Abstract
The rheological properties of cysteine-containing elastin-like polypeptide (Cys-ELP) solutions and Cys-ELP hydrogels are reported. The Cys-ELP solutions exhibit a surprisingly high apparent viscosity at low shear rate. The high viscosity is attributed to the formation of an interfacial cross-linked “skin” at the sample surface, rather than the bulk of the Cys-ELP solution. At higher shear rate, the interfacial cross-linked film breaks, and its influence on the viscosity of the Cys-ELP solution can be ignored. Cys-ELP hydrogels are formed by mixing Cys-ELP and hydrogen peroxide (H2O2). At fixed concentration of Cys-ELP, the gelation time can be tuned by the concentration of H2O2. Cys-ELP hydrogels have the typical characteristics of covalent cross-linked networks, as the storage moduli are larger than the loss moduli and are independent of frequency in dynamic oscillatory frequency sweep experiments. The plateau moduli obtained from linear frequency sweep experiments are much lower than those estimated from the number of thiol groups along the Cys-ELP chain, indicating that only a small fraction of thiols form elastically active cross-links. From the small value of the fraction of elastically active cross-links, the Cys-ELP hydrogel is concluded to be an inhomogenous network. Under steady shear, a 2.5 wt% Cys-ELP hydrogel shear thickens at shear rates lower than that necessary for fracture.
Introduction
Hydrogels are three-dimensional polymer networks capable of imbibing a significant amount of water or biological fluids. They are a promising class of biomaterials that are used extensively in the biomedical field, with applications including drug delivery and tissue engineering. 1–3 In the field of drug delivery, research interest has now shifted from hydrogel implants to injectable hydrogels formed by in situ chemical polymerization or by sol-gel phase transition, as injectable materials offer several advantages including patient comfort and cost reduction. An ideal injectable biomaterial should meet the following requirements: the system is in a sol state before administration; gelation starts to happen immediately after injection; the gel is biodegradable and the breakdown products are bioresorbable and nontoxic.4–8
Elastin-like polypeptides (ELPs) are a class of artificial polypeptides composed of the pentameric repeat Val-Pro-Gly-Xaa-Gly (where Xaa is a guest residue that is any amino acid except Pro) and are so named because this motif recurs in the tropoelastin gene of most vertebrates. ELPs are increasingly utilized for many biomedical applications, as they are nontoxic, biodegradable, and show good pharmacokinetics.9–13 We recently reported an ELP that has periodic Cys residues at the 4th guest residue position (Cys-ELP) forms hydrogels under mild oxidative conditions.14 The potential applications of Cys-ELP as an injectable biomaterial depend on the rheological properties of both the injectable solution (viscosity under shear) and of the oxidized gel (moduli, gelation time, and dynamic mechanical properties). Herein, we investigate the rheological properties of Cys-ELP solutions and Cys-ELP hydrogels as a function of their composition.
Experimental Section
Materials
The recombinant synthesis of Cys-ELP and control ELPs that do not contain Cys residues without cysteine units (denoted as ELP throughout the paper to distinguish them from the Cys-ELP) from a plasmid-borne synthetic gene in E. coli were reported in our previous paper. 14 The Cys-ELP used in this work has 160 pentapeptide repeats, Val-Pro-Gly-Xaa-Gly, where the guest residue Xaa are Ala:Val:Cys in the ratio of 14:1:1 so that 10 Cys residues are periodically distributed along the polypeptide chain. The molecular weight of this Cys-ELP is 62525 g/mol.14 The control ELP also has 160 pentapeptide repeats of Val-Pro-Gly-Xaa-Gly, where the guest residue Xaa are Ala, Gly, and Val residues in the ratio of 8:7:1. The molecular weight of this ELP is 61223 g/mol.14 30 wt% hydrogen peroxide (H2O2) solution and phosphate-buffered saline (PBS) were purchased from Calbiochem of EMD Millipore (La Jolla, CA).
Preparation of Cys-ELP Solution
In this work, a 3.9 wt% Cys-ELP batch solution is first prepared by recombinant synthesis. The batch solution was stored at −80 °C prior to use. The frozen batch solution was thawed in a water bath at room temperature for 5–10 min and then kept in ice. Finally, an aliquot of the 3.9 wt% Cys-ELP batch solution was diluted with PBS buffer to prepare a buffered solution of the desired Cys-ELP concentration (from 0 wt% to 3.9 wt%).
Preparation of Cys-ELP hydrogels
Cys-ELP hydrogels were formed by mixing a Cys-ELP solution with appropriate amounts of H2O2 solution. H2O2 accelerates the formation of disulfide bonds in the Cys-ELP, thereby permitting the formation of a crosslinked network. 14 For rheological measurements, Cys-ELP hydrogels were made by mixing freshly prepared H2O2 solutions and Cys-ELP solutions in PBS using a pipette to ensure good mixing. The samples were then transferred immediately to the rheometer for characterization.
Rheological Measurements
Rheological data were obtained on an AR G2 rheometer (TA Instruments). A concentric cylinder geometry and 8 mm parallel plates were used to characterize the rheological properties of Cys-ELP solutions and Cys-ELP hydrogels, respectively. For rheological measurement of Cys-ELP hydrogels, mineral oil was used at the edge of the samples to prevent evaporation of water in the hydrogels and to prevent air oxidation of the cysteines. To characterize the gelation kinetics of Cys-ELP hydrogels, time-sweep experiments were performed at a frequency of 3 rad/s at strains within the linear region. Strain sweeps were performed across a strain range of 0.001 to 200 at 10 rad/s. Dynamic oscillatory frequency sweeps from 0.1 to 10 rad/s were carried out at appropriate strains within the linear region. Steady shear measurements were performed over a range of shear rates between ~10−3 and ~103 s−1. The experiments were performed at 25 °C.
