Abstract
Thyme (Thymus vulgaris L., Lamiaceae) is an aromatic and medicinal plant that has been used in folk medicine, phytopharmaceutical preparations, food preservatives, and as an aromatic ingredient. The effect of Thymus vulgaris essential oil (TEO) and its isolated constituents thymol and cavacrol (CVL) were studied in the following experimental models: ear edema, carrageenan-induced pleurisy, and chemotaxis in vitro. In the pleurisy model, TEO, CVL, and thymol significantly inhibited inflammatory edema. However, only TEO and CVL inhibited leukocyte migration. In the in vitro chemotaxis experiment, CVL inhibited leukocyte migration, whereas thymol exerted a potent chemoattractant effect. In the ear edema model, CVL (10 mg/ear), applied topically, reduced edema formation, exerting a topical anti-inflammatory effect. Thymol did not reduce edema formation but rather presented an irritative response, probably dependent on histamine and prostanoid release. Our data suggest that the antiinflammatory effects of TEO and CVL are attributable to the inhibition of inflammatory edema and leukocyte migration.
1. Introduction
Thyme (Thymus vulgaris L., Lamiaceae), a small subshrub native to the western Mediterranean region of Europe, has a long history of use and is a chemically variable species [1]. In folk medicine, some Thymus spp. are used for their antihelminthic, expectorant, antiseptic, antispasmodic, antimicrobial, antifungal, antioxidative, antivirotic, carminative, sedative, and diaphoretic effects. They are usually administered by infusion or are used externally in baths to cure rheumatic and skin disease [2, 3]. Reports indicate that the volatile oils of thyme are among the main essential oils used in the food industry and in cosmetics as preservatives and antioxidants [1].
Thymus vulgaris essential oil (TEO) is a mixture of monoterpenes. The main compounds of this oil are the natural terpenoid thymol and its phenol isomer carvacrol (CVL) [4, 5], which have antioxidative, antimicrobial, antitussive, expectorant, antispasmodic, and antibacterial effects [6–10]. Terpenoids, flavonoid aglycones, flavonoids glycosides, and phenolic acids were also found in Thymus spp. [11].
Several studies have been performed with plant extracts [4, 12, 13], but few studies have evaluated the effects of TEO and its isolated constituents in the inflammatory response. In the present study, the effects of TEO and its isolated components thymol and CVL were studied in experimental models of ear edema, carrageenan-induced pleurisy, and chemotaxis in vitro.
2. Materials and Methods
2.1. Plant Material and Isolation of the Essential Oil
2.1.1. Plant Material
The fresh leaves of Thymus vulgaris L. were collected from the Profa Irenice Silva Medicinal Plant Garden on the campus of the State University of Maringá, Paraná, Brazil. The leaves were identified and authenticated by botanist Maria Aparecida Sert. A voucher specimen was deposited in the Herbarium of the Department of Botany, State University of Maringá (no. 11329).
2.1.2. Isolation of the Essential Oil
The leaves of Thymus vulgaris were extracted by conventional steam distillation using a Clevenger-type apparatus for 2 h. The obtained essential oil was dried over sodium sulphate and stored at 4°C in dark vials until tested. The yield of TEO was 1.76% v/w. Thymol and CVL were isolated from TEO as fractions of hydrodistillated oil.
2.2. Analysis of the Essential Oil and Compound Identification
2.2.1. Gas Chromatography-Mass Spectrometry
Gas chromatography (GC) was performed with a Thermo Electron Corporation Focus GC model under the following conditions: DB-5 capillary column (30 m × 0.32 mm, and 0.50 mm); column temperature, 60°C (1 min) to 180°C at 3°C/min; injector temperature, 220°C; detector temperature, 220°C; split ratio, 1 : 10; carrier gas, He; flow rate, 1.0 mL/min. The volume injected (1 μL) was diluted in chloroform (1 : 10). The GC/mass spectrometry (MS) analysis was performed with a Quadrupole mass spectrometer (DSQ II model, Thermo Electron Corporation) that operated at 70 V. The identification of the individual compounds was based on comparisons of their GC retention indices (RI) on an apolar column and comparisons with the mass spectra of authentic standards purchased from Sigma-Aldrich and literature data [14]. The retention indices (RI) were obtained with reference to n-alkane C7H16–C44H90 series (supelco-Bellefonte USA, UK).
2.2.2. Nuclear Magnetic Resonance
The Nuclear Magnetic Resonance (NMR) was used to prove the chemical structure of the essential oil constituents identified by CG-MS. 1H (300.06 MHz) and 13C nuclear magnetic resonance (NMR; 75.45 MHz) spectra were recorded in a deuterated chloroform (CDCl3) solution using a Mercury-300BB spectrometer, with δ (ppm) and spectra referenced to CDCl3 (δ 7.27 for 1H and δ 77.00 for 13C) as the internal standard.
2.3. Animals
Male Wistar rats (weighing 180–220 g) and male Swiss mice (weighing 25–30 g) were provided by the Central Animal House of the State University of Maringá. The animals were housed at 22 ± 2°C under a 12 h/12 h light/dark cycle. Prior to the experiments, the animals were fasted overnight, with water provided ad libitum. The experimental protocols were approved by the Ethical Committee in Animal Experimentation of the State University of Maringá (CEAE/UEM 066/2010).
2.4. Bioassays for Cytotoxic Activity
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-2H-tetrazolium bromide) assay is based on the mitochondrial enzyme reduction of tetrazolium dye that detects and determines cell viability. The leukocytes were obtained from the peritoneal cavity of mice 4 h after zymosan injection (1 mg/cavity, i.p.). Briefly, the cells (5 × 105 cells/well) were exposed to CVL (1, 3, 10, 30, and 90 μg/mL), thymol (1.5, 15, and 150 μg/mL, dissolved in dimethyl sulfoxide [DMSO]), or TEO (1, 3, 10, 30, and 90 μg/mL) for 90 min at 37°C in 5% CO2. A volume of 10 μL of MTT (5 mg/mL; Sigma) was added to each well. After 2 h, 150 μL of the supernatant was removed, and 100 μL DMSO was added to each well. The cells were incubated at 25°C for an additional 10 min, and absorbance was measured using a Biochrom Asys Expert plus microplate reader (Asys) at a wavelength of 540 nm. The values of the blank wells were subtracted from each well of treated and control cells. The percentage of viability was determined by the following formula:
| (1) |
2.5. Acute Toxicity Test
Fasted mice were orally treated with TEO. The doses progressively increased to determine the dose necessary to produce lethality in 50% of the animals (LD50). The mice were observed for 7 days following the treatments. Food and water were provided ad libitum throughout the experiment. The number of mice that died within the study period was noted for each group. The LD50 was calculated according to the literature [15]. An equivalent dose of vehicle was administered to the control group.
