Abstract
Background/Aims
Hepatitis C virus (HCV) infection is a global medical problem. The current standard treatment of chronic hepatitis C (CHC), pegylated interferon plus ribavirin, is prolonged, expensive, has serious side effects and, at best, is only 50% effective. Silymarin is a natural antioxidant often used by patients with CHC, although its efficacy for decreasing HCV levels or ameliorating CHC remains uncertain. HCV infection is associated with increased hepatic oxidative stress, and one of the antioxidant enzymes which protect cells against this stress is heme oxygenase-1 (HO-1).
Methods
We investigated effects of silymarin on HCV and HO-1 gene expression in Huh-7 cells, CNS3, and 9-13 cells (the latter two stably expressing HCV-proteins).
Results
Silymarin significantly down-regulated HCV core mRNA (by 20% - 36%) and protein (by 30%-60%) in CNS3 cells. In contrast, silymarin did not decrease HCV NS5A mRNA or protein expression in 9-13 cells. HO-1 mRNA was up-regulated (60%-400%) by silymarin in Huh-7, CNS3 and 9-13 cells, whereas Bach1 and Nrf2 mRNA levels were not affected. The effect of silymarin to down-regulate HCV core was not related to changes in the Jak-Stat signaling pathway.
Conclusions
Silymarin may be of benefit in CHC, although prospective, randomized, controlled trials are needed to be certain.
Keywords: Bach1, heme oxygenase-1, hepatitis C, interferon, silymarin
Introduction
Approximately 170 million people around the world are infected by hepatitis C virus (HCV) (4), and in the United States about 4 million persons are infected. Most HCV infections become chronic and for nearly half of these patients this condition is gradually progressive, sometimes leading to cirrhosis, end-stage liver disease and/or hepatocellular carcinoma. The HCV genome is a 9.6-kilobase positive single-strand RNA molecule. It contains both 5′ and 3′ non-translated regions (NTRs) that flank an open reading frame, which encodes a single polyprotein of around 3010 amino acids, that is processed into structural (C, E1, E2) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins (5, 13, 21, 25).
The mechanism of injury in hepatitis C includes oxidative stress as a result of altered mitochondrial function, which is thought to be a direct effect of HCV core protein (17, 29). The interaction of the HCV core protein with mitochondria, and the subsequent oxidation of the glutathione pool and complex I inhibition, can be an important cause of the oxidative stress seen in chronic hepatitis C (18). It has been also shown that HCV NS5A protein disturbs intracellular calcium levels and triggers an increase in mitochondrial ROS formation (11). Due to this increase, antioxidant therapy may have beneficial effects in hepatitis C patients.
Cells are protected against oxidative stress by numerous intracellular antioxidant compounds and by diverse antioxidant enzymes. Most of these enzymes can be up-regulated by various physical, chemical, and biological agents that induce oxidative stress. One of these inducible enzymes is heme oxygenase-1 (HO-1). HO-1 protein (∼32 kDa) catalyzes the degradation of heme to release free iron and equimolar amounts of carbon monoxide and biliverdin, the latter of which in mammals is converted to bilirubin by the enzyme biliverdin reductase (36, 37). A multiplicity of DNA-binding proteins interacts with regions that contain multiple antioxidant response elements (ARE). Among these are Nrf2 and Bach1 proteins which heterodimerize with the small Maf proteins (16, 28). Nrf2 has been associated with HO-1 gene activation in response to multiple agents (33). Bach1 binding factor plays an important role in the negative regulation of HO-1 transcription.
The current standard therapy for chronic hepatitis C (CHC) is a combination of pegylated interferon (IFN) and ribavirin (27), but the response to treatment varies depending on viral and host characteristics, especially the viral genotype. The combination therapy is effective in ∼50% of people with chronic hepatitis C, but many patients fail to respond to or relapse following treatment. In addition to the high cost of the treatment and its low availability in many parts of the world, this therapy causes numerous unpleasant side effects, some of which may be serious (9). HCV-infected patients often consider the use of complementary and alternative medicine (CAM) as an alternative to, or in conjunction with, IFN-α and ribavirin treatment. One of these alternative medications, silymarin (SI), is consumed by many patients with CHC (32). It is a mixture of plant flavonoids which are extracted from the seeds and fruits of the milk thistle, Silybum marianum (12), comprised mainly of three structural isomers, silybinin, silidianin and silichristin (38). Flavonoids such as SI have been used, either as such, or as part of several chemically complex preparations. A number of therapeutic and curative properties have been ascribed to flavonoids and many of them have been incorporated into popular folk remedies.
Complementary and alternative medications, such as SI for liver disease, have been widely used around the world for a long time with purported benefits and with few reported side effects, although strong scientific evidence for its benefits is modest, at best.
The possible hepatocyte membrane stabilizing, regeneration-promoting and iron binding properties of SI may be of benefit in hepatitis C (26). Overall, studies that have analyzed the possible beneficial role of SI in hepatitis C treatment have not reported any beneficial effects on serum viral levels. However, a recent study (31) in vitro showed that SI exerts anti-viral and anti-inflammatory effects in liver cells, suggesting that it may assist in the management of patients with chronic hepatitis C. The biochemical mechanism(s) of action of SI is (are) not yet well understood, however, it is possible that there exists a relationship between the effects of SI and the JAK-STAT pathway to decrease the viral load in vitro (31). In this work, we investigated effects of SI on expression of the HCV RNA and core and NS5A proteins and on HO-1 in human liver cells.
