Abstract
Two studies of variables affecting voluntary ethanol consumption by adolescent male and female rats are reported. Sprague-Dawley (SD) and spontaneously hypertensive rats (SHRs) were compared in Experiment 1. Starting on postnatal day 30 all had 24-hr access to 2%, then 4%, and then 6% ethanol, followed by 1-hr access to the 6% until intake stabilized. During the 1-hr access SHR females consumed more ethanol than all other groups. In Experiment 2 the same procedure was used to compare SD groups prenatally exposed to nicotine, with controls. Nicotine-exposed females consumed more ethanol during 1-hr access than both nicotine-exposed and control males; but after using water intake as a covariate, the differences were not significant. These data show that deprivation conditions need to be considered when generalizing the results of voluntary consumption studies, and that estrogens may be a modulator of addictive behavior.
Keywords: Addiction, Dopamine, Ethanol, Nicotine, Sex differences, SHRs, Voluntary Consumption
Alcohol abuse is associated with increased morbidity and mortality in the United States of America [4], and worldwide [84]. Adolescents and college students are particularly vulnerable [3;42;49;76;80;86]. In their often-cited review Hawkins et al. [26] separated the risk factors for adolescent and young adult alcohol and substance abuse into two broad categories: contextual factors “…which provide the legal and normative expectations for behavior.” (p. 65); and individual and interpersonal factors such as physiological characteristics, a family history of alcoholism, conduct problems, hyperactivity, and the early initiation of drug use.
According to Grant [24] one of the strongest predictors of alcohol or other drug abuse later in life is early use of alcohol or other addictive substances. Jernigan [27] reported that the levels of drinking and bingeing among girls have risen faster than boys. Although women have abused alcohol less than men, and have a lower lifetime prevalence (7-12%) of alcoholism compared to men (20%) [32;84], data show that over the past 30 years the age of first alcohol use among females has been declining, and that the rate of initiation of alcohol use among the youngest females (10-14 year olds) is now equal to that of males [25]. Zilberman et al. [87] also suggested that there is a gender convergence in the prevalence rates of alcohol use initiation among adolescents. They stated that women's pattern of experiencing alcohol-related problems at a later age than men is rapidly changing; and that the increase in alcohol-related disorders among women, and their reduced age of initiation, suggest that the sexes may become equal in this regard. A recent review by Lynch [32] indicated that the rates of initiation of drug and alcohol use are now comparable between boys and girls. These are particularly disturbing findings since women take less time than men to progress from initial use of a substance (including alcohol) to dependence on that substance [33]. Additionally, when women drink to excess they are at a higher risk than men for developing alcohol related health problems, including damage to their brain and heart, alcohol related liver disease [13], disruption of their menstrual cycle, and reduced bone mass [17]. Drinking alcohol during pregnancy can also have serious consequences such as fetal alcohol syndrome [54;55].
In this paper we present the findings from two investigations of variables affecting the voluntary intake of ethanol by adolescent male and female rats: strain (Experiment 1), and gestational exposure to an addictive substance (nicotine, Experiment 2). Spear and Varlinskaya [74] indicated that many of the effects of ethanol found in human adolescents (e.g., attenuated sensitivity) are also found in many other species, including the laboratory rat. The similarities between the species provide sufficiently promising evidence of face and construct validity to support the use of animal models as tools for the study of the effects of alcohol. Animal models also provide an opportunity to control experimental variables that can influence sex-related differences in alcohol consumption [67].
Experiment 1
There has been growing interest and evidence showing the importance of genetic risk factors in the development of alcoholism [67]. Investigations of genetic links have gone beyond simply looking within families that have an alcoholic parent or grandparent. It is now recognized that genetic risk factors include disorders such as Attention Deficit Hyperactivity Disorder (AD/HD) [5]. Faraone [21], and Faraone et al. [22] presented compelling evidence that AD/HD has a genetic component; and several recently published studies [39;78;81] have shown AD/HD to be a risk factor for alcohol and drug abuse. In a prospective study Molina and Pelham [37] also showed that AD/HD in childhood was associated with increased risk of use and abuse of alcohol.
The spontaneously hypertensive rat (SHR) is a genetically hyperactive strain that has been regarded as a suitable model for studying aspects of human AD/HD because they exhibit all the behavioral characteristics of that disorder: impaired sustained attention, motor impulsiveness, and hyperactivity [58;59]. In the first experiment we compared the volitional intake of ethanol by adolescent male and female SHRs with male and female Sprague-Dawley (SD) rats.
There is considerable evidence to suggest that aspects of AD/HD behavior may result from an imbalance between increased noradrenergic and decreased dopaminergic regulation of neural circuits that involve the prefrontal cortex [59] and alterations in gene expression [12]. The SHRs appear to have decreased dopamine (DA) activity in the prefrontal cortex, along with increased noradrenergic activity [58]. Alterations in dopaminergic activity when exposed to alcohol are considered a major mechanism in the development of alcoholism and addiction [62].
Just as alcohol consumption varies among the human population, differences are also found between strains of rats. For example, Da Silva et al. [10] measured the voluntary choice of various concentrations of a continuously available ethanol solution versus water by Floripa genetically high and low anxious, Lewis, and SHR adult male and female rats. Their most surprising finding was that SHRs, a strain that they referred to as the least anxiety prone of the three strains, consumed more ethanol than the Lewis rats, even though the latter are known to present more anxiety-like behaviors than SHRs [10;51], and to be addiction prone [2;10]. The data also showed that, regardless of strain, female rats consumed 46% more ethanol than males. As a partial replication of the 2004 study [10], Da Silva et al. [11] compared adult male Lewis and SHRs in volitional ethanol consumption. The results replicated the 2004 experiment: SHRs consumed more ethanol than Lewis rats.
Ethanol activates the mesolimbic dopaminergic system, increases the firing of DA neurons in the ventral tegmental area, and may function as a learning signal associated with such natural reinforcers under conditions of deprivation and novelty [see 72]. Blanchard and associates [6;7] have reported that ethanol intake produced significantly greater increases in extracellular DA in the nucleus accumbens of female, than in male rats. The increased consumption of ethanol by SHRs, relative to other strains [10;11], may be a result of their hypodopaminergic activity [59], and abnormal DA Transporter [82]; which may alter their mesolimbic DA response to ethanol.
In the present experiment we used a limited-access procedure [77] to compare the voluntary intake of ethanol of adolescent male and female SHR and SD rats. We were particularly interested in consumption following ethanol deprivation, 1-hr (limited) access, as a reflection of the animals' addiction to alcohol [71]. Based on the results of Da Silva et al. [10;11] we expected SHRs, regardless of sex, to consume more ethanol than the SDs. However it was also possible, and would be consistent with Da Silva's findings [10] and those of Blanchard and associates [6;7], that the effects of sex and strain would interact. Due the paucity of studies of ethanol consumption by female rats in general, and SHR females in particular, more specific hypotheses regarding the proposed interaction would be speculative.
Method
A total of 40 rats were purchased from Charles River Laboratories, Wilmington, MA for the present experiment. Half were SHRs (10 males & 10 females) and the other half SDs (10 males & 10 females) rats. Starting on postnatal (PND) day 30 we used the limited-access procedure [77] to gradually introduce the rats to the ethanol solutions; and provide a measure of their volitional intake following ethanol deprivation, as an indication of their degree of addiction [71]. The research protocol was reviewed and approved by the SUNY Cortland Institutional Animal Care and Use Committee, and conformed to the NIH Guide for the use of Laboratory Animals.
