Abstract
Objectives. To determine whether there are racial/ethnic differences in the willingness of SLE patients to receive CYC or participate in clinical trials, and whether demographic, psychosocial and clinical characteristics contribute to these differences.
Methods. Data from 120 African-American and 62 white lupus patients were evaluated. Structured telephone interviews were conducted to determine treatment preferences, as well as to study characteristics and beliefs that may affect these preferences. Data were analysed using serial hierarchical multivariate logistic regression and deviances were calculated from a saturated model.
Results. Compared with their white counterparts, African-American SLE patients expressed less willingness to receive CYC (67.0% vs 84.9%, P = 0.02) if their lupus worsened. This racial/ethnic difference remained significant after adjusting for socioeconomic and psychosocial variables. Logistic regression analysis showed that African-American race [odds ratio (OR) 0.29, 95% CI 0.10, 0.80], physician trust (OR 1.05, 95% CI 1.00, 1.12) and perception of treatment effectiveness (OR 1.40, 95% CI 1.22, 1.61) were the most significant determinants of willingness to receive CYC. A trend in difference by race/ethnicity was also observed in willingness to participate in a clinical trial, but this difference was not significant.
Conclusion. This study demonstrated reduced likelihood of accepting CYC in African-American lupus patients compared with white lupus patients. This racial/ethnic variation was associated with belief in medication effectiveness and trust in the medical provider, suggesting that education about therapy and improved trust can influence decision-making among SLE patients.
Keywords: systemic lupus erythematosus, racial inequities, patient decision-making, patient preferences, cyclophosphamide
Introduction
SLE is three to four times more common among African-Americans than among whites. At the time of SLE diagnosis, there are already differences between African-American and non-African-American patients. In the LUMINA (Lupus in Minority populations: Nature vs Nurture) cohort, African-American lupus patients were more likely to have organ system involvement, more active disease, higher frequencies of auto-antibodies, lower levels of social support and more abnormal illness-related behaviours compared with white lupus patients [1]. African-Americans also scored lower on multiple measures of socioeconomic status compared with whites. Other studies have shown that mortality rates are markedly higher [2, 3] and outcomes from kidney disease are worse [4] among African-American compared with white lupus patients.
Thus racial/ethnic differences exist in the incidence, disease course and outcomes of SLE, making new strategies to address these problems a high priority. According to an Institute of Medicine report on racial inequities in US health care, a significant body of research demonstrates variation in the rates of medical procedures by race/ethnicity after controlling for insurance status, income, age and clinical conditions [5]. The report indicates that US racial and ethnic minorities are less likely to receive certain procedures and are more likely to experience lower quality of health services. The report concludes that addressing racial and ethnic disparities in health care will require increased awareness of disparities in health care systems, care processes and patient-level factors.
In this age of shared doctor–patient decision-making, improving the evidence base regarding patient-level factors, particularly preferences for treatment, and the methods for eliciting these are necessary to improve health care quality [6]. Recent studies have demonstrated differences in treatment choices between African-Americans and whites in medical conditions such as coronary artery disease, cervical cancer and end-stage OA [7–9]. In addition, the extent to which minority patients convey reluctance to accept proven treatments can contribute to health disparities [5]. Once identified, interventions can be designed to address racial/ethnic differences in patient decision-making. However, no study has examined racial/ethnic differences in treatment preferences among lupus patients.
The primary objective of this study is to determine whether there are differences between African-American and white SLE patients’ willingness to (i) receive an immunosuppressive medication (i.e. CYC) when clinically indicated or (ii) participate in a clinical trial involving a novel, experimental medication. A second objective is to identify demographic, psychosocial and clinical characteristics associated with treatment preferences among SLE patients. Finally, we seek to determine whether demographic, psychosocial and clinical characteristics explain racial/ethnic differences in either measure of treatment preference.
We hypothesize that compared with their white counterparts, African-American SLE patients will be less willing to accept aggressive medical management with CYC or participate in clinical trials involving novel, experimental medications. We also hypothesize that certain factors, including educational attainment, lack of familiarity with treatment and mistrust of the medical establishment contribute to these differences.
Methods
This cross-sectional study utilized the model of Bowling and Ebrahim [6] and followed the strategies of Ibrahim et al. [10] and Suarez-Almazor et al. [9], who conducted a series of studies to determine the factors most important to racial and ethnic differences in joint replacement surgery. Confidentiality was maintained by assigning participant numbers instead of using participant names on survey and evaluation forms. We obtained institutional review board approval (University of Chicago IRB Protocol #16912A) and participants’ informed consent.