2.6. Carrageenan-Induced Pleurisy in Rats
This test was performed according to the technique described by Vinegar et al. [16]. The animals were orally pretreated with TEO (250, 500, or 750 mg/kg), CVL (100, 200, or 400 mg/kg), and thymol (100, 200, or 400 mg/kg). Indomethacin (5 mg/kg) and celecoxib (10 mg/kg) were used as standard drugs. Control rats received only water. One hour later, all of the animals, with the exception of the normal group, received an intrapleural injection of carrageenan (200 μg/animal). Four hours later, the animals were euthanized, and the pleural exudate was collected. The volume was determined, and the pleural cavity was washed with 2.0 mL phosphate-buffered saline (PBS) that contained EDTA. The exudate volume was measured, and a 50 μL aliquot was used to determine the number of leukocytes in a Neubauer chamber. For total leukocyte count, red blood cells were lysed by adding Turk's solution. For differential cell counting, the fluid was centrifuged at 2500 rotations per minute for 10 min. Exudate smears were prepared, dried, fixed, and stained with May-Grunwald-Giemsa. The numbers of mononuclear and polymorphonuclear leukocytes in the exudate were determined by optical microscopy, with 100 cells counted per slide. The results are expressed as mean ± SEM.
2.7. In Vitro Chemotaxis
To evaluate the effects of CVL and thymol on chemotaxis, leukocytes were obtained from the peritoneal cavity of mice 4 h after zymozan injection (1 mg/cavity, i.p). The cell number was adjusted to 1 × 106 cells/mL in RPMI medium containing 0.1% bovine serum albumin (BSA). The chemotaxis assay was performed using a 48-well microchemotaxis plate (Neuro Probe), in which the chambers were separated by a polyvinylpyrrolidone-free polycarbonate membrane (5 μm pore size). The chemoattractants N-formyl methionyl leucyl phenylalanine (fMLP; 10−6 M) and leukotriene B4 (LTB4; 10−8 M) and a negative control (RPMI 1640) were placed in the lower chamber. A leukocyte suspension (1 × 106 cells/mL) pretreated with thymol (0.3, 1, 3, 10, 30, or 90 μg/mL) and CVL (0.3, 1, 3, 10, 30, or 90 μg/mL) for 30 min was then placed in the upper chamber. The cells were allowed to migrate into the membrane for 1 h at 37°C in 5% CO2. Following incubation, the membrane was washed in PBS, fixed in methanol, and stained with Instant Prov. The membrane area of each well was scored using light microscopy to count the intact cells present in five random fields. The results are expressed as the mean number of leukocytes per field and are representative of three separate experiments.
To evaluate the chemoattractant effect of thymol on leukocyte chemotaxis, thymol was tested at concentrations of 1.5, 15, and 150 μg/mL. The cells were obtained from the peritoneal cavity as described above. Thymol or RPMI 1640 were placed in the lower chamber. The leukocyte suspension (1 × 106 cells/mL) was placed in the upper chamber, and the chemotaxis assay was performed as described above.
2.8. Topical Ear Edema
Cutaneous inflammation was induced by the application of 5% croton oil (10 μL) in acetone (vehicle) to the inner surface of the right ear in mice. The left ear received an equal volume of vehicle. CVL (10, 20, and 40 mg/ear), thymol (10 mg/ear), indomethacin (0.5 mg/ear), dexamethasone (0.1 mg/ear), and vehicle were topically applied to the right ear 1 h before croton oil application. Four hours after the inflammatory stimulation, the mice were sacrificed, and a plug (7 mm diameter) was removed from both the treated and untreated ears (n = 10). Edema was measured as the weight difference between the two plugs. The data are expressed as the mean ± SEM weight of the ears.
Thymol as a topical irritative was also tested. Ten microliters of thymol was applied to the right ears of mice, and the left ears received an equal volume of vehicle (acetone). The animals were treated with indomethacin (5 mg/kg, orally) or promethazine (5 mg/kg, i.p.) 60 min before the thymol application. Croton oil was used as a positive control. Ear edema was determined 30, 60, 120, 180, and 240 min after the inflammatory stimulation and is expressed as the increase in ear thickness measured with an electronic micrometer (Digimess) before and after the induction of the inflammatory response. The micrometer was applied near to the tip of the ear just distal to the cartilaginous ridges, and the thickness was determined in micrometers. To minimize the variations caused by using this technique, a single investigator made the measurements throughout all of the experiments. The data are expressed as the mean ± SEM ear measurements.
2.9. Myeloperoxidase Activity
Myeloperoxidase (MPO) activity was assayed in the supernatant of homogenates of the ear sections (untreated controls and animals treated with CVL, thymol, and 0.1 mg dexamethasone) [17]. The ears were placed in 50 mM potassium phosphate buffer (pH 6.0) that contained 0.5% hexadecyl trimethyl ammonium bromide (1 mL/50 mg of tissue; Sigma, St. Louis, MO, USA) in a Potter homogenizer. The homogenate was shaken in a vortex mixer and centrifuged for 5 min. Ten microliters of the supernatant was added to each well in triplicate in a 96-well microplate. Two hundred microliters of the buffer solution that contained 16.7 mg O-dianisidine dihydrochloride (Sigma), 90 mL double-distilled water, 10 mL potassium phosphate buffer, and 50 μL of 1% H2O2 was added. The enzyme reaction was stopped by the addition of sodium acetate. Enzyme activity was determined by the absorbance measured at 460 nm using a microplate spectrophotometer (Spectra Max Plus).
2.10. Statistical Analysis
The data are expressed as the mean ± SEM for each group. The data were statistically analyzed using one-way variance analysis followed by Tukey's test. Differences were considered significant at P < 0.05.
3. Results and Discussion
The essential oils obtained from the leaves of Thymus are rich in monoterpene phenols, especially thymol and CVL [5]. The chemical composition of TEO was investigated using GC-MS and NMR. The results of the GC-MS analysis (Figure 1) showed a predominance of CVL (45.5%), α-terpineol (22.9%), and endo-Borneol (14.3%). The percentages of the major components and their retention indices are summarized in Table 1.
Figure 1.