Materials and methods
Chemicals and antibodies
SI and interferon from human leukocytes (IFN) were from Sigma (Saint Louis, MO, USA). Dimethyl sulfoxide (DMSO) was purchased from Thermo Fisher Scientific Inc. (Rockford, IL, USA). A 100 mM SI stock solution was prepared in DMSO and filtered through a 0.2 micron filter. Interferon stock solution (∼1 × 103 units/mL) was prepared fresh in deionized water.
Mouse monoclonal antibody against HCV core protein was purchased from Affinity BioReagents, Inc (ABR, Golden, CO, USA). Mouse monoclonal antibody against HCV NS5A was purchased from Virogen Corporation (Watertown, MA, USA). Goat anti-GAPDH polyclonal antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Rabbit anti-STAT1 monoclonal and rabbit anti-PhosphoStat1 polyclonal antibodies were from Cell Signaling Technology (Danvers, MA, USA). Enhanced chemiluminescence (ECL)-Plus western blotting detection reagent was from Amersham Biosciences (Piscataway, NJ, USA).
Cell culture and treatment
Wild type (WT) human hepatoma Huh-7 cell line was purchased from the Japan Health Research Resources Bank (Osaka, Japan). The CNS3 cell line was a gift from R. Bartenschlager. This line stably expresses HCV core to the amino terminal NS3 (amino acid residues 1 through 1233 of the Con1 isolate; Gene Bank accession number AJ238799). It was generated by transduction of Huh-7 cells with a retroviral vector (pRV-CNS3-IGZ) which carries an expression cassette for HCV core-E1-E2-p7-NS2-NS3 regions (7) (Figure 1A). The 9-13 cell line was also from R. Bartenschlager. These cells carry a stably replicating HCV (NS3-5B) region (22) (Figure 1B). The three cell lines were maintained in low glucose Dulbecc’s modified Eagle’s medium (DMEM) supplemented with 100 U/mL of penicillin, 100 μg/mL streptomycin, and 10% (v/v) fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA). For CNS3 and 9-13 cells, additional selection antibiotics 10 μg/mL Zeocin (Invitrogen, Carlsbad, CA, USA) and 1mg/mL G418 (Gibco, Grand Island, NY, USA) respectively, were added to the culture media.
Figure 1.

Schematic Representation of the HCV-Expressing Constructs Used in this work (Also see Ref 10). A. Construct used in CNS3 cells; B. Construct used in 9-13 cells.
Colorimetric MTT assay
Cellular health and viability of treated Huh-7, CNS3 and 9-13 cells were assessed by measuring the conversion of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) to MTT formazan (Sigma). The absorbance was measured on a Synergy HT microtiter plate reader (Biotek Instruments Winooski, VT), at a wavelength of 570 nm with background subtraction at 690 nm. Decreases in absorption were taken as an index of cellular cytotoxicity.
RNA isolation and quantitative RT-PCR
Total RNA from treated cells was isolated by use of TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA). The RNA concentration and purity were determined by measuring absorbance at 260/280 nm. Reverse transcription was performed on 1 μg of total RNA into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real time RT-PCR was performed using a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories) and iQTM SYBR Green Supermix (Bio-Rad). Sequence specific primers for HCV core, HO-1 and glyceraldehyde-3-phosphate dehydrogenase were designed as described (10, 34). Primers used for HCV NS5A were: forward primer, 5′- CGG ACG TAG CAG TGC TCA CTT C and reverse primer, 5′- CGG AAG CTG GGA TGG TCA AAC. Fold change values were calculated by comparative Ct values analysis after normalizing for the quantity of GAPDH mRNA in samples.
Protein preparation and western blotting
Cells were grown to near confluence and washed with PBS, lysed in a buffer containing 1% Triton X-100 with PBS and Halt Protease Inhibitor Cocktail (Pierce Chemicals, Rockford, IL). Protein concentrations were measured using the BCA method. Total proteins (30-50 μg) were separated on 4-15% gradient SDS-PAGE (Bio-Rad) and electrophoretically transferred onto an Immun-Blot PVDF (Bio-Rad). The membranes were blocked for 1 h in PBS containing 5% nonfat dry milk, and then incubated for 1 h with the primary antibody at room temperature. The dilutions of the primary antibodies were as follows: 1:1000 for anti-HCV core antibody; 1:1000 for anti-HCV NS5A antibody; 1:500 for anti-STAT1 and anti-Phospho-STAT1 antibodies; 1:1000 for anti-GAPDH antibody. After four washes with 0.1% Tween 20 in PBS (PBS-T), the membranes were incubated for 1 h with a secondary antibody (anti-rabbit, anti-goat or anti-mouse IgG; dilution 1:10,000). Finally, the membranes were washed four times with PBS-T, and the bound antibodies were visualized with the ECL-Plus chemiluminescence system. A computer based imaging system (Kodak, Rochester, NY) was used to measure the relative optical density of each specific band obtained after Western blotting.
Statistical analysis of data
The experiments were performed at least three times with similar results. Except for Western blots, every experiment included at least triplicate samples for each treatment group. The results shown are the means and SE of three separate determinations. Initial data analyses were performed to indicate whether data were normally distributed, which was found to be the case. Therefore, appropriate parametric statistical procedures were performed. Comparisons were performed by analysis of variance with the Kruskal-Wallis procedure for pair-wise comparisons. Statistical analyses were performed with JMP 6 software (SAS Institute, Cary, NC). Values of p < 0.05 were considered significant.