Participants
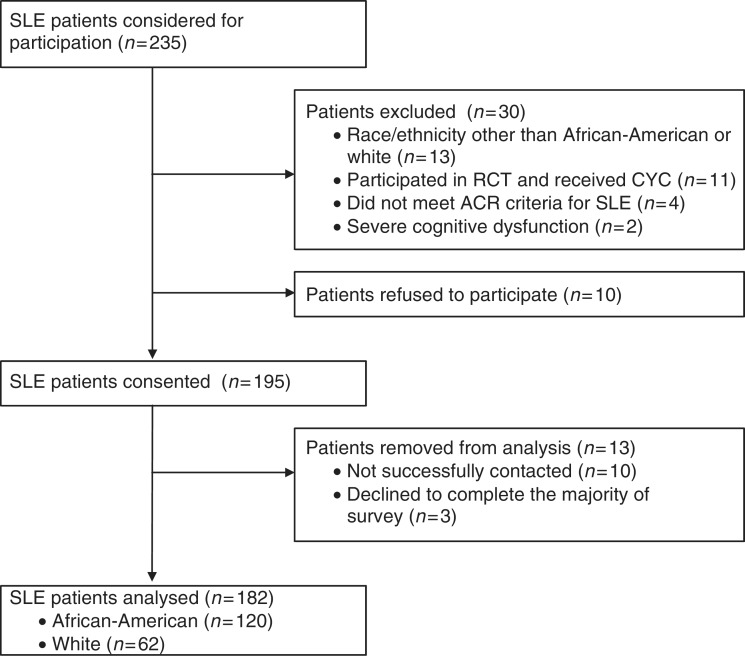
All patients who received their usual lupus care in the University of Chicago Rheumatology Clinic were recruited during clinic visits from November 2009 through December 2010. Only those who fulfilled the ACR SLE criteria (≥4 of 11) were included. The following were exclusion criteria: age <18 years, race other than African-American or white, history of taking CYC, history of participating in an interventional clinical trial involving a new medication and severe cognitive dysfunction. Patients who were not successfully contacted or failed to complete the majority of the survey were not included in the analysis (Fig. 1).
Fig. 1.
SLE patients recruited.
Survey questionnaire
Informed consents were obtained and telephone interview appointment dates were arranged during patient clinic visits. Sociodemographic and treatment preference data were obtained from structured telephone interviews. Following Bowling and Ebrahim’s [6] model of treatment decision-making, we asked questions regarding specific variables that have been postulated and/or shown to explain racial or ethnic differences in treatment preferences in other medical conditions. The standardized survey is attached (see supplementary data, available at Rheumatology Online).
Patient characteristics
The following self-reported demographic information was collected: race/ethnicity, age, educational attainment, household income, employment status, medical insurance and marital status. The following personality and psychological characteristics were measured: Center for Epidemiologic Studies Depression (CES-D) self-reported depression scale [11] (range 1–53), prayer reliance [12] (range 0–2 for efficacy, 0–3 for usage) and Domain-Specific Risk Attitude Scale in health and safety [13] (range 10–50). The Multidimensional Health Locus of Control [14] was determined in the following subscales: internal, chance and powerful others (range 6–36, each subscale).
Aggressive medical management preferences
After providing information regarding CYC and clinical trials, agreement with the following statements was measured: ‘If my lupus becomes more severe, seriously attacking my lung, heart, kidney or brain and if my doctor recommended it, then I would be willing to receive cyclophosphamide’ and ‘If my lupus gets worse and if my doctor recommended it, then I would be willing to participate in a lupus research clinical trial that may involve the use of a new, experimental medication.’ Measures of familiarity with CYC treatment (range 0–3), as well as perceptions of risk (range 3–15) and effectiveness (range 6–30) of CYC treatment are based on previously used measures pertaining to joint replacement surgery [9, 10] but were modified to reflect CYC administration in lupus patients.
Setting, role preferences and experience
Participants were asked whether they preferred seeing a rheumatologist of their own race, sex or approximate age. Patient perceived physician participatory decision-making (PDM) style [15] (range 0–100) and duration of physician–patient relationship were measured. Trust in physicians was determined using Hall’s Trust in Physicians Scale [16] (range 11–55) as well as patient perceived discrimination [17] (range 4–20).
Disease context
Standardized measures were used to assess disease severity during the telephone interviews and chart abstraction. These measures included the SLEDAI [18], SLICC Damage Index [19], Charlson Comorbidity Index [20], number/type of major immunosuppressants used and disease duration.
Pilot testing
Pilot testing was performed to determine the practicality of conducting the telephone survey and to receive feedback regarding the survey format and content. An early version of the survey was used during pilot testing and was administered to volunteer lupus patients (three white and two African-American) from the Lupus Foundation. The original survey was then modified to the current version based upon preliminary results. Based upon feedback, we developed a response card containing potential answers to the survey questions. Each subject was given this card upon consenting to participate.
Reliability and validity tests
The reliability and validity of several of the measures used have never been tested in lupus patients. Other measures were also modified from the original forms. Hence the following reliability and validity tests were conducted.
The Cronbach α coefficient formula was used to test the internal consistency of survey components with multiple items. An internal consistency reliability score of ≥0.70 was considered ideal.
