Abstract
Classical behavioral tests in animal models of trigeminal neuropathic pain measure reflexive responses that are not necessarily measures of pain. To overcome the problem, we created a chronic constrictive nerve injury rat model of pain (CCI) by ligation of the infraorbital nerve (ION), and applied the orofacial operant test to assess behavioral responses to mechanical and cold stimulation in these rats. Animals were trained to voluntarily contact their facial region to a mechanical or a cold stimulation module in order to access sweetened milk as a positive reward. ION-CCI rats displayed aversive behaviors to innocuous mechanical stimuli, as indicated by a significant decrease in both contact time and the numbers of long contact events in comparison with sham group. For cold stimulation, ION-CCI rats displayed aversive behaviors to both innocuous (17 °C) and noxious cold temperatures (12 °C and 5 °C), as indicated by a significant decrease in both contact time and the numbers of long contact events at the cooling temperatures. The decreases of the contact time and numbers in ION-CCI rats were partially abolished by morphine. Our orofacial operant test demonstrates mechanical allodynia, cold allodynia, and hyperalgesia in rats with chronic trigeminal nerve injury. The neuropathic pain in ION-CCI rats was partially alleviated by morphine. Thus, orofacial operant test provides a desirable behavioral assessment method for preclinical studies of chronic trigeminal neuropathic pain.
Keywords: Trigeminal neuropathic pain, Mechanical allodynia, Cold allodynia, Behavioral assessment, Operant behavior
1. Introduction
Trigeminal Neuralgia is the most common debilitating orofacial neuropathic pain disorder [1–2]. It is often manifested with thermal and mechanical allodynia and hyperalgesia [2–3]. For example, trigeminal neuralgia patients can suffer severe pain triggered by a gentle air puff on their faces. This neuropathic pain disorder, among various others (e.g. temporomandibular disorders), can be difficult to treat since the pathophysiology is not well understood [2]. To better study neuropathic pain, Bennet and Xie first developed the chronic constriction injury model (CCI) in rats [4]. This method consists of tying ligatures around a sciatic nerve trunk to produce neuropathic pain states including thermal and mechanical allodynia and hyperalgesia in rat hindpaws. Orofacial regions are innervated by the trigeminal nerve system, the cranial sensory system that shares many similarities to the sciatic nerves. However, the trigeminal system has features different from the sciatic nerves. For example, trigeminal nerve branches that innervate dental pulps of the teeth are exclusively nociceptors [5]. The study of trigeminal neuropathic pain has been facilitated by adopting the sciatic CCI method to the infraorbital nerve (ION) [6–7]. The ION, the entire second division of the trigeminal nerve, is exclusively sensory and covers the most common distribution for trigeminal neuralgia in orofacial region.
In most previous studies, classical behavioral tests such as von Frey filament poking for mechanical stimulation were applied to orofacial regions [6–8]. ION-CCI animals have demonstrated behavioral alterations including paw licking, face-rubbing, limb-guarding, vocalization, grooming [6, 9]. However, these are unlearned behaviors that are reflexive responses mediated by brainstem since these behaviors could be seen in decerebrate animals [10]. Although useful in preclinical studies, these reflex behaviors do not provide information on a higher order cerebral function and are not necessarily measures of pain [11]. There are also technical concerns on the previous orofacial behavioral tests, including stress of animals in restrained condition, anticipation of stimulation as animals can visualize probes approaching to them, and investigator bias. These problems may account for the large variations in previous orofacial pain behavioral tests.
In view of the problems of classical behavioral tests, Neubert et al has developed an orofacial operant test system[12]. Operant tests of pain use a conflict paradigm to allow animals to make a choice between receiving a positive reward (drinking sweetened milk) or escaping aversive stimuli [12], and animals have control over the amount of nociceptive stimulation and can modify its behavior based on cerebral cortical processing [13–14]. Therefore, operant behavioral responses are not simple reflexive responses and are considered to be better indicator of pain in comparison with classical behavioral tests. Orofacial operant tests have been used to characterize thermal pain in normal rats following noxious heat or cold stimulation [15–16], thermal and mechanical pain in rats with facial inflammation induced by injection of carrageenan and capsaicin [12, 17]. To the best of our knowledge, there is no reported study applying orofacial operant behavior paradigm to study trigeminal neuropathic pain.
2. Materials and Methods
Male Sprague-Dawley rats (280–380 g) were used in this study. All animals were exposed to light 12 hours per day; food and water were available ad libitum. Protocol for the maintenance and use of the experimental animals were approved by the Laboratory Animal Medical Services and Institutional Animal Care and Use Committee at the University of Cincinnati. These were carried out in accordance with the NIH regulations on animal use.
Animals initially underwent two weeks of pre-surgical adaptation training utilizing the Ugo Basile Orofacial Stimulation Test System® (Comerio VA, Italy)after a 12 hour fasting food period. Rats were placed in a standard rat cage with a plastic divider to create two rooms, the testing room and the companion room. In the anterior aspect of the cage there was an Ugo Basile apparatus with a drinking window for the rat head to enter and acquire a reward (milk) located on the opposing aspect of the drinking window. Nestle Carnation® Sweetened Condensed Milk was diluted with deionized water to 30% and placed in a cylindrical plastic container with metal nipple drinker. The apparatus also consisted of a mechanical or a thermal module but the module was removed during adaptation training period. An infrared photo-beam was built on the exterior aspect of the drinking window and wired to a computer to automatically detect head accessing the feeding tube. Depending on the type of the experiment, the animals were subjected to either no stimulus during the adaptation training period, mechanical, or thermal stimulus when it attempted to poke its head through the drinking window. For the adaptation training, the orofacial apparatus was used without stimulation modules. The training was started by placing a rat in the testing room and another one in the companion room. After the rats were given 10 minutes to familiarize themselves with their environment, the drinking window was opened and the testing rat was subsequently timed for 10 minutes to allow drinking the milk.