Results
Cytotoxicity of silymarin in CNS3, 9-13 and Huh7 cells
Because it is important to establish that the effects of SI on HCV expression are due to its properties (as opposed to possible SI-induced cytotoxic effects on the cells), we initially determined the appropriate dose of SI alone in the cell lines. Figures 2A, 2B and 2C show the effects of different doses of SI on mitochondrial activity in Huh-7, CNS3 and 9-13 cells as measured by the MTT assay. SI concentrations of 100-200 μM had no effect on cell viability, whereas concentrations of 300 μM (9-13 cells) and 500 μM (CNS3, 9-13 and Huh-7 cells) caused significant cytotoxicity in the cells (p<0.05). Although 300 μM SI appeared to be non-toxic to the cells by the MTT assay, cell growth inhibition was detected when cell density was observed microscopically. Therefore, in subsequent experiments, we used SI concentrations not exceeding 200 μM.
Figure 2.

Effects of silymarin on cell cytotoxicity. A) Huh-7, B) CNS3 and C) 9-13 cells were incubated with the indicated concentrations of silymarin for 24 or 48 h, and the cell viability was measured with the MTT assay. Asterisks denote significant differences from the vehicle-only control (DMSO), p<0.05. Data represent means ± SE of quadruplicate determinations.
Dose response studies of mRNA and proteins levels in CNS3 and 9-13 cells treated with silymarin
In preliminary studies, the optimal exposure time for SI treatment was found to be 48 h for CNS3 cells and 24 h for 9-13 cells (data not shown). In CNS3 cells, HCV core mRNA levels were down-regulated at both of the SI concentrations tested (Figure 3A). 200 μM SI significantly down-regulated HCV core mRNA (0.44 fold) more than 100 μM did (Figure 3A) at 48 h. Compared to the control, both 100 μM and 200 μM SI down-regulated HCV core protein in CNS3 cells at 48 h (Figure 3B). In 9-13 cells, HCV NS5A mRNA (Figure 3C) and protein levels (Figure 3D) were up-regulated by SI, in contrast to what was seen in CNS3 cells (Figure 3 A and B).
Figure 3.
Comparative effects of silymarin and IFN on expression of HCV core and NS5A in CNS3 and 9-13 cells. The cells were grown to near confluence and the medium was changed to 5% FBS plus DMEM, then treated with 100 or 200 uM Silymarin (SI), 10U Interferon (IFN) as a positive control or with vehicle alone (DMSO). A) At 48 h, silymarin down-regulated HCV core mRNA (by 20% - 36%) to a similar extent as IFN. B) HCV core protein levels normalized to GAPDH. 45 ug of protein were loaded on a 4-15% SDS-polyacrylamide gel and transferred to a PVDF membrane. SI decreased levels of HCV core protein (by 30%-60%) in CNS3 cells. C) In contrast, SI did not decrease HCV NS5A mRNA, or D) HCV NS5A protein levels normalized to GAPDH. Data for mRNA levels are means ± SE of triplicate determinations. *differs from control (DMSO), p<0.05.
Effects of silymarin on HO-1, Bach1 and Nrf2 mRNA levels in Huh-7, CNS3 and 9-13 cells
HO-1 mRNA levels were significantly up-regulated by most of the SI concentrations tested in the three cell lines tested, with 200 μM showing the highest levels of HO-1 mRNA induction (Figure 4). It is known that HO-1 can be up-regulated markedly by a variety of chemical and physical perturbations. To explore the possible mechanism by which SI up-regulates HO-1 mRNA levels, the levels of Bach1 and Nrf2 transcription factors, which are known regulators of HO-1 gene expression, were measured. We found that SI did not affect expression of Bach1 or Nrf2 mRNA levels in Huh-7 cells at 24h (Figure 4A). In CNS3 and 9-13 cells, there was no clear relationship between SI-induced changes in Bach1 and Nrf2 and the induction of HO-1 mRNA (Figure 4B and 4C). Although in CNS3 cells, 200 μM SI significantly up-regulated Nrf2 mRNA, it did so to a smaller extent than HO-1 mRNA up-regulation (Figure 4B). In 9-13 cells, there was a significant dose-dependent up-regulation of HO-1 mRNA levels, with no significant change in mRNA levels of Bach1 or Nrf2. These results suggest that changes in Bach1 or Nrf2 mRNA levels are not required for SI-induced HO-1 gene expression.
Figure 4.

Effects of silymarin on HO-1, Bach1 and Nrf2 gene expression. Cells were grown to near confluence and medium was changed to DMEM plus 5% FBS. Vehicle only (DMSO), 100 or 200 μM Silymarin (SI) were added to the cells for 24 h or 48 h.HO-1 levels were significantly up-regulated by SI (A, B, C). SI did not affect expression of Bach1 or Nrf2 in Huh-7 cells (A). There was no clear relationship between SI-induced changes in Bach1 or Nrf2 and the induction of HO-1 mRNA in either CNS3 or 9-13 cell lines (B, C). Results are means ± SE of triplicate determinations. *Differs from control (DMSO), p<0.05.