To confirm the number of dimensions underlying the different set of multi-item variables compared, confirmatory factor analyses were performed. Factors with Eigen values ≥1 were retained. After running each factor analysis, factor loadings were orthogonally rotated. Proportions indicating the relative weight of each factor in the total variance were noted.
Correlational analyses were used to quantify the construct validity of certain measures. Pearson’s correlation coefficient scores and their P-values were calculated.
Statistical analysis
Racial/ethnic differences in willingness to accept CYC or participate in a clinical trial involving new, experimental medications for SLE were analysed using χ2 analysis. Parametric and non-parametric tests were used to compare the demographic, psychosocial and clinical characteristics between subjects who did and did not agree to CYC administration or clinical trial participation. Categorical variables were compared by χ2 analysis or Fisher’s exact test. Continuous variables were compared by a two-sample t-test or Wilcoxon rank sum test.
To determine the most significant determinants of (i) willingness to receive CYC administration and (ii) willingness to participate in a clinical trial involving a novel, experimental medication, logistic regression analyses were performed and their deviances from a saturated model were calculated. Willingness was defined as agreement with the aggressive medical management preference statement (i.e. selecting strongly agree or agree). Deviances were then compared using χ2 analysis to determine the best model; significance was set at the 5% level. To be parsimonious, only variables shown to be related to preferences for either measure of aggressive medical management (P ≤ 0.05) were considered.
Logistic regression models were also performed to evaluate the relationship between patient preferences and race/ethnicity, adjusted for patient characteristics. The initial model in these analyses included only race/ethnicity as the independent variable. Patient characteristics and beliefs that may mediate this relationship, based on bivariate analyses (P ≤ 0.05) and theoretical models, were then serially added to subsequent models to determine whether these covariates may explain the difference between the racial/ethnic groups’ treatment preferences. A linear association between each potential mediating variable with the log-odds of each dependent variable was also checked; continuous variables were converted into categorical variables with non-linear associations [21].
Finally, data were analysed using ordinal logistic regression models and results were similar to logistic regression models (data not shown). Logistic regression was preferred over ordinal logistic regression for simplicity, ease of interpretability and theoretical soundness. All analyses were performed using STATA 11.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 235 SLE patients were initially considered for participation in the study. One hundred and ninety-five were eligible and consented to participate. Data from 120 African-American and 62 white patients were evaluated; 92.3% were women (Fig. 1).
Participants’ sociodemographic and clinical characteristics are shown in Table 1. Statistically significant differences were observed between the racial/ethnic groups. African-American SLE patients, compared with white SLE patients, were less likely to have more education than a high-school degree (64.2% vs 83.9%, P < 0.01), were less likely to be employed (38.5% vs 56.5%, P = 0.02) and were more likely to have lower incomes (33.6% vs 5.4% with annual income of <$10 000, P < 0.001). Although African-American patients had a higher Charlson Comorbidity Index mean score than white patients (2.34 vs 1.85, P = 0.03), the mean SLEDAI score, SLICC Damage Index score, disease duration and number of immunosuppressant agents used did not differ.
Table 1.
SLE patient sociodemographic and clinical characteristics by racial/ethnic group
| Characteristic | African-American | White | P-valuea |
|---|---|---|---|
| Number of subjects, n | 120 | 62 | |
| Age, mean (s.d.), years | 41.58 (12.70) | 45.24 (11.94) | 0.06 |
| More than HS graduate, n (%) | 77 (64.2) | 52 (83.9) | <0.01 |
| Income, n (%) | <0.001 | ||
| <$10 000 | 40 (33.6) | 3 (5.4) | |
| $10 001–30 000 | 32 (26.9) | 5 (8.9) | |
| $30 001–50 000 | 23 (19.3) | 10 (17.9) | |
| >$50 000 | 24 (20.2) | 38 (67.9) | |
| Employed, n (%) | 46 (38.5) | 35 (56.5) | 0.02 |
| With private medical insurance, n (%) | 42 (35.0) | 52 (83.9) | <0.001 |
| Married, n (%) | 37 (30.8) | 41 (66.1) | <0.001 |
| SLEDAI, mean (s.d.) | 3.32 (3.39) | 2.95 (2.71) | 0.52 |
| SLICC Damage Index, mean (s.d.) | 2.02 (2.19) | 1.71 (2.56) | 0.22 |
| Charlson Comorbidity, mean (s.d.) | 2.34 (1.43) | 1.85 (1.28) | 0.03 |
| CES-D, mean (s.d.) | 19.48 (12.26) | 15.92 (12.32) | 0.04 |
aSignificance level of the χ2 (or Fisher’s exact) statistic for categorical variables and two-tailed t-test (or Wilcoxon’s rank sum test) for continuous variables. HS: high school.