In addition to the softened water from the automatic watering system, a 14-oz drinking bottle containing an ascending series of ethanol concentrations was placed on their home cages 24 hrs per day (continuous access). This fade-in series started with 2% ethanol for the first 4 days, followed by 4% for the next 4 days, and then 6% for the remainder of this phase of the experiment. Bottles were weighed to the nearest 0.1 g, at the same time daily. When consumption of the 6% ethanol leveled off (no greater than a 20% change in the group mean across 3 days) the 6% ethanol was removed and then presented on the next and subsequent days for only 1 hr (limited access), at the same time daily, until consumption again reached the stability criterion of no greater than a 20% change in the group mean across 3 days. Water from the automatic system remained available. The ethanol bottles were weighed prior to and following each 1-hr limited-access session.
On the day following the limited access to ethanol sessions all the groups were placed on a 23-hr water deprivation schedule. We measured their water intake, as above, during daily 1-hr limited-access sessions for the next 5 days to determine if ethanol intake was related to inherent differences in water intake among the groups. Ethanol was not available during those days. Body weights were measured weekly during the continuous access to ethanol, and every two days during the limited access to ethanol and water deprivation phases of the procedure.
Statistics
All the data were analyzed using t-tests or univariate ANOVAs. Statistica for Windows, version 6.0 [75] was used for the statistical calculations. Significant ANOVA effects were followed up by the Tukey HSD Multiple Comparisons test, using procedures outlined by Maxwell and Delaney [35]. The alpha level was set at 0.05 for all statistical tests. All data are presented as means ± the standard error of the mean (SEM). Rosenthal et al. [57] stated that data should be analyzed in a way that provides the most interpretable interactions, which then allows making the most meaningful follow-up comparisons. Therefore, in both experiments the data were treated as from four separate groups, and the ANOVAs followed up by mainly same-sex (planned) comparisons [3;57].
Results
Each rats daily intake of ethanol during all the sessions was computed in grams per kilogram of body weight (g/kg).
Fade-in series
The average daily amounts of ethanol consumed by each animal during the fade-in series were calculated. Figure 1 presents the groups' means and SEMs across the four days of continuous access to the 2% solution (left panel), across the four days of continuous access to the 4% solution (middle panel), and those across the 12 days of continuous access to the 6% solution that it took for all the groups to reach the criterion (right panel). Both SHR groups consumed relatively more than the corresponding SD comparison groups; and the ethanol intake of all the groups was lower with the 2% than the 4% and 6% solutions.
Figure 1.
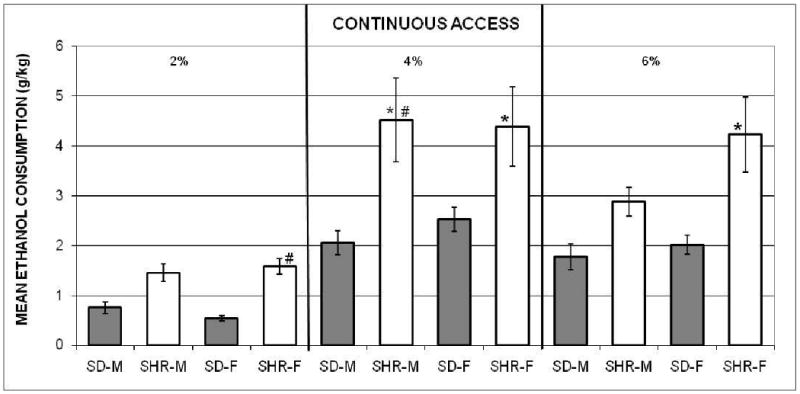
The mean amounts (± SEMs) of 2% ethanol (left panel), 4% ethanol (middle panel), and 6% ethanol (right panel), adjusted for body weights, consumed by the spontaneously hypertensive male (SHR-M) and female (SHR-F), and Sprague-Dawley male (SD-M) and female (SD-F) rats during the continuous-access to ethanol (fade-in procedure) in Experiment 1. *indicates significant difference from SD groups at same ethanol concentration. # indicates significant difference within the same group at different ethanol concentrations.
A two-way mixed ANOVA was computed using each rat's mean across the three ethanol concentrations, for each group. The main effects of groups, F(3, 36) = 7.44, and concentration, F(2, 72) = 50.61, were significant. These main effects were qualified by a significant interaction, F(6, 72) = 2.42. Tukey HSD comparisons of the ethanol intake by the groups within and across concentrations were used to clarify the interaction. They indicated that there were no significant differences among the groups during the period with the 2% solution; and no sex differences within-strain at each solution. However, the male and female SHR groups consumed more ethanol during the four days with the 4% solution than both of the SD comparison groups; and ethanol intake by SHR females (SHR-F), but not the SHR males (SHR-M) was greater than that by SD males (SD-M) and SD females (SD-F) during the period with the 6% solution.
The post-hoc comparisons for each group across solutions showed that the amounts of ethanol consumed by SHR–M were greater with the 4% than the 2% and 6% solutions. The SHR-F consumed less of the 2% than the 4% and 6% solutions. The SD-F consumed less 2% than 4%; but intake by the SD-M did not differ across solution concentrations.
Limited access to 6% ethanol
Inspection of the data indicated that the 1-hr daily amounts consumed by all the groups stabilized on day 7. The group means and SEMs during the six days just after intake had stabilized are shown in Figure 2. In contrast with the fade-in data, the SHR–F consumed relatively more ethanol during the limited-access phase than the control females and both male groups. A one-way ANOVA computed with each animal's 6-day mean produced a significant treatment effect, F(3, 36) = 22.43. The HSD comparisons confirmed that the mean ethanol intake of SHR-F (collapsed across days) was higher than that of the other three groups, which did not differ from each other.
Figure 2.
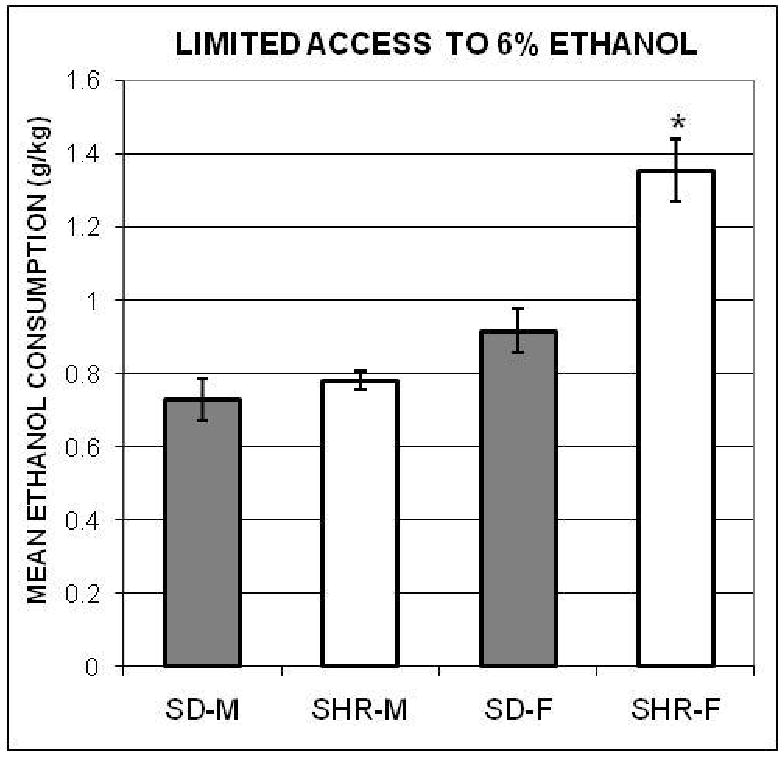
The mean amounts of 6% ethanol (± SEMs), adjusted for body weights, consumed by the spontaneously hypertensive male (SHR-M) and female (SHR-F), and Sprague-Dawley male (SD-M) and female (SD-F) rats during the 1-hr access to ethanol procedure in Experiment 1. *indicates significant difference from all other groups.