Results
Rheological properties of Cys-ELP solution
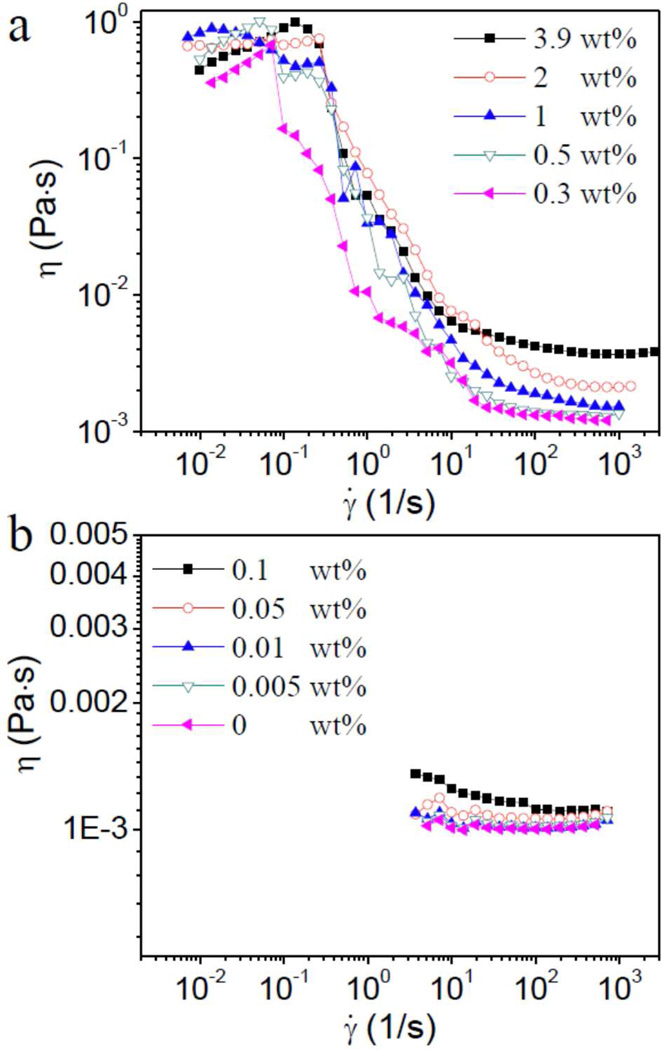
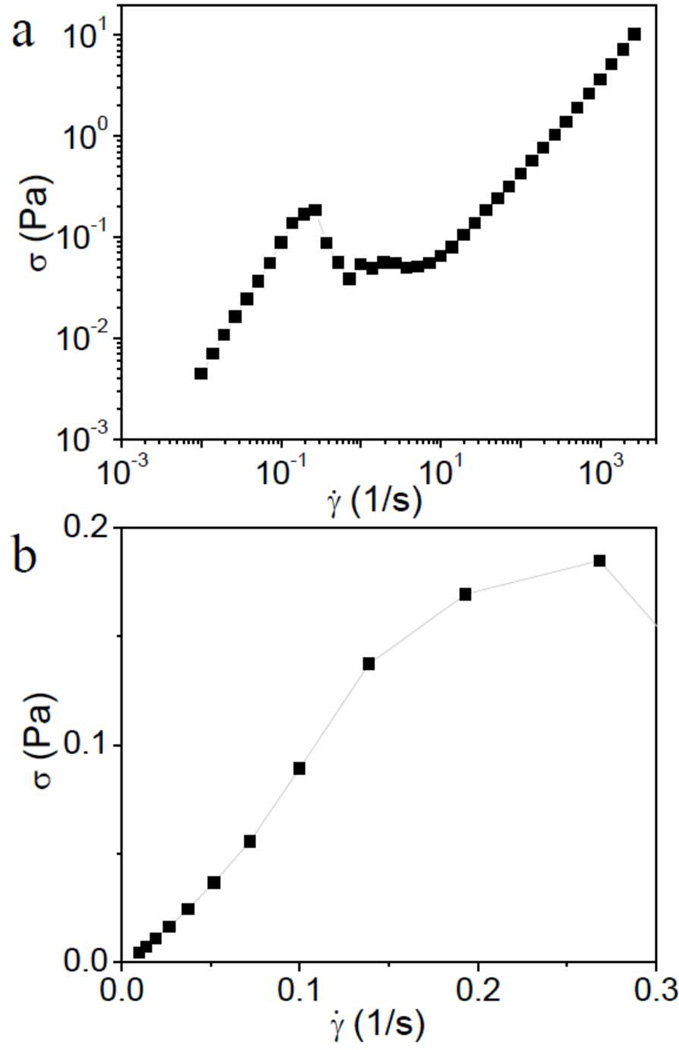
The steady shear behavior of different concentrations of Cys-ELP in PBS is shown in Figure 1. For samples with concentrations of Cys-ELP from 0.3 wt% to 3.9 wt% (Figure 1a), an unexpectedly high apparent viscosity (~0.8 Pa•s) and shear thickening – characterized by an increase in viscosity with shear rate– are observed at low shear rate (from ~10−2 to ~10−1 s−1). The possible mechanism of shear thickening is discussed later in the text. As the shear rate is further increased from ca. 10−1 to 102 s−1, an apparent shear thinning – characterized by an apparent decrease in viscosity with shear rate – is observed. As discussed below, the torque is nearly constant over this apparent shear thinning regime, and the behavior likely involves the motion of an intact sample (or part of the sample) and it is the friction over the cylinder wall that is being measured. This regime is not the primary focus of this paper and is discussed in more detail in the Supporting Information (Figure S1). At a yet higher shear rate of ~103 s−1, the steady shear viscosity of the samples enters a Newtonian regime in which it does not depend on the shear rate. The viscosity of the solutions in the Newtonian regime increases with the concentration of Cys-ELP. For Cys-ELP samples with a concentration of less than 0.1 wt% (Figure 1b), the viscosity at low shear rate is not shown because the torque is close to the sensitivity limit of the instrument. In Figure S2 (Supporting Information), the steady shear results of analogous ELP solutions (0 wt% to 3.9 wt%) are shown, and, unlike their cysteine-containing counterparts, they do not exhibit an apparent high viscosity at low shear rate during steady shear experiments. An analysis of the shear stress versus steady shear rate for the 3.9 wt% Cys-ELP solution is shown in Figure 2. In Figure 2b, no yield-stress is observed at low shear rate, so the fluid is not a Bingham fluid.
Figure 1.
Steady shear viscosity (η) of different concentrations of Cys-ELP in PBS as a function of shear rate (γ̇). (a) Concentrations of Cys-ELP ranging from 0.3 wt% to 3.9 wt%; (b) concentrations of Cys-ELP range from 0 wt% to 0.1 wt%.
Figure 2.
Shear stress (σ) versus shear rate (γ̇) for 3.9 wt% Cys-ELP solution during steady shear experiments. (a) All range of shear rate in logarithmic plot; (b) low shear rate in linear plot.