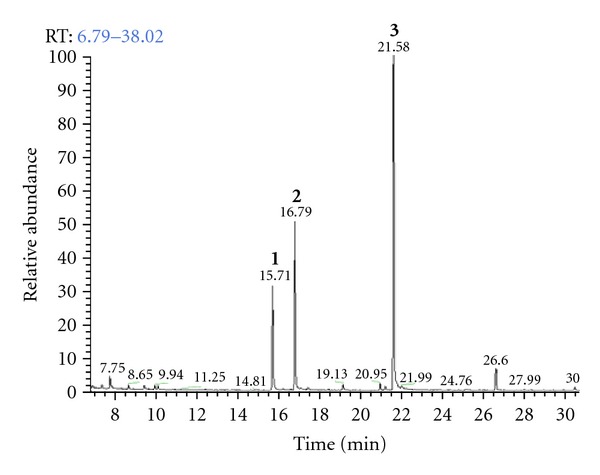
GC chromatogram of Thymus vulgaris L. essential oil. Percentage data were obtained by gas chromatography-mass spectrometry (GC-MS). Peak (1) endo-Borneol (14.3%); (2) α-Terpineol (22.9%); (3) Carvacrol (45.5%).
Table 1.
Percentual chemical composition of Thymus vulgaris leaves essential oil.
| Retention time | Retention indexa | Compound | Percentual (%) | Identification |
|---|---|---|---|---|
| 6.8 | 945 | Solvente | — | MSb |
| 7.8 | 960 | α-Pinene | 1.9 | MS, NMRd |
| 8.7 | 1022 | p-Cymene | 0.6 | MS, NMR |
| 9.9 | 1022 | Limonene | 0.6 | MS, NMR |
| 10.1 | 1028 | γ-Terpinene | 1.1 | MS, NMR |
| 11.3 | 1056 | Linalool | 0.2 | MS, NMR |
| 14.8 | 1143 | Camphor | 0.1 | MS, NMR |
| 15.7 | 1163 | endo-Borneol | 14.3 | MS, NMR |
| 16.2 | 1175 | 4-Terpineol | 0.7 | MS, NMR |
| 16.8 | 1187 | α-Terpineol | 22.9 | MS, NMR |
| 19.1 | 1240c | Carvacrol methyl ether | 1.3 | MS, NMR |
| 21.0 | 1289c | Thymol | 0.9 | MS, NMR |
| 21.6 | 1297c | Carvacrol | 45.5 | MS, NMR |
| 24.8 | 1417 | Geranyl acetate | 0.3 | MS, NMR |
| 26.6 | 1417 | Caryophyllene | 3.2 | MS, NMR |
| 30.8 | 1580 | Unknown | 0.8 | MS, NMR |
| 33.2 | 1580 | Caryophyllene oxide | 2.9 | MS, NMR |
| 35.3 | 1637 | Germacrene-D | 1.8 | MS, NMR |
| 38.0 | 1708 | Unknown | 0.1 | MS, NMR |
|
| ||||
| Total identified | 99.2 | |||
aRI: Retention index relative to a homologous series of n-alkanes on the DB-5 capillary column.
bMass spectrometry.
cCalculated considering retention time of 22.65 minutes.
dNuclear magnetic resonance.
In the cell viability assay, the treatments were tested at different concentrations. TEO at concentrations of 1, 3, 10, 30, and 90 μg/mL showed cell viability of 88%, 82%, 90%, 92%, and 75%, respectively. CVL at concentrations of 1, 3, 10, 30, and 90 μg/mL showed cell viability of 90%, 90%, 90%, 88%, and 87%, respectively. Thymol at concentrations of 1.5, 15, and 150 μg/mL showed cell viability of 83%, 97%, and 95%, respectively. Our data indicated that TEO, CVL, and thymol did not present in vitro cytotoxicity at any of the concentrations tested.
In the acute toxicology study, TEO was tested orally at doses of 2000 mg/kg, 3000 mg/kg, and 4000 mg/kg. The LD50 value of TEO was 4000 mg/kg. All of the doses used in the present study were lower than the observed LD50 values. Consequently, no apparent behavioral side effects were observed in the animals during our studies. The high LD50 values also suggest that TEO is relatively safe and nontoxic. Therefore, we studied the effects of TEO, CVL, and thymol on the inflammatory response evaluated by antiedematogenic activity and leukocyte migration.
Acute inflammation, typically characterized by redness, swelling, pain, and heat, is one of the most important defense mechanisms against invading pathogens. Lipopolysaccharide (LPS) can active monocytes, neutrophils, and macrophages [18] and induce an oversecretion of various proinflammatory and toxicity-mediating molecules, such as tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), eicosanoids, and nitric oxide (NO) [19]. Prostaglandins (PGs) and NO are two important proinflammatory mediators. The inhibition of the production of PGs and NO via the inhibition of their synthases (i.e., cyclooxygenase 2 [COX-2] and inducible nitric oxide synthase [iNOS], resp.) has been demonstrated to be beneficial in the treatment of inflammatory disease [20]. Anti-inflammatory drugs, such as steroids and nonsteroidal anti-inflammatory drugs (NSAIDs), have numerous adverse side effects, such as gastrointestinal discomfort, the inhibition of platelet aggregation, and liver and kidney toxicity [21]. Therefore, the search for natural products with fewer side effects has been increasingly important.
Different mechanisms are well known to be involved in the genesis of inflammatory reactions. The development of inflammatory edema induced by carrageenan is characterized by an initial stage (1-2 h) and is dependent on the release of histamine, serotonin, and bradykinin, followed by a later stage (3-4 h) that is maintained principally by the release of kinins, lysozymes, and prostanoids [22, 23]. Eicosanoids promote the chemotaxis of neutrophils, and they induce the biosynthesis of elastase, collagenase, and other compounds. These enzymes break down structural proteins into peptides. Consequently, vascular permeability and hydrostatic pressure increase, resulting in edema and the migration of neutrophils to the damaged tissue [24]. COX-2, an inducible enzyme found in activated inflammatory cells, plays a crucial role in cytokine production and prostanoid mediator release. The inhibition of COX-2 protein expression has been used to evaluate the anti-inflammatory effects of compounds in vivo and in vitro [19, 25, 26]. TNF-α, a key mediator of the inflammatory response, stimulates innate immune responses by activating T-cells and macrophages that stimulate the release of other inflammatory cytokines. TNF-α is also a mediator of carrageenan-induced inflammation and is able to enhance the further release of kinins and leukotrienes [27]. Nitric oxide has been shown to play an important role in both the regulation of vascular permeability and cell migration induced by proinflammatory agents, including carrageenan [28, 29].