Table 2 shows patient preferences and perceptions regarding aggressive treatment. While more white subjects indicated a willingness to participate in a clinical trial involving a new, experimental medication compared to African-Americans, this difference was not statistically significant (80.7% vs 68.7%, P = 0.10). In contrast, more whites than African-Americans were willing to receive CYC if their lupus worsened and if their doctor recommended the treatment (84.9% vs 67.0%, P = 0.02). No significant racial/ethnic differences were observed in the perceptions of effictiveness and risk of CYC.
Table 2.
SLE patient preferences and perceptions regarding aggressive treatment
| African-American | White | P-valuea | |
|---|---|---|---|
| Willing to receive CYC, n (%)b | 63 (67.0) | 45 (84.9) | 0.02 |
| Familiarity, mean (s.d.) | 0.36 (0.67) | 0.57 (0.82) | 0.10 |
| Perception of effectiveness, mean (s.d.) | 20.67 (4.37) | 21.25 (4.27) | 0.44 |
| Perception of risk, mean (s.d.) | 11.14 (2.11) | 11.11 (2.33) | 0.95 |
| Willing to participate in a clinical trial involving a new medication, n (%)c | 79 (68.7) | 46 (80.7) | 0.10 |
aSignificance level of the χ2 statistic for categorical variables and two-tailed t-test (or Wilcoxon’s rank sum test) for continuous variables. bAmong patients who had never received CYC (n = 147, 94 African-American and 53 white). cAmong patients who had never participated in a clinical trial involving a new medication (n = 172, 115 African-American and 57 white).
Table 3 demonstrates patient health attitudes and beliefs. Compared with whites, African-Americans were more likely to believe that prayer is helpful for their lupus (P < 0.001) and to utilize prayer to cope with their disease (P < 0.01). In addition, African-American patients were more likely than whites to believe that their health outcomes are controlled by their own internal actions (P < 0.01) and by powerful others (P < 0.01). They also reported higher trust in physicians than white patients (P = 0.01).
Table 3.
Participant beliefs and attitudes according to racial/ethnic group
| Belief/attitude | African-American | White | P-valuea |
|---|---|---|---|
| Number of subjects, n | 120 | 62 | |
| Prayer efficacy, n (%) | <0.001 | ||
| No help | 3 (2.5) | 9 (14.5) | |
| Some help | 24 (20.0) | 24 (38.7) | |
| Much help | 93 (77.5) | 29 (46.8) | |
| Prayer frequency, n (%) | <0.01 | ||
| Never | 8 (6.7) | 11 (17.7) | |
| Monthly | 16 (13.3) | 14 (22.6) | |
| Weekly | 19 (15.8) | 15 (24.2) | |
| Daily | 77 (64.2) | 22 (35.5) | |
| Locus of control, mean (s.d.) | |||
| Internal | 26.06 (5.22) | 23.52 (5.37) | <0.01 |
| Chance | 19.16 (6.44) | 17.44 (4.99) | 0.07 |
| Powerful others | 24.95 (5.32) | 22.48 (5.37) | <0.01 |
| Physician’s PDM style, mean (s.d.) | 66.25 (24.49) | 73.52 (22.46) | 0.05 |
| Trust in physicians, mean (s.d.) | 39.43 (7.39) | 36.16 (8.96) | 0.01 |
| Perceived discrimination, mean (s.d.) | 10.47 (3.23) | 9.69 (3.41) | 0.14 |
aSignificance level of the χ2 (or Fisher’s exact) statistic for categorical variables and two-tailed t-test (or Wilcoxon’s rank sum test) for continuous variables.
Reliability and validity of measures
Reliability
Supplementary Table S1 (available as supplementary data at Rheumatology Online) shows the Cronbach α coefficient values of several multi-item components of the survey.
Correlational analyses
Willingness to participate in a clinical trial positively correlated with willingness to receive CYC (r = 0.24, P = 0.001). Perceived effectiveness negatively correlated with perceived risk of CYC treatment (r = −0.32, P < 0.001). Trust in physicians negatively correlated with perceived discrimination in the medical setting (r = −0.60, P < 0.001).
Factor analyses
The results of the factor analyses are shown in supplementary Table S2 (available as supplementary data at Rheumatology Online).
(1) Beliefs about CYC. Effectiveness of treatment items all loaded on Factor 1, which accounted for 70% of the variance. Familiarity with CYC items loaded on Factor 2, which accounted for 23% of the variance.
(2) Trust in physicians and perceived discrimination. All trust in physicians items loaded on Factor 1, which accounted for 86% of the variance. All perceived discrimination items loaded on Factor 2, which accounted for 13% of the variance.
Preferences: bivariate analyses
Table 4 shows the patient characteristics and beliefs that were significantly related to patients’ CYC treatment preference. Compared with SLE patients unwilling to receive the medication, those willing to receive CYC tended to have higher perceived effectiveness (22.12 vs 17.44, P < 0.001) and lower perceived risk (10.92 vs 11.72, P = 0.05) of treatment. Willingness to receive CYC was also significantly associated with a higher trust in physicians score (39.08 vs 35.79, P = 0.03), but not with physician PDM style score, locus of control or perceived discrimination in medicine.