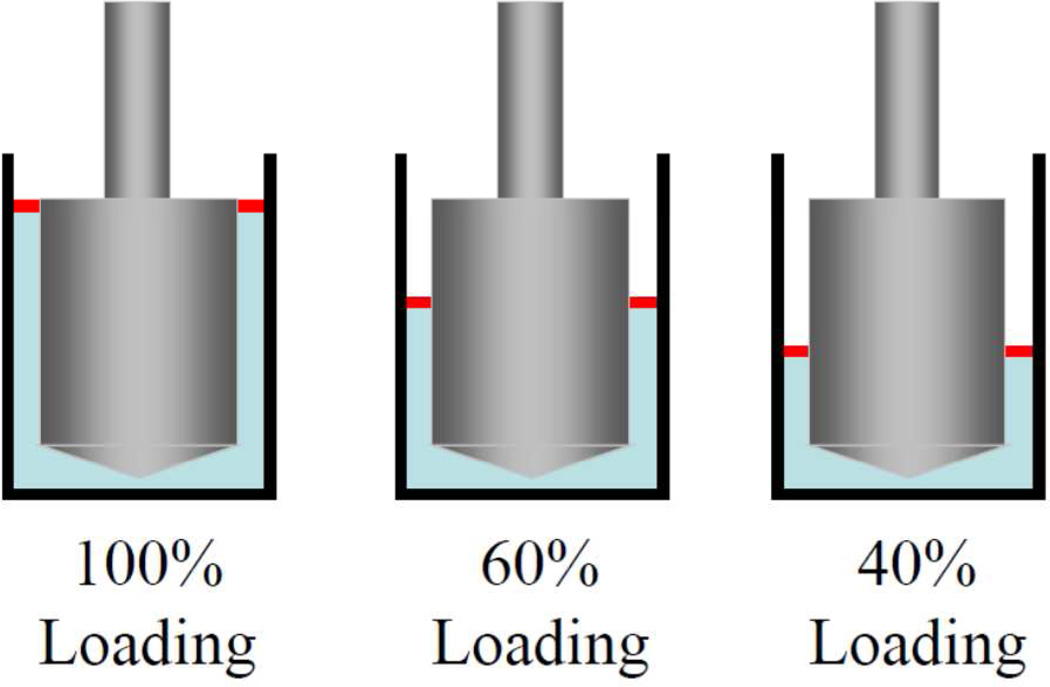
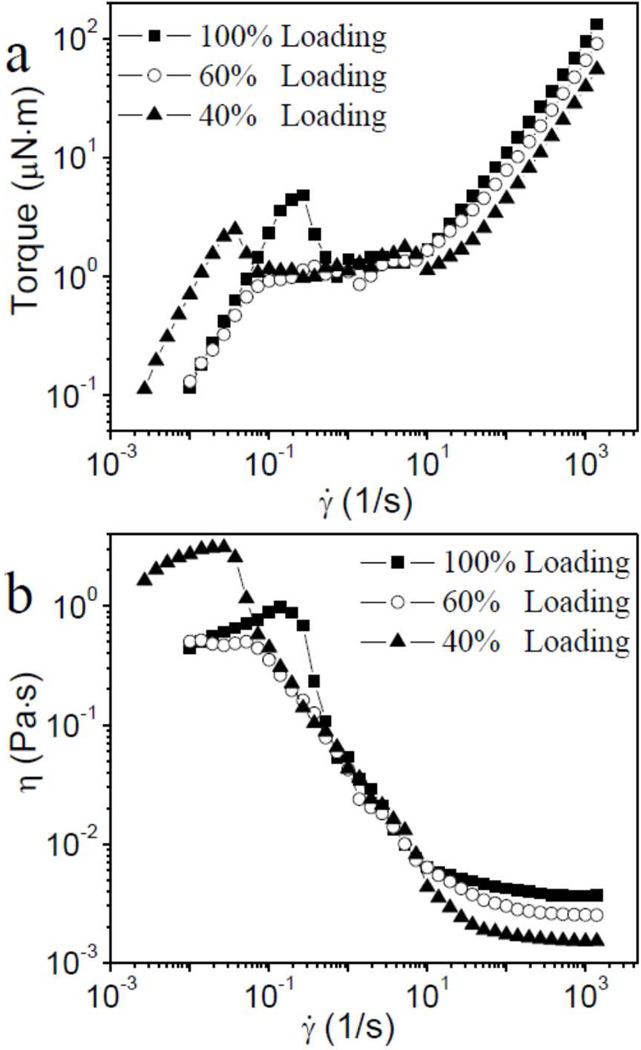
The thiol groups in the cysteine units along the Cys-ELP chain can be oxidized in air to form disulfide bonds, 14 and we hypothesized that the apparent high viscosity at low shear rate in Figure 1 might be due to a cross-linked film at the air-liquid interface, rather than the bulk Cys-ELP solution. To test this hypothesis, we fixed the area of the air-liquid interface while simultaneously varying the contact area of the force-detecting surface of the rheometer by loading different volumes of sample into the concentric cylinder geometry, as shown in Scheme 1. The steady shear results of 3.9 wt% Cys-ELP solution with different loading volume are shown in Figure 3. As the relative volume of the sample is decreased from 100% to 40% (Scheme 1), the torque measured at low shear rate (e.g., 0.02 s−1) does not decrease (Figure 3a), and, accordingly, the apparent shear viscosity at that shear rate does not decrease (Figure 3b). This confirms that the steady shear behavior at low shear rate is dominated by the surface, and not the bulk of the Cys-ELP solution. The selective partitioning of the Cys-ELPs to the air-liquid interface would come with two effects, both of which are observed in these samples. First, the bulk concentration required for surface layer gelation would be lower than that necessary for gelation in the bulk, and the formation of an interfacial layer occurs at Cys-ELP concentrations as low as 0.3 wt%, vs. approximately 2.2 wt% in the bulk (see below). Second, the torque observed under shear should be nearly independent on bulk Cys-ELP concentration above a critical value necessary to form a fully cross-linked surface layer, and this torque saturation is also observed (Figure 3a).
Scheme 1.
Schematic of different volume of loaded Cys-ELP solution in the concentric cylinder geometry of the rheometer. The contact area of samples with the inner cylinder, which in turn is connected to the torque transducer, is decreased by decreasing the volume of the loaded samples (from 100% to 40% of the fully loaded volume). The air-liquid interface area (red) remains constant.
Figure 3.
(a) Torque during steady shear of different volumes of 3.9 wt% Cys-ELP solution loaded in a concentric cylinder geometry (Scheme 1) as a function of the shear rate (γ̇); (b) Apparent steady shear viscosity (η), not corrected for sample volume, of different volumes of 3.9 wt% Cys-ELP solution (Scheme 1) as a function of the shear rate (γ̇).
In contrast to the low shear rate behavior, the torque in the high shear rate Newtonian regime (e.g., at 103 s−1) decreases as the volume of loaded sample decreases. In this case, the torque exerted on the inner cylinder is proportional to the contact area, and thus it reflects a bulk property of the loaded sample.15 We conclude that the cross-linked Cys-ELP film at the air-liquid interface breaks under higher stresses (the apparent shear thinning regime in Figure 1), beyond which point the contribution of the interfacial cross-linked film to the viscosity of the samples is minimal.