The pleurisy model is used to screen anti-inflammatory drugs. Exudate accumulation in the pleural cavity and leukocyte migration can be evaluated [16]. In the pleurisy model, TEO at doses of 250, 500, and 750 mg/kg significantly reduced inflammatory exudates. At a dose of 750 mg/kg, TEO reduced the number of migrated cells (Table 2). The groups treated with indomethacin and celecoxib exhibited a reduction in inflammatory exudates but not a reduction in leukocyte migration. CVL and thymol significantly reduced the volume of pleural inflammatory exudates by 47.3% and 34.2%, respectively, at a dose of 400 mg/kg. CVL decreased the number of migrated cells at doses of 100, 200, and 400 mg/kg. Thymol, however, was not able to reduce cell migration (Tables 3 and 4). These data indicate that TEO, CVL, and thymol significantly inhibited inflammatory edema, but only TEO and CVL exerted inhibitory effects on leukocyte migration to the injury site. Our data corroborate previous studies that demonstrated the anti-inflammatory effects of different essential oils (i.e., inhibition of inflammatory edema and chemotaxis) [30–34]. CVL acts as a suppressor of COX-2 and activator of peroxisome proliferator-activated receptors [35]. Our data suggest that CVL may inhibit prostanoid release because CVL had effects that were similar to indomethacin and celecoxib (i.e., inhibition of COX-1 and -2) [36] (Table 3).
Table 2.
Effect of Thymus vulgaris essential oil (TEO) treatment on exudate volume and leukocytes number 4 hours after carrageenan injection (200 μg/pleural cavity) in rats.
| Group | Exudate volume (mL) | Inhibition (%) | (cells/mm3) × 103 | ||
|---|---|---|---|---|---|
| Total leukocytes | MN | PMN | |||
| Normal | 0.16 ± 0.01 | 6700 ± 450 | 1800 ± 160 | 4900 ± 390 | |
| Control | 0.76 ± 0.03b | 55650 ± 1860b | 8831 ± 644b | 46819 ± 1399b | |
| Indomethacin (5 mg/kg) | 0.33 ± 0.02a,b | 56.7 | 60250 ± 7600b | 10162 ± 1137b | 50088 ± 6989b |
| Celecoxib (10 mg/kg) | 0.43 ± 0.03a,b | 43.3 | 42350 ± 3536b | 6972 ± 1047b | 35378 ± 3774b |
| TEO | |||||
| 250 mg/kg | 0.62 ± 0.02a,b | 18.4 | 52696 ± 2558b | 10786 ± 1156b | 41910 ± 2646b |
| 500 mg/kg | 0.61 ± 0.03a,b | 19.7 | 54729 ± 3090b | 7457 ± 924.9b | 47272 ± 2354b |
| 750 mg/kg | 0.53 ± 0.04a,b | 30.2 | 41417 ± 2125a,b | 5873 ± 415.7b | 35543 ± 1801a,b |
Data are mean ± SEM of 8–10 animals/group. Indomethacin and celecoxib administered orally were used as reference anti-inflammatory drugs (positive controls). Normal: animals that received injection of saline in the cavity (normal group), Control: animals that received injection of carrageenan in the cavity (negative control). MN: mononuclears cells. PMN: polimorphonuclears cells. a P < 0.05 compared to control group; b P < 0.05 compared to normal group (ANOVA, Tukey's test).
Table 3.
Effect of carvacrol treatment on exudate volume and leukocytes number 4 hours after carrageenan injection (200 μg/pleural cavity) in rats.
| Group | Exsudate volume (mL) | Inhibition (%) | (cells/mm3) × 103 | ||
|---|---|---|---|---|---|
| Total leukocytes | MN | PMN | |||
| Normal | 0.16 ± 0.01 | 6700 ± 450 | 1800 ± 160 | 4900 ± 390 | |
| Control | 0.76 ± 0.03b | 55650 ± 1860b | 8831 ± 644b | 46819 ± 1399b | |
| Indomethacin (5 mg/kg) | 0.33 ± 0.02a,b | 56.7 | 60250 ± 7600b | 10162 ± 1137b | 50088 ± 6989b |
| Celecoxib (10 mg/kg) | 0.43 ± 0.03a,b | 43.3 | 42350 ± 3536b | 6972 ± 1047b | 35378 ± 3774b |
| Carvacrol | |||||
| 100 mg/kg | 0.68 ± 0.01b | — | 32250 ± 3256a,b | 4522 ± 635.3b | 27728 ± 2696b |
| 200 mg/kg | 0.65 ± 0.02b | — | 38625 ± 2617a,b | 5544 ± 430.9b | 33081 ± 2171b |
| 400 mg/kg | 0.40 ± 0.05a,b | 47.3 | 33583 ± 2548a,b | 4914 ± 353.8b | 28669 ± 2211a,b |
Data are mean ± SEM of 8–10 animals/group. Indomethacin and celecoxib administered orally were used as reference anti-inflammatory drugs (positive controls). Normal: animals that received injection of saline in the cavity (normal group), Control: animals that received injection of carrageenan in the cavity (negative control). MN: mononuclears cells. PMN: polimorphonuclears cells. a P < 0.05 compared to control group; b P < 0.05 compared to normal group (ANOVA, Tukey's test).
Table 4.
Effect of thymol treatment on exudate volume and leukocytes number 4 hours after carrageenan injection (200 μg/pleural cavity) in rats.
| Group | Exsudate volume (mL) | Inhibition (%) | (cells/mm3) × 103 | ||
|---|---|---|---|---|---|
| Total leukocytes | MN | PMN | |||
| Normal | 0.16 ± 0.01 | 6700 ± 450 | 1800 ± 160 | 4900 ± 390 | |
| Control | 0.76 ± 0.03 | 55650 ± 1860b | 8831 ± 644b | 46819 ± 1399b | |
| Indomethacin (5 mg/kg) | 0.33 ± 0.02a | 56.7 | 60250 ± 7600b | 10162 ± 1137b | 50088 ± 6989b |
| Celecoxib (10 mg/kg) | 0.43 ± 0.03a | 43.3 | 42350 ± 3536b | 6972 ± 1047b | 35378 ± 3774b |
| Thymol | |||||
| 100 mg/kg | 0.73 ± 0.06 | — | 42306 ± 2429b | 5563 ± 403.6b | 36742 ± 2216b |
| 200 mg/kg | 0.65 ± 0.03 | — | 46900 ± 2931b | 6609 ± 683.8b | 40290 ± 2357b |
| 400 mg/kg | 0.54 ± 0.05a | 34.2 | 47600 ± 2987b | 6286 ± 380.3b | 41313 ± 2979b |
Data are mean ± SEM of 8–10 animals/group. Indomethacin and celecoxib administered orally were used as reference anti-inflammatory drugs (positive controls). Normal: animals that received injection of saline in the cavity (normal group), Control: animals that received injection of carrageenan in the cavity (negative control). MN: mononuclears cells. PMN: polimorphonuclears cells. a P < 0.05 compared to control group; b P < 0.05 compared to normal group (ANOVA, Tukey's test).