Effects of the combined treatment of silymarin and interferon on HCV core and NS5A expression in CNS3 and 9-13 cells
To our surprise, IFN alone did not affect levels of HCV core mRNA or protein, and there was no evidence of any additive or synergistic effect of IFN in combination with SI, compared with results for SI alone (Figure 5A and 5B). Interferon down-regulated HCV NS5A mRNA (Figure 5C) but not protein (Figure 5D). There was no evidence of any significant treatment interaction between IFN and SI in 9-13 cells. With the exception of 50U IFN, all treatments decreased significantly HCV core mRNA expression (Figure 5A); however, the combination of both compounds did not show any evidence of enhanced antiviral effects. HO-1 mRNA levels were significantly increased with 200 μM SI and with the mixtures, but the highest levels were obtained with 200 μM SI alone. SI alone or with IFN has similar effects on HCV core protein levels in CNS3 cells (Figure 5B). 100 μM SI had a modest effect to down-regulate HCV core protein; however 200 μM SI and 50U IFN+100 μM SI decreased HCV core protein levels by ∼50% (Figure 5B). 100 and 200 μM SI significantly increased HCV NS5A mRNA, as demonstrated previously (Figure 5C). However combining SI with IFN did not down-regulate HCV NS5A mRNA. The combined treatment significantly up-regulated NS5A mRNA expression compared to DMSO alone. 50U IFN significantly down-regulated HCV NS5A mRNA. SI concentrations significantly up-regulated HO-1 mRNA levels in a dose dependent fashion. IFN treatment alone did not increase HO-1 levels, however the combinations of SI plus IFN significantly up-regulated HO-1 mRNA. All treatments up-regulated HCV NS5A protein expression. In contrast with the mRNA levels, HCV NS5A protein was up-regulated by 50U IFN (D).
Figure 5.
Effects of the combination of silymarin and IFN on HCV core and NS5A expression in CNS3 and 9-13 cells. CNS3 and 9-13 cells were treated with DMSO, 100 μM SI, 200 μM SI, 10U IFN+100 μM SI, 50U IFN+100 μM SI or 50U IFN as shown. After treatments for the times shown (24 or 48h) total RNA was isolated and HCV core or NS5A, HO-1 and GAPDH mRNA’s were measured by qRT-PCR. For protein analysis cells were treated as described before. 35μg [B] or 45 μg [D] of cell proteins were loaded and separated by SDS-PAGE, levels of HCV core and NS5A (upper bands) and control GAPDH proteins (lower bands) were detected by Western Blot analysis (B, D). A. Levels of HCV core and HO-1 mRNAs: HO-1 mRNA levels were significantly increased with 200 μM SI and with the mixtures, but the highest levels were obtained with 200 μM SI alone. B. Western blots of HCV core and GAPDH proteins. Lanes 1-6 correspond to the six treatment conditions shown in Part A. C. Levels of HCV NS5A and HO-1 mRNAs. D. Western blots of HCV NS5A and GAPDH proteins. Lanes 1-6 correspond to the six treatment conditions shown in Part C. DMSO=control, SI=Silymarin, IFN=Interferon, *Differs from control, p<0.05. Data represent means ± SE for triplicate determinations (A, C).
HCV regulation by silymarin is not related to changes in the Jak-Stat Pathway
To explore a possible mechanism by which SI could down-regulate HCV core protein, the levels and phosphorylation status of Stat1 were assessed. Figure 6A shows that in CNS3 cells, SI had no effect on either Stat1 or phospho-Stat1 (Y-701), suggesting that down-regulation of HCV core protein its not related to the Jak-Stat signaling pathway. IFN treatment (Figure 6A, lane 3) modestly increased levels of both total Stat1 and Stat1 (Y701). Treatment with SI inhibited HCV core protein expression but IFN treatment did not (Fig. 6A). As shown in Figure 6B, 200 μM SI treatment of 9-13 cells did not change the basal levels of total Stat1 or Stat1 (Y701). IFN markedly increased protein levels of total Stat1 and Stat1 tyrosine (Y701). HCV NS5A was not down-regulated by either treatment.
Figure 6.