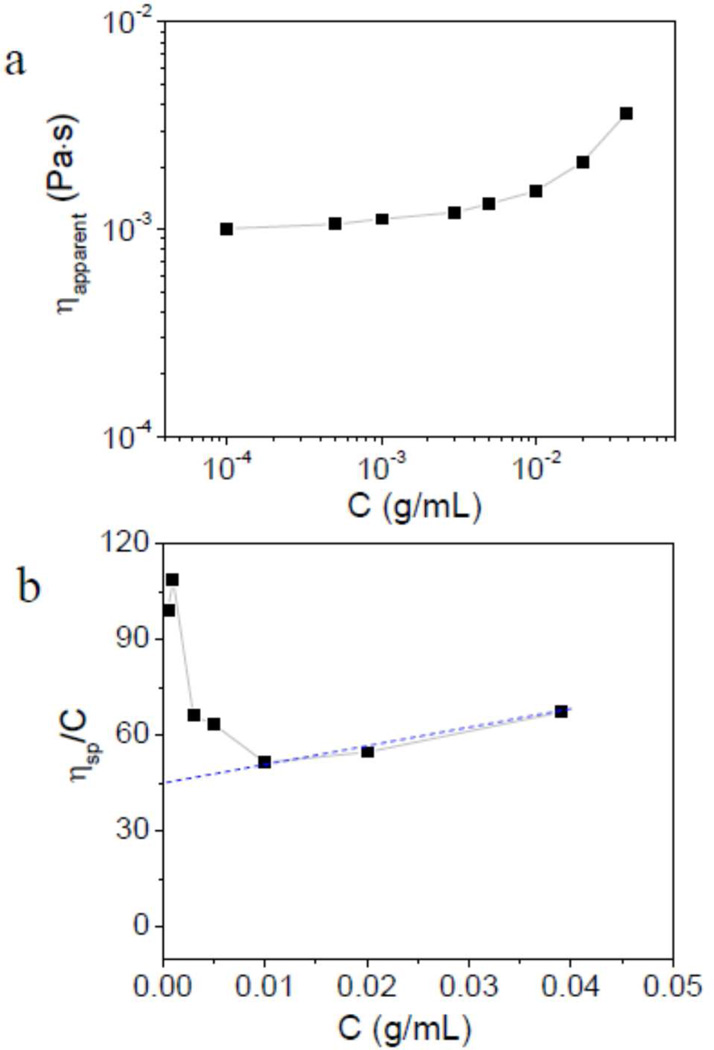
As discussed above, the viscosity in the Newtonian regime reflects the viscosity of bulk Cys-ELP solution without any disulfide bond forming. As the cross-linking of Cys-ELP solution at the air-liquid interface is hard to avoid, the apparent viscosities (ηapparent) of Cys-ELP solution in the high shear Newtonian regime in Figure 1 are used as the viscosities of the bulk Cys-ELP solution. In Figure 4a, ηapparent versus the concentration of Cys-ELP solution is shown. For 5×10−5 g/mL and 1×10−4 g/mL Cys-ELP solution, the viscosity is almost the same as that of pure PBS buffer. As the concentration of Cys-ELP solution is increased, ηapparent increases. Figure 4b shows the plot of reduced viscosity (ηsp/C) versus concentration (C) of Cys-ELP solution. ηsp is the specific viscosity and is defined as ηsp= (η/ηs) −1, where η is the viscosity of polymer solution (ηapparent here) and ηs is the viscosity of pure PBS buffer here. The intrinsic viscosity ([η]) is obtained by the Huggins equation:16
| (1) |
where K′ is the Huggins constant. In Figure 4b, the reduced viscosity (ηsp/C) has a linear relationship with concentration of Cys-ELP (C) from 0.010 g/mL (1.0 wt%) to 0.039 g/mL (3.9 wt%). However, as concentration of Cys-ELP (C) decreases below 1.0 wt%, the reduced viscosity (ηsp/C) increases and does not have a linear relationship with C. Similar results are obtained for ELP solutions (data not shown). This phenomenon is well known for aqueous polymer solutions, 17–19 and the high value of ηsp/C in dilute solution is thought to be caused by the absorption of polymer to the geometry. 17–19 Following previous treatments, 17–19 the data from the linear range in Figure 4b (C ranges from 0.010 g/mL to 0.039 g/mL) is used to obtain the intrinsic viscosity [η]. In Figure 4b, the [η] of Cys-ELP solution is determined to be ~45.0 mL/g. Experimentally, the overlap concentration C* (as [η]C =1) and the critical concentration of entanglement Ce (as [η]C = 4) are used to distinguish the concentration regimes: the dilute regime as C<C*([η]C<1); the semidilute unentangled regime as C*<C<Ce (1<[η]C<4); and the semidilute entangled regime as C>Ce (4<[η]C<10).16,20,21 The overlap concentration C* determined in this manner is about 0.022 g/mL (or 2.2 wt%), and so the three different concentrations of Cys-ELP solution (2.5 wt%, 3.5 wt%, and 4.5 wt%) used below are in the semidilute unentangled regime. We note that although most naturally occurring polypeptides are polyelectrolytes, the genetically engineered sequence of Cys-ELP contains no charged residues, and so the only charged groups on the ~62 kDa polymer are the terminal amine and carboxylic acid groups. The Debye screening length in PBS is approximately 0.7 nm, the minimal contribution of these two charges on the overlap concentration C* is neglected. While surface adsorption and charged end groups might have a minor influence on the derived value of C*, we note that the value obtained here is in excellent agreement with that obtained on a similar system previously using photon correlation spectroscopy.22
Figure 4.
(a) Apparent viscosity of bulk Cys-ELP solution (ηapparent) versus the mass concentration (C) of Cys-ELP solution; (b) Reduced viscosity of bulk Cys-ELP solution (ηsp/C) versus the mass concentration (C) of Cys-ELP solution. Dotted line is used for fitting.
Interchain cross-linking is favored relative to intrachain cross-linking above the overlap concentration, promoting network formation. 20,23 This value of C* is therefore consistent with our previous observation that a Cys-ELP hydrogel is formed for samples with concentrations of Cys-ELP above 2.5 wt% and appropriate amounts of H2O2, while no hydrogel is formed for 1.0 wt% Cys-ELP solution, as characterized by the inverted tube test (Figure S3, Supporting Information).14
Rheological properties of Cys-ELP hydrogel
As discussed above, to form a cross-linked Cys-ELP hydrogel, the concentration of a Cys-ELP solution should be above the overlap concentration (2.2 wt%). Thus, three different concentrations of Cys-ELP solution (2.5 wt%, 3.5 wt%, and 4.5 wt%) were used here to form the Cys-ELP hydrogel. To determine the critical concentration of H2O2 solution necessary to form a Cys-ELP hydrogel, six different concentrations of H2O2 solution from 0.01 wt% to 3 wt% were used. To reduce the influence of oxygen in the air on the formation of disulfide bonds in the Cys- ELP hydrogels, mineral oil was applied to the edge of samples to restrict the contact of the sample with air.