Polymorphonuclear leukocyte recruitment is an essential factor in the acute inflammatory process, acting as first-line-defense cells in the initiation and resolution phases of this process [37]. In situations in which uncontrolled infiltration of these cells occurs, they can become the main aggressor factor. Under such conditions, pharmacological interventions with drugs that are able to modulate leukocyte recruitment may present an interesting therapeutic possibility.
The present study also evaluated the effects of thymol and CVL at different concentrations on leukocyte chemotaxis in vitro. The chemoattractants fMLP (10−6 M) and LTB4 (10−8 M) were used. CVL at doses of 0.3, 1, 3, 10, 30, and 90 μg/mL significantly reduced (P < 0.05) neutrophil migration in response to fMLP stimulation (20.07 ± 1.02%, 14.79 ± 1.79%, 25.28 ± 2.51%, 29.48 ± 2.21%, 42.92 ± 2.06%, and 52.23 ± 1.75%, resp.) and LTB4 stimulation (19.82 ± 2.50%, 24.81 ± 1.66%, 20.35 ± 1.18%, 30.41 ± 0.61%, 36.44 ± 1.54%, and 61.11 ± 1.82%, resp.; Figures 2(a) and 2(b)). Thymol at doses of 0.3, 1, 3, 10, 30, and 90 μg/mL did not reduce leukocyte migration in vitro in response to fMLP and LTB4 stimulation (Figures 2(c) and 2(d)). However, thymol at concentrations of 1.5, 15, and 150 μg/mL was a potent chemoattractant agent (Figure 3). CVL and thymol did not affect leukocyte viability at the concentrations tested, suggesting that the direct effects of the treatments on the inhibition of leukocyte chemotaxis did not occur because of the toxic effects that induce cell death.
Figure 2.
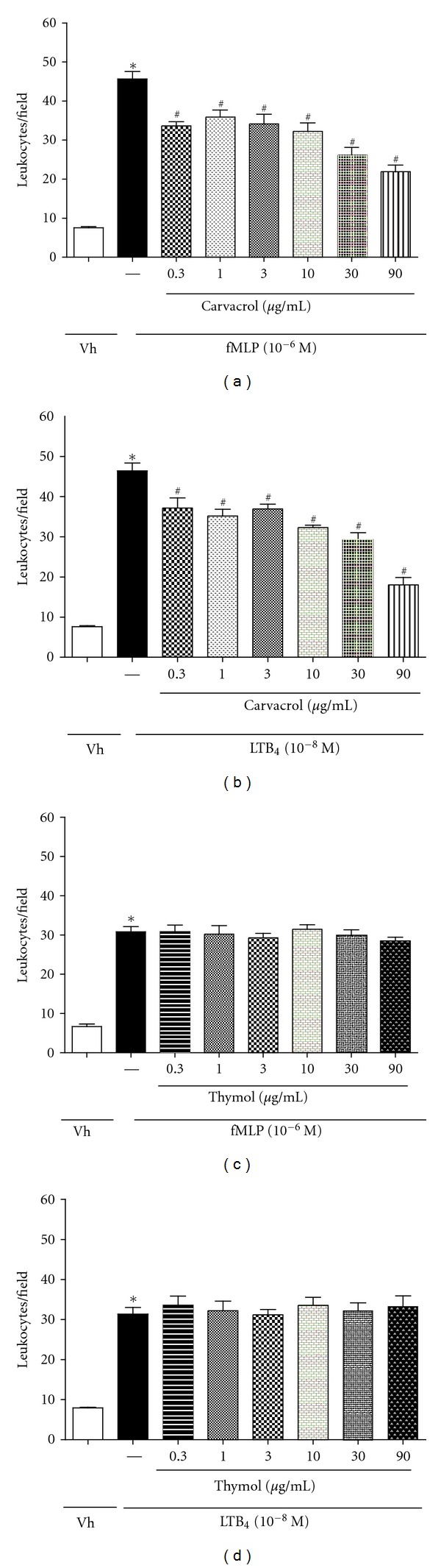
Effect of CVL and thymol on in vitro leukocyte chemotaxis. Leukocytes were obtained from zymosan-induced peritonitis (200 μg/cavity) and stimulated with fMLP (10−6) or LTB4 (10−8) after 30 min of CVL (a, b) or thymol (c, d) treatments at doses of 0.3, 1, 3, 10, 30 and 90 μg/mL. Values are mean ± SEM (n = 5) and are representative of three independent experiments. *P < 0.05 versus Vh (vehicle), # P < 0.05 versus group of leukocytes stimulated with fMLP or LTB4.
Figure 3.
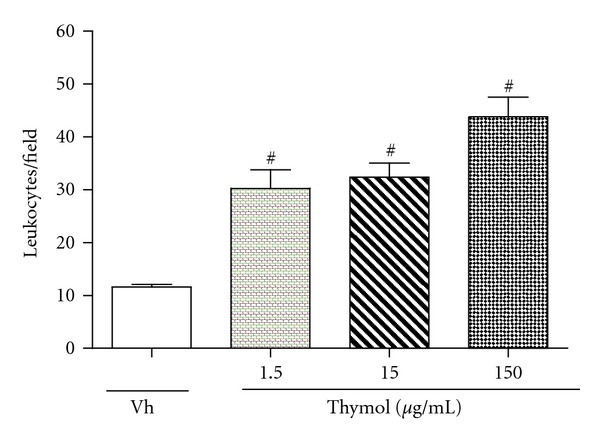
Thymol used as a chemotactic agent in concentrations of 1.5, 15, and 150 μg/mL. Leukocytes were obtained from zymosan-induced peritonitis (200 μg/cavity). Values are mean ± SEM (n = 5) and are representative of three independent experiments. # P < 0.05 versus Vh (vehicle).
Only CVL was able to inhibit in vitro chemotaxis induced by fMLP and LTB4, suggesting that CVL and thymol exert their effects on leukocyte chemotaxis through different mechanisms. Leukotriene is a potent chemotactic agent derived from arachidonic acid [38]. fMLP is a chemotactic agent involved in the release of cytokines. Upon binding to its G-protein-coupled receptor, it activates multiple signaling cascade pathways [39]. These pathways include the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI-3K) cascades, which are important for the development of the functional responses of neutrophils in inflammation [40, 41]. Our data suggest that CVL may act by inhibiting cytokines and leukotrienes, and these mediators are likely not involved in the mechanism of action of thymol.