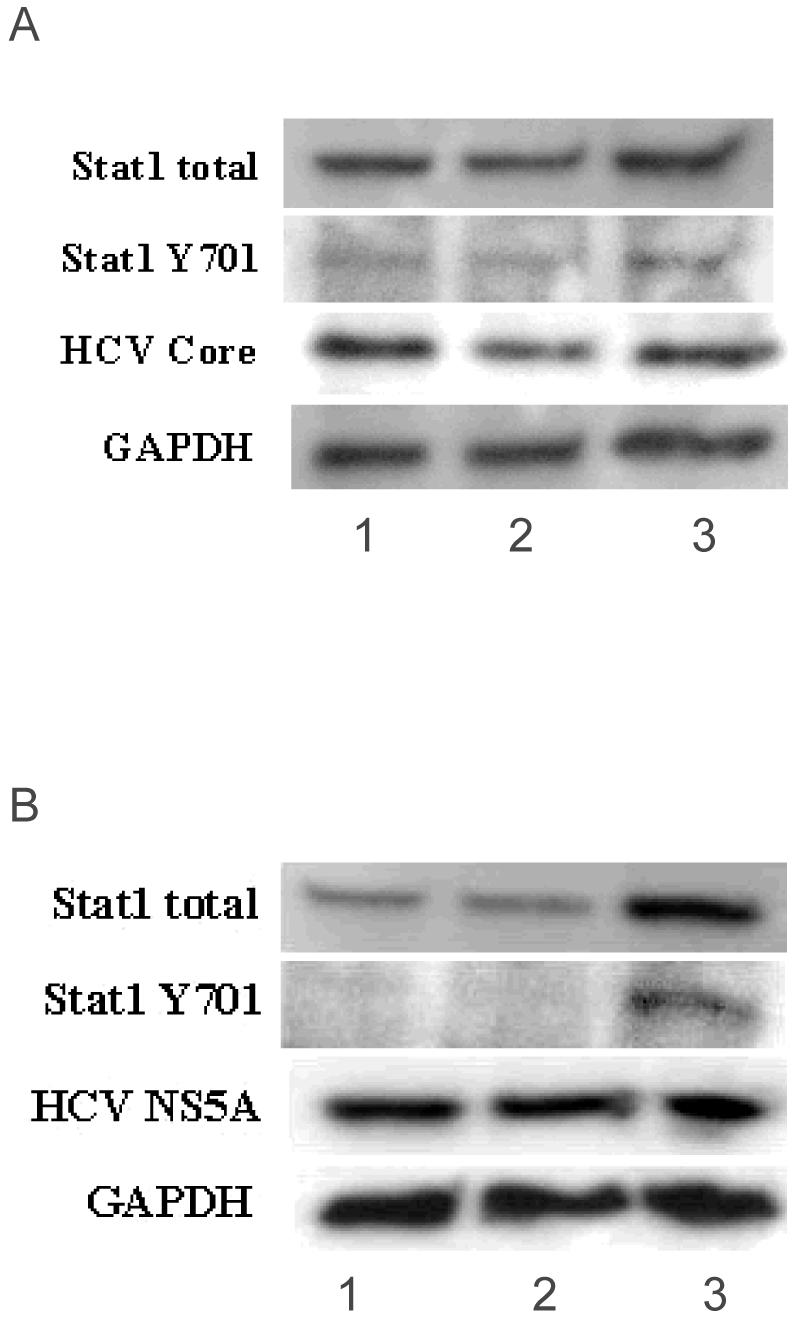
HCV regulation by silymarin is not related to the Jak-Stat Pathway. CNS3 cells (A) and 9-13 cells (B) were grown for 48 and 24 h respectively, in the presence of 1=Control, 2=200 μM SI and 3=10 U/mL IFN (CNS3) or 50 U/mL IFN (9-13). 50 μg and 38 μg (CNS3 and 9-13 cells respectively) of protein were loaded and separated by SDS-PAGE and levels of Stat1 total, Stat1 phosphorylated on tyrosine (Y701), HCV core, HCV NS5A and/or GAPDH proteins were detected by Western Blot analysis. A) SI treatment of CNS3 cells did not affect basal levels of total Stat1 or Stat1 (Y701). B) SI treatment of 9-13 cells showed that the basal levels of total Stat1 remain constant and no effect was seen in phosphorylation of Stat1 (Y701). [According to the time course experiments performed in CNS3 and 9-13 cells, the maximum effects of silymarin and IFN were at 48 and 24 h respectively, in each cell line, with no cytotoxic effects detected (Data not shown).]
Discussion
SI is a time-honored herbal remedy/complementary and alternative medication to treat acute and chronic liver diseases (8, 12, 14, 26, 30, 35) including chronic hepatitis C (32). Despite this broad use, the exact molecular mechanism by which SI confers hepatoprotection is not known. Recent findings indicate that a “standardized” preparation of SI showed anti-inflammatory actions via inhibition of NF-kB-induced transcription in human liver cell cultures, inhibition of inflammatory cytokine induction in human peripheral blood mononuclear cells, and direct antiviral effects against HCV infection (31). The data presented in the current work extend these findings, showing that another preparation of SI possesses antiviral effects on HCV core expression and stimulatory effects on the antioxidant and cytoprotective enzyme HO-1.
With recent advances, including the HCV expressing constructs used here, renewed effort has been directed towards developing drugs that inhibit HCV replication. Previous HCV molecular studies had been hampered due to a lack of an efficient cell culture system which could produce an infectious virion. In this study, two different cell lines that express HCV proteins were analyzed: CNS3 cells which contain the structural proteins and part on the nonstructural proteins of the HCV, and 9-13 cells which express only nonstructural HCV proteins (NS3-NS5). In these cells and wt Huh-7 cells, SI at concentrations of 100 and 200 μM was not toxic. Although 300 μM was not overtly toxic to the cells, it seemed to be an inhibitor of cell growth, possibly by the same mechanism as has been shown in previous studies on the inhibition of cell growth by SI (20, 39). Although it was shown that a “standardized” preparation of SI was effective at lower concentrations (20.7 and 41.9 μmoles/L) (31) than those used here, it is important to note that different molecular forms of SI, different HCV-expressing constructs, and different cell lines will likely have different effects on cellular and HCV-mediated responses.
The antiviral effects of SI are shown by the decrease of HCV core mRNA (Figures 3A and 5A) and protein (Figures 3B and 5 B) in SI-treated CNS3 cells. Surprisingly, IFN did not decrease either HCV core mRNA or protein as expected. Instead, the levels of HCV core in IFN-treated cells were similar to the levels in cells not otherwise treated or tested with SI. Western blots showed that IFN did not down-regulate HCV core protein, even in combination with SI. This lack of IFN antiviral effect may be due to the presence of HCV structural proteins (core-E1-E2) which impair IFN-mediated antiviral activity, and/or to other effects of these proteins. HCV core protein, in particular, has numerous adverse effects on host hepatocytes (1-3, 19, 23). For example, the HCV core protein (a.) impairs IFN-induced signal transduction via induction of SOCS3 expression and inhibits nuclear translocation of STAT1(2) (Fig. 6A) resulting in IRF-1 repression (3); (b.) inhibits IFN-induced transcription of antiviral genes by decreasing binding of ISGF3 to the ISRE (23); (c.) with other HCV proteins, modulates transacting factors of Jak/Stat signaling pathway (1); and that is sufficient for immunosuppression, prolonged viremia, and increased mortality in murine models (19). Thus, our results may be a reflection of these effects of HCV core protein.