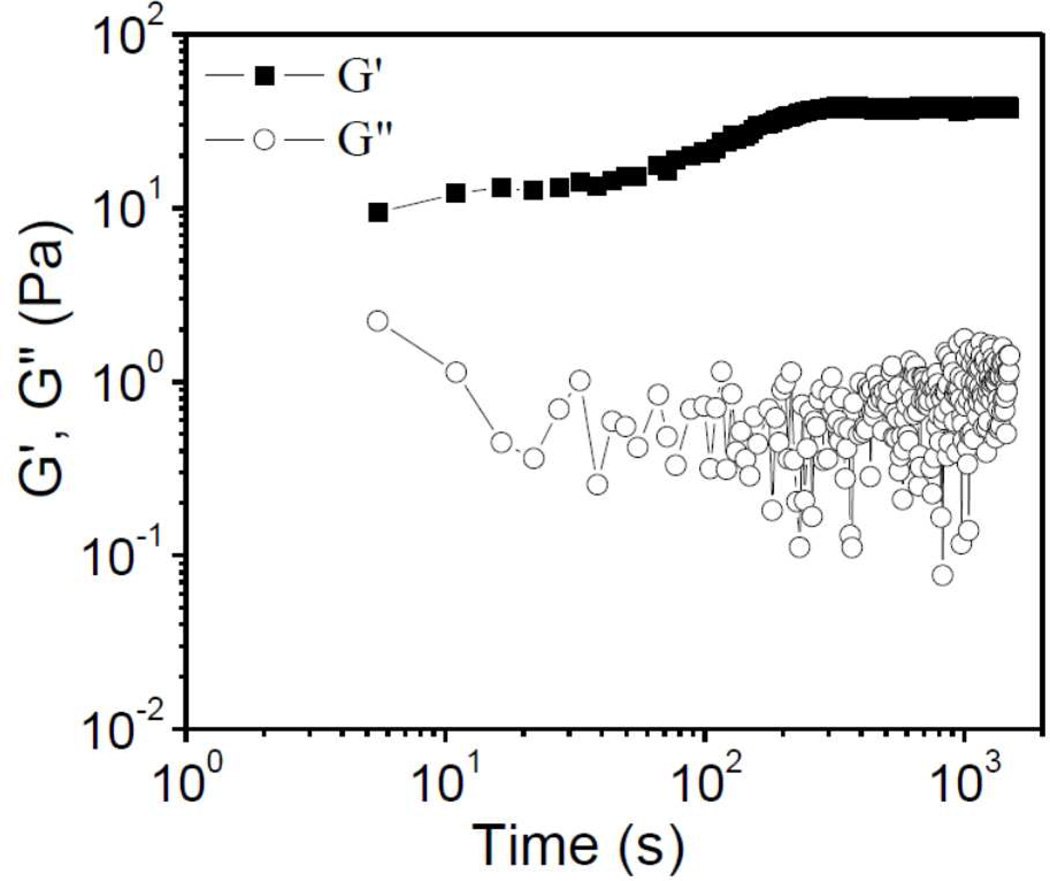
In a covalent cross-linked network, the storage modulus G΄ is larger than the loss modulus G˝ under linear oscillatory deformation,24 and time sweep experiments can be used to determine the evolution of G΄ and G˝ during the cross-linking.11 The time at which G΄ equals or exceeds G˝ is called the gelation time.12 In Figure 5, the time sweep experiment of a 2.5 wt% Cys-ELP solution after addition of 0.3 wt% H2O2 is shown. The value of G΄ is larger than G˝ from the start of the measurement, indicating that a network structure is formed immediately, and it is therefore hard to determine the exact gelation time in this case. G΄ increases with time from ~5 s to ~300 s and reaches a plateau value after ~300 s, whereas G˝ fluctuates with time and no apparent increase is observed. These results suggest that the thiol residues within the Cys-ELP hydrogel continue to cross-link over the time until G΄ reaches its plateau value, and we denote the time at which G΄ reaches its plateau as the equilibrium time. The equilibrium time is 300 s for the hydrogel formed from 2.5 wt% Cys-ELP and 0.3 wt% H2O2. All subsequent linear oscillatory frequency sweep data for Cys-ELP hydrogels with 2.5 wt% Cys-ELP and 0.3 wt% H2O2, described below, were obtained after the equilibrium time had passed to ensure that the Cys-ELP hydrogel has a stable network structure. The gelation time of a sample with 2.5 wt% Cys-ELP and 0.01 wt% H2O2 is about 600 s, while other samples form a gel at the beginning of experiments and the exact gelation time is hard to determine (< 5s). For a 2.5 wt% Cys-ELP solution with 0.01 wt%, 0.03 wt%, 0.1 wt%, 0.2 wt%, and 3 wt% H2O2, the equilibrium time is about 1200 s, 600 s, 300 s, 300 s, and 10 s, respectively (Figures S4–S8, Supporting Information). From the results in Figures S4–S8, we know that the critical concentration of H2O2 solution for samples with 2.5 wt% Cys-ELP to ensure gelation is as low as 0.01 wt%.
Figure 5.
Evolution of storage (G΄) and loss (G˝) moduli during the gelation of 2.5 wt% Cys-ELP solution with 0.3 wt% H2O2. Scanning frequency is 3 rad/s, strain is 5% (linear regime).
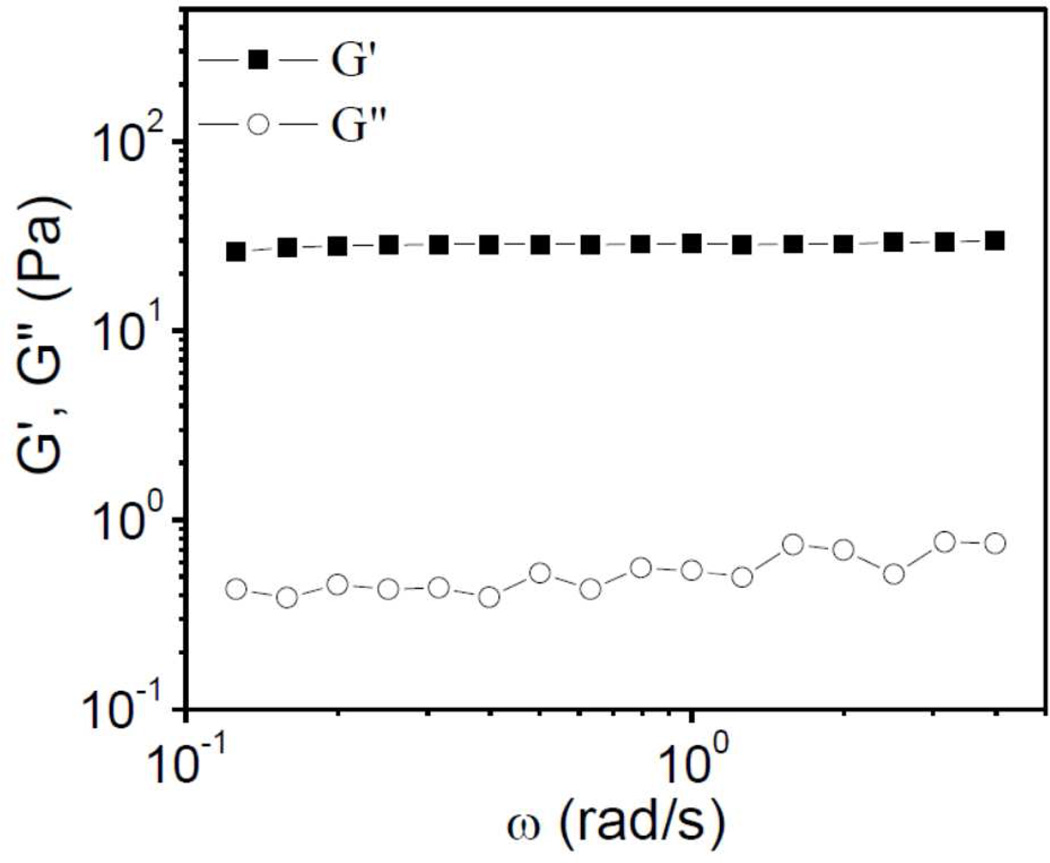
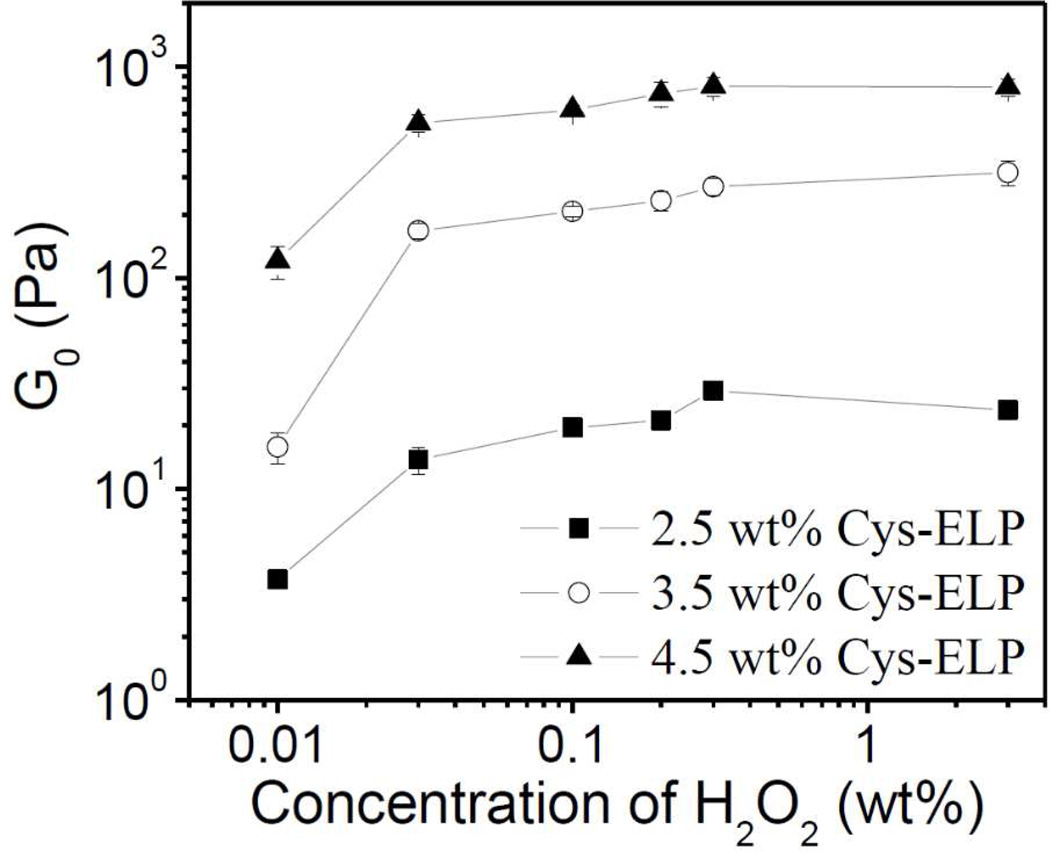