After 2 weeks of the pre-surgical adaptation training, the rats were divided into two groups: sham and ION-CCI (Infraorbital nerve ligation). In the ION-CCI group a chronic constriction nerve injury model was created using unilateral ligation of the infraorbital nerve as described previously [7] In brief, the rats were anesthetized with intraperitoneal injection of ketamine/xylazine cocktail (100 mg/kg). The skin above the right eye was shaved and the rat head was immobilized. A 2-cm curvilinear incision was made superior to the right orbital cavity. A meticulous dissection was made, and the muscle and fascia was retracted laterally. The infraorbital nerve can be found approximately 1 cm down against the floor of the maxillary bone. The nerve was freed from the surrounding connective tissues and two ligatures were made approximately 5 mm apart with a 5-0 absorbable chromic gut suture Superion®. The incision was closed with 6-0 non-absorbable braided silk suture. The sham groups also had a similar surgery, but without any ligatures. The nerve was freed from the surrounding connective tissue and the incision closed. After a 2-week healing period, the rats underwent a 2-week period of post-surgical adaption training performed in the same manner as the pre-surgical adaptation training.
Subsequently, experiments were performed utilizing the mechanical module or thermal module during post-operative period of 4 to 8 weeks. The mechanical module was custom made. It consisted of a cassette with ten tungsten wires placed 3 mm apart from each other and at 8 mm from the opening hole to the cassette held to produce a bending force from the drinking window of the apparatus. The proximal tips of these wires were coated with a drop of ethyl 2-cranaocrylate to create blunt tips. For mechanical stimulation experiments, the animal’s face contacted the tungsten wires of mechanical module as it projected its head through the hole in the apparatus in order to drink milk located on the exterior aspect of the drinking window. In the thermal module there was a surrounding metal tubing at the opening enclosed with circulating ethylene glycol (Sigma Aldrich, US) made in a 50/50 mixture with distilled water. The temperatures of the circulating ethylene glycol solution were controlled by a thermal circulating bath unit. The distance between the metal tube and the nipple of the milk bottle was 14 mm. For thermal stimulation, thermal module was set at 24 °C, 17 °C, 12 °C, or 5 °C. The animal’s orofacial region was shaved and subjected to different cold stimuli by contacting the metal tube as it poked its head through the hole to obtain the milk. To test the effects of morphine on mechanical and cold sensitivity of ION-CCI rats, animals were administered morphine (s.c., 0.5 mg/kg) 30 min prior to the orofacial operant tests. At different days these animals were also administered saline as control and then orofacial operant tests were performed in the same manner. Similar to the adaptation training, all experiments with mechanical or thermal module were preceded by a 12 hour fasting period, 10 minutes for the rats to be familiarized with testing environment, and a subsequent 10 minutes to allow for orofacial operant behavioural assessment.
The events of head pokes were detected by the infrared photo-beam, recorded by a computer, and analysed by the Oro software (Ugo Basile, Comerio VA, Italy). This computer software recorded and analysed several variables of the rat’s behaviour including the total time the beam was broken also defined as the contact time, the count, which can also be described as number of contacts, and the maximum as well as the minimum contact time. All Data were analysed by the one way ANOVA test. Post-hoc comparisons were made using the Duncan’s test. Significant value for this statistical method was at P < 0.05.
3. Results
3.1. Experimental settings and general observations
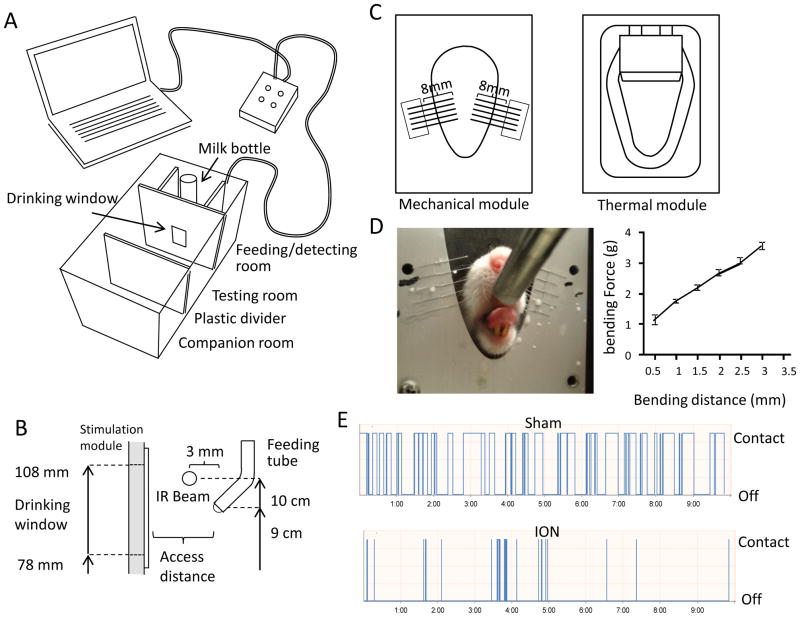
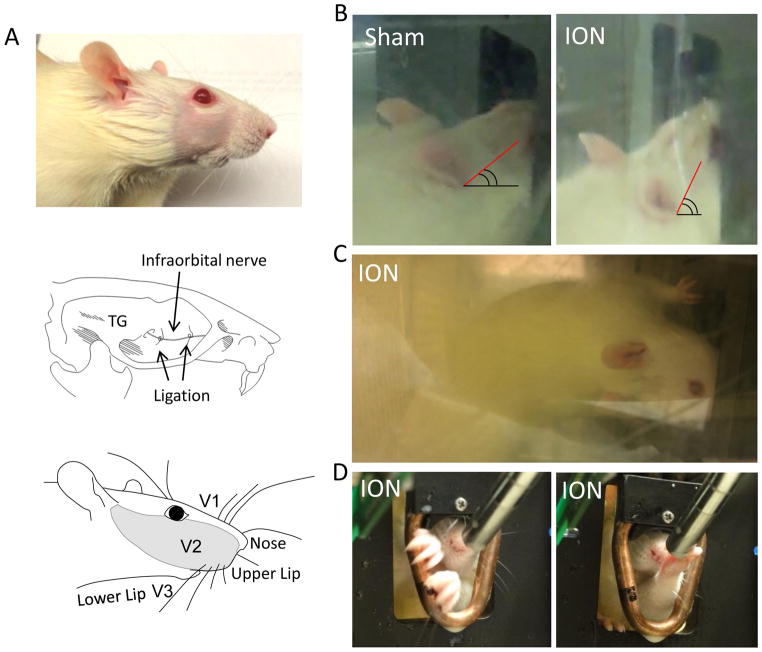
We modified the Ugo Basile Orofacial Stimulation Test System® to create a companionship environment (Figure 1A) that would expedite the adaptation of the testing rat and promote a consistent drinking behavior. When stimulation modules were placed in the drinking window, drinking behaviors were found to be also affected by the positions of the nipple of milk bottle and infrared beam. The locations of these parts were tested in preliminary studies and the optimal distances were maintained (Figure 1B) in each set of experiments. A custom made mechanical stimulation module was used for the mechanical stimulation (Figure 1C left). The module consisted of a cassette that anchored tungsten wire filaments. The length of each filament was set to 8 mm to produce desirable bending forces (Figure 1D). Our preliminary study, performed at 2 mm intervals, confirmed that the filament length of 8 mm created the optimum behavior results that could well differentiate ION-CCI group from sham group (Figure 1E). At filament length of 6 mm, all rats had excessive restriction and at 10 mm they would drink the milk with comfort such that differences between sham and