IFN binds to cellular receptors that dimerize and cause the activation of Janus-activated and tyrosine kinases (JAK), which subsequently phosphorylate the cytoplasmic signal transducers and activators of transcription (STAT) protein. This JAK/STAT pathway activates the transcription of multiple IFN-stimulated genes (ISG,) which encode proteins that interfere with virus replication, protein synthesis and assembly (15). One hypothesis is that SI might activate the JAK-STAT pathway in a manner analogous to that of IFN. To gain insight into whether SI-mediated down-regulation of HCV core in CNS3 cells could be related with the JAK-STAT pathway, we measured Stat1 and Stat1 phosphorylation on tyrosine (Y701) protein levels in CNS3 and 9-13 cells (Fig. 6). We failed to observe SI-dependent changes either in total or phosphorylated Stat1 protein levels. This suggests that SI down-regulation of HCV core is not related to activation of the Jak-Stat signaling pathway. Thus, more investigation is needed to elucidate the possible mechanisms of SI down-regulation of HCV core in CNS3 cells.
Polyak et al (31) reported recently that HCV NS5A protein expression is down-regulated by a SI in Huh7.5.1 transfected with JFH1. In our 9-13 cells, we did not observe any down-regulation on HCV NS5A mRNA or protein. These differences may be due to a number of factors, including 1) the different cell lines used in each study. [We used cells expressing only the nonstructural HCV proteins (9-13), while others used a full length HCV genome (JFH1). Different HCV-related proteins can give rise to different responses and the presence of one protein can modulate the activity of the other proteins. Polyak et al used Huh7.5.1 cells containing the full-length HCV infectious JFH1 virion, while we used Huh7 cells. The JFH1 system is based on an HCV 2a genotype while the 9-13 cell line is based on the HCV 1b genotype. Different genotypes of HCV show differences of 20-30% in their sequences that differ in their responses to treatment.] 2) We also used a different SI formulation. They treated the cells with a “standardized” SI, whereas we used a commercial SI preparation, which may have different chemical properties that may lead to different cellular responses.
The lack of any antiviral effects of SI on HCV NS5A mRNA or protein levels may be due to the ability of NS5A to produce a resistance effect on the cells, thereby inactivating the possible mechanism by which SI produces an antiviral effect in CNS3 cells. HCV NS5A can serve as an inhibitor of IFN-induced antiviral activity, and thus play a role in development of resistance to IFN treatment in patients with chronic hepatitis C (40). In contrast, other studies show that cell clones carrying replicons with HCV NS5A sequences from IFN-responder and IFN-nonresponder patients, did not reveal significant differences on HCV RNA replication to IFN-treatment, providing no evidence that NS5A protein contributes to resistance of HCV replication to IFN (24). Results presented here show that in contrast to CNS3 cells, IFN treatment of 9-13 cells led to a significant down-regulation of the HCV NS5A mRNA and protein. Also, in contrast to the HCV core down-regulation in CNS3 cells, SI treatment did not decrease HCV NS5A expression. The reasons why SI down-regulated HCV core but not HCV NS5A in these cell lines is a subject that we currently studying.
In the three cell lines used in these studies (CNS3, 9-13 and wild type Huh-7 cells), SI up-regulated HO-1 gene expression significantly. The maximum up-regulation depends on the cell line, the time of exposure, and the SI concentrations used. In CNS3 and 9-13 cells, HO-1 gene expression was not up-regulated by IFN treatment, and the levels of HO-1 in such cells were the same as, or lower than, the controls. When cells were treated with the combination of SI and interferon, a significant increase in HO-1 mRNA was observed with both cell lines; however, this increase appears to be solely due to SI, because no synergistic effect was observed in presence of IFN plus SI.
It is known that HO-1 is a highly inducible enzyme which is transcriptionally regulated by a large number of chemical and physical factors. Two transcription factors, Bach1 and Nrf2, in particular, have been shown to be important to the heme-mediated induction of HO-1 expression (6, 10, 33, 34). We did not find a clear relation between these transcription factors and the SI-mediated up-regulation of HO-1. Therefore, another mechanism would likely be involved to account for how SI induces the expression of HO-1. Oxidative stress plays an important role in various diseases, including viral infection and chronic inflammation. HCV gene expression in particular can increase the levels of reactive oxygen species (ROS) (10, 11). Thus, the effects of SI to up-regulate HO-1, a well known antioxidant and cytoprotective enzyme may be important in the global effects of SI on HCV infection.
There are few published clinical trials that evaluate the usefulness of SI as a treatment for chronic hepatitis C. However, studies in which SI is administered to cell cultures expressing HCV proteins and RNA can potentially give us valuable clues to understand whether SI treatment positively affects HCV infection and, if so, the mechanism by which this occurs. This work is but one step to elucidate the possible beneficial roles of SI on HCV infection.