Limited access to water
The amounts of water consumed daily by each animal (adjusted for body weight) during the 5-days of limited access to water were calculated and a one-way ANOVA was computed with each rat's mean over those five days. The result was significant, F(3, 36) = 5.70. The post-hoc comparisons revealed a pattern (see Figure 3) that is different from the limited access to ethanol data.
Figure 3.
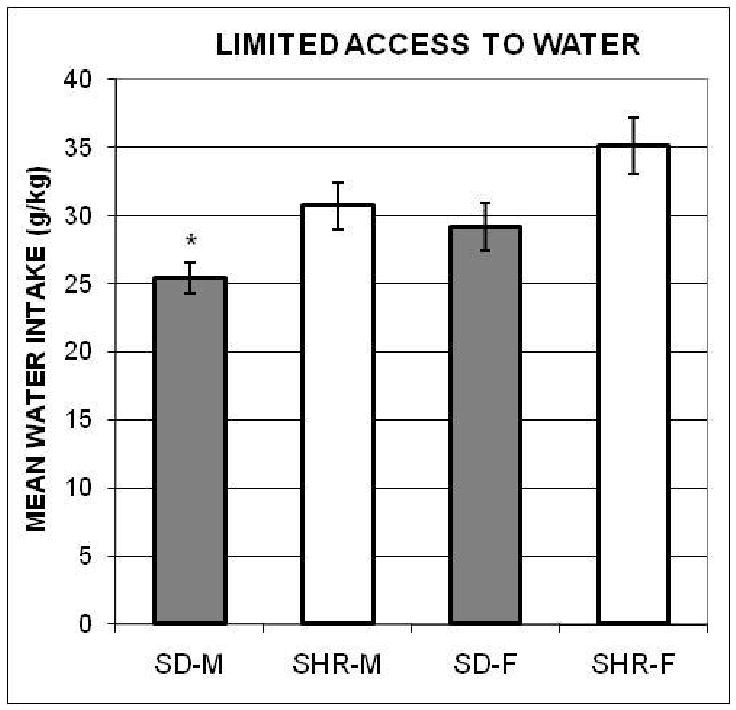
The mean amounts of water (± SEMs), adjusted for body weights, consumed by the spontaneously hypertensive male (SHR-M) and female (SHR-F), and Sprague-Dawley male (SD-M) and female (SD-F) rats during the 1-hr access to water control procedure in Experiment 1. *indicates significant difference from SHR-F.
To check on the possible effects of differences in 1-hr water intake among the groups we computed an ANCOVA with the limited access to ethanol data from all groups, using their 1-hr access to water data as the covariate (r = 0.6, p < 0.05). The results remained significant, F(3, 35) = 14.04, p = 0.000004; and the post-hoc comparisons confirmed that the mean 6% ethanol intake of SHR-F was higher than of the other three groups, which did not differ.
Experiment 2
Although cigarettes deliver more than 4000 chemicals that separately or together may affect human health, nicotine has been identified as the addictive component and it is the central drug associated with abnormalities in fetal brain development [16;19]. Nicotine has been reported to induce abnormalities in cell proliferation and cell differentiation in mice and rats [40]. Abnormalities in cell proliferation and differentiation have been shown to lead to regionally specific abnormalities in cell number and macromolecular content [40;66].
Nicotine also exerts its effects on various neurotransmitter systems. In fact, the dopaminergic and noradrenergic systems have been found to be hypoactive and hyporesponsive to exogenous stimulation after prenatal exposure to nicotine, at PND 30 [41;63;64]. Slotkin et al. [65] also found abnormalities in cell development of rats exposed in utero. Muneoka et al. [38] found reduced levels of dopaminergic innervation in the neocortex of offspring of dams exposed to nicotine from gestational days (G) 7-20. Other research has shown that prenatal exposure to nicotine can lead to reductions in DA in the ventral tegmental area and striatum of 14 day old rats [53], and prenatal nicotine treatment was also shown to produce a significant reduction in nicotine-induced release of norepinephrine [63].
Several investigators have shown that exposure to nicotine affects alcohol consumption by rats. Potthoff et al. [50] reported increased ethanol intake in female albino rats implanted with a device that slowly released nicotine. Blomqvist et al. [8] found that subchronic nicotine treatment increased the intake of 6% ethanol by medium-preferring adult male Wistar rats (the total fluid intake of those animals was 25-65% ethanol during previous screening tests for ethanol preference). Prior subcutaneous (SC) injections of nicotine have also been shown to increase subsequent ethanol consumption [69]; and enhance lever pressing for drops of 12% ethanol [29].
In the second experiment we examined the ethanol consumption by adolescent male and female SD rats that were exposed to nicotine only during gestation. In a review of associated brain actions Pogun [48] acknowledged the bases for expected sex differences in the effects of nicotine exposure on behavior. Acheson et al. [1] found that transdermal nicotine treatment increased alcohol consumption in men, but decreases occurred with women. Both Smith, Horan et al. [69] and Lê et al. [29] ran male rats. Potthoff et al. [50] used females. In light of their findings, those of Muneoka et al. [38], Richardson and Tizabi [53], and Ribary and Lichtensteiger [52] showing that prenatal exposure to nicotine reduces dopaminergic activity, and the connection to the development of addiction by Sari et al. [62], we expected the nicotine-exposed male and female rats to drink more of the ethanol solutions than their unexposed counterparts. And, based on the results of Experiment 1 with SHRs, and considering the sex differences in brain mechanisms reviewed by Pogun [48], we expected the effects of prenatal nicotine exposure to interact with the sex of the animals.
Method
The 20 male and 20 female SD rats whose ethanol consumption we measured were the offspring of dams that were either exposed to nicotine during gestation (SD-Nicotine, n = 10), or to a matched vehicle control (SD-Control, n = 10). The exposure manipulations for all the dams took place at SUNY Upstate Medical University. The timed pregnant SD females, obtained from Charles River Laboratories, Wilmington, MA, arrived on G5. Nicotine administration (nicotine hydrogen tartrate salt) was via an osmotic pump (Azlet: 2ML2) set to deliver 5.0 mg/kg/day (yields plasma nicotine levels equivalent to 2 packs of cigarettes daily) from G6 to delivery. The SD-control group received vehicle alone. All animals had continuous access to standard lab chow and water. Pumps were installed on the morning of G6 using aseptic procedures. To insert the pump, a 2-mm incision was made on the shaved back of an anesthetized dam (Fluothane). Sterile forceps were inserted into the wound and used to make a 6-mm pocket into which the pump was placed. The incision was closed with wound clips. Females were returned to a heated cage and monitored until recovery.