ION-CCI groups were not as significant as those at 8 mm. Once this optimal length of filament was established, experiments were carried out at several access distances, defined as the distance between the mechanical module to the metal nipple drinker, to determine the optimum access distance at which the mechanical stimulation altered orofacial operant behavior of the ION-CCI rats but least affected the sham rats. The second part of the study was to test the outcome of innocuous and noxious cold allodynia on orofacial operant behaviors by using a thermal stimulation module (Figure 1C right). In order to create an orofacial region that is more sensitive to cooling temperatures, the sham and ION-CCI group rat’s facial area were shaved (Figure 2A Top panel) one day before experimentation. The orofacial region is innervated by infraorbital nerve from V2 branch of trigeminal nerve (Figure 2A middle panel). The ligation of infraorbital nerve (Figure 2A middle panel) would produce nerve injury to affect the sensations of orofacial region (shaded area in Figure 2A bottom panel) to thermal and mechanical stimuli.
Figure 1. Settings of orofacial operant behavioral test system for mechanical and thermal stimulation.
A) Diagram of the automated orofacial operant behavioral test system. It consists of a feeding/detecting room, a testing room, and a companion room. The feeding/detecting room contains a milk bottle, an infrared (IR) beam for motion detection, and stimulation modules. The testing room is a compartment where a testing rat is placed in during orofacial operant test. The companion room is a room for another rat that serves as a companion for the testing rat. It is separated from the testing room by a transparent plastic divider. B) Side view and settings of the feeding/detecting room. The distance from stimulation module to the nipple of metal feeding tube is adjustable. All other parts are at the fixed position indicated in the diagram. C) Mechanical (left panel) and thermal (right panel) modules that are positioned inside the drinking window for testing either mechanical or thermal sensitivity, respectively. For the mechanical module, each stimulation filament has an 8-mm segment that can be bended toward the nipple of milk bottle. D) An example shows a rat that was drinking milk while its orofacial regions were contacting the stimulating filaments of mechanical stimulation module (left panel). The bending force of the filaments in the mechanical module was shown to be linearly proportion to the distance of displacement (right panel). E) Two sample traces show the automatic recordings of drinking behavior in a duration of 10 min of a sham rat (upper trace) and a rat following infraorbital nerve ligation (ION-CCI, lower trace).
Figure 2. Drinking postures of sham and ION-CCI rats.
A) Illustration of infraorbital nerve ligation (ION-CCI) and affected region (V2). B) Drinking postures of a sham and an ION-CCI rat with the mechanical module. The head position of ION-CCI rat was tilt up to try to avoid contacting V2 regions with the stimulation filaments within the drinking window. C) Another ION-CCI rat showed unusual drinking posture. The rat turned his head (also body) up-side down and used its V3 regions to push away mechanical stimulation filaments in order to access the nipple of milk bottle. D) An ION-CCI rat (right side ligation) tiled its head to left side to avoid contacting the right side of the thermal module (12 °C) while trying to drink milk (left panel). Right panel shows another ION-CCI rat who learned later to use its left front paw to take milk from the nipple.
As we tested the animals under the influence of mechanical and thermal stimuli, there were some general drinking behaviors of the sham and ION-CCI rat groups that were observed and are noteworthy. During the experiments with mechanical or thermal modules, the sham rats had longer contact time per attempts to drink milk while the ION-CCI rats had shorter contact time per attempts (Figure 1E). Several interesting body postures and abnormal behaviors were observed only in ION-CCI rats. All ION-CCI rats had a unique face posture as they would tilt their heads up to contact the mandible first (V3 region) during tests (Fig. 2B right). However, control rats had direct facial (V2 region) contact with mechanical and cold stimuli condition (Figure 2B left). During the mechanical module experiment in ION-CCI rats, two rats demonstrated an inverted head posture to avoid the mechanical stimulation to orofacial regions (Figure 2C). Similarly, during experiments with cold module, ION-CCI rats avoided contact of ipsilateral ION-CCI side (right side) of their face to the cooling tube of thermal module (Fig 1D left). Another interesting behavior occurred during experiments with cold module that was set at 5°C, 2 ION-CCI rats drank with their front paw (Figure 2D, right panel). They performed this by extending their front paw to the metal nipple and contacting the milk with their front paw and subsequently bringing their paw to the mouth to lick the milk off their paw. This learned behavior only occurred after a number of tests.