To demonstrate the topical effect of thymol and CVL in vivo, we evaluated inflammatory ear edema induced by croton oil. Croton oil is an irritant agent that causes cell damage and activates phospholipase A2, which releases arachidonic acid from the cell plasma membrane. From arachidonic acid, the production of prostaglandins by COX-1 and COX-2 and leukotrienes by 5-lipoxygenase occurs. Prostaglandins and leukotrienes are inflammatory mediators involved in edema and leukocyte migration [42]. Croton oil application to the right ear in mice induced an apparent inflammatory response 4 h later. An increase in the weight of the ears was observed. Indomethacin (0.5 mg/ear) and dexamethasone (0.1 mg/ear) significantly inhibited ear edema by 44% and 36%, respectively (P < 0.05). CVL did not reduce ear edema at concentrations of 20 and 40 mg/ear, but ear edema was reduced at the lowest concentration (10 mg/ear), similar to the 37.2% reduction observed with dexamethasone (P < 0.05; Figure 4(a)).
Figure 4.
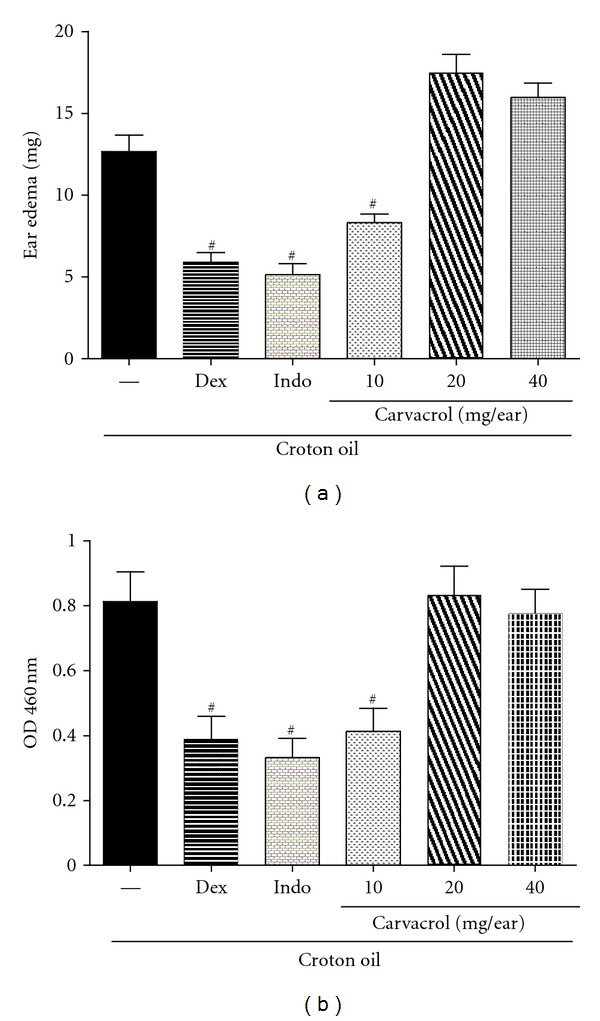
Effect of CVL on ear edema (a) and myeloperoxidase activity (MPO) (b) induced by croton oil in ear tissues from mice. The animals (n = 9) were treated topically with carvacrol, indomethacin (Indo), or dexamethasone (Dex) 1 h before croton oil application (10 μL/ear). Dex (0.1 mg/ear) or Indo (0.5 mg/ear) were used as anti-inflammatory drugs (positive control). The right ears received only the vehicle (Basal). Data are mean ± SEM weight of the ears (a) or MPO activity (b), 4 hours after application of croton oil, # P < 0.05, compared to the control group (croton oil) (ANOVA, Tukey's test).
The present study showed that CVL at 10 mg/ear has an antiedematogenic effect when administered topically, similar to the observations with dexamethasone and indomethacin. In this experimental model, these treatments inhibited both fluid extravasation and cellular influx, indirectly reflected by a reduction in MPO activity. The enzyme MPO is found in the azurophilic granules of neutrophils and other cells of myeloid origin and is considered a marker of polymorphonuclear leukocyte influx into inflamed tissues. Therefore, MPO inhibition may result in an anti-inflammatory effect [23]. CVL (10 mg/ear), indomethacin (0.5 mg/ear), and dexamethasone (0.1 mg/ear) significantly inhibited the activity of this enzyme (43.8%, 52.0%, and 38.3%, resp.; P < 0.05; Figure 4(b)). Our data showed that CVL effectively inhibited chemotaxis in vitro but also had antiedematogenic and antichemotactic effects in vivo (pleurisy test) when administered systemically.
As shown in Figure 5, the ear edema formation intensities were similar between croton oil and thymol, suggesting similar responses to both irritative agents. Ear edema induced by croton oil involves the activation of phospholipase A2 and biosynthesis of prostaglandins and leukotrienes [43, 44]. To compare the probable irritative mechanism of the topical effect of thymol and croton oil, the following experiments were performed. The animals were treated with indomethacin (5 mg/kg, p.o.) and promethazine (5 mg/mL, i.p.) 60 min before the application of thymol. Croton oil was used as a positive control. To evaluate the participation of histamine in this inflammatory response, promethazine was used as an antihistaminic reference drug. Ear edema was determined 30, 60, 120, 180, and 240 min after the inflammatory stimulation. The time-course analysis of thymol revealed an increase in edema volume at 60 min (Figure 6). This response could be attributable to the release of different autacoids, including histamine. Essential oils and their isolated compounds can promote the release of histamine and other mediators, acting as irritative agents [45]. Indomethacin treatment effectively reduced the formation of ear edema induced by thymol, whereas promethazine treatment only inhibited the early stage of edema. Our results indicate that the edematogenic response of thymol is partially dependent on histamine and prostanoids. Therefore, our results suggest that thymol has an irritative effect, similar to the effect observed with croton oil.
Figure 5.
