SUMMARY
The Fat pathway controls both planar cell polarity (PCP) and organ growth [1, 2]. Fat signaling is regulated by the graded expression of the Fat ligand Dachsous (Ds), and the cadherin-domain kinase Four-jointed (Fj). The vectors of these gradients influence PCP [1], whereas their slope can influence growth [3, 4]. The Fj and Ds gradients direct the polarized membrane localization of the myosin Dachs, which is a crucial downstream component of Fat signaling [5–7]. Here we show that re-polarization of Dachs by differential expression of Fj or Ds can propagate through the wing disc, which indicates that Fj and Ds gradients can be measured over long range. Through characterization of tagged genomic constructs, we show that Ds and Fat are themselves partially polarized along the endogenous Fj and Ds gradients, providing a mechanism for propagation of PCP within the Fat pathway. We also identify a biochemical mechanism that might contribute to this polarization by showing that Ds is subject to endoproteolytic cleavage, and that the relative levels of Ds isoforms are modulated by Fat.
RESULTS and DISCUSSION
Propagation of Fat-PCP through the wing disc
Two complementary mechanisms for coordination of PCP have been suggested [8]. One relies upon local interactions that enable the polarity of one cell to be coupled to that of its neighbors. The other relies upon organ-wide gradients, whose vectors could be interpreted by each cell. Two PCP pathways have been identified in metazoans, a Frizzled-dependent pathway (Fz-PCP), and a Ds- and Fat-dependent pathway (Fat-PCP) [8]. Fat-PCP is regulated by Ds and Fj gradients, but it has remained unclear whether coupling mechanisms that coordinate polarity between adjacent cells also contribute to Fat-PCP.
Dachs is polarized along the Fj and Ds gradients (Fig. 1B–E) [4, 5]. Dachs localization can be altered by manipulating Fj or Ds expression, but initial experiments only examined Dachs repolarization within cells with altered Fj or Ds [5, 7]. We reasoned that if the polarity of neighboring cells is coupled, then the influence of Fj or Ds could propagate through a tissue. This was examined using a tagged dachs transgene (AyDachs:Cit) that could be expressed in clones independently of manipulations of Fj or Ds. AyDachs:Cit was crossed into flies in which Ds or Fj expression were either increased (using UAS transgenes) or decreased (using UAS-RNAi transgenes) within posterior cells under hedgehog (hh)-Gal4 control.
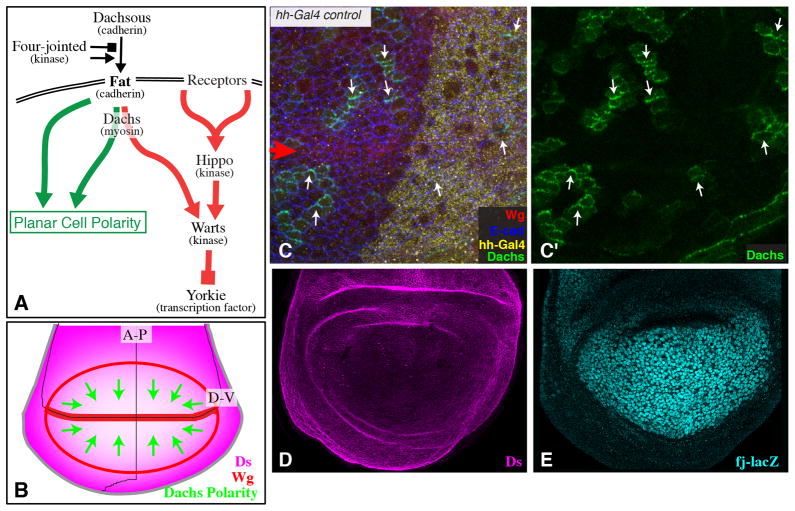
Fig. 1. Fat signaling and Dachs polarity in the wing disc.