3.2. Baseline of orofacial operant test before and after surgery
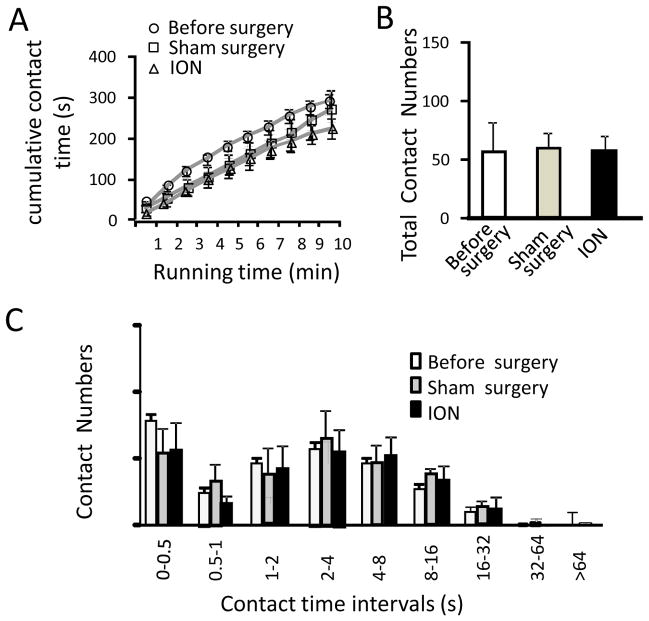
Before surgery all animals were first trained for 2 weeks (10 sessions) to drink milk through the drinking window that had no stimulation modules. Although contact time was short with a large variation among these animals in the earlier sessions of this pre-surgical adaptation training (not shown), the total contact time reached a stable level of about 300 s, 50% of the total testing time (600 s) in the last 3 sessions. Two week after surgical creation of sham (n = 7) and ION-CCI (n = 7) groups, animals continuously received training without stimulation module for two weeks (post-surgical adaptation training). The last sessions of the pre- and post-surgical adaption trainings were used as the baselines of orofacial operant test. Figure 3 shows the characterization of baseline orofacial operant tests in these animals before and after surgery in both sham and ION-CCI groups. We analyzed cumulative contact time (Figure 3A), total contact numbers (Figure 3B), and contact number histogram in sub-divided contact time intervals (Figure 3C). These baseline orofacial operant parameters were not significantly modified in either group when simulation modules were not placed in the drinking window.
Figure 3. Baseline of orofacial operant tests before and after surgery.
A) Baseline cumulative contact time of orofacial operant tests before surgery (open circles, n = 14 rats), after sham surgery (open squares, n = 7), and after ION-CCI (open triangles, n = 7). B) Baseline total contact numbers before surgery (open bars, n = 14 rats), after sham surgery (gray bars, n = 7), and after ION-CCI (dark bars, n = 7). C) Baseline contact number histogram before surgery (open bars, n = 14 rats), after sham surgery (gray bars, n = 7), and after ION-CCI (dark bars, n = 7). Baseline tests were performed without stimulation module in the drinking window. Data represent Mean ± SEM.
3.3. Effects of mechanical stimulation on orofacial operant behaviors
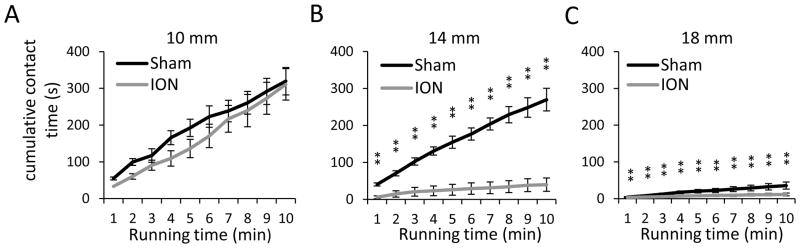
We performed orofacial operant tests on sham and ION-CCI groups with mechanical module to study effects of mechanical stimulation on their orofacial operant behaviors. These experiments were performed on rats between 4 weeks to 8 weeks after surgery. The reason that we waited for 4 weeks to perform orofacial operant test with stimulation modules was to avoid the potential influence of postoperative pain not related to neuropathy. Three bending force conditions were tested, which were achieved by setting the access distances of 10, 14, or 18 mm between the mechanical module and the nipple of the milk bottle. At 10 mm, animal orofacial regions just touched filaments while they drank milk with filament bending distance being minimum (< 0.5 mm). Bending force produced by this minimal bending distance was in the range of 0 to 1 g (Figure 1D). The sham and ION-CCI rats had similar cumulative contact time (Figure 4A) at 10 mm access distance. The total contact time was not significantly different between the sham (319 ± 37 s, n = 7) and the ION-CCI group (311 ± 45 s, n = 7) (Figure 4A). At 14 mm access distance, animals had to press the filament using their orofaicial region in order to access the nipple of milk bottle and the bending distances during their head movement for milk drinking is estimated to be 0.5 to 3 mm, which corresponds to bending force of 1 to 4 g (Figure 1D) in each filament. The cumulative contact time of ION-CCI group was significantly shorter than that of sham group at each time point at 14 mm access distance (Figure 4B, n = 7, P < 0.01). For example, the total contact time was 40 ± 18 s (n = 7) for ION-CCI group and 270 ± 31 s (n = 7) for sham group, and the total contact time of ION-CCI group was significantly shorter than that of the sham group (P < 0.01, Figure 4B). At 18 mm access distance, the bending distance was 5 to 8 mm, which corresponded to the bending force of 5 to 9 g in each filament. The cumulative contact time of ION-CCI group was significantly shorter than that of sham group at 18 mm access distance (Figure 4C, n = 7, P < 0.01). Also, compared to baseline, the cumulative contact time for both sham and ION-CCI at 18 mm access distance was significantly shorter at every time point (compare Figure 4C with Figure 3A, n = 7, P < 0.01).