Birth was expected on G21. Within 12 hours of birth all experimental animals were surrogate fostered to non-experimental (females not exposed to nicotine) dams. One female and one male pup randomly selected from each litter was sent to SUNY Cortland at PND 25, thereby forming four groups of 10 pups (N = 40). Starting on PND 30 we used the same procedure [77] as described in Experiment 1 above (fade-in series involving continuous access, followed by 1-hr, limited access, to ethanol and then to water). The research protocol was reviewed and approved by the Committee for the Humane Use of Animals at SUNY Upstate Medical University and the SUNY Cortland Institutional Animal Care and Use Committee. It conformed to the NIH Guide for the use of Laboratory Animals.
Results
Body weights of the dams
The mean initial body weight of the SD-Nicotine females upon arrival was 214.5 ± 7.21 g; and for the SD-Control females that mean was 216.0 ± 8.16 g. The difference was not significant, t(19) < 1. The mean body weights 3 days prior to delivery was 310 ± 9.91 g and 361 ± 7.10 g for the SD-Nicotine and SD-Control dams, respectively. The difference was significant, t(19) = - 4.18. Thus, the mean amounts of weight gained was 95.5 ± 8.62 g for the SD-Nicotine females and 145.0 ± 7.92 g for the SD-Control females. That difference was also significant, t(19) = - 4.23.
Litter size
The mean litter size of the SD-Nicotine dams was 12.7 pups; and that of the SD-Control dams was 11 pups. An independent groups t-test showed that the apparent difference was not significant.
Body weights of the pups
The mean body weight at the beginning of the fade-in procedure (PND 30) of the Nicotine males (NIC-M) was 99.8 ± 2.96 g, the Control males (CON-M) was 105.5 ± 2.13 g, the Nicotine females (NIC-F) was 72.3 ± 1.85 g, and the Control females (CON-F) was 70.9 ± 1.87 g. An ANOVA computed using each pup's body weight was significant, F(3, 36) = 69.4. The post-hoc comparisons showed that both male groups weighed more that both female groups; the apparent same-sex differences were not significant.
Fade-in series
Figure 4 depicts the average amounts of ethanol consumed by each group (g/kg) across the four days of continuous access to the 2% solution (left panel), the four days to the 4% solution (middle panel), and the 12 days to the 6% solution that it took for all the groups to reach the criterion (right panel). The NIC-F consumed relatively more than both male groups; and the ethanol intake of all the groups was lower with the 2% than the 4% and 6% solutions.
Figure 4.
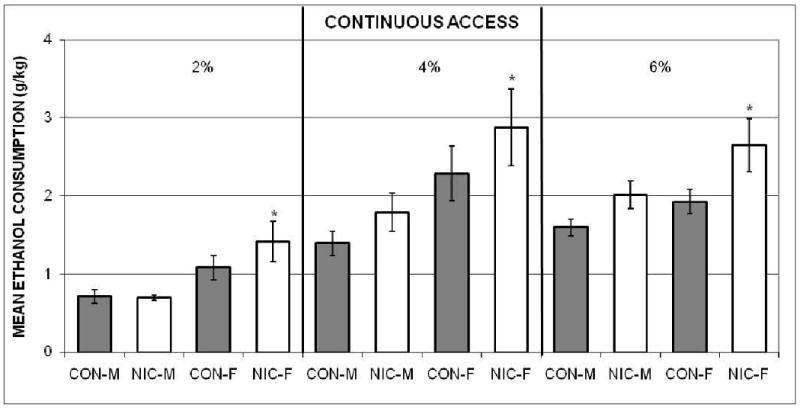
The mean amounts (± SEMs) of 2% ethanol (left panel), 4% ethanol (middle panel), and 6% ethanol (right panel), adjusted for body weights, consumed by the Nicotine-exposed male (NIC-M) and female (NIC-F), and vehicle control male (CON-M) and female (CON-F) groups during the continuous-access to ethanol (fade-in procedure) in Experiment 2. *indicates significant difference from both male groups.
A two-way mixed ANOVA was computed using each rat's mean across the three ethanol concentrations for each group, as described above. Only the main effects of groups, F(3, 36) = 4.93, and concentrations, F(2, 72) = 59.5, were significant. The post-hoc comparisons collapsed across concentrations confirmed that NIC-F consumed more ethanol than both the NIC-M and CON-M, but not the CON-F. The Multiple Comparisons test also established that the mean amounts consumed, collapsed across groups, were less with the 2% than both the 4% and 6% concentrations. Intake of the latter two did not differ.
Limited access to 6% ethanol
The group means and SEMs during days 6 to 11, after intake had stabilized (on day 6), are represented in Figure 5. As during the fade-in series, NIC-F consumed more ethanol (g/kg) than both NIC-M and CON-M, but not CON-F. The ANOVA computed with each animal's 6-day mean was significant, F(3, 36) = 5.80. The post-hoc comparisons substantiated that the mean ethanol intake by NIC-F was higher than that of the two male groups; but not CON-F. Mean consumption by NIC-M, CON-M and CON-F were not different.
Figure 5.
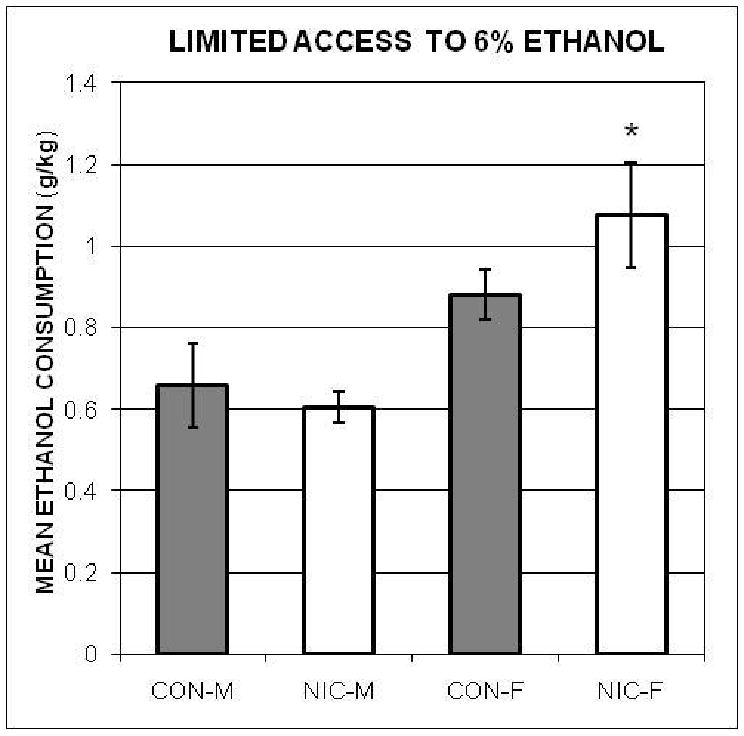
The mean amounts of 6% ethanol (± SEMs), adjusted for body weights, consumed by the Nicotine-exposed male (NIC-M) and female (NIC-F), and vehicle control male (CON-M) and female (CON-F) groups during the 1-hr access to ethanol procedure in Experiment 2. *indicates significant difference from both male groups. However, a subsequent ANCOVA with water consumption as the covariate showed that it was no longer significant.