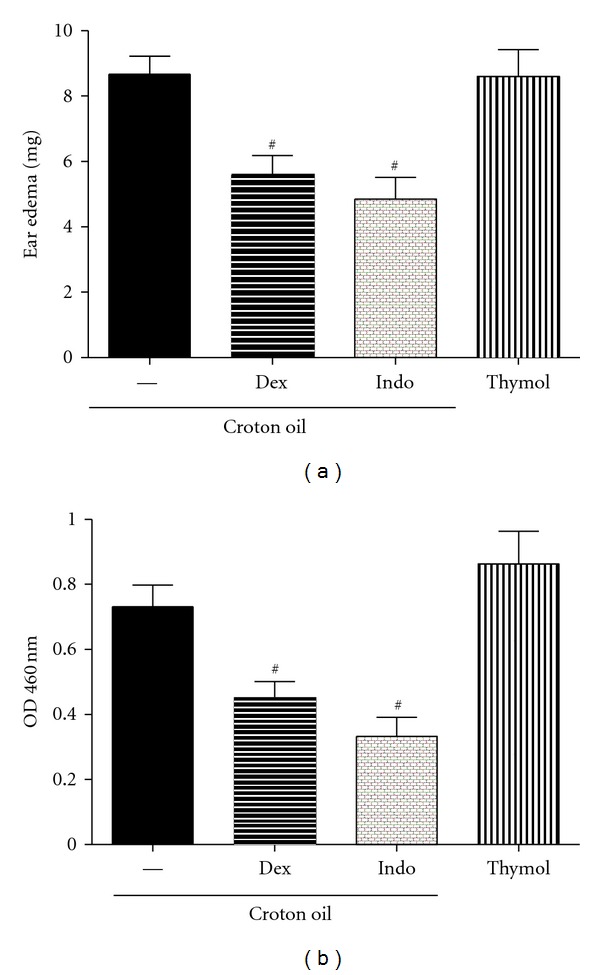
Effect of thymol on ear edema (a) and myeloperoxidase activity (MPO) (b) in ear tissues from mice. The animals (n = 9) were treated topically with indomethacin (Indo) or dexamethasone (Dex) 1 h before croton oil application (10 μL/ear). The group treated with thymol did not received the croton oil. Dex (0.1 mg/ear) and Indo (0.5 mg/ear) were used as anti-inflammatory drugs (positive control). The right ears received only the vehicle. Data are mean ± SEM weight of the ears (a) or MPO activity (b), 4 hours after application of croton oil or thymol. # P < 0.05, compared to the control group (croton oil) (ANOVA, Tukey's test).
Figure 6.
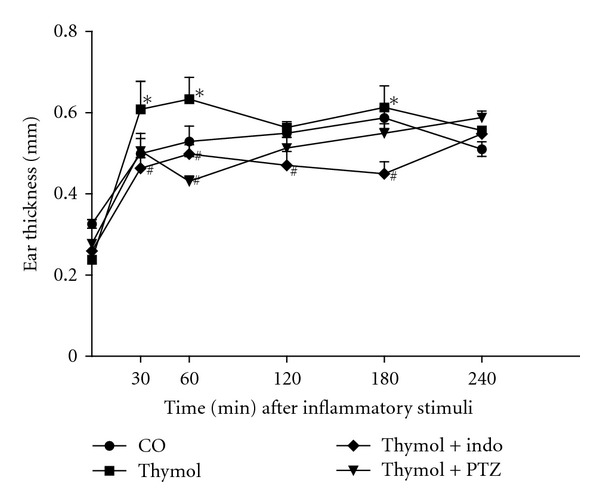
Ear edema induced by thymol (10 mg/ear). The animals (n = 8) were treated with indomethacin (Indo) (5 mg/Kg, v.o.) or promethazine (PTZ) (5 mg/Kg, i.p.) 60 minutes beforethe thymol application. The croton oil (CO) was used for positive control. The ear edema was determined in 30, 60, 120, 180, and 240 minutes after inflammatory stimuli. Data are mean ± SEM of the ear thickness, # P < 0.05 compared to thymol group. *P < 0.05 compared to the positive control (ANOVA, Tukey's test).
Thus, our results are consistent with the literature and showed that TEO has anti-inflammatory effects in vivo. However, the isolated compounds thymol and CVL showed antagonist effects. Thymol has an irritative effect that likely involves histamine, prostanoids, and other inflammatory mediators. CVL may be the compound responsible for the anti-inflammatory effects of TEO, demonstrated by chemotaxis in vitro. The present study contributes to the growing evidence of the anti-inflammatory effects of natural products. Overall, our data support the hypothesis that the inhibitory effect of CVL on leukocyte migration contributes to its anti-inflammatory action, in addition to the irritant effect of thymol.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
This study was supported by grants from CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Brazil. The authors thank Mr. Jailson Araujo Dantas and Mrs. Celia Regina Miranda for technical assistance.
References
- 1.Zarzuelo A, Crespo E. The medicinal and non-medicinal uses of thyme. In: Stahl-Biskup E, Saez F, editors. Thyme: The Genus Thymus. Medicinal and Aromatic Plants—Industrial Profiles. New York, NY, USA: Taylor & Francis; 2002. pp. 263–292. [Google Scholar]
- 2.Soliman KM, Badeaa RI. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food and Chemical Toxicology. 2002;40(11):1669–1675. doi: 10.1016/s0278-6915(02)00120-5. [DOI] [PubMed] [Google Scholar]
- 3.Rustaiyan A, Masoudi S, Monfared A, et al. Volatile constituents of three Thymus species grown wild in Iran. Planta Medica. 2000;66(2):197–198. doi: 10.1055/s-0029-1243136. [DOI] [PubMed] [Google Scholar]
- 4.Amiri H. Essential oils composition and antioxidant properties of three Thymus species. Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/728065.728065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickavar B, Mojab F, Dolat-Abadi R. Analysis of the essential oils of two Thymus species from Iran. Food Chemistry. 2005;90(4):609–611. [Google Scholar]
- 6.Höferl M, Buchbauer G, Jirovetz L, et al. Correlation of antimicrobial activities of various essential oils and their main aromatic volatile constituents. Journal of Essential Oil Research. 2009;21(5):459–463. [Google Scholar]
- 7.Youdim KA, Damien Dorman HJ, Deans SG. The antioxidant effectiveness of thyme oil, α-tocopherol and ascorbyl palmitate on evening primrose oil oxidation. Journal of Essential Oil Research. 1999;11(5):643–648. [Google Scholar]
- 8.Barnes J, Anderson LA, Philipson JD. Herbal Medicines. 3rd edition. London, UK: Pharmaceutical Press; 2007. [Google Scholar]
- 9.Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of Applied Microbiology. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 10.ESCOP. ESCOP Monographs: The Scientific Foundation for Herbal Medicinal Products. 2nd edition. The European Scientific Cooperative on Phytotherapy in collaboration with Georg Thieme; 2007. [Google Scholar]
- 11.Vila R. Flavonoids and further polyphenols in the genus Thymus . In: Stahl-Biskup E, Saez F, editors. Thyme: The Genus Thymus. Medicinal and Aromatic Plants—Industrial Profiles. New York, NY, USA: Taylor and Francis; 2002. p. p. 75. [Google Scholar]
- 12.El-Nekeety AA, Mohamed SR, Hathout AS, Hassan NS, Aly SE, Abdel-Wahhab MA. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon. 2011;57(7-8):984–991. doi: 10.1016/j.toxicon.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Asbaghian S, Shafaghat A, Zarea K, Kasimov F, Salimi F. Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris . Natural Product Communications. 2011;6(1):137–140. [PubMed] [Google Scholar]
- 14.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4th edition. Carol Stream, Ill, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 15.Lorke D. A new approach to practical acute toxicity testing. Archives of Toxicology. 1983;54(4):275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 16.Vinegar R, Truax JF, Selph JL. Some quantitative temporal characteristics of carrageenin induced pleurisy in the rat. Proceedings of the Society for Experimental Biology and Medicine. 1973;143(3):711–714. doi: 10.3181/00379727-143-37397. [DOI] [PubMed] [Google Scholar]