Figure 4. Contact time of orofacial operant test with mechanical stimulation.
A–C) Cumulative contact time over the test period of 10 min at the access distance of 10 (A), 14 (B), and 18 mm (C). Dark line, sham group (n = 7); gray line, ION-CCI group (n = 7). The experiments were performed with mechanical module placed in the drinking window. The access distance is the distance between the nipple of milk bottle and the mechanical stimulation module. Data represent Mean ± SEM; * p < 0.05; ** p < 0.01.
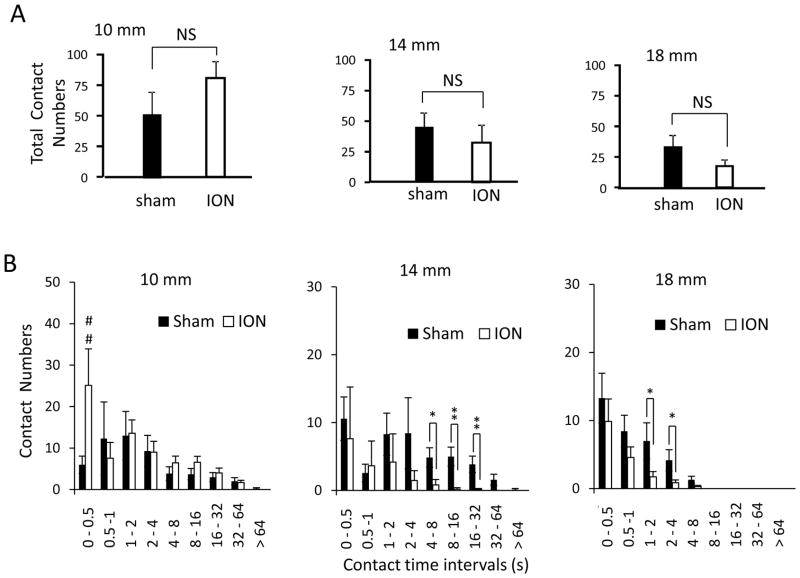
At each of the three access distances tested, the total contact numbers in ION-CCI group was not found to be significantly different between the sham and ION-CCI groups (Figure 5A). This indicates that the significant reduction of total contact time in ION-CCI group shown in Figure 4B&C was not due to the decreases of animals’ attempts to drink milk. To gain an insight into the cause for the reduction of cumulative contact time, we analyzed distribution of contact numbers in different contact time intervals (Figure 5B). At 10 mm access distance, contact numbers were not significantly different between sham and ION-CCI group in each contact time interval except the shortest contact time interval of 0–0.5 s at which ION-CCI group had higher contact numbers (25 ± 10, n= 7) than sham group (6 ± 3, n = 7, P < 0.05, Figure 5B first panel). At 14 mm access distance, ION-CCI group showed significantly lower contact numbers in 4–8, 8–16 s, and 16–32 s contact time intervals in comparison with sham group (Figure 5B, second panel). For example, at contact time interval of 16–32 s, ION-CCI group only had contact numbers of 0.1 ± 0.1 (n = 7) whereas sham group had contact number of 3.8 ± 1.2 (n = 7, P < 0.01). At 18 mm access distance, both sham group and ION-CCI group were unable to contact in the time interval of 8–16 s and longer time intervals (Figure 5B, third panels). However, ION-CCI group showed significantly less contact in the time intervals of 1–2 and 2–4 s (n =7, P < 0.05) compared to the sham group (n = 7). These results indicated that mechanical stimulations with access distance of 14 mm or longer were less tolerable at a longer duration for ION-CCI group in comparison with sham group.
Figure 5. Contact numbers of orofacial operant test with mechanical stimulation.
A) Total contact numbers of sham (n = 7) and ION-CCI (n = 7) groups at the access distance of 10 (1st panel), 14 (2nd panel), and 18 mm (3rd panel). B) Contact number histogram at access distance of 10 (1st panel), 14 (2nd panel), and 18 mm (3rd panel) in sham (n = 7) and ION-CCI (n = 7) groups. The experiments were performed with mechanical module in the drinking window. The access distance is the distance between the nipple of milk bottle and the mechanical stimulation module. Data represent Mean ± SEM; NS, no significant difference; * p < 0.05; ** p < 0.01.