Limited access to water
The amounts of water consumed daily by each animal (adjusted for body weight) during the 5-days of limited access to water were calculated. The ANOVA computed using each rat's mean over those five days was significant, F(3, 36) = 9.81. The post-hoc comparisons indicated that both NIC-F and CON-F consumed more water that NIC-M and CON-M; and that there were no significant same-sex differences in water intake (see Figure 6).
Figure 6.
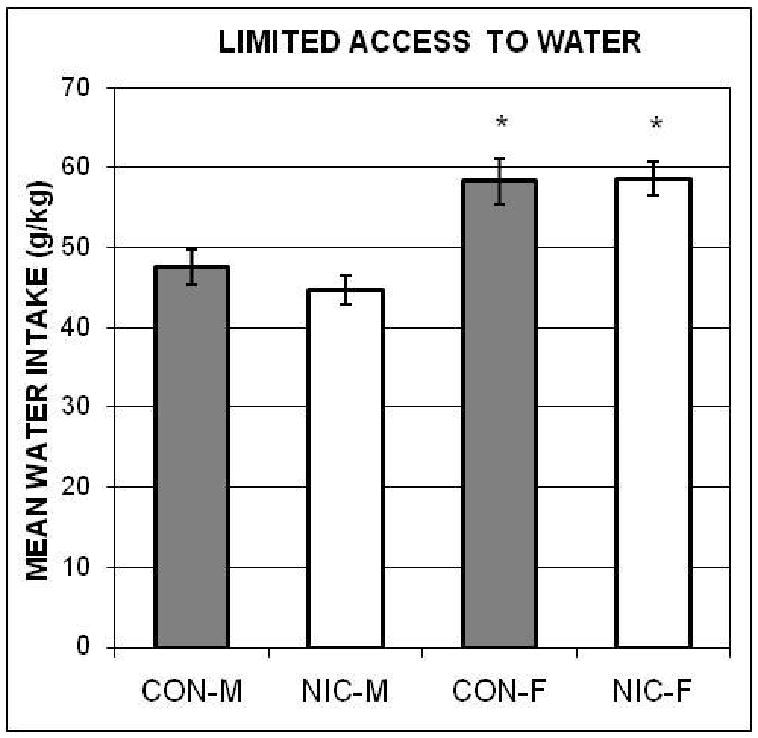
The mean amounts of water (± SEMs), adjusted for body weights, consumed by the Nicotine-exposed male (NIC-M) and female (NIC-F), and vehicle control male (CON-M) and female (CON-F) groups during the 1-hr access to water control procedure in Experiment 2. *indicates significant difference from both male groups.
Because both female groups consumed more water during the limited access to water phase than both male groups, one could argue that the difference in ethanol consumption between NIC-M and NIC-F was due to sex differences in thirst. Therefore, as above, we computed an ANCOVA with the groups' 1hr access to water data as the covariate (r = 0.49, p < 0.05). It showed that the result was no longer significant, F(3, 35) = 2.16, p = 0.11.
Discussion
The hypothesis tested in Experiment 1 that SHRs would consume more ethanol than SDs was supported. This strain difference was evident during the fade-in series (continuous access). The male and female SHR groups consumed more ethanol than both of the SD comparison groups during the four days with the 2% and 4% concentrations of ethanol. However, ethanol intake by SHR females, but not SHR males, was greater than that by SD males and females during the fade-in series with the 6% solution.
These results partially agree with those of Da Silva et al. [10]. They found that females, regardless of strain, consumed more 4% ethanol (v/v) than males. Vendruscolo et al. [79] found similar sex differences in the voluntary consumption of 10% ethanol (v/v) by SHRs under continuous access conditions. We did not find a main effect of sex. However, besides concentration differences, the fact that we used adolescent (PND 30 at start) animals, Da Silva et al. used adults (about PND 90 at start), and Vendruscolo et al. began 20 days of measuring ethanol consumption at about PND 70, needs to be considered [73].
The present results also are consistent with those reported by Soeters et al. [71]. Using the same limited access procedure [77] they found no difference in ethanol consumption by male SHRs compared to male Wistar-Kyoto rats. They did not study females.
The strain differences observed during the limited-access phase are especially interesting. When the female SHRs had access to the 6% ethanol solution for only 1 hr a day, they consumed significantly more than all of the other groups. That difference in SHR groups occurred following withdrawal (deprivation) from ethanol prior to the limited-access phase. Ethanol deprivation can modify the pattern of consumption by each sex [28], which may reflect differences in craving. Further investigation is needed to explain why a similar sex difference in consumption following ethanol withdrawal did not occur with the SD groups. Both organizational and activational influences of hormones could be involved [79]. Perhaps an as yet unidentified interactive effect of estrogen on the SHRs' altered dopaminergic activity may explain this strain difference.
Published accounts of SHR brain-behavior relationships [60;61;82] have been based almost exclusively on males [also see 79]. Further studies are needed to elucidate possible genetic modifications in the dopaminergic activity of SHR females; and if their estrogen activity may be affected. Therefore an explanation of the new and perhaps most important finding from Experiment 1 that a sex difference in ethanol intake by SHRs occurred following ethanol withdrawal would be speculation based on incomplete and sometimes contradictory expositions of alterations in their mesolimbic DA system. Ethanol intake changes the sensitivity of DA receptors [31] in the nucleus accumbens and striatum. Chronically high levels of estrogens decrease mesolimbic DA [15], and alcohol consumption increases mesolimbic DA levels [14]. Blanchard and associates [6;7] found that ethanol at low to moderate doses (the 6% ethanol solution is considered to be a moderate dose), produced significantly greater increases in extracellular DA in the nucleus accumbens of female, than male rats. Thus, a reasonable interpretation of our limited-access data may be that because the SHRs have a genetic mutation involving the modulation of brain DA [12;59;60;82], ethanol-induced DA release may make ethanol intake relatively more attractive for SHR females, especially following ethanol deprivation. Alternatively, ethanol deprivation may produce a more negative emotional state in SHR females that may be alleviated by ethanol.
The results of Experiment 2 partially supported the hypothesis that the effects of exposure to nicotine during gestation on voluntary ethanol consumption would depend upon the sex of the rat. The exposure increased consumption by females, but not males. During the fade-in series NIC-F drank more than both NIC-M and CON-M. The apparent difference between NIC-F and CON-F was not significant.
Although the original ANOVA computed with data from the limited-access to 6% ethanol showed that NIC-F drank more than both NIC-M and CON-M; the result of the ensuing ANCOVA with water consumption as the covariate was not significant. Consequently we cannot assert a specific effect of gestational nicotine exposure on subsequent ethanol consumption, but only a general effect of nicotine on drinking behavior. And we cannot be certain that we are not committing a Type II error by discarding the effects of nicotine on ethanol consumption during limited access—we just cannot distinguish between these effects and those on general fluid consumption under 23-hr deprivation conditions.
