Abstract
Objectives. This study aims to examine the utility of von Willebrand factor (vWF) as a biomarker in lcSSc, in particular the ability of vWF to predict the future development of disease manifestations in this disease.
Methods. vWFAg concentrations were measured in the serum of patients with lcSSc at baseline and at 3 years, during the QUINs trial [Prevention of Vascular Damage in Scleroderma with Angiotensin-Converting Enzyme (ACE) Inhibition]. %DLCO, %KCO, %FVC, pulmonary artery pressure (PAP) estimation by echocardiography, Raynaud’s attack frequency, Raynaud’s severity, digital ulcer frequency, urinary protein excretion, estimated glomerular filtration rate (eGFR), modified Rodnan skin score and Medsger disease activity score were also measured at baseline and 3 years.
Results. Baseline serum vWF concentrations were related to concurrent Medsger disease activity score, %DLCO, %FVC, urinary protein excretion, eGFR and PAP >30 mmHg. In logistic regression models, baseline serum vWF concentrations were able to predict the future development of elevated PAP by echocardiography (PAP >40 mmHg, P = 0.001).
Conclusions. Pulmonary artery hypertension is a life-threatening complication of lcSSc. vWF is a marker of endothelial cell activation. Raised serum concentrations of vWF in lcSSc increase the risk of developing subsequent elevation in PAP. Therefore screening patients with lcSSc for vWF may identify a group at risk of developing PAH. These patients could potentially be targeted with agents that stabilize the endothelium, e.g. statins.
Keywords: vWF, von Willebrand factor, systemic sclerosis, pulmonary artery hypertension
Introduction
SSc is a connective tissue disease characterized by fibrosis, together with activation and dysfunction of the immune system and endothelium. Endothelial injury is thought to be central to the pathogenesis of this condition [1]. Von Willebrand factor (vWF), a circulating glycoprotein synthesized by endothelial cells and megakaryocytes, acts as a carrier protein for the coagulation factor, factor VIII. It promotes adhesion of platelets to the subendothelium following vascular injury and has been validated as a marker of endothelial injury [2]. In previous small-scale studies, plasma vWF has been reported to be increased in SSc and potentially correlates with disease severity [3, 4]. No studies have previously examined the utility of vWF as a predictor of future disease manifestations.
Since the successful introduction of angiotensin-converting enzyme (ACE) inhibitors for the treatment of SSc renal crisis, pulmonary problems such as fibrosis and PAH are now the most serious complications [5]. Pulmonary artery hypertension (PAH) is more common in lcSSc associated with ACAs and can occur after the patient has suffered from the disease for many years. The morbidity and mortality from this complication is significant [6].
Recently, a number of treatments have become available that can improve the prognosis in established PAH [7]. There is now an imperative to determine, as early as possible, when pulmonary hypertension is developing [8] as an essential prerequisite for clinical trials to determine the impact of early intervention on outcome.
The QUINs randomized placebo-controlled trial [Prevention of Vascular Damage in Scleroderma with Angiotensin-Converting Enzyme (ACE) Inhibition] was set up to investigate the potential utility of quinapril in preventing digital ulcers in a large cohort of patients with lcSSc. We undertook this substudy to investigate the utility of vWF as a biomarker in this condition, measuring the vWF antigen at 0 and 3 years together with a range of clinical parameters at 12 weekly or annual intervals over the 3 years.
Methods
Patients
Two hundred and thirteen patients from 20 different centres across England and Wales with lcSSc [9] were recruited as part of the QUINs study. Approval for the study was gained from the Multicentre Research Ethics Committee (MREC Wales 00/09/19), and all centres gained local ethics committee approval. Patients gave written informed consent according to the Declaration of Helsinki.
vWF: antigen measurements
The vWF antigen (vWF:Ag) was measured at a single central lab (Aintree University Hospital) in sera of patients at baseline; follow-up measurements were taken after at least 2 years and no more than 3 years. vWF:Ag was measured using the STA Liatest Immuno-Turbidimetric assay. The detection threshold for the assay is 2%. The intra-assay reproducibility for the assay is 1.9–2.7% [coefficient of variance (CV)]. The inter-assay reproducibility of the assay is 2.9–4.5% (CV). The assay correlates well with ELISA procedures (r = 0.982).