3.4. Effects of innocuous and noxious cold on orofacial operant behaviors
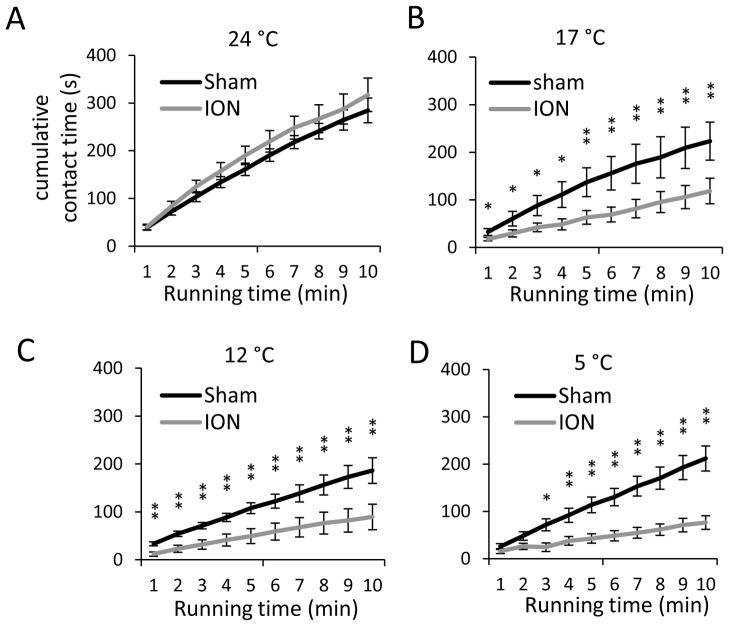
We performed orofacial operant test with thermal module to study effects of innocuous and noxious cold stimuli on orofacial operant behaviors. The temperatures of the contact surface of thermal module were set to three distinct thermal categories at 24 °C as neutral temperature, 17 °C as cooling, and 12 °C and 5 °C as noxious cold stimuli. At 24 °C (Figure 6A) the cumulative contact time was not significantly different between the sham (n = 7) and ION-CCI groups (n = 6). The total contact time at the end of the 10-min test session were 285 ± 26 s (n = 7) for sham group and 317 ± 35 s (n = 7) for ION-CCI group (Figure 6A). These results were similar to the baseline levels shown in Figure 3A. At 17 °C (Figure 6B), the cumulative contact time for ION-CCI group was significantly less than the sham group throughout each time point. For example, the total contact times at the end of the 10-min test session were 224 ± 40 s (n = 7) for sham group and 119 ± 27 s (n = 7, P < 0.01) for ION-CCI group (Figure 6B). We also compared the cumulative contact time between 17 °C and 24 °C. For ION-CCI group, throughout each time point the cumulative contact time at 17 °C (Figure 6B) was significantly less than that at 24 °C (Figure 6A, P < 0.05). For sham group, no significant differences were found between the two temperatures. These results indicate that the temperature of 17 °C is innocuous for sham group, but noxious for ION-CCI group (i.e. cold allodynia). At 12 °C, the cumulative contact time of ION-CCI group was significantly less than the sham groups through each time point (Figure 6C). For example, the total contact times at the end of the 10-min test session were 186 ± 27 s (n = 7) for sham group and 89 ± 27 s (n = 7, P < 0.01) for ION-CCI group (Figure 6C). For both ION-CCI and sham groups, contact time also showed significantly less at 12 °C (Figure 6C) than at 24 °C (Figure 6A, P < 0.05). These results indicate that cold temperature of 12 °C is noxious for sham group (physiological cold pain) and that ION-CCI group showed hyperalgesia to noxious cold. Similar to the results at 12°C, cold hyperalgesia was observed in ION-CCI group and physiological cold pain was seen in sham group at 5 °C (Figure 6D).
Figure 6. Contact time of orofacial operant test with cold stimulation.
A–D) Cumulative contact time over the test period of 10 min at 24°C (A), 17°C (B), 12°C (C), and 5°C (D). Dark line, sham group (n = 7); gray line, ION-CCI group (n = 6). The experiments were performed with thermal module placed in the drinking window. The temperatures are the surface temperatures of the thermal contact tube. Data represent Mean ± SEM; * p < 0.05; ** p < 0.01.
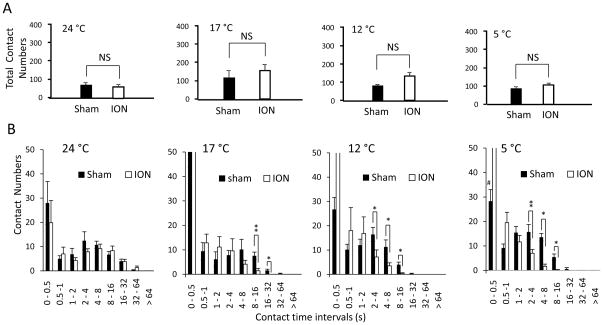
Total contact numbers were not found to be significantly different between the sham and ION-CCI groups at each temperature tested (Figure 7A). We analyzed distribution of contact numbers in different contact time intervals (Figure 7B). At 24 °C the contact numbers in different contact time intervals were not significantly different between sham and ION-CCI groups (Figure 7B 1st panel, n = 7 for sham and 6 for ION-CCI). At 17 °C the ION-CCI group had significantly lower contact numbers at 8–16 s and 16–32 s contact time interval than those in sham group (Figure 7B 2nd panel, n = 7 for sham and 6 for ION-CCI, P < 0.05). At 12 °C the ION-CCI group had significantly lower contact number at 2–4, 4–8, and 8–16 s contact time intervals (Figure 7B 3rd panel, n = 7 for sham and 6 for ION-CCI, P < 0.05). At 5 °C the ION-CCI group had significantly lower contact number at 2–4, 4–8, and 8–16 s contact time intervals (Figure 7B 4th panel, n = 7 for sham and 6 for ION-CCI, P < 0.05). However, the ION-CCI group showed significantly increased contact number at 0.5~1 second interval (P < 0.05). These results indicate that longer time contact was less tolerable for ION-CCI at both innocuous and noxious cold in comparison with sham group.
Figure 7. Contact numbers of orofacial operant test with cold stimulation.
A) Total contact numbers of both sham (n = 7) and ION-CCI (n = 6) groups at 24, 17, 12, and 5 °C. B) Contact number histogram at temperatures of 24, 17, 12, and 5 °C in sham (n = 7) and ION-CCI (n = 6) groups. The experiments were performed with thermal module placed in the drinking window. The temperatures are the surface temperatures of the thermal contact tube. Data represent Mean ± SEM; NS, no significant difference; * p < 0.05; ** p < 0.01.