The present findings during the continuous access phase (fade-in series) are consistent with those of Potthoff et al. [50]. They reported that continuous administration of nicotine produced elevated consumption of 10% ethanol by female rats. However the present results do not agree with Blomqvist et al. [8], Smith, Horan et al. [69], and Lê et al. [29] because only our nicotine-exposed female offspring showed a relative increase in ethanol ingestion. Perhaps age, ethanol concentration, nicotine dose and exposure-time differences are involved. Nicotine exposure in the present study was 5.0 mg/kg/day delivered to the dams from G6 to delivery. The fade-in ethanol series for the offspring began on PND 30. In contrast, Blomqvist et al. [8] started daily 1.0 mg/kg SC injections 10 days prior to measuring the effect on the intake 6% ethanol by their adult (100-day old) male Wistar rats. Smith, Horan et al. [69] administered 1.0 mg/kg injections to their 250-g (approximately PND 57) male Wistar rats 30 min prior to giving them 1-hr access to 5%, then 8%, then 10% ethanol over a 20-day period. And Lê et al. [29] gave different groups of nicotine-treated male Long-Evans rats either 0.2, 0.4, or 0.8 mg/kg prior to daily operant self-administration of 12% ethanol sessions starting at about PND 70. In contrast, Smith, Kelly et al. [68] found no effect on consumption of 2%, 5%, 8%, and 10% ethanol solutions by either SD males or females implanted SC on PND 35 with 15- or 25-mg, 21-day time-release nicotine pellets, compared to placebo-implanted controls. Their presentation of the series of ethanol solutions began on PND 53. Further investigations are needed to elucidate the effects of all these variables. Inconsistent developmental exposure times to both nicotine and ethanol may be responsible for the discrepant findings in the literature.
Although we did not monitor the dams' food intake, the fact that the initial body weights of the groups were equal, and that the SD-Control dams gained more weight during gestation than the SD-Nicotine dams, suggests that the nicotine exposure may have reduced food intake. That result is consistent with reports of nicotine-induced anorexia [e.g. 36] and malnutrition during gestation. However, there was no difference in the pups' weight between groups at PND 30, the start of the ethanol procedure. This suggests that plausible reduced food intake by the SD-Nicotine females may not have adversely affected the nicotine-exposed pups' early development. It is also possible that a weight deficit in those offspring at birth could have been alleviated by the start of the behavioral procedure [30].
Previous investigations of the effect of gestational exposure to nicotine on the pups' body weights have varied with dose [44;45], sex of the offspring [46;47], and whether the pups were raise by their biological mothers or unexposed foster dams [see 30;44;45]. For example Paulson et al. [45] exposed pregnant SD rats to two different doses of Smokeless Tobacco via oral gavage. The offspring were then fostered to unexposed dams. They found a reduction in the body weights of pups exposed in utero to the higher dose, equivalent to 12 mg/kg/day, compared to the unexposed pups, until PND 29. However, the corresponding weight deficit in the pups exposed to the lower dose, equivalent to 3.99 mg/kg/day, and closest to ours (5.0 mg/kg/day), was observed only up to PND 8. In their subsequent study [44], with the pups raised by their biological mothers, these authors observed the opposite effect in pups exposed to the 3.99 mg/kg/day during gestation; i.e. higher weights starting on PND 6 and persisting through PND 30 and 42, compared to unexposed controls. For a table summarizing recent findings see [30].
In the present study all pups were fostered to dams that were not exposed to nicotine. This procedure avoided the possible confounding effects of further exposure during lactation by possibly malnourished dams, that may affect the quality of the milk and maternal care. Thus the present experimental animals were only exposed in utero.
We also did not find that exposing gravid rats to nicotine resulted in reduced litter sizes, compared to controls. Previous observations of the effects of nicotine exposure during gestation on litter sizes have been inconsistent. For example Witschi et al., [83] found a 25% reduction in average litter size following exposure to sidestream smoke during the first half of pregnancy that produced relatively low doses of nicotine (authors estimated that plasma levels reached about 25% of those observed following exposure to 1.75 mg/kg/day). More recently Mahlière et al. [34] reported that groups that received a nicotine pellet subcutaneously, delivering a dose rate corresponding to 6 mg/kg/day (closer to the present study) throughout gestation, had relatively reduced litter sizes. However, the present results are consistent with the more frequent reports that rodent litter size was not affected by prenatal exposure to nicotine [20;30;56;see 85]; or by exposure to cigarette smoke, [9]. For instance Xiao et al. [85] used a procedure similar to ours (implanted minipumps delivering a nicotine dose equivalent to 6 mg/kg/day from G4 to delivery), and observed that litter sizes were not affected. The reasons for the inconsistency in litter size effects are not readily apparent.
Several authors have provided explanations of the underlying neurobiological mechanisms which may account for the observed increase in alcohol consumption resulting from exposure to nicotine. Acheson et al. [1], Funk et al. [23], Lê et al. [29], Pogun [48], and Söderpalm et al. [70], and others stated that the reinforcing effects of alcohol are enhanced by nicotine. Funk et al. presented evidence that (a) both drugs operate through the mesolimbic DA system, and both stimulate DA release in the nucleus accumbens, (b) a cross-tolerance between them has been demonstrated in animal models and humans, (c) nicotinic receptors are involved in the rewarding effects of alcohol, and (d) there may be a genetic basis for their interaction. Olausson et al. [43] concluded that nicotine-induced increases in ethanol intake may be due to a decrease in inhibitory control. Söderpalm et al. [70] showed that blockade of nicotinic acetylcholine receptors (nAChR) in the ventral tegmental area with mecamylamine, a nicotinic antagonist, reduced ethanol consumption. However, in addition to the central effects, nicotine-induced increases in ethanol consumption may also be due to intermittent blockade of these receptors in the autonomic nervous system [18].
The review of sex differences in the effects of nicotine by Pogun [48] may help explain the increased ethanol consumption by exposed females, compared to exposed males, observed during the continuous access phase in the present study. She stated that systemic nicotine treatment produces a higher increase in extracellular DA in the nucleus accumbens with female, than with male rats. Analyses of rats' urine have shown that there are sex differences in nicotine metabolism, half life, and availability. Although female rodents have a higher nAChR density than males; chronic nicotine exposure produces a up-regulation of these receptors in males, but not females. Pogun also indicated that progesterone and the estrogens have effects on both dopaminergic and nicotinic cholinergic systems that may influence the behavioral effects of nicotine.
In conclusion, the present findings provide evidence of sex differences in the voluntary consumption of ethanol following ethanol withdrawal, as a reflection of addictive behavior in SHRs; as well as sex differences in consumption of continuously available ethanol by adolescent SDs following gestational exposure to nicotine. These data demonstrate that deprivation conditions, such as employed in the limited-access procedure, need to be considered when generalizing the results of animal studies of addictive behavior. Because the observed effects were greater in females rather than males, they may shed light on possible contributing factors (other than social) to the increased rates of alcoholism among women.