A) Simplified schematic of the Fat signaling pathway. Fat is regulated by Dachsous and Four-jointed. Fat regulates Hippo signaling (red arrows) through Dachs, and regulates PCP signaling (green arrows) partly through Dachs and partly independently of Dachs. B) Schematic of wing disc with the Ds gradient indicated in magenta, and the direction of Dachs polarization indicated by green arrows. The A-P and D-V compartment boundaries are indicated by thin black lines, and Wg expression is indicated in red. C) Dachs:Cit polarization in a wing disc with wild-type Ds and Fj expression, stained for Wg (red), hh-Gal4 (yellow, revealed by Dcr2 staining from a UAS-Dcr2 transgene), and E-cadherin (E-cad, blue, outlines all cells). The polarity of Dachs localization is indicated by small white arrows pointing in the direction of Dachs:Cit membrane localization. Large Red arrow points to the D-V Wg stripe. D) Ds protein staining in a wild-type wing disc. E) Fj expression in a wild-type wing disc, revealed by a fj-lacZ transgene.
Within the medial wing (near the hh expression border), Dachs is preferentially detected along the sides of cells closest to the dorsal-ventral (D-V) boundary, which can be identified by expression of Wingless (Wg) (Fig. 1B,C). When Ds was over-expressed in posterior cells, Dachs was re-localized in nearby anterior cells, defining a re-oriented vector of polarization (Fig. 2A). This re-polarization was detected not only in adjacent cells, but also a few cells away, reflecting a propagation of altered PCP from the Ds expression boundary into the anterior compartment. (Fig. 2A).
Fig. 2. Non-autonomous repolarization of Dachs by boundaries of Fj or Ds expression.
A–B) Examples of wing discs with clones of cells expressing Dachs:Cit (green) from an AyDachs:Cit transgene. These discs are stained for expression of Wg (red, marks D-V boundary, highlighted by large red arrow), hh-Gal4 (yellow, revealed by expression of Dcr2 from a UAS-Dcr2 transgene), and E-cad (blue, outlines cells). The polarity of Dachs localization is indicated by small arrows pointing in the direction of Dacsh:Cit membrane localization; white arrows indicate normal polarity, yellow arrows indicate repolarization. Panels marked prime show the Dachs:Cit channel only from the image to the left. These animals also express either: A) UAS-ds, or B) UAS-fj. C) For each of 4 genotypes, comprising animals expressing the indicated transgenes under hh-Gal4 control, and in each of 3 regions (distal half of the wing pouch, proximal half of the wing pouch, or dorsal hinge), we identified anterior Dachs:Cit-expressing clones near (within 8 cells) of the A-P compartment boundary. In each disc, the number of cells to the farthest Dachs:Cit clone with evident repolarization towards or away from the compartment boundary was recorded (green). Because the clone frequency was relatively low, only a few relative positions are represented in each wing disc, but a representative sampling was achieved by scoring many discs (for each genotype, between 50 and 100 clones were scored in total). In addition, to set an upper limit on the extent of repolarization we also scored the closest Dachs:Cit clones without evident repolarization (red). The range of cell numbers obtained in each case is presented using a “box and whiskers plot”, where the box indicates the range of cell distances in the middle 50% of the distribution, and the line within the box indicates the mean value (if no line is visible the mean overlapped the number of cells represented by the 25% or 75% value, and if no box is visible it indicates that the 25% and 75% values overlapped the mean). Lines outside the box extend to the maximum and minimum distances obtained in that class. D–G) Schematics of wing discs, with Ds, Wg, and hh-Gal4 expression indicated. Green arrows indicate direction and relative range of Dachs repolarization. See also Fig. S1
Repolarization of Dachs within anterior cells was also detected when Fj was over-expressed in posterior cells (Fig. 2B), or when Ds or Fj were depleted from posterior cells (Fig. S1), and this repolarization could be detected at a distance. When Fj was depleted or Ds was over-expressed, Dachs was relocalized to the sides of cells farthest from the anterior-posterior (A-P) boundary (Figs 2A,S1B). Conversely, when Fj was over-expressed or Ds was depleted, Dachs was relocalized to the sides of cells closest to the A-P boundary (Figs 2B,S1A). This parallels wild type, because Dachs normally accumulates on the sides of cells closest to where Fj is highest and Ds is lowest.
To quantify the distance over which Dachs could be repolarized, AyDachs:Cit clones were scored in UAS-ds, UAS-fj, UAS-RNAi-ds, or UAS-RNAi-fj expressing wing discs. These clones were subdivided into distal wing, proximal wing, or dorsal hinge. Within each disc, the number of cells from the A-P boundary to the farthest cell with evident Dachs repolarization was recorded, as was the distance to the closest cell in which Dachs polarity appeared normal. This revealed that the range of repolarization varied from zero to six cells, depending upon the genotype and clone location (Fig. 2C–G).