3.5. Effects of morphine on orofacial operant behaviors in ION-CCI rats
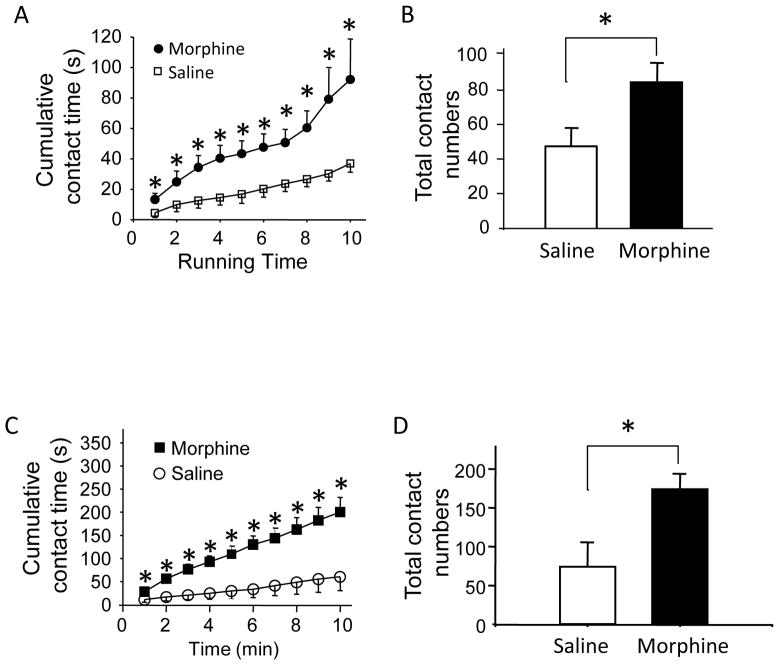
We determined if orofacial operant behavioral test could demonstrate the effects of morphine on orofacial neuropathic pain in our ION-CCI rats. For mechanical sensitivity, contact time in ION-CCI rats that received saline injection (Fig. 8A) was similar to that without injecting anything (Fig. 4B). However, following the administration of morphine (0.5 mg/kg) to these ION-CCI rats, contact time was significantly longer than that with saline (Fig. 8A). For example, at the end of 10-min session, the contact time was 37 ± 6 s (n = 7) after administration of saline and increased to 92 ± 26 s (n = 7, p < 0.05) after morphine. When total contact numbers were compared, morphine significantly increased contact numbers in the ION-CCI rats, from 47 ± 11 with saline to 84 ± 10 with morphine (n = 7, p < 0.05). For cold sensitivity, we determined effects of morphine on orofacial operant test with thermal module set at 5 °C. Administration of saline did not significantly affect the contact time of ION-CCI rats (Fig. 8C) when compared with the ION-CCI rats without injecting anything (Fig. 6D). However, morphine significantly increased contact time in the cold orofacial operant test (Fig. 8C). For examine, at the end of 10-min session, the contact time was 60 ± 30 s (n = 7) after saline administration and increased to 200 ± 32 s (n = 6, p < 0.05) after morphine. The total contact numbers after morphine were also significantly increased, from 74 ± 31 with saline to 174 ± 20 with morphine (n = 6, p < 0.05).
Figure 8. Effects of Morphine on orofacial operant test in ION-CCI rats.
A&B) Cumulative contact time (A) and total contact numbers (B) of orofacial operant test with mechanical stimulation module. Access distance of mechanical module was set at 14 mm. C&D) Cumulative contact time (C) and contact numbers (D) of orofacial operant test with thermal stimulation module (5 °C). ION-CCI rats were administrated with saline or morphine. Data represent Mean ± SEM, * p < 0.05.
4. Discussion
The behavioral outcomes with orofacial operant tests in this study strongly suggest the presence of chronic orofacial neuropathic pain manifested by mechanical allodynia, cold allodynia, and cold hyperalgesia in the ION-CCI rats. This result well resembles the main symptoms of chronic trigeminal neuropathic pain in human patients. Furthermore, morphine was shown to partially alleviate mechanical and cold pain in ION rats, a result validating the suitability of our orofacial operant tests for future preclinical study to identify new and more effective drugs for chronic trigeminal neuropathic pain. To our knowledge, this is the first report using the orofacial operant tests to assess chronic orofacial neuropathic pain in animals following ION-CCI.
The orofacial operant tests presented in this study are investigator independent, performed by animals voluntarily, and animal behaviors are recorded automatically. Therefore, animals had less stress and anticipation in our study than those using classical pain behavioral assessment methods such as testing mechanical sensitivity with von Frey filaments. More importantly, the animals’ operant behaviors in our study are the integrative results of sensory stimulation and cortical processing rather than simple noxious reflex seen in the classical pain behavioral assessments. Thus, our orofacial operant tests on ION-CCI rats is a desirable approach for assessing chronic orofacial neuropathic pain with the outcomes that well resembles trigeminal neuropathy in clinical settings.
A previous study using orofacial operant test showed that noxious heat decreased contact time and that the heat response was sensitized under the acute inflammatory condition induced by carrageenan [12] or capsaicin [17]. Orofacial operant test also demonstrated the sensitization of mechanical response in rats after injection of capsaicin into facial region to produce acute inflammation [17]. In our study, we focused on mechanical and cold hypersensitivity in our ION-CCI rats since these two types of sensory abnormalities are more prominent features seen in patients with trigeminal neuropathic pain [2]. We delineated that our ION-CCI animals had orofacial mechanical allodynia as validated with a significant reduction of contact time (Figure 4B&C). Although the total contact number was not significantly affected, we found that the numbers of long-contacts became significantly reduced in ION-CCI rats in comparison with the sham group, a result that displayed the behavioral conflict between positive reward and nociceptive stimulation for ION-CCI rats and thereby indicated the presence of mechanical allodynia in these animals.