We next consider the linear rheological properties of equilibrated Cys-ELP hydrogels. In Figure 6, the linear oscillatory frequency sweep results of Cys-ELP hydrogel with 2.5 wt% Cys-ELP and 0.3 wt% H2O2 are shown. The storage modulus (G΄) is larger than the loss modulus (G˝), and both G΄ and G˝ are independent of frequency, across the experimental range, further confirming that a cross-linked network is formed.24 From the plateau value of G΄ in Figure 6, the plateau modulus (G0) is obtained. Similar frequency sweep results were obtained for Cys-ELP hydrogels formed under different conditions, and G0 of samples with different concentrations of Cys-ELP and H2O2 solution are summarized in Figure 7. At a fixed concentration of Cys-ELP, G0 increases as the concentration of H2O2 is increased from 0.01 wt% to 0.03 wt%, but higher H2O2 concentrations have little or no effect on G0. At fixed concentrations of H2O2, G0 increases with concentration of Cys-ELP, providing the opportunity to tune hydrogel stiffness.
Figure 6.
Storage (G΄) and loss (G˝) moduli as a function of frequency (ω) for a Cys-ELP hydrogel formed from 2.5 wt% Cys-ELP and 0.3 wt% H2O2.
Figure 7.
Equilibrium plateau modulus (G0) of Cys-ELP hydrogels as a function of the concentration of H2O2 for different concentrations of Cys-ELP.
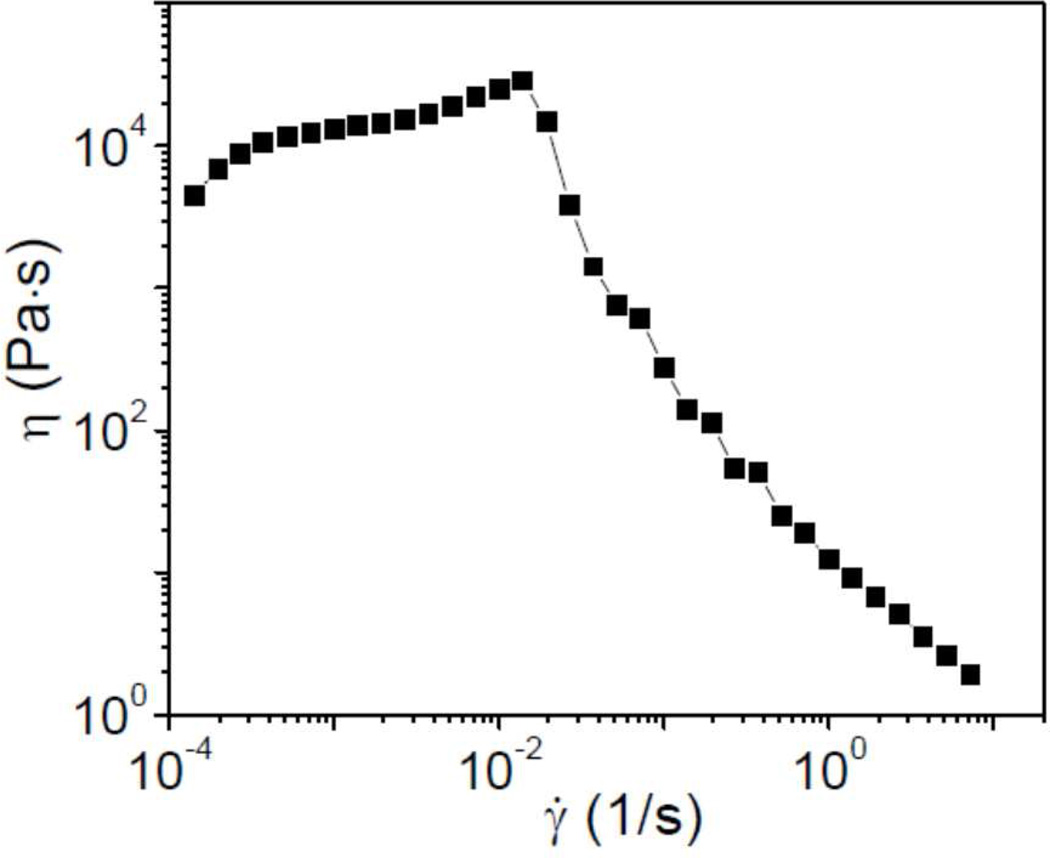
The steady shear behavior of the equilibrated Cys-ELP hydrogel formed from 2.5 wt% Cys-ELP and 0.3 wt% H2O2 is shown in Figure 8. The viscosity increases with shear rate (shear thickening) from 10−4 to ~10−2 s−1, before dropping precipitously with further increase in shear rate. The similarity of this behavior in the Cys-ELP hydrogels to that observed (although at a lower magnitude) in the Cys-ELP solutions (Figure 1) further supports the formation of an air-oxidized cross-linked film in the latter system. The shear thinning of the networks is inferred to be caused by network fracture,23 as the gels are broken into pieces under these conditions. The shear thickening mechanism is less obvious. We rule out additional cross-linking due to ongoing oxidation, because the steady shear experiments are started only after the 300 s equilibration time has elapsed and the concentration of free thiol groups in the hydrogel is determined to be very small and can be ignored.14 To date, the potential mechanisms for shear thickening of polymer networks have been classified into two main categories. The first ascribes shear thickening to the nonlinear high tension along stretched polymer chains beyond the Gaussian range. 25–27 The second mechanism attributes shear thickening to a shear induced increase in number of elastically active chains.28–30 The Cys-ELP hydrogels are covalently cross-linked, and a reorganization of network structure without fracture seems unlikely. We therefore infer that the main contribution to the shear thickening behavior is likely nonlinear high tension along stretched polymer chains, but did not investigate this mechanism further.