Table 4.
Bivariate analysis of SLE patient characteristics and attitudes by CYC treatment preferencea variables
| Characteristic | Unwilling to receive CYC | Willing to receive CYC | P-valueb |
|---|---|---|---|
| Number of subjects, n | 39 | 108 | |
| Race/ethnicity, n (%) | 0.02 | ||
| African-American | 31 (33.0) | 63 (67.0) | |
| White | 8 (15.1) | 45 (84.9) | |
| Medical insurance, n (%) | 0.03 | ||
| Without private | 24 (35.3) | 44 (64.7) | |
| With private | 15 (19.0) | 64 (81.0) | |
| Marital status, n (%) | 0.01 | ||
| Married | 11 (16.4) | 56 (83.6) | |
| Other (single, divorced, widowed) | 28 (35.0) | 52 (65.0) | |
| Perception of effectiveness, mean (s.d.) | 17.44 (3.79) | 22.12 (3.82) | <0.001 |
| Perception of risk, mean (s.d.) | 11.72 (2.33) | 10.92 (2.10) | 0.05 |
| Trust in physicians, mean (s.d.) | 35.79 (9.15) | 39.08 (7.64) | 0.03 |
aAmong patients with no history of taking CYC. bSignificance level of the χ2 (or Fisher’s exact) statistic for categorical variables and two-tailed t-test (or Wilcoxon’s rank sum test) for continuous variables.
Married patients were more willing than single, divorced or widowed patients to participate in a clinical trial involving an experimental medication (80.8% vs 66.7%, P = 0.04). Willingness to participate in a clinical trial was also associated with having a lower internal locus of control score (24.75 vs 26.74, P = 0.03) and believing that having a rheumatologist of the same race (P = 0.02) and age (P = 0.05) are very unimportant. Those willing to participate in a clinical trial also had a significantly higher perceived physician PDM style mean score (72.20 vs 56.74, P < 0.001). Yet, clinical trial participation was unrelated to physician–patient relationship duration, trust in physicians or perceived discrimination.
Preferences: multivariate analyses
Determinants of preferences
Multivariate logistic regression analysis showed that the following variables were the most significant determinants of the odds of willingness to receive CYC: African-American race [odds ratio (OR) 0.29, 95% CI 0.10, 0.80)], trust in physicians (OR 1.05, 95% CI 1.00, 1.12) and perceptions of effectiveness of CYC (OR 1.40, 95% CI 1.22, 1.61).
Similarly, a separate logistic regression analysis demonstrated that the following were significant determinants of the odds of willingness to participate in a clinical trial: internal health locus of control (OR 0.93, 95%CI 0.86, 0.99), physician PDM style (OR 1.03, 95% CI 1.01, 1.04), lack of physician race preference (OR 1.56, 95% CI 1.07, 2.28) and marital status (OR 2.30, 95% CI 1.06, 5.00).
Race/ethnicity and preferences
Consistent with the bivariate analysis, when asked whether they would consider CYC treatment if their lupus became more severe and if their doctor recommended it, African-American patients were less willing than white patients to receive the treatment (OR 0.36, 95% CI 0.15, 0.86). When income, private medical insurance and marital status were added to the model, this OR migrated slightly away from the null. Addition of the CES-D measure, prayer efficacy and internal locus of control led to further movement of the OR away from the null. When perception of medication effectiveness and trust in physicians measures were added, this racial/ethnic difference (OR 0.27, 95% CI 0.06, 1.26) was no longer statistically significant (Table 5).
Table 5.
Logistic regression of willingness to receive CYC, with serial addition of sociodemographic variables, clinical context, personality traits and other patient beliefsa
| Variable, OR (95% CI) | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Race/ethnicity | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| African-American | 0.36 (0.15, 0.86) | 0.32 (0.10, 1.00) | 0.26 (0.08, 0.91) | 0.27 (0.06, 1.26) |
| Incomeb | 0.98 (0.59, 1.61) | 1.11 (0.64, 1.93) | 1.19 (0.60, 2.37) | |
| Insurance | ||||
| Without private | 1.00 | 1.00 | 1.00 | |
| With private | 1.50 (0.45, 4.97) | 1.50 (0.42, 5.34) | 1.27 (0.30, 5.43) | |
| Marital status | ||||
| Not married | 1.00 | 1.00 | 1.00 | |
| Married | 1.42 (0.57, 3.54) | 1.34 (0.50, 3.61) | 1.37(0.33, 5.59) | |
| Depression, CES-Dc | ||||
| Quartile 1 | 1.00 | 1.00 | ||
| Quartile 2 | 1.97 (0.63, 6.17) | 3.43 (0.78, 15.09) | ||
| Quartile 3 | 5.73 (1.30, 25.17) | 9.83 (1.28, 75.25) | ||
| Quartile 4 | 1.87 (0.48, 7.25) | 2.59 (0.41, 16.35) | ||
| Prayer efficacy | ||||
| No help | 1.00 | 1.00 | ||
| Some help | 0.94 (0.14, 6.45) | 0.61 (0.06, 6.23) | ||
| Much help | 1.29 (0.19, 8.59) | 0.58 (0.06, 5.72) | ||
| Internal HLCc | ||||
| Quartile 1 | 1.00 | 1.00 | ||
| Quartile 2 | 1.04 (0.35, 3.08) | 0.79 (0.18, 3.38) | ||
| Quartile 3 | 1.52 (0.43, 5.33) | 1.47 (0.30, 7.18) | ||
| Quartile 4 | 3.09 (0.83, 11.52) | 1.68 (0.31, 9.10) | ||
| Perceived effectivenessb,c | 1.47 (1.23, 1.75) | |||
| Trust in physiciansc | ||||
| Quartile 1 | 1.00 | |||
| Quartile 2 | 8.97 (1.85, 43.46) | |||
| Quartile 3 | 1.66 (0.36, 7.68) | |||
| Quartile 4 | 3.00 (0.59, 15.30) |
aAmong patients with no history of taking CYC. bPer unit difference. cHigher score indicates more of the concept being measured. HLC: health locus of control.