Supplementary Material
AKNOWLEDGEMENTS
We thank R. Bartenschlager for kindly supplying the HCV replicon constructs used in this work.
Supported by a grant (DK RO1 38825) and contracts (DK NO1 29236 and UO1 DK 06193) from NIH (NIDDK) to Herbert L. Bonkovsky.
Abbreviations used
- ARE
Antioxidant response elements
- Bach1
BTB and CNC homology 1
- BCA
Bicinchoninic acid
- CAM
Complementary and alternative medicine
- CHC
Chronic hepatitis C
- DMSO
Dimethyl sulfoxide
- E-1
Internal ribosome entry site of the encephalomyocarditis virus
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HO-1
Heme oxygenase-1
- IFN-α
Interferon-alpha
- IRF
Interferon response factor
- ISGF
Interferon stimulated gene factor
- ISRE
Interferon stimulated response element
- JAK
Janus kinase
- JFH-1
Japanese fulminant hepatitis-1
- MAPK
Mitogen-activated protein kinase
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- Neo
Neomycin phosphotransferase gene
- NF-κB
Nuclear factor-kappa B
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NTR
Non-translated region
- PBS
Phosphate-buffered saline
- PVDF
Polyvinylidene fluoride
- qRT-PCR
Quantitative real time polymerase chain reaction
- ROS
Reactive oxygen species
- SDS
Sodium dodecyl sulfate
- SI
Silymarin
- SOCS
Suppressor of cytokine signaling
- STAT
Signal transducers and activators of transcription
- Zeo
Zeocin resistance gene
LITERATURE CITED
- 1.BASU A, MEYER K, RAY RB, RAY R. Hepatitis C virus core protein modulates the interferon-induced transacting factors of Jak/Stat signaling pathway but does not affect the activation of downstream IRF-1 or 561 gene. Virology. 2001;288:379–390. doi: 10.1006/viro.2001.1100. [DOI] [PubMed] [Google Scholar]
- 2.BODE JG, LUDWIG S, EHRHARDT C, ALBRECHT U, ERHARDT A, SCHAPER F, HEINRICH PC, HÄUSSINGER D. IFN-alpha antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003;17:488–90. doi: 10.1096/fj.02-0664fje. [DOI] [PubMed] [Google Scholar]
- 3.CICCAGLIONE AR, STELLACCI E, MARCANTONIO C, MUTO V, EQUESTRE M, MARSILI G, RAPICETTA M, BATTISTINI A. Repression of Interferon Regulatory Factor 1 by Hepatitis C Virus Core Protein Results in Inhibition of Antiviral and Immunomodulatory Genes. Journal of Virology. 2007;81:202–214. doi: 10.1128/JVI.01011-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COLLIER L, OXFORD J. Human Virology. Oxford University Press; UK: 2006. [Google Scholar]
- 5.CHOO QL, WEINER AJ, OVERBY LR, BRADLEY DW, HOUGHTON M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 6.MARTIN D, ROJO A, SALINAS M, DIAZ R, GALLARDO G, ALAM J, RUIZ DE GALARRETA CM, CUADRADO A. Regulation of Heme Oxygenase-1 Expression through the Phosphatidylinositol 3-Kinase/Akt Pathway and the Nrf2 Transcription Factor in Response to the Antioxidant Phytochemical Carnosol. The Journal of Biological Chemistry. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 7.DINEV D, JORDAN BW, NEUFELD B, LEE JD, LINDEMANN D, RAPP UR. Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells. Eur Mol Biol Org Rep. 2001;2:829–834. doi: 10.1093/embo-reports/kve177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FLORES K, ROSEN H, BENNER K. The use of naturopathic remedies for chronic liver diseases [letter] Am J Gastroenterol. 1996;91:2654–2655. [PubMed] [Google Scholar]
- 9.FRIED MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 10.GHAZIANI T, SHAN Y, LAMBRECHT R, DONOHUE S, PIETSCHMANN T, BARTENSCHLAGER R, BONKOVSKY HL. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. Journal of Hepatology. 2006;45:1–8. doi: 10.1016/j.jhep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 11.GONG G, WARIS G, TANVEER R, SIDDIQUI A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci. 2001;98:9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HAVSTEEN B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 13.HJIKATA M, MIZUSHIMA H, TANJI Y, KOMODA Y, HIROWATARI Y, AKAGI T, KATO N, KIMURAN K, SHIMOTOHNO K. Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci USA. 1993;90:10773–7. doi: 10.1073/pnas.90.22.10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HOH C, BOOCOCK D, MARCZYLO T, SINGH R, BERRY DP, DENNISON AR, HEMINGWAY D, MILLER A, WEST K, EUDEN S, GARCEA G, FARMER PB, STEWARD WP, GESCHER AJ. Pilot Study of Oral Silibinin, a Putative Chemopreventive Agent, in Colorectal Cancer Patients: Silibinin Levels in Plasma, Colorectum, and Liver and their Pharmacodynamic Consequences. Clinical Cancer Research. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 15.HOOFNAGLE JH, SEEFF LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–51. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 16.ITOH K, CHIBA T, TAKAHASHI S, ISHII T, IGARASHI K, KATOH Y, OYAKE T, HAYASHI N, SATOH K, HATAYAMA I, YAMAMOTO M, NABESHIMA Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 17.MENGSHOL JA, GOLDEN-MASON L, ROSEN HR. Mechanisms of Disease: HCV-induced liver injury. Nature Clinical Practice Gastroenterology & Hepatology. 2007;4:622–633. doi: 10.1038/ncpgasthep0961. [DOI] [PubMed] [Google Scholar]
