436–44. doi: 10.1016/j.jprot.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–71. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Zwaal RF, Comfurius P, Bevers EM. Scott syndrome, a bleeding disorder caused by defective scrambling of membrane phospholipids. Biochim Biophys Acta. 2004;1636:119–28. doi: 10.1016/j.bbalip.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 105.O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 106.Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomized controlled trials. Br J Haematol. 2004;126:139–52. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 107.Morel O, Morel N, Freyssinet JM, Toti F. Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets. 2008;19:9–23. doi: 10.1080/09537100701817232. [DOI] [PubMed] [Google Scholar]
- 108.Lawrie AS, Harrison P, Cardigan RA, Mackie IJ. The characterization and impact of microparticles on haemostasis within fresh-frozen plasma. Vox Sang. 2008;95:197–204. doi: 10.1111/j.1423-0410.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 109.Matijevic N, Kostousov V, Wang YW, et al. Multiple levels of degradation diminish hemostatic potential of thawed plasma. J Trauma. 2011;70:71–9. doi: 10.1097/TA.0b013e318207abec. discussion 79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krailadsiri P, Seghatchian J, Macgregor I, et al. The effects of leukodepletion on the generation and removal of microvesicles and prion protein in blood components. Transfusion. 2006;46:407–17. doi: 10.1111/j.1537-2995.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 111.Lawrie AS, Cardigan RA, Williamson LM, et al. The dynamics of clot formation in fresh-frozen plasma. Vox Sang. 2008;94:306–14. doi: 10.1111/j.1423-0410.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 112.George JN, Pickett EB, Heinz R. Platelet membrane microparticles in blood bank fresh frozen plasma and cryoprecipitate. Blood. 1986;68:307–9. [PubMed] [Google Scholar]
- 113.Akram A, Teitel JM, Freedman JJ, Gross PL. Hemosta sis in factor VIII deficient mice is improved after the infusion of monocyte-derived microparticles [abstract] J Thromb Haemost. 2007;5(Suppl 2):P-M-118. [Google Scholar]