For UAS-ds and UAS-RNAi-fj, the range of repolarization was longest within the distal wing and shortest within the hinge (Figs 2C–G). Conversely, for UAS-fj and UAS-RNAi-ds, the repolarization range was longest within the hinge and shortest within the distal wing. Since Ds expression is normally highest proximally and lowest distally, whereas Fj is highest distally and lowest proximally (Fig. 1D,E) these results imply that the range of repolarization depends upon the difference in expression. This suggests that there is a gradual dissipation of repolarization: a larger expression difference would generate a stronger repolarization of neighboring cells, which propagates farther than an initially weaker repolarization. In addition, longer extents of repolarization were observed in proximal regions, particularly within the hinge, as compared to distal regions. For example, both expression of UAS-ds in the distal wing, and UAS-RNAi-ds in the hinge, result in strong differences in Ds expression, but the range of repolarization was longer in the hinge (Fig. 2C).
We confirmed these effects of Ds and Fj on Dachs polarization through an independent approach involving quantitative image analysis. By taking advantage of a slight offset between the membrane localization of Dachs versus E-cadherin, we were able to computationally derive a mean vector of Dachs polarization within individual cells. This was used to score anterior Dachs polarization in discs with altered Ds or Fj expression in posterior cells. This analysis confirmed that Dachs could be repolarized at a distance, with differences in the extent of repolarization between distal and proximal cells (Fig. S1D–G).
The observation that Fat-PCP can propagate through tissues establishes that differences in Fj or Ds expression can be sensed by cells at a distance through coupling of polarization between neighboring cells. This implies that gradients can be measured across a tissue, rather than just between neighboring cells.
Polarization of Ds and Fat
One potential mechanism for propagation of Fat-PCP is polarization of Fat and Ds. Over-expression or mutation of Fat or Ds can re-localize its binding partner (i.e. Ds or Fat, respectively) [5, 9–12]. Similarly, mutation or over-expression of Fj can alter localization of Ds and Fat [5, 9–11, 13]. However, these manipulations cause strong changes in relative expression, and it had remained uncertain whether Ds and Fat are normally polarized within endogenous gradients. We could not discern polarization by direct examination of protein staining. Detecting polarization by making clones of cells expressing a tagged protein, as described above for Dachs, would obscure any normal polarization of Ds and Fat, because of the re-localization of Fat or Ds that occurs when their binding partner is over-expressed. Thus, we employed a more complex approach involving Bac clones expressing tagged forms of genomic transgenes [14].
A 39 kb genomic fat construct with a V5-tag at the Fat N-terminus has been described [15]. We generated an equivalent untagged genomic fat construct, and both untagged and HA-tagged Ds genomic constructs, comprising 109 kb surrounding ds (Fig. S2A–D). These constructs were inserted at the same location, using phiC31-mediated recombination [16]. They encode functional proteins, as they can rescue their respective mutants. We constructed flies heterozygous for the untagged and tagged versions; they contain four copies of fat or ds (two from the wild-type allele, and two from the transgenes), but this does not result in any visible phenotypes. Induction of recombination between the tagged and untagged transgenes results in clones of cells that either have 2 tagged transgenes and no untagged transgenes, or 2 untagged transgenes and no tagged transgenes (Fig. S2E), with the total copy number of Fat or Ds unaltered. We then examined the staining of tagged proteins along the edges of clones of cells with only untagged Fat or Ds (Figs 3A,F, S3E–G). We also stained for the endogenous proteins to ensure that differences detected reflected polarization, as opposed to differences in focal planes or expression levels, and considered the intensity of staining of a clone marker (GFP), so that comparisons would be made between cells with equal numbers of tagged transgenes.