- 18.KORENAGA M, WANG T, LI Y, SHOWALTER L, CHAN T, SUN J, WEINMAN S. Hepatitis C Virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. The Journal of Biological Chemistry. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 19.LARGE MK, KITTLESEN DJ, HAHN YS. Suppression of Host Immune Response by the Core Protein of Hepatitis C Virus: Possible Implications for Hepatitis C Virus Persistence. J Immunol. 1999;162:931–8. [PubMed] [Google Scholar]
- 20.LINDENBACH B, MEULEMAN P, PLOSS A, VANWOLLEGHEM T, SYDER A, MCKEATING J, LANFORD R, FEINSTONE S, MAJOR M, LEROUX-ROELS G, RICE C. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Natl Acad Sci USA. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LOHMANN V, KOCH JO, BARTENSCHLAGER R. Processing pathways of the hepatitis C virus proteins. J. Hepatol. 1996;24:11–19. [PubMed] [Google Scholar]
- 22.LOHMANN V, K. F, KOCH J, HERIAN U, THEILMANN L, BARTENSCHLAGER R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 23.LUCAS SD. Hepatitis C Virus Core Protein Down-Regulates Transcription of Interferon-Induced Antiviral Genes. The Journal of Infectious Diseases. 2005;191:93–9. doi: 10.1086/426509. [DOI] [PubMed] [Google Scholar]
- 24.AUS DEM SIEPEN M, LOHMANN V, WIESE M, ROSS S, ROGGENDORF M, VIAZOV S. Nonstructural protein 5A does not contribute to the resistance of hepatitis C virus replication to interferon alpha in cell culture. Virology. 2005;336:131–136. doi: 10.1016/j.virol.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 25.MARION ME, FEINSTONE SM. Molecular virology of hepatitis C. Hepatology. 1997;25:1527–1538. doi: 10.1002/hep.510250637. [DOI] [PubMed] [Google Scholar]
- 26.MASINI A, CECCARELLI D, GIOVANNINI F, MONTOSI G, GARUTI C, PIETRANGELO A. Iron induced oxidant stress leads to irreversible mitochondrial dysfunctions and fibrosis in the liver of chronic iron-dosed gerbils. The effect of silybin. J Bioenerg Biomembr. 2000;32:174–182. doi: 10.1023/a:1005512014280. [DOI] [PubMed] [Google Scholar]
- 27.MCHUTCHISON JG, FRIED MW. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin. Liver. Dis. 2003;7:149–161. doi: 10.1016/s1089-3261(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 28.MOTOHASHI H, SHAVIT JA, IGARASHI K, YAMAMOTO M, ENGEL JD. The world according to Maf. Nucleic Acids Res. 1997;25:2953–2959. doi: 10.1093/nar/25.15.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.OKUDA M, LI K, BEARD MR, SHOWALTER LA, SCHOLLU F, LEMON S. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 30.DAVIS-SEARLES PR, NAKANISHI Y, KIM N-C, GRAF TN, OBERLIES NH, WANI MC, WALL ME, AGARWAL R, KROLL DJ. Milk thistle and prostate cancer: differential effects of pure flavonolignans from silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 31.POLYAK S, MORISHIMA C, SHUHART M, WANG C, LIU Y, LEE D. Inhibition of T cell inflammatory cytokines, hepatocyte NF-kB signaling, and HCV infection by standardized silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 32.SEEFF LB, CURTO TM, SZABO G, EVERSON GT, BONKOVSKY HL, DIENSTAG JL, SHIFFMAN ML, LINDSAY KL, LOK AS, DI BISCEGLIE AM, LEE WM, GHANY MG, HALT-C TRIAL GROUP Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605–12. doi: 10.1002/hep.22044. [DOI] [PubMed] [Google Scholar]
- 33.SHAN Y, LAMBRECHT R, DONOHUE S, BONKOVSKY HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. The FASEB Journal. 2006;20:E2258–E2267. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 34.SHAN Y, LAMBRECHT RW, GHAZIANI T, DONOHUE SE, BONKOVSKY HL. Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: insights from studies with small interfering RNAs. J Biol Chem. 2004;279:51769–51774. doi: 10.1074/jbc.M409463200. [DOI] [PubMed] [Google Scholar]
- 35.SINGH R, MALLIKARJUNA G, SHARMA G, DHANALAKSHMI S, TYAGI A, CHAN D, AGARWAL C, AGARWAL R. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappaB-mediated inducible chemoresistance. Clin Cancer Res. 2004;10:8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 36.TENHUNEN R, MARVER HS, SCHMID R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.TENHUNEN R, MARVER HS, SCHMID R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- 38.VALENZUELA A, GARRIDO A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994;27:105–112. [PubMed] [Google Scholar]
- 39.WAKITA T, PIETSCHMANN T, KATO T, DATE T, MIYAMOTO M, ZHAO Z, MURTHY K, HABERMANN A, KRÄUSSLICH HG, MIZOKAMI M, BARTENSCHLAGER R, LIANG TJ. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.PAN Y, WEI M, KANG L, WANG Z, FANG J, ZHU Y, WU J. NS5A protein of HCV enhances HBV replication and resistance to interferon response. Biochem Biophys Res Commun. 2007;359:70–75. doi: 10.1016/j.bbrc.2007.05.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