Acknowledgments
This research was funded in part by a specified grant to J. P. Lombardo through the SUNY Cortland Alumni Foundation, by NIH grant R21MH/NS66191 to S.V. Faraone, and by NIH-NIAAA Grant R01AA014871 to S.L. Youngentob. We are grateful to our research assistants Whitney Creager, Angel Medina, and Melissa Morales. Without their diligent and careful work this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David F. Berger, State University of New York College at Cortland
John P. Lombardo, State University of New York College at Cortland
Joshua A. Peck, State University of New York College at Cortland
Stephen V. Faraone, SUNY Upstate Medical University
Frank A. Middleton, SUNY Upstate Medical University
Steven L. Youngentob, SUNY Upstate Medical University
References
- 1.Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- 2.Ballesteros-Yanez I, Ambrosio E, Perez J, Torres I, Miguens M, Garcia-Lecumberri C, DeFelipe J. Morphine self-administration effects on the structure of cortical pyramidal cells in addiction-resistant rats. Brain Res. 2008 Sep 16;1230:61–72. doi: 10.1016/j.brainres.2008.06.128. [DOI] [PubMed] [Google Scholar]
- 3.Berger DF, Sagvolden T. Sex differences in operant discrimination behaviour in an animal model of Attention- Deficit Hyperactivity Disorder BBR. 1998;94:73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- 4.Beyers JM, Toumbourou JW, Catalano RF, Arthur MW, Hawkins JD. A cross-national comparison of risk and protective factors for adolescent substance use: the United States and Australia. J Adolesc Health. 2004;35:3–16. doi: 10.1016/j.jadohealth.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry. 1998 7:59. 4–16. [PubMed] [Google Scholar]
- 6.Blanchard BA, Glick SD. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats. Recent Dev Alcohol. 1995;12:231–241. doi: 10.1007/0-306-47138-8_15. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- 8.Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996 Oct 31;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 9.Carmines EL, Gaworski CL, Faqi AS, Rajendran N. In utero exposure to 1R4F reference cigarette smoke: evaluation of developmental toxicity. Toxicol Sci. 2003;75:134–147. doi: 10.1093/toxsci/kfg155. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva GE, Ramos A, Takahashi RN. Comparison of voluntary ethanol intake by two pairs of rat lines used as genetic models of anxiety. Braz J Med Biol Res. 2004;37:1511–1517. doi: 10.1590/s0100-879x2004001000010. [DOI] [PubMed] [Google Scholar]
- 11.Da Silva GE, Vendruscolo LF, Takahashi RN. Effects of ethanol on locomotor and anxiety-like behaviors and the acquisition of ethanol intake in Lewis and spontaneously hypertensive rats. Life Sci. 2005 Jun 24;77:693–706. doi: 10.1016/j.lfs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Dasbanerjee T, Middleton FA, Berger DF, Lombardo JP, Sagvolden T, Faraone SV. A comparison of molecular alterations in environmental and genetic rat models of ADHD: a pilot study. Am J Med Genet B Neuropsychiatr Genet. 2008 Dec 5;147B:1554–1563. doi: 10.1002/ajmg.b.30877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaud LL, Alele P, Ritu C. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15:41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- 14.Di Chiara G. Alcohol and dopamine. Alcohol Health Res World. 1997;21:108–114. [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont A, Di PT, Gagne B, Barden N. Effects of chronic estrogen treatment on dopamine concentrations and turnover in discrete brain nuclei of ovariectomized rats. Neurosci Lett. 1981 Feb 23;22:69–74. doi: 10.1016/0304-3940(81)90287-1. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- 17.Emanuele MA, Wezeman F, Emanuele NV. Alcohol's effects on female reproductive function. Alcohol Res Health. 2002;26:274–281. [PMC free article] [PubMed] [Google Scholar]
- 18.Ericson M, Engel JA, Soderpalm B. Peripheral involvement in nicotine-induced enhancement of ethanol intake. Alcohol. 2000;21:37–47. doi: 10.1016/s0741-8329(99)00099-3. [DOI] [PubMed] [Google Scholar]
- 19.Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von BR. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci. 2008 Dec 17;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faraone SV. Genetics of childhood disorders: XX. ADHD, Part 4: is ADHD genetically heterogeneous? J Am Acad Child Adolesc Psychiatry. 2000;39:1455–1457. doi: 10.1097/00004583-200011000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular Genetics of Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- 24.Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield SF. Women and alcohol use disorders. Harv Rev Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- 27.Jernigan D. The USA: alcohol and young people today. Addiction. 2005;100:271–273. doi: 10.1111/j.1360-0443.2005.01006.x. [DOI] [PubMed] [Google Scholar]
- 28.Juarez J, Barrios DeTE. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- 29.Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- 30.LeSage MG, Gustaf E, Dufek MB, Pentel PR. Effects of maternal intravenous nicotine administration on locomotor behavior in pre-weanling rats. Pharmacol Biochem Behav. 2006;85:575–583. doi: 10.1016/j.pbb.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liljequist S. Changes in the sensitivity of dopamine receptors in the nucleus accumbens and in the striatum induced by chronic ethanol administration. Acta Pharmacol Toxicol (Copenh) 1978;43:19–28. doi: 10.1111/j.1600-0773.1978.tb02227.x. [DOI] [PubMed] [Google Scholar]
- 32.Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- 33.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 34.Mahliere S, Perrin D, Peyronnet J, Boussouar A, Annat G, Viale JP, Pequignot J, Pequignot JM, Dalmaz Y. Prenatal nicotine alters maturation of breathing and neural circuits regulating respiratory control. Respir Physiol Neurobiol. 2008 Jun 30;162:32–40. doi: 10.1016/j.resp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Maxwell SE, Delaney HD. Designing experiment and analyzing data. Belmont, Ca: Wadsworth Publishing; 1990. [Google Scholar]
- 36.Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav. 2001 Sep 1;74:169–176. doi: 10.1016/s0031-9384(01)00540-6. [DOI] [PubMed] [Google Scholar]
- 37.Molina BS, Pelham WE., Jr Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 38.Muneoka K, Nakatsu T, Fuji J, Ogawa T, Takigawa M. Prenatal administration of nicotine results in dopaminergic alterations in the neocortex. Neurotoxicol Teratol. 1999;21:603–609. doi: 10.1016/s0892-0362(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 39.Murphy KR, Barkley RA, Bush T. Young adults with attention deficit hyperactivity disorder: subtype differences in comorbidity, educational, and clinical history. J Nerv Ment Dis. 2002;190:147–157. doi: 10.1097/00005053-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Nasrat HA, Al-Hachim GM, Mahmood FA. Perinatal effects of nicotine. Biol Neonate. 1986;49:8–14. doi: 10.1159/000242503. [DOI] [PubMed] [Google Scholar]
- 41.Navarro HA, Mills E, Seidler FJ, Baker FE, Lappi SE, Tayyeb MI, Spencer JR, Slotkin TA. Prenatal nicotine exposure impairs beta-adrenergic function: persistent chronotropic subsensitivity despite recovery from deficits in receptor binding. Brain Res Bull. 1990;25:233–237. doi: 10.1016/0361-9230(90)90066-9. [DOI] [PubMed] [Google Scholar]