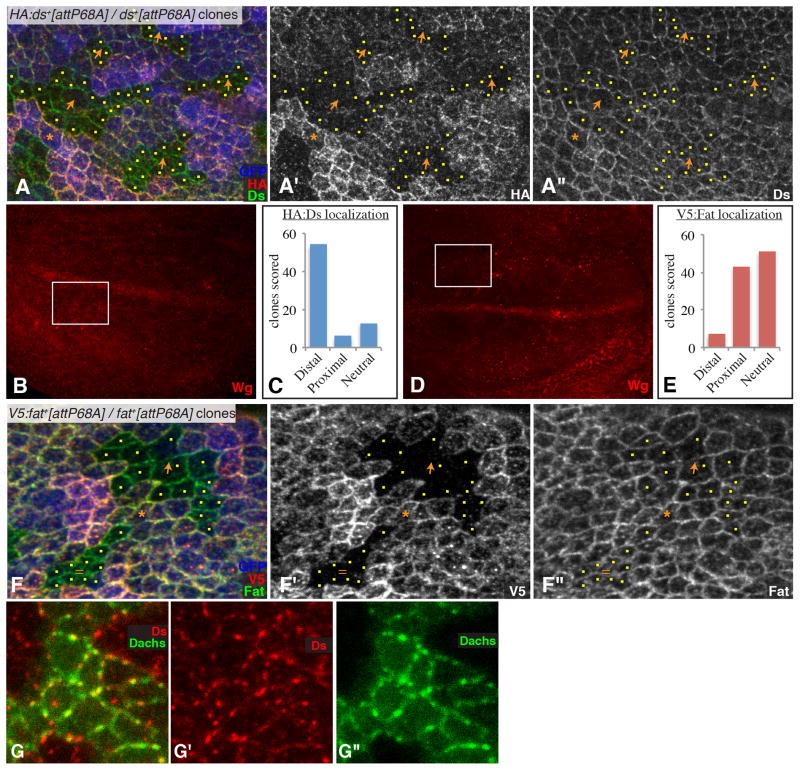
Fig. 3. Polarization of Fat and Ds localization in the wing.
A) Example of a Ubi-GFP attB-P[acman-HA:ds+] FRT80/attB-P[acman-ds+] FRT80 clones in a wing disc, stained for Ds (green/white) and HA (red/white) and labeled by GFP (blue) as indicated. Orange arrows indicate observed polarity of HA:Ds localization, yellow dots mark cells at clone edges. Orange asterisk marks a cell expressing tagged Ds that has unlabeled cells on both its distal and proximal sides; this situation is rare but when it occurs Ds polarization is evident. B) Shows the same wing disc as in (A), but at lower magnification and with Wg expression visible (red), the white rectangle identifies the location of the image in (A). C) Summarizes the results of blind scoring of 73 clones for HA:Ds polarization. D) Shows the same wing disc as in (F), but at lower magnification and with Wg expression visible (red), the white rectangle identifies the location of the image in (F). E) Summarizes the results of blind scoring of 101 clones for V5:Fat polarization. F) Example of a Ubi-GFP attB-P[acman-V5:fat+] FRT80/attB-P[acman-fat+] FRT80 clones in a wing disc, stained for Fat (green/white) and V5 (red/white) and labeled by GFP (blue) as indicated. Orange arrows indicate observed polarity of V5:Fat localization, yellow dots mark cells at clone edges. Equal sign indicates a clone where Fat was scored as not significantly polarized. Orange asterisk marks a cell expressing tagged Fat that has unlabeled cells on both distal and proximal edges, a slight polarization of Fat is evident. G) Close-up of a portion of a wing disc with a Dachs:Cit-expressing clone (green), Stained for expression of Ds (red). Panels marked by prime symbols show individual stains of the image to the left. See also Fig. S2
In preliminary analysis, Ds protein appeared polarized, with stronger HA staining detected on cells proximal to unlabeled clones than on cells distal to unlabeled clones (Figs 3A,B, S3F). Since this staining comes from neighboring cells (Fig. S2E), it is indicative of a polarized, distal membrane localization for Ds. Conversely, Fat did not appear polarized. To confirm this, images of 80–100 clones from both the tagged-Fat and tagged-Ds experiments were collected, assigned random numbers, and then scored blind (ie, without knowledge of whether Ds or Fat was being scored). In this blind scoring, 74% of Ds clones were scored as revealing a polarization of Ds localization towards the distal side of cells, 8% were scored as revealing a proximal polarization, and 18% were scored as revealing a lack of polarization (Fig. 3C). By contrast, 7% of Fat clones were scored as revealing a polarization of Fat localization towards the distal side of cells, 43% were scored as revealing a proximal polarization, and 50% were scored as revealing a lack of polarization (Fig. 3E). Polarization of Ds or Fat could also sometimes be identified when a single cell expressing a tagged transgene was bordered on both proximal and distal sides by cells lacking tagged transgenes (Fig. 3A,F).