- 17.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. Journal of Investigative Dermatology. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 18.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 19.Nantel F, Denis D, Gordon R, et al. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. British Journal of Pharmacology. 1999;128(4):853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogdan C. Nitric oxide and the immune response. Nature Immunology. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 21.Batlouni M. Nonsteroidal anti-inflammatory drugs: cardiovascular, cerebrovascular and renal effects. Arquivos Brasileiros de Cardiologia. 2010;94(4):522–563. doi: 10.1590/s0066-782x2010000400019. [DOI] [PubMed] [Google Scholar]
- 22.Crunkhorn P, Meacock SC. Mediators of the inflammation induced in the rat paw by carrageenin. British Journal of Pharmacology. 1971;42(3):392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niemegeers CJ, Verbruggen FJ, Janssen PA. Effect of various drugs on carrageenan-induced oedema in the rat hind paw. Journal of Pharmacy and Pharmacology. 1964;16:810–816. doi: 10.1111/j.2042-7158.1964.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 24.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacology and Therapeutics. 2002;96(2-3):67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Soyoola E, Chanmugam P, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. Journal of Biological Chemistry. 1992;267(36):25934–25938. [PubMed] [Google Scholar]
- 26.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. Journal of Leukocyte Biology. 1993;54(2):171–178. [PubMed] [Google Scholar]
- 27.Tonussi CR, Ferreira SH. Tumour necrosis factor-α mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain. 1999;82(1):81–87. doi: 10.1016/S0304-3959(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 28.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. British Journal of Pharmacology. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira SH, Vane JR. New aspects of mode of action of non-steroid anti-inflammatory drugs. Annual Review of Pharmacology. 1974;14:57–73. [Google Scholar]
- 30.Siani AC, Ramos MFS, Menezes-de-Lima O, Jr., et al. Evaluation of anti-inflammatory-related activity of essential oils from the leaves and resin of species of Protium . Journal of Ethnopharmacology. 1999;66(1):57–69. doi: 10.1016/s0378-8741(98)00148-2. [DOI] [PubMed] [Google Scholar]
- 31.Vendruscolo A, Takaki I, Bersani-Amado LE, Dantas JA, Bersani-Amado CA, Cuman RKN. Antiinflammatory and antinociceptive activities of zingiber officinale roscoe essential oil in experimental animal models. Indian Journal of Pharmacology. 2006;38(1):58–59. [Google Scholar]
- 32.Takaki I, Bersani-Amado LE, Vendruscolo A, et al. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. Journal of Medicinal Food. 2008;11(4):741–746. doi: 10.1089/jmf.2007.0524. [DOI] [PubMed] [Google Scholar]
- 33.Nogueira de Melo GA, Grespan R, Fonseca JP, et al. Rosmarinus officinalis L. essential oil inhibits in vivo and in vitro leukocyte migration. Journal of Natural Medicines. 2011;14:944–946. doi: 10.1089/jmf.2010.0159. [DOI] [PubMed] [Google Scholar]
- 34.Nogueira de Melo GA, Grespan R, Fonseca JP, et al. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. Journal of Natural Medicines. 2011;65(1):241–246. doi: 10.1007/s11418-010-0479-5. [DOI] [PubMed] [Google Scholar]
- 35.Hotta M, Nakata R, Katsukawa M, Hori K, Takahashi S, Inoue H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. Journal of Lipid Research. 2010;51(1):132–139. doi: 10.1194/jlr.M900255-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature. 1971;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 37.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual Review of Immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 38.Samuelsson B, Dahlen SE, Lindgren JA. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 39.Haribabu B, Richardson RM, Verghese MW, Barr AJ, Zhelev DV, Snyderman R. Function and regulation of chemoattractant receptors. Immunologic Research. 2000;22(2-3):271–279. doi: 10.1385/IR:22:2-3:271. [DOI] [PubMed] [Google Scholar]
- 40.Mócsai A, Jakus Z, Vántus T, Berton G, Lowell CA, Ligeti E. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. Journal of Immunology. 2000;164(8):4321–4331. doi: 10.4049/jimmunol.164.8.4321. [DOI] [PubMed] [Google Scholar]
- 41.Coffer PJ, Geijsen N, M’Rabet L, et al. Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function. Biochemical Journal. 1998;329(1):121–130. doi: 10.1042/bj3290121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kremmyda L, Tvrzicka E, Stankova B, Zak A. Fatty acids as biocompounds: their role in human metabolism, helth and disesse—a review. Part 2: fatty acid physiological roles and applications in human health and disese. Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czech Republic. 2011;155(3):195–218. doi: 10.5507/bp.2011.052. [DOI] [PubMed] [Google Scholar]
- 43.Kondoh H, Sato Y, Kanoh H. Arachidonic acid metabolism in cultured mouse keratinocytes. Journal of Investigative Dermatology. 1985;85(1):64–69. doi: 10.1111/1523-1747.ep12275349. [DOI] [PubMed] [Google Scholar]
- 44.Fuerstenberger G, Marks F. Early prostaglandin E synthesis is an obligatory event in the induction of cell proliferation in mouse epidermis in vivo by the phorbol ester TPA. Biochemical and Biophysical Research Communications. 1980;92(3):749–756. doi: 10.1016/0006-291x(80)90767-6. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes ES, Passos GF, Medeiros R, et al. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea . European Journal of Pharmacology. 2007;569(3):228–236. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]