When asked whether they would consider participating in a research clinical trial that may involve the use of an experimental medication if their lupus worsened and if their doctor recommended it, 68.7% of African-Americans and 80.7% of whites said they would. However, this difference did not reach statistical significance (OR 0.52, 95% CI 0.24, 1.13). The relationship between race/ethnicity and willingness to participate in a clinical trial remained statistically insignificant despite adjustment for patient socioeconomic status, clinical variables and psychosocial beliefs.
Discussion
Our study is the first to show that race/ethnicity, trust in physicians and perceptions of effectiveness of the medication were the most significant determinants of willingness to receive CYC, which is considered the gold standard in controlling severe lupus [22]. In our cohort, African-American SLE patients were less willing than white SLE patients to receive CYC, a finding consistent with previous studies of treatment preferences by race/ethnicity. In one study, African-American patients were more reluctant than white patients to undergo cardiac therapeutic procedures [7] while in other studies minority OA patients were less willing than their white counterparts to consider joint replacement surgery [9, 10, 23]. In our study, the racial/ethnic difference in willingness of lupus patients to receive CYC remained significant despite adjustment for potentially confounding sociodemographic and psychosocial variables. However, when trust in physicians and perception of medication effectiveness were accounted for, this finding was no longer significant, suggesting that these variables mediate this observed racial/ethnic difference.
Trust in physicians appears to be a more relevant factor in SLE patients’ treatment preferences for CYC than race/ethnicity. The significance of patient trust in physicians in determining patient preferences has been observed in other studies. In a study of Hispanic, African-American and white patients with knee OA, physician trust was a major determinant of racial and ethnic variation in joint replacement consideration by patients [9]. In a study of cardiac patients, patient level of medical mistrust was an independent predictor of patient satisfaction [17], which has been linked with patient compliance and utilization of health services [5]. Similarly, in a study of African-Americans’ perceptions of breast cancer treatment, mistrust of the medical establishment was a significant concern [24].
Racial/ethnic variations in treatment preferences have also been associated with perception of treatment benefits. Racial and ethnic variations in willingness to undergo knee replacement were highly attributed to patient expectations about procedure success in a cohort of OA patients [9]. In a separate study, African-American OA patients’ reluctance to consider joint replacement was attributed to their expectations of hospital course, pain and function following surgery [10]. These findings suggest patient beliefs may be influenced by patient education, as well as by improved physician–patient interactions [25].
Poor responses to standard drugs by African-Americans with lupus make participation in clinical trials vital [26, 27]. The literature is contradictory on whether African-Americans are as willing to participate in health research as non-Hispanic whites [28–31]. In our study, fewer African-Americans than whites expressed a willingness to participate in a clinical trial, but this difference was not statistically significant. A type II error may explain this lack of significance because the racial/ethnic difference was actually quite large (80.7% vs 68.7%). The estimated power for the comparison of proportions of African-American and white SLE patients willing to participate in a clinical trial in our study was small (0.31) and a larger sample size may have yielded a significant result. We found that the major determinants of preferences for participation in a lupus clinical trial included internal health locus of control and marital status. Understandably, a low sense of internal control over one’s health may predict a higher likelihood of voluntary participation in a research trial involving an experimental medication. On the other hand, the presence of a partner may encourage participation, knowing that a support system is available in the event of an adverse outcome.
Other determinants of SLE clinical trial participation included high perceived physician PDM style and lack of physician race preference. Indeed, greater physician PDM and patient-centred care have both been associated with better patient satisfaction and patient compliance [32]. In addition, African-Americans were found to be less willing to participate in research if they attribute high importance to physician race when seeking routine medical care [28].