- 42.Nelson TF, Naimi TS, Brewer RD, Wechsler H. The state sets the rate: the relationship among state-specific college binge drinking, state binge drinking rates, and selected state alcohol control policies. Am J Public Health. 2005;95:441–446. doi: 10.2105/AJPH.2004.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001 Apr 6;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- 44.Paulson RB, Shanfeld J, Vorhees CV, Cole J, Sweazy A, Paulson JO. Behavioral effects of smokeless tobacco on the neonate and young Sprague Dawley rat. Teratology. 1994;49:293–305. doi: 10.1002/tera.1420490409. [DOI] [PubMed] [Google Scholar]
- 45.Paulson RB, Shanfeld J, Vorhees CV, Sweazy A, Gagni S, Smith AR, Paulson JO. Behavioral effects of prenatally administered smokeless tobacco on rat offspring. Neurotoxicol Teratol. 1993;15:183–192. doi: 10.1016/0892-0362(93)90014-f. [DOI] [PubMed] [Google Scholar]
- 46.Peters DA, Tang S. Sex-dependent biological changes following prenatal nicotine exposure in the rat. Pharmacol Biochem Behav. 1982;17:1077–1082. doi: 10.1016/0091-3057(82)90497-x. [DOI] [PubMed] [Google Scholar]
- 47.Peters DA, Taub H, Tang S. Postnatal effects of maternal nicotine exposure. Neurobehav Toxicol. 1979;1:221–225. [PubMed] [Google Scholar]
- 48.Pogun S. Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. Int J Psychophysiol. 2001;42:195–208. doi: 10.1016/s0167-8760(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 49.Pope HG, Jr, Ionescu-Pioggia M, Pope KW. Drug use and life style among college undergraduates: a 30-year longitudinal study. Am J Psychiatry. 2001;158:1519–1521. doi: 10.1176/appi.ajp.158.9.1519. [DOI] [PubMed] [Google Scholar]
- 50.Potthoff AD, Ellison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- 51.Ramos A, Kangerski AL, Basso PF, Da Silva SantosJE, Assreuy J, Vendruscolo LF, Takahashi RN. Evaluation of Lewis and SHR rat strains as a genetic model for the study of anxiety and pain. Behav Brain Res. 2002 Feb 1;129:113–123. doi: 10.1016/s0166-4328(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 52.Ribary U, Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989;248:786–792. [PubMed] [Google Scholar]
- 53.Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 54.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 55.Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998;22:252–258. [PubMed] [Google Scholar]
- 56.Romero RD, Chen WJ. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol Biochem Behav. 2004;78:675–681. doi: 10.1016/j.pbb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 57.Rosenthal R, Rosnow RL, Rubin DB. Contrasts and effect sizes in behavioral research: a correlational approach. New York: Cambridge University Press; 2000. [Google Scholar]
- 58.Russell V, Allie S, Wiggins T. Increased noradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder--the spontaneously hypertensive rat. Behav Brain Res. 2000;117:69–74. doi: 10.1016/s0166-4328(00)00291-6. [DOI] [PubMed] [Google Scholar]
- 59.Russell VA. Hypodopaminergic and hypernoradrenergic activity in prefrontal cortex slices of an animal model for attention-deficit hyperactivity disorder --- the spontaneously hypertensive rat. BBR. 2002 Mar 10;130:191–196. doi: 10.1016/s0166-4328(01)00425-9. [DOI] [PubMed] [Google Scholar]
- 60.Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct. 2005 Jul 15;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M. Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005 Jun 1;57:1239–1247. doi: 10.1016/j.biopsych.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seidler FJ, Levin ED, Lappi SE, Slotkin TA. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Brain Res Dev Brain Res. 1992 Oct 23;69:288–291. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- 64.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- 65.Slotkin TA, Lappi SE, Seidler FJ. Impact of fetal nicotine exposure on development of rat brain regions: critical sensitive periods or effects of withdrawal? Brain Res Bull. 1993;31:319–328. doi: 10.1016/0361-9230(93)90224-y. [DOI] [PubMed] [Google Scholar]
- 66.Slotkin TA, Orband-Miller L, Queen KL, Whitmore WL, Seidler FJ. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J Pharmacol Exp Ther. 1987;240:602–611. [PubMed] [Google Scholar]
- 67.Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002;111:124–133. [PubMed] [Google Scholar]
- 68.Smith AM, Kelly RB, Chen WJ. Chronic continuous nicotine exposure during periadolescence does not increase ethanol intake during adulthood in rats. Alcohol Clin Exp Res. 2002;26:976–979. doi: 10.1097/01.ALC.0000021176.13538.55. [DOI] [PubMed] [Google Scholar]
- 69.Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- 70.Söderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. BBR. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 71.Soeters HS, Howells FM, Russell VA. Methylphenidate does not increase ethanol consumption in a rat model for attention-deficit hyperactivity disorder-the spontaneously hypertensive rat. Metab Brain Dis. 2008;23:303–314. doi: 10.1007/s11011-008-9098-1. [DOI] [PubMed] [Google Scholar]
- 72.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 73.Spear LP. Alcohol Research & Health. Vol. 24. The Journal Of The National Institute On Alcohol Abuse And Alcoholism; 2000. Modeling adolescent development and alcohol use in animals; pp. 115–123. [PMC free article] [PubMed] [Google Scholar]
- 74.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- 75.StatSoft. STATISTICA for Windows. [6.1]. 2003. Tulsa, OK: StatSoft, Inc; 2003. Ref Type: Computer Program. [Google Scholar]
- 76.Steptoe A, Wardle J, Bages N, Sallis JF, Sanabria-Ferrand PA, Sanchez M. Drinking and driving in university students: an international study of 23 countries. Psychology & Health. 2004;19:527–540. [Google Scholar]
- 77.Stromberg MF, Volpicelli JR, O'Brien CP. Effects of naltrexone administered repeatedly across 30 or 60 days on ethanol consumption using a limited access procedure in the rat. Alcohol Clin Exp Res. 1998;22:2186–2191. [PubMed] [Google Scholar]
- 78.Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 79.Vendruscolo LF, Izidio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behav Pharmacol. 2008;19:21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- 80.Weitzman ER, Chen YY. Risk modifying effect of social capital on measures of heavy alcohol consumption, alcohol abuse, harms, and secondhand effects: national survey findings. J Epidemiol Community Health. 2005;59:303–309. doi: 10.1136/jech.2004.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilens TE, Biederman J. Alcohol, drugs, and attention-deficit/ hyperactivity disorder: a model for the study of addictions in youth. J Psychopharmacol. 2006;20:580–588. doi: 10.1177/0269881105058776. [DOI] [PubMed] [Google Scholar]
- 82.Williams J, Sagvolden G, Taylor E, Sagvolden T. Dynamic behavioural changes in the Spontaneously Hyperactive Rat: 1. Control by place, timing, and reinforcement rate. Behav Brain Res. 2009 Mar 17;198:273–282. doi: 10.1016/j.bbr.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 83.Witschi H, Lundgaard SM, Rajini P, Hendrickx AG, Last JA. Effects of exposure to nicotine and to sidestream smoke on pregnancy outcome in rats. Toxicol Lett. 1994;71:279–286. doi: 10.1016/0378-4274(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 84.World Health Organization. Global Status Report on Alcohol. Geneva: 1999. [Google Scholar]
- 85.Xiao D, Huang X, Lawrence J, Yang S, Zhang L. Fetal and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J Pharmacol Exp Ther. 2007;320:654–661. doi: 10.1124/jpet.106.113332. [DOI] [PubMed] [Google Scholar]
- 86.Zaborskis A, Sumskas L, Maser M, Pudule I. Trends in drinking habits among adolescents in the Baltic countries over the period of transition: HBSC survey results, 1993-2002. BMC Public Health. 2006;6:67. doi: 10.1186/1471-2458-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22:61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]