Thus, Ds is polarized towards the distal sides of cells. However, in contrast to Dachs, for which membrane staining is normally detected on only one side of a cell, membrane staining of Ds is often detected on both sides of a cell, but at unequal levels. Fat also appears to be polarized, although its polarization is weaker, such that it often falls below our ability to discern it. One possible explanation for this is that Ds expression is relatively low within the wing pouch (Fig. 1D), whereas Fat expression is relatively high [17]; a polarized localization that is coupled to Ds-Fat binding would thus affect a greater fraction of the available Ds than of the available Fat. The more robust polarization of Dachs compared to Fat and Ds suggests that there is an amplification mechanism that operates downstream of Fat and Ds localization to enhance Dachs polarization.
The polarization of Ds and Fat identifies modulation of their localization as a potential mechanism for propagation of Fat-PCP. Moreover, detection of this polarization in wild type implies that it normally makes a contribution to PCP. Since proximal and distal cells are characterized by differences in the levels of Ds and Fat, the hypothesis that polarization of Ds and Fat contributes to propagation of PCP suggests explanations for the greater range of Dachs repolarization in the hinge compared to the distal wing. For example, if propagation of Fat-PCP depends upon Ds, then repolarization could be more extensive where Ds expression is higher than where it is lower.
The distal localization of Ds in wing cells parallels the distal localization of Dachs. Moreover, both Dachs and Ds often appear to have a punctate localization profile at the membrane, rather than smooth continuous staining [4]. Their correlated localization extends to these puncta (Fig. 3G). However, Ds is not required for Dachs membrane localization, because strong, unpolarized membrane localization of Dachs is detected in ds mutant discs (Fig. S3B), just as it is in fat mutants [5].
Processing of Ds and its modulation by Fat
In Fz-PCP signaling, maintenance and propagation of polarity depends both upon intercellular binding between distinct of membrane complexes, and an intracellular, mutual antagonism between these complexes [18]. Intercellular binding between Fat and Ds has been described [9–13]. To investigate the possibility of intracellular antagonism between Fat and Ds, we asked whether there are post-translational modifications of one protein that depend upon the other. An influence of Ds on Fat phosphorylation has been described, but it only influences Fat-Hippo signaling, not Fat-PCP [15, 19].
Examination of Ds protein in wing disc lysates revealed that in addition to a band near the top of the gel, which could represent full length Ds (Ds-FL), two smaller bands, with mobilities corresponding to approximately 150 kd (Ds-C150) and 210 kd (Ds-C210), were detected (Fig. 4A). These bands were confirmed as Ds polypeptides by their absence from lysates of ds mutant discs (the antisera also recognizes non-specific bands (NS), which are present in ds mutants). These bands represent C-terminal fragments, since the Ds antisera is directed against the cytoplasmic domain [20]. There are several potential mechanisms by which smaller Ds polypeptides could be generated, such as proteolytic cleavage, alternative transcription starts, or alternative splicing. A prediction of the endoproteolytic cleavage hypothesis is the existence of complementary N-terminal Ds polypeptides. This was tested using our genomic HA-tagged Ds transgene. In these animals, anti-HA recognized a band near the top of the gel with the same mobility as the largest band detected by anti-Ds (Ds-FL), and also two smaller polypeptides, with mobilities of approximately 270 kd (Ds-N270) and 220 kd (Ds-N220) (Fig. 4B). These observations suggest that Ds can be endoproteolytically processed at one of two alternative sites, a more N-terminal site, leading to Ds-N220 and Ds-C210 polypeptides, and a more C-terminal site, leading to Ds-N270 and Ds-C150 polypeptides (Fig. 4C). In most of the wing disc, the anti-Ds and anti-HA localization profiles were not distinguishable (Fig. S3D–F). The overall similarity between HA and Ds staining patterns suggests that the N- and C terminal halves of Ds remain associated.