Figure 8.
Steady shear viscosity (η) versus shear rate (γ̇) for Cys-ELP hydrogels formed from 2.5 wt% Cys-ELP and 0.3 wt% H2O2.
The strain sweep behavior of the equilibrated Cys-ELP hydrogel formed from 2.5 wt% Cys-ELP and 0.1 wt% H2O2 was also examined. As shown in the supporting information, a linear response regime was observed for both G΄ and G˝ at low strain, while strain hardening was observed in G΄ and G˝ at intermediate strain and strain softening of G΄ and G˝ at higher strain (Figure S9). The nonlinear strain sweep results are similar to the nonlinear steady shear results shown in Figure 6, and although we do not pursue it further here, we therefore infer that the strain hardening is also caused by nonlinear high tension along stretched polymer chains.31
Discussion
Solution Properties
We have previously demonstrated in animal experiments that Cys-ELP solutions are potentially valuable as injectable biomaterial platforms by using dual-needle simultaneous injection with an oxidizing H2O2 solution.14 The rheological properties of Cys-ELP solutions are directly relevant to their potential use in this regard. In particular, the sol before administration should be of sufficiently low viscosity to allow injection with minimal pain, and our results show that at concentrations that are suitable for rapid gelation, the bulk solution properties are quite favorable. The initial viscosities of Cys-ELP solution are low (10−3 – 10−2 Pa•s) and the solutions are Newtonian up to shear rates of 103 s−1. Indeed, Lys-containing ELPs reported previously as a potential hydrogel required concentrations in excess of 20 wt%, chemical cross-linkers, and also heating.11,12 The Cys-ELP presented herein formed a hydrogel faster than the previously reported ELPs, without any exogenous cross-linking chemistry other than the H2O2, at lower concentration of Cys-ELP (the minimum is 2.5 wt%), and under physiological conditions. The quantitative measurements are supported further by qualitative observations: in practice, the solutions were easy to handle and to load into the geometry of the rheometer. The Cys-ELP solutions are therefore comparable to non-cysteine bearing ELP solutions employed with success previously.
Our studies did uncover, however, a potential complication in the form of an air-oxidized, cross-linked “skin” at the air-liquid interface. This skin could provide significant resistance to flow, with apparent relative viscosity increases of 2–3 orders of magnitude or higher, depending on sample surface-to-volume ratio. While various strategies for inhibiting oxidation might be envisioned, the rheological studies demonstrated a practical solution to surface film formation. The skins are easily broken by shear, and so compromised samples could be “pre-sheared” immediately prior to injection, for example by repeatedly drawing into and out of a syringe. Such mechanical action restores the solution rheology to its intrinsic properties.
Hydrogel Properties
The properties of the networks during and following gelation also impact their potential utility. For modest concentration of H2O2 (< 3 wt%), the gelation kinetics are fast – too fast, in many cases, to be characterized in the rheometer. Injectable biomaterials need to gel rapidly in order to form a depot and/or scaffold in vivo, and the gelation times exhibited by Cys-ELP are rapid enough to be useful for biomedical applications. Furthermore, the mechanical strength of a hydrogel is an important physical parameter for controlling its degradability and the release of drugs. The present rheological study reveals that the mechanical strength is tunable by changing the concentrations of Cys-ELP and H2O2, which offers a flexible platform for the design of injectable hydrogels for medical application. 3 wt% of H2O2 is generally used as a common, mild oxidative antiseptic for wound cleaning because it is well-known to be rapidly metabolized to non-toxic products, oxygen and water, in vivo. The lower concentration of H2O2 required to form hydrogels in this system is an advantage for its potential biomedical application.
Hydrogel Structure
From the frequency sweep results of samples, the plateau moduli (G0) are obtained from the constant value of G΄ at high frequency (Figure 7), and the number density of elastically active chains (ν) can be calculated by the following equation: 20
| (2) |
where kB is the Bolzmann constant and T is the temperature. The maximum theoretical number of cross-links per unit volume (ν0) in the samples is calculated from the concentration of Cys-ELP and number of thiol groups per Cys-ELP chain. We refer to the ratio of apparent to theoretical active chains, ν/ν0, as the fraction of elastically active chains. According to the results in Figure 7, ν/ν0 ranges from 0.1% to 9% for samples with different concentrations of Cys-ELP and H2O2 solution (Figure S10, Supporting Information). The small value of ν/ν0 indicates that only a small fraction of thiol groups along the Cys-ELP chain form elastically active (interchain) cross-links. In our previous work, the content of thiol groups in the hydrogel (2.5 wt% Cys-ELP and 0.3 wt% H2O2 solution) was quantified using 5,5’-dithiobis(2-nitrobenzoic acid) with L-cysteine as a standard, and we found that the fraction of free thiol groups left unreacted in the as-formed hydrogel is very small and can be ignored.14 So we conclude that most of thiol groups along Cys-ELP chain form inactive intrachain cross-links.