Clinical measurements
12 weekly measurements
Measurements included Rodnan skin score, digital ulcers, frequency and severity of RP on a visual analogue scale (VAS), a composite measurement of disease extent and severity using the Medsger score [10].
Annual measurements
The estimated glomerular filtration rate (eGFR), 24-h urinary protein excretion, %DLCO, %FVC and %KCO were measured. Pulmonary artery pressure (PAP) was measured by an experienced operator at each site using echocardiography at baseline and at 3 years. PAP was taken to be the tricuspid gradient (mmHg) plus an estimate of right atrial pressure (10 mmHg).
Statistics
Regression models were fitted to predict values recorded at 3 monthly or yearly intervals from the baseline value of the variable, treatment (placebo or quinapril) and the baseline vWF:Ag value. vWF:Ag levels correlated weakly with age (correlation coefficient = 0.168, P = 0.018) so all regression models were adjusted for age at the baseline visit. Robust Huber–White standard errors of the regression coefficients were used to take account of the observed correlations between repeated assessments of the same patient. High estimated PAP was analysed as a binary variable (yes/no) due to the way that the data were recorded during the study, i.e. PAP values were not quantified in 126 out of 323 patients with normal PAP <30 mmHg. A logistic regression model was applied to binarized PAP. No adjustments were made for multiple comparisons.
Results
vWF levels at follow-up visits did not differ significantly (P = 0.42) between the two randomized treatment groups (placebo vs quinapril) after adjusting for baseline vWF and age.
Relationships with vWF at baseline
There is no significant relationship between baseline vWF:Ag and baseline values of concurrent/recent ulcers, Raynaud’s attack frequency VAS score, Raynaud’s severity VAS score or skin score. However, there was a significant relationship between baseline vWF:Ag and the Medsger score (a composite measurement of disease severity) (β = 0.0087, 95% CI 0.0025, 0.0149, P = 0.006). There was a significant relationship between baseline vWF:Ag and baseline urinary protein excretion (β = 0.0005, 95% CI 0.003, 0.0007, P < 0.001) and baseline eGFR (β = 0.034, 95% CI 0.007, 0.060, P = 0.02).
There was a significant relationship between baseline vWF:Ag and baseline %FVC (β = −0.076, 95% CI − 0.119, −0.033, P = 0.001), %DLCO (β = −0.079, 95% CI −0.119, −0.039, P < 0.001) and with baseline PAP of >30 mmHg (odds ratio = 0.998, 95% CI 0.977, 0.9998, P = 0.05) but not with baseline PAP of >40 mmHg (n = 8) (odds ratio = 0.996, 95% CI 0.978, 1.015, P = 0.71).
vWF:Ag as a predictor of future outcomes
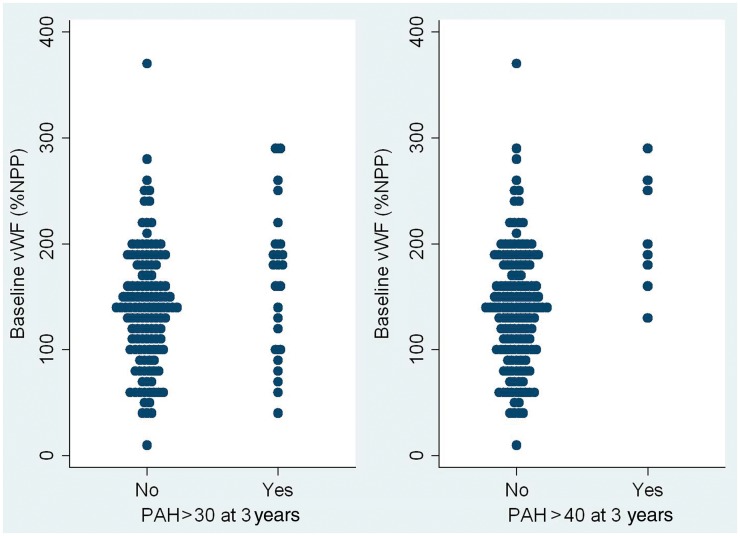
Two alternative definitions for raised PAP were explored: PAP > 30 mmHg and PAP > 40 mmHg. Logistic regression models to predict high PAP values during the follow-up from high baseline PAP, randomized treatment during the trial (quinapril or placebo) and baseline vWF:Ag indicate that baseline vWF:Ag is a predictor of PAP > 40 mmHg (P = 0.001) and approached significance for prediction of PAP > 30 mmHg (P = 0.11) (Fig. 1). In regression models, baseline vWF:Ag was not a significant predictor of outcome for Raynaud’s frequency, Raynaud’s severity, new ulcer formation or skin score or any of the other clinical outcomes recorded during the follow-up (Table 1).