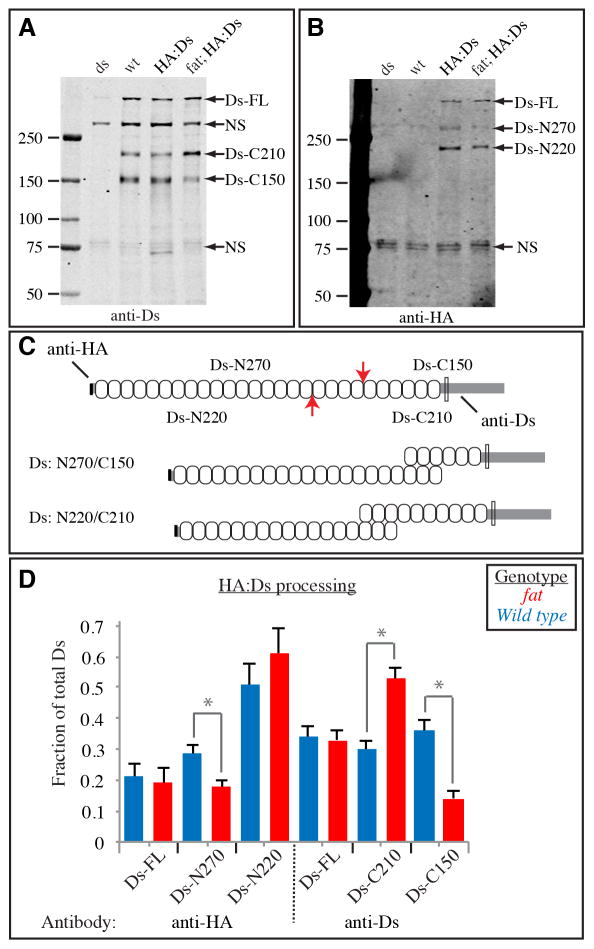
Figure 4. Endoproteolytic processing of Ds.
A,B) Western blot on lysates of wing discs, from ds mutant (dsUA071/ds36D), wild-type, HA:Ds-expressing, and HA:Ds-expressing fat mutant (fat8/fatG-rv). The left lane contains size markers of the indicated molecular weights. The presumed identities of bands in other lanes are indicated by the labels to the right. The same membrane is depicted in both panels, A shows the results of anti-Ds staining, B shows the results of anti-HA staining. C) Schematic of Ds protein. Upper indicates the approximate locations of endoproteolytic cleavage sites (red arrows) and the resulting polypeptides, and the epitopes of antisera used. Ovals indicate cadherin domains, the transmembrane domain is indicated by the thin rectangle. Lower indicates the Ds isoforms that could result from cleavage at the two different sites. D) Quantitation of Ds processing. The fraction of Ds in each of the three bands detected by each antisera was calculated by summing the intensities of all bands. Bars show the average results from six Western blots on three independently prepared lysates, error bars indicate standard error of the mean. The influence of Fat on Ds processing was significant by t test evaluation of the differences between the fractions of the isoforms indicated by gray bars and asterisks (Ds-N270, P=0.025, Ds-C210, P=0.0008, Ds-C150, P=0.0004). For Ds-N220 the difference was not significant (P=0.36). See also Fig. S3.
The total amount of Ds was not affected by fat mutation (Fig. S3C). However, absence of fat had a significant effect on Ds isoforms, as Ds-C210 increased, whereas Ds-C150 decreased (Fig. 4A,D). The observation that these are affected in opposite ways is consistent with the hypothesis that Ds is subject to alternative, mutually exclusive processing pathways. More importantly, it suggests that Fat modulates this processing. When the N-terminal half of Ds was examined, Ds-N270 was decreased in fat mutants, consistent with a stoichiometric relationship between Ds-N270 and Ds-C150 (Fig. 4B–D). A subtle increase in Ds-N220 was observed, but it was not statistically significant (Fig. 4D). This lack of effect on Ds-N220 does not fit the simple model of alternative processing, and there may be additional effects of fat on Ds-N220. Although further studies will be needed to clarify the mechanism by which these Ds polypeptides are generated, and their significance to Fat signaling, the observation that Fat influences the distribution of Ds isoforms is significant in that it identifies a post-translational influence of Fat on Ds that might contribute to Ds polarization.
Establishment of polarity by Ds and Fj gradients
Our observation that differences in Fj or Ds expression can alter Fat PCP at a distance, and that Ds, and to a lesser extent Fat, are polarized within the wing, together with other recent studies [21, 22], imply that establishment of polarity in the Fat PCP system relies not just upon direct interpretation of Fj and Ds gradients, but also upon amplification and propagation of PCP. To achieve this, PCP models incorporate both asymmetric intercellular signaling, and antagonistic intracellular interactions between complexes that localize to distinct sides [18, 23]. Intercellular binding between Ds and Fat is well established, but on its own this would not propagate polarity from cell to cell. However, incorporation of a local, intracellular antagonism of Ds by Fat activity could polarize Ds localization, which could then enable Fat-PCP to propagate. We hypothesize that Fat regulates Ds by influencing production or stability of processed Ds isoforms.