In our cohort, African-American and white SLE patients differed in several ways. Specifically, African-American participants had less education, lower household incomes, and higher co-morbidity and depression scores than white participants. Similar racial/ethnic differences have been seen in other SLE cohorts [1, 33, 34]. In contrast to other SLE cohorts [35, 36], however, lupus disease activity and damage index scores were not significantly different between racial/ethnic groups in our study. This may be due to the fact that only lupus patients in the outpatient clinic were recruited. In this setting, lupus patients with more severe disease may have been less inclined to participate in the study.
African-American SLE patients were more likely to acknowledge the helpfulness of prayer in the treatment of the disease, not unlike African-American OA patients [37]. Compared with whites, African-American lupus patients were also more likely to believe that the outcomes of their disease could be attributed by their own actions and by powerful others such as family, friends and medical professionals. They had higher locus of control scores in the internal and powerful others subscales, inconsistent with findings in OA patients [9]. The low internal consistency reliability scores of these measures of control warrant further investigation. Our African-American patients also reported higher trust in physicians than their white counterparts. In contrast, studies in patients with cardiac [17] and other rheumatological disease [38, 39] in other centres did not find this racial/ethnic difference. This finding may be specific to our patient population. Alternatively, this may also be a form of reporting bias, as all telephone interviews were conducted by physicians.
Our study has certain limitations. As those who receive care at the University of Chicago Rheumatology Clinic are primarily African-American and white, we did not have an opportunity to evaluate the preferences of SLE patients from other racial/ethnic groups, including Hispanic Americans and Asian Americans. Our results are also based on data from patients who agreed to participate in the study. If those who refused to participate and those who were not reached by phone differed from our cohort in significant ways, then the generalizability of our results would be reduced. Most surveys were also conducted by a single physician interviewer (E.V.) and response bias may be present among patients of this interviewer (12% of sample). However, we found no differences in patient race/ethnicity, preference for CYC treatment or preference for clinical trial participation among patients of the primary interviewer compared with others. To address the issues of external validity and selection bias, similar studies should be performed at other sites and include both physician and non-physician interviewers. Finally, our measures of decision-making were also framed in a hypothetical manner, therefore we cannot predict patients’ choices if they were actually faced with the decision of accepting CYC or participating in a clinical trial.
This study showed that African-American lupus patients may be less likely than their white lupus counterparts to participate in a clinical research trial, although this difference did not quite reach statistical significance. Similarly, African-American lupus patients were less willing to receive a potent yet potentially toxic immunosuppressive agent. However, this racial/ethnic difference disappeared after adjusting for perceived effectiveness of CYC and trust in medical providers. These findings suggest that efforts should be made to increase awareness of the effectiveness of CYC and to enhance trust in medical providers. Such steps may lead to more effective therapy among all lupus patients and reduce persistent racial/ethnic disparities in lupus morbidity and mortality.
Supplementary data
Supplementary data are available at Rheumatology Online.
Funding: ACR/Research and Education Foundation Rheumatology Scientist Development Award.
Disclosure statement: E.R.V. is funded by the ACR/Research and Education Foundation Rheumatology Scientist Development Award. All other authors have declared no conflicts of interest.
References
- 1.Alarcon GS, Friedman AW, Straaton KV, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus. 1999;8:197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 2.Ward MM, Pyun E, Studenski S. Long-term survival in systemic lupus erythematosus. Patient characteristics associated with poorer outcomes. Arthritis Rheum. 1995;38:274–83. doi: 10.1002/art.1780380218. [DOI] [PubMed] [Google Scholar]
- 3.Trends in deaths from systemic lupus erythematosus—United States, 1979–1998. MMWR Morb Mortal Wkly Rep. 2002;51:371–4. [PubMed] [Google Scholar]
- 4.Korbet SM, Schwartz MM, Evans J, Lewis EJ. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18:244–54. doi: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
- 5.Smedley B, Stith A, Nelson A, editors. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC, USA: National Academies Press; 2003. [PubMed] [Google Scholar]
- 6.Bowling A, Ebrahim S. Measuring patients’ preferences for treatment and perceptions of risk. Qual Health Care. 2001;10(Suppl 1):i2–8. doi: 10.1136/qhc.0100002... [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedlis SP, Fisher VJ, Tice D, et al. Racial differences in performance of invasive cardiac procedures in a Department of Veterans Affairs Medical Center. J Clin Epidemiol. 1997;50:899–901. doi: 10.1016/s0895-4356(97)00089-9. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JB, McCormack LA. Time trends in late-stage diagnosis of cervical cancer. Differences by race/ethnicity and income. Med Care. 1997;35:1220–4. doi: 10.1097/00005650-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Suarez-Almazor ME, Souchek J, Kelly PA, et al. Ethnic variation in knee replacement: patient preferences or uninformed disparity? Arch Intern Med. 2005;165:1117–24. doi: 10.1001/archinte.165.10.1117. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Differences in expectations of outcome mediate African American/white patient differences in ‘willingness’ to consider joint replacement. Arthritis Rheum. 2002;46:2429–35. doi: 10.1002/art.10494. [DOI] [PubMed] [Google Scholar]