Fig. 1.
Dot plot illustrating the relationship between baseline vWF:Ag and estimated PAP >30 mmHg and 40 mmHg at 3 years (or the end of the study).
Table 1.
Regression coefficients predicting outcome from baseline vWF:Ag, adjusted for baseline values
| Outcome | Baseline vWf:Ag regression coefficienta | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|
| PAP >30 mmHgb | 1.007 | 0.998, 1.017 | 0.11 | |
| PAP >40 mmHgb | 1.022 | 1.009, 1.035 | 0.001 | |
| Number of new or recent digital ulcers | 0.0009 | −0.0003, 0.0021 | 0.15 | |
| Raynaud’s attack frequency VAS | −0.0185 | −0.0651, 0.0282 | 0.44 | |
| Raynaud’s disease severity VAS | −0.0192 | −0.0627, 0.0243 | 0.39 | |
| Modified Rodnan skin score | 0.0025 | −0.0035, 0.0086 | 0.42 | |
| Percentage of expected FVC | −0.0150 | −0.0410, 0.0111 | 0.26 | |
| Percentage of expected DLCO | −0.0099 | −0.0356, 0.0159 | 0.45 | |
| Percentage of expected KCO | −0.0102 | −0.0367, 0.0162 | 0.45 | |
| eGFR | 0.0006 | −0.0200, 0.0211 | 0.96 | |
| Urinary protein, g/24 h | 0.0002 | −0.0002, 0.0007 | 0.30 | |
| Medsger disease severity score | 0.0009 | −0.0043, 0.0060 | 0.75 |
aRegression coefficients indicate the change in outcome per unit increase in vWFconcentration. Each regression coefficient is adjusted for age, randomized treatment (quinapril or placebo) and the baseline value of the outcome variable. Robust Huber–White sandwich estimators of the standard errors of the regression coefficients were used to construct the 95% CI, which take account of the observed correlation between repeated assessments on the same patient. bOdds ratio from a logistic regression model.
Discussion
This study, involving a large, well-characterized cohort of patients, was suitably placed to explore the utility of vWF:Ag as a biomarker in lcSSc. Previous smaller studies have suggested that plasma vWF:Ag may be raised in SSc compared with healthy controls and reflect disease severity [3, 4]. This study seems to confirm the latter, as vWF:Ag levels were related to the Medsger disease score that reflects the overall disease extent and severity. The relationship, however, is weak, and this may reflect the underlying problems in using vWF as a biomarker: levels are highly variable in the normal population and may be altered by factors such as exercise and ABO blood group [11]. A recent study in a small group of patients with SSc has suggested that vWF propeptide may be more useful as a marker of disease activity, but this assay was not available at the time this study commenced [12].
In this study we examined in particular the relationships with disease manifestations presumed to be directly a consequence of endothelial injury and dysfunction: number of ulcers, Raynaud’s severity and attack frequency, urinary protein excretion and raised PAP. It is interesting to note that baseline vWF did correlate with urinary protein excretion and PAP. vWF did not correlate with Raynaud’s severity and frequency, and this is likely to be due to the subjective and imprecise nature of these assessments. Of note, the average vWF:Ag levels decreased (mean change 15.9, 95% CI 5.8, 26.0, P = 0.002) over the 3 years of the study. This may reflect a decrease in endothelial injury over time, in keeping with the observations that inflammation, the presumed stimulus for endothelial injury, is greatest earlier in the disease.
Strikingly, we have identified the utility of baseline vWF:Ag to predict the future development of raised PAP. Previous studies have shown that vWF:Ag levels are elevated in patients with concurrent primary PAH and that levels are responsive to treatment [13, 14]. This suggests that endothelial injury does play a significant role in this condition.