The propagation of polarity means that Fat-PCP is influenced not only by the local gradient, but also by differential expression at a distance. Strong repolarization of Dachs was dependent upon having substantial differences in expression. Notably, strong differences in expression of both Fj and Ds normally occur in the proximal wing (Fig. 1D,E), and these differences have significant effects on Fat activity [9, 24]. Both our measures of the range of Dachs repolarization, and mathematical modeling [25], suggest that the Fj/Ds expression boundary in the proximal wing would not be sufficient to direct Fat-PCP across 30 or more cells, as would be required at late third instar. However, at early third instar, when the developing wing is small, a mechanism that propagates PCP from an expression boundary for several cells could in principle be sufficient to establish PCP throughout the wing. Once established, the mechanisms that allow Fat-PCP to propagate could also help maintain Fat-PCP as the wing grows. In this case, the Fj and Ds boundaries at the edge of the developing wing would be the main drivers of polarity, rather than the shallow gradients of their expression within the wing itself.
Supplementary Material
HIGHLIGHTS.
Differential expression of Fj or Ds can modulate Dachs polarization at a distance
Fat and Ds are normally polarized, providing a means for propagation of PCP
Ds protein is processed, and this processing may be modulated by Fat
Our observations imply that Fj and Ds gradients are measured across several cells
Acknowledgments
We thank the Developmental Studies Hybridoma Bank, the Bloomington stock center, and Mike Simon for antibodies and Drosophila stocks, Binnaz Staley for the UAS-Dachs:Cit plasmid, Cordelia Rauskolb for Figure panels 1E and S3A,B and for comments on the manuscript. This research was supported by NIH grant 2R01GM078620 and the Howard Hughes Medical Institute.
Footnotes
Experimental Procedures and four figures are included within the supplemental material.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas C, Strutt D. Developmental dynamics: an official publication of the American Association of Anatomists. 2011. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. [DOI] [PubMed] [Google Scholar]
- 2.Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 3.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008;105:14897–14902. doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 6.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 7.Mao Y, Tournier AL, Bates PA, Gale JE, Tapon N, Thompson BJ. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes & Development. 2011;25:131–136. doi: 10.1101/gad.610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development (Cambridge, England) 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho E, Irvine KD. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 11.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 12.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 13.Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of Fat:Dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Irvine KD. Processing and phosphorylation of the Fat receptor. Proc Natl Acad Sci U S A. 2009;106:11989–11994. doi: 10.1073/pnas.0811540106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Y, Kucuk B, Irvine KD. Drosophila lowfat, a novel modulator of Fat signaling. Development. 2009;136:3223–3233. doi: 10.1242/dev.036152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strutt H, Strutt D. Asymmetric localisation of planar polarity proteins: Mechanisms and consequences. Seminars in Cell & Developmental Biology. 2009;20:957–963. doi: 10.1016/j.semcdb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Sopko R, Silva E, Clayton L, Gardano L, Barrios-Rodiles M, Wrana J, Varelas X, Arbouzova NI, Shaw S, Saburi S, et al. Phosphorylation of the tumor suppressor fat is regulated by its ligand Dachsous and the kinase discs overgrown. Curr Biol. 2009;19:1112–1117. doi: 10.1016/j.cub.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Axelrod JD, Simon MA. Regulation of Frizzled by Fat-like Cadherins during Planar Polarity Signaling in the Drosophila Compound Eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 21.Brittle A, Thomas C, Strutt D. Planar Polarity Specification through Asymmetric Subcellular Localization of Fat and Dachsous. Current biology: CB. 2012 doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, Marchand R, Bardet PL, Marcq P, Graner F, et al. Mechanical Control of Morphogenesis by Fat/Dachsous/Four-Jointed Planar Cell Polarity Pathway. Science. 2012 doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- 23.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 24.Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burak Y, Shraiman BI. Order and stochastic dynamics in Drosophila planar cell polarity. PLoS computational biology. 2009;5:e1000628. doi: 10.1371/journal.pcbi.1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.