We have to note that hydrogels are necessarily heterogeneous, and, in all cases, there must be some steady-state fraction of discrete (uncrosslinked) polymers and cross-linked polymer aggregates in the network.20, 32, 33 The presence of such “non-networked” fragments should not affect the main features of this analysis, because they do not contribute substantially to the bulk viscoelastic properties of the samples. 32 Additionally, we point out that the heterogeneity means that the value of ν/ν0 as calculated above underestimates the true average number of active cross-links per polymer chain within the active network structure, because it is the ratio of the number of active cross-links (all of which are in the active network) to the total number of polymer chains (some of which are in the active network, and some of which are not). 33
Conclusion
The rheological properties of cysteine-containing elastin-like polypeptide (Cys-ELP) solutions and Cys-ELP hydrogels are reported. Cys-ELP solutions form a cross-linked film at the air-liquid interface due to interfacial air oxidation, and the apparent high viscosity of Cys-ELP solutions at low shear rate is mainly attributed to the interfacial cross-linked film rather than to the bulk Cys-ELP solution. At higher shear rate, the interfacial cross-linked film is broken, and the influence of the interfacial cross-linked film on the viscosity of Cys-ELP solution can be excluded. The overlap concentration of Cys-ELP (2.2 wt%) is calculated from the apparent viscosity of Cys-ELP solution in the Newtonian regime at high shear rate, and this concentration provides an important benchmark for solution concentrations that are necessary in order to achieve subsequent chemically triggered gelation.
Hydrogen peroxide (H2O2) is used to accelerate the disulfide cross-linking to form a Cys-ELP hydrogel. Cys-ELP hydrogels have the typical characteristic of covalent cross-linked networks, as the storage moduli are larger than the loss moduli and moduli do not exhibit frequency dependence during linear frequency sweep. The plateau moduli obtained from the linear frequency sweep experiments are much lower than that estimated from the total number of thiol groups along the Cys-ELP chain, which indicates that only a small fraction of thiol groups form elastically active cross-linkers. Cys-ELP hydrogels also show shear thickening during steady shear experiments. The mechanisms of shear thickening are inferred to be mainly caused by the non-Gaussian stretching of polymer chains.
The combination of solution properties, gelation kinetics, and ultimate hydrogel moduli and network structure suggest that the Cys-ELPs are promising candidates for biomedical applications, as they are compatible with the criteria required of an injectable biomaterial. In that pursuit, the ability to engineer structural variations in the Cys-ELP component through recombinant DNA techniques and the complementary ability to tune the ultimate properties by optimizing the concentrations of both Cys-ELP and hydrogen peroxide provide an opportunity to tune the properties of the system for a desired application.
Supplementary Material
Acknowledgment
S. L. C. acknowledges the financial support of NSF (CHE-0646670) and NIH (R01-EB-001037). A. C. acknowledges the financial support of NIH grant R01-GM-061232 and R01-EB-000188. D. A. acknowledges a grant from the Science and Technology Foundation of Japan.
Footnotes
Supporting Information Available: Torque versus shear rate during steady shear experiments for different concentrations of Cys-ELP solution, steady shear results of ELP solution, inverted tube test results of sol-gel transition for different concentration of Cys-ELP with different concentration of H2O2, time sweep results for 2.5 wt% Cys-ELP with different concentration of H2O2, strain sweep results of the equilibrated Cys-ELP hydrogel formed from 2.5 wt% Cys-ELP and 0.1 wt% H2O2, fraction of elastically active chains in the Cys-ELP hydrogels. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wichterle O, Lim D. Nature. 1960;185:117–118. [Google Scholar]
- 2.Hoffman AS. Adv. Drug. Deliv. Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 3.Kopecek J. Biomaterials. 2007;28:5185–5192. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatefi A, Amsden B. J. Control. Release. 2002;80:9–28. doi: 10.1016/s0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 5.Packhaeuser CB, Schnieders J, Oster CG, Kissel T. Eur. J. Pharm. Biopharm. 2004;58:445–455. doi: 10.1016/j.ejpb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Kretlow JD, Klouda L, Mikos AG. Adv. Drug. Deliv. Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Ding J. Chem. Soc. Rev. 2008;37:1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MK, Lee DS. Macromol. Biosci. 2010;10:563–579. doi: 10.1002/mabi.200900402. [DOI] [PubMed] [Google Scholar]
- 9.Meyer DE, Chilkoti A. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 10.McHale MK, Setton LA, Chilkoti A. Tissue. Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 11.Lim DW, Nettles DL, Setton LA, Chilkoti A. Biomacromolecules. 2007;8:1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim DW, Nettles DL, Setton LA, Chilkoti A. Biomacromolecules. 2008;9:222–230. doi: 10.1021/bm7007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fluegel S, Fischer K, McDaniel JR, Chilkoti A, Schmidt M. Biomacromolecules. 2010;11:3216–3218. doi: 10.1021/bm100965y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asai D, Xu DH, Liu WG, Quiroz FG, Callahan DJ, Zalutsky MR, Craig SL, Chilkoti A. Biomaterials. 2012;33:5456–5458. doi: 10.1016/j.biomaterials.2012.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada S, Wirtz D, Coulombe PA. J. Struct. Biol. 2003;143:45–55. doi: 10.1016/s1047-8477(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 16.Hong PD, Chou CM, He CH. Polymer. 2001;42:6105–6112. [Google Scholar]
- 17.Ohrn OE. J. Polym. Sci. 1955;17:137–140. [Google Scholar]
- 18.van Oene H, Cragg LH. Nature. 1961;191:1160–1161. [Google Scholar]
- 19.Yang HY, Zhu PP, Peng CL, Ma SL, Zhu QR, Fan CG. Eur. Polym. J. 2001;37:1939–1942. [Google Scholar]
- 20.Rubinstein M, Colby RH. Polymer Physics. New York: Oxford University Press; 2003. [Google Scholar]
- 21.Liu YG, Jun YG, Steinberg V. J. Rheol. 2009;53:1069–1085. [Google Scholar]
- 22.San Biagio PL, Madomia F, Trapane TL, Urry DW. Chem. Phys. Lett. 1988;145:571–574. [Google Scholar]
- 23.Xu DH, Hawk J, Loveless DM, Jeon SL, Craig SL. Macromolecules. 2010;43:3556–3565. doi: 10.1021/ma100093b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishinari K. Progr. Colloid Polym. Sci. 2009;136:87–94. [Google Scholar]
- 25.Marrucci G, Bhargava S, Cooper SL. Macromolecules. 1993;26:6483–6488. [Google Scholar]
- 26.Serero Y, Jacobsen V, Berret JF, May R. Macromolecules. 2000;33:1841–1847. [Google Scholar]
- 27.Ma SX, Cooper SL. Macromolecules. 2001;34:3294–3301. [Google Scholar]
- 28.Tam KC, Jenkins RD, Winnik MA, Bassett DR. Macromolecules. 1998;31:4149–4159. [Google Scholar]
- 29.Regalado EJ, Selb J, Candau F. Macromolecules. 1999;32:8580–8588. [Google Scholar]
- 30.Witten J TA, Cohen MH. Macromolecules. 1985;18:1915–1918. [Google Scholar]
- 31.Xu DH, Craig SL. Macromolecules. 2011;44:7478–7488. doi: 10.1021/ma201386t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu DH, Craig SL. Macromolecules. 2011;44:5465–5472. doi: 10.1021/ma200096s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flory PJ. Principles of Polymer Chemistry. Ithaca, NY: Cornell University Press; 1953. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.