This study is the first to report, importantly, that an increase in vWF is predictive of the future development of elevated PAP in patients with lcSSc. This may allow patients at risk of developing this life-threatening manifestation of the disease to be identified early. Of note, treatment with pravastatin significantly lowered vWF:Ag levels in patients with SSc. This was presumed to reflect a protective effect on the vascular endothelium [15]. It may be that prophylactic treatment of patients with raised vWF:Ag may reduce the risk of developing PAH, though this hypothesis remains to be tested. Statin use was not routinely recorded in our population.
Although echocardiography has its limitations in the assessment of PAP, it has been validated as an appropriate screening tool [16]. Three patients in this study had a right heart catheter to establish a more accurate assessment of PAP. Each had confirmed PAH and all three patients had very high levels of vWF:Ag at baseline: 201, 253 and 259% normal pooled plasma.

Supplementary Material
Acknowledgements
Many thanks to the QUINs Trial Study Group.
Funding: The study was funded by the Arthritis Research Campaign (now Arthritis Research UK) (Arthritis Research UK grant number 13479). Pfizer donated the quinapril for this study.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Fleming JN, Schwartz SM. The pathology of scleroderma vascular disease. Rheum Dis Clin North Am. 2008;34:41–55, vi. doi: 10.1016/j.rdc.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255–65. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 3.Herrick AL, Illingworth K, Blann A, et al. Von Willebrand factor, thrombomodulin, thromboxane, beta-thromboglobulin and markers of fibrinolysis in primary Raynaud's phenomenon and systemic sclerosis. Ann Rheum Dis. 1996;55:122–7. doi: 10.1136/ard.55.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheja A, Eskilsson J, Akesson A, Wollheim FA. Inverse relation between plasma concentration of von Willebrand factor and CrEDTA clearance in systemic sclerosis. J Rheumatol. 1994;21:639–42. [PubMed] [Google Scholar]
- 5.Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54. doi: 10.1016/s0889-857x(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 6.Hachulla I, Carpentier P, Gressin V, et al. Risk factors for death and the 3-year survival of patients with systemic sclerosis: the French ItinerAIR-Sclerodermie study. Rheumatology. 2009;48:304–8. doi: 10.1093/rheumatology/ken488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin VV. Survival in patients with pulmonary artery hypertension. Eur J Clin Invest. 2006;36(Suppl 3):10–5. doi: 10.1111/j.1365-2362.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 8.Vachiery JL, Coghlan G. Screening for pulmonary arterial hypertension in systemic sclerosis. Eur Respir Rev. 2009;18:162–9. doi: 10.1183/09059180.00003209. [DOI] [PubMed] [Google Scholar]
- 9.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- 10.Medsger TA, Silman A, Steen VD, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–67. [PubMed] [Google Scholar]
- 11.Jenkins PV, ODonnell J. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46:1836–44. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 12.Scheja A, Akesson A, Geborek P, et al. Von Willebrand factor propeptide as a marker of disease activity in systemic sclerosis (scleroderma) Arthritis Res. 2001;3:178–82. doi: 10.1186/ar295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geggel RL, Carvalho AC, Hoyer LW, Reid LM. von Willebrand factor abnormalities in primary pulmonary hypertension. Am Rev Respir Dis. 1987;135:294–9. doi: 10.1164/arrd.1987.135.2.294. [DOI] [PubMed] [Google Scholar]
- 14.Rossi R, Nuzzo A, Lattanzi A, Coppi F, Modena MG. Sildenafil improves endothelial function in patients with pulmonary hypertension. Pulm Pharmacol Ther. 2008;21:172–7. doi: 10.1016/j.pupt.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa S, Yasuda S, Amengual O, et al. Protective effect of pravastatin on vascular endothelium in patients with systemic sclerosis: a pilot study. Ann Rheum Dis. 2006;65:1118–20. doi: 10.1136/ard.2005.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sweiss NJ, Hushaw L, Thenappan T, et al. Diagnosis and management of pulmonary hypertension in systemic sclerosis. Curr Rheumatol Rep. 2010;12:8–18. doi: 10.1007/s11926-009-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.