- 11.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population applied psychological measurement. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 12.Bill-Harvey D, Rippey RM, Abeles M, Pfeiffer CA. Methods used by urban, low-income minorities to care for their arthritis. Arthritis Care Res. 1989;2:60–4. doi: 10.1002/anr.1790020207. [DOI] [PubMed] [Google Scholar]
- 13.Weber E, Blais A-R, Betz N. A domain-specific risk-attitude scale: measuring risk perceptions and risk behaviors. J Behav Decis Mak. 2002;15:263–90. [Google Scholar]
- 14.Wallston KA, Wallston BS, DeVellis R. Development of the Multidimensional Health Locus of Control (MHLC) scales. Health Educ Monogr. 1978;6:160–70. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan SH, Gandek B, Greenfield S, Rogers W, Ware JE. Patient and visit characteristics related to physicians’ participatory decision-making style. Results from the Medical Outcomes Study. Med Care. 1995;33:1176–87. doi: 10.1097/00005650-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hall MA, Camacho F, Dugan E, Balkrishnan R. Trust in the medical profession: conceptual and measurement issues. Health Serv Res. 2002;37:1419–39. doi: 10.1111/1475-6773.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146–61. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- 18.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 19.Gladman DD, Urowitz MB, Goldsmith CH, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–13. doi: 10.1002/art.1780400506. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Szklo M, Nieto FJ. Epidemiology: beyond the basics. Sudbury, MA, USA: Jones and Bartlett; 2007. Stratification and adjustment: multivariate analysis in epidemiology; pp. 227–96. [Google Scholar]
- 22.Boumpas DT, Austin HA, 3rd, Fessler BJ, et al. Systemic lupus erythematosus: emerging concepts. Part 1: renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med. 1995;122:940–50. doi: 10.7326/0003-4819-122-12-199506150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Byrne MM, Souchek J, Richardson M, Suarez-Almazor M. Racial/ethnic differences in preferences for total knee replacement surgery. J Clin Epidemiol. 2006;59:1078–86. doi: 10.1016/j.jclinepi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Masi CM, Gehlert S. Perceptions of breast cancer treatment among African-American women and men: implications for interventions. J Gen Intern Med. 2008;24:408–14. doi: 10.1007/s11606-008-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashton CM, Haidet P, Paterniti DA, et al. Racial and ethnic disparities in the use of health services: bias, preferences, or poor communication? J Gen Intern Med. 2003;18:146–52. doi: 10.1046/j.1525-1497.2003.20532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51:1188–95. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 27.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int. 2006;69:1846–51. doi: 10.1038/sj.ki.5000243. [DOI] [PubMed] [Google Scholar]
- 28.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248–56. doi: 10.1016/s1047-2797(01)00265-4. [DOI] [PubMed] [Google Scholar]
- 29.Gifford AL, Cunningham WE, Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346:1373–82. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 30.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3:e19. doi: 10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynia MK, Gamble VN. Mistrust among minorities and the trustworthiness of medicine. PLoS Med. 2006;3:e244; author reply e5. doi: 10.1371/journal.pmed.0030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper LA, Roter DL. Patient-provider communication: the effect of race and ethnicity on process and outcomes of healthcare. Smedley B, Stith A, Nelson A eds. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC, USA: National Academies Press, 2003:552–93. [PubMed] [Google Scholar]
- 33.Ginzler EM, Diamond HS, Weiner M, et al. A multicenter study of outcome in systemic lupus erythematosus. I. Entry variables as predictors of prognosis. Arthritis Rheum. 1982;25:601–11. doi: 10.1002/art.1780250601. [DOI] [PubMed] [Google Scholar]
- 34.Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant. 2003;18:2039–46. doi: 10.1093/ndt/gfg345. [DOI] [PubMed] [Google Scholar]
- 35.Alarcon GS, McGwin G, Jr, Bartolucci AA, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797–806. doi: 10.1002/1529-0131(200112)44:12<2797::aid-art467>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Alarcon GS, Calvo-Alen J, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis. 2006;65:1168–74. doi: 10.1136/ard.200X.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ang DC, Ibrahim SA, Burant CJ, Siminoff LA, Kwoh CK. Ethnic differences in the perception of prayer and consideration of joint arthroplasty. Med Care. 2002;40:471–6. doi: 10.1097/00005650-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Berrios-Rivera JP, Street RL, Jr, Garcia Popa-Lisseanu MG, et al. Trust in physicians and elements of the medical interaction in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum. 2006;55:385–93. doi: 10.1002/art.21988. [DOI] [PubMed] [Google Scholar]
- 39.Groeneveld PW, Kwoh CK, Mor MK, et al. Racial differences in expectations of joint replacement surgery outcomes. Arthritis Rheum. 2008;59:730–7. doi: 10.1002/art.23565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.