We used orofacial operant test to study cold sensitivity in our ION-CCI rats because cold allodynia is also a common symptom of chronic trigeminal neuropathic pain [2]. A previous study using orofacial operant test in normal rats showed that neither contact numbers nor contact time were significantly changed until cooling temperatures dropped down to -4 °C, but menthol, a TRPM8 agonist could sensitize cold responses at 10 °C [16]. It is unclear why noxious cold at 10 and 2 °C did not significantly modify animals’ orofacial operant behavioral in the previous study [16]. However, in our study we observed a significant reduction of contact time at 12 °C and 5 °C, indicating that our method is more sensitive than the previous study [16] and our results better represent physiological pain responses to noxious cold. The discrepancy between the previous study and ours could be due to the differences in the settings of orofacial operant test apparatus. Cold sensitivity has not been studied previously with orofacial operant tests under any types of pathological pain conditions. We showed that ION-CCI resulted in cold allodynia as manifested by the decrease of contact time and the numbers of long contact at the cooling temperature of 17 °C. Our ION-CCI rats also showed cold hyperalgesia as tested at noxious cold temperatures of 12 and 5 °C. In a previous study by us using CCI rats with sciatic nerve ligation, we have shown that the up-regulation of TRM8 in L5 DRG neurons is a molecular mechanism of the cold allodynia in sciatic CCI rats [18]. It is conceivable that chronic trigeminal nerve injury may also result in the up-regulation of TRPM8 channels in trigeminal neurons to result in cold allodynia and hyperalgesia.
We have shown in our ION-CCI rats that both mechanical allodynia and cold hyperalgesia were alleviated by morphine as indicated by the increases of contact time and total contact numbers in these animals. However, the mechanical allodynia in our ION-CCI rats appears to be only partially relieved by morphine since the contact time after morphine remained to be less than that of sham group or that of ION-CCI group without mechanical module. It is possible that the dose of morphine used in our study was not high enough to completely suppress nociceptive transmission provoked by mechanical stimulation. Alternatively, the inability to completely relieve the pain behaviors by morphine is the nature of orofacial neuropathic pain in our ION-CCI rats. In fact, orofacial neuropathic pain is known not to be fully response to the treatment with opioids in human patients [19–20].
In summary, our study for the first time indicates that orofacial operant test is a suitable behavioral assessment for chronic trigeminal neuropathic pain. This behavioral assessment could provide a useful approach for both basic and translational research on chronic orofacial neuropathic pain.
Research Highlights.
The orofacial operant test was used to assess chronic pain in rats with infraorbital nerve injury.
Operant behaviors revealed mechanical and cold allodynia and cold hyperalgesia in these rats.
Operant behaviors also revealed pain relief by morphine in these trigeminal pain animals.
Orofacial operant test is a desirable method for studying chronic trigeminal neuropathic pain.
Acknowledgments
We thank J. Neubert for his scientific and technical advice at the early stage of this study, J. Strong for comments on an earlier version of this manuscript. This work was supported by a NIH grant DE018661 to J.G.G
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennetto L, Patel NK, Fuller G. Trigeminal neuralgia and its management. BMJ. 2007;334:201–5. doi: 10.1136/bmj.39085.614792.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakrzewska JM, McMillan R. Trigeminal neuralgia: the diagnosis and management of this excruciating and poorly understood facial pain. Postgrad Med J. 2011;87:410–6. doi: 10.1136/pgmj.2009.080473. [DOI] [PubMed] [Google Scholar]
- 3.Iwata K, Imamura Y, Honda K, Shinoda M. Physiological mechanisms of neuropathic pain: the orofacial region. Int Rev Neurobiol. 2011;97:227–50. doi: 10.1016/B978-0-12-385198-7.00009-6. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GJ, Xie YK. A Peripheral Mononeuropathy in Rat That Produces Disorders of Pain Sensation Like Those Seen in Man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 5.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–8. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 6.Vos BP, Strassman AM, Maciewicz RJ. Behavioral Evidence of Trigeminal Neuropathic Pain Following Chronic Constriction Injury to the Rats Infraorbital Nerve. Journal of Neuroscience. 1994;14:2708–23. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kernisant M, Gear RW, Jasmin L, Vit JP, Ohara PT. Chronic constriction injury of the infraorbital nerve in the rat using modified syringe needle. J Neurosci Methods. 2008;172:43–7. doi: 10.1016/j.jneumeth.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu M, Aita M, Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: behavioral, neural, and glial reactions. J Pain. 2008;9:1036–48. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos BP, Hans G, Adriaensen H. Behavioral assessment of facial pain in rats: face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat’s infraorbital nerve. Pain. 1998;76:173–8. doi: 10.1016/s0304-3959(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 10.Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–43. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- 11.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 12.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–95. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Vierck CJ, Jr, Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neuroscience. 2003;119:223–32. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 14.Mauderli AP, Acosta-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. J Neurosci Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- 15.Rossi HL, Neubert JK. Effects of hot and cold stimulus combinations on the thermal preference of rats. Behav Brain Res. 2009;203:240–6. doi: 10.1016/j.bbr.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi HL, Vierck CJ, Jr, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Mol Pain. 2006;2:37. doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolan TA, Hester J, Bokrand-Donatelli Y, Caudle RM, Neubert JK. Adaptation of a novel operant orofacial testing system to characterize both mechanical and thermal pain. Behav Brain Res. 2011;217:477–80. doi: 10.1016/j.bbr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xing H, Chen M, Ling J, Tan W, Gu JG. TRPM8 mechanism of cold allodynia after chronic nerve injury. J Neurosci. 2007;27:13680–90. doi: 10.1523/JNEUROSCI.2203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 20.Eide PK, Jorum E, Stubhaug A, Bremnes J, Breivik H. Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain. 1994;58:347–54. doi: 10.1016/0304-3959(94)90129-5. [DOI] [PubMed] [Google Scholar]