Abstract
Presenilin-1 (PS1) is a transmembrane protein that is in many cases responsible for the development of familial Alzheimer’s disease. PS1 is widely expressed in embryogenesis and is essential for neurogenesis, somitogenesis, angiogenesis, and cardiac morphogenesis. To further investigate the role of PS1 in the brain, we inactivated the PS1 gene in Wnt1 cell lineages using the Cre-loxP recombination system. Here we show that conditional inactivation of PS1 in Wnt1 cell lineages results in congenital hydrocephalus and subcommissural organ abnormalities, suggesting a possible role of PS1 in the regulation of cerebrospinal fluid homeostasis.
1. Introduction
Presenilin-1 (PS1) is a transmembrane protein expressed ubiquitously in human and mouse tissues (Sherrington et al., 1995; Lee et al., 1996), and is in many cases responsible for the development of early-onset familial Alzheimer’s disease (Cruts and Van Broeckhoven, 1998a,b). Full-length PS1 undergoes endoproteolytic cleavage, generating stable N- and C-terminal fragments (NTF and CTF) that interact with other proteins to form a macromolecular complex with γ-secretase activity. This enzyme is required for the regulation of intramembrane proteolysis of amyloid precursor protein (APP), Notch, and cadherins (De Strooper et al., 1999; Marambaud et al., 2003; Koo and Kopan, 2004). PS1 also has an important role in the turnover of β-catenin, a molecule essential in Wnt signaling and cell adhesion (Kang et al., 2002; Gottardi and Gumbiner, 2004).
Earlier studies of PS1-knockout null mice have contributed to our understanding of the in vivo developmental functions of PS1 in neurogenesis, somitogenesis, angiogenesis, and cardiac morphogenesis (Shen et al., 1997; Wong et al., 1997; Handler et al., 2000; Koizumi et al., 2001; Yuasa et al., 2002; Nakajima et al., 2003, 2004). The role of PS1 in the perinatal and postnatal stages, however, has not been examined because PS1 null mice die perinatally. A new approach using the Cre-loxP system allow for the production of mice that conditionally lack PS1 and examination of the PS1 function during the perinatal and postnatal periods (Yu et al., 2001; Saura et al., 2004; Nakajima et al., 2009).
Hydrocephalus is typically divided into noncommunicating or communicating subtypes (Fishman 1992). Noncommunicating hydrocephalus is caused by an obstruction within the ventricular system, such as a tumor, that prevents cerebrospinal fluid (CSF) proximal to the obstruction from draining into the subarachnoid space, where it is reabsorbed into the venous sinuses. Communicating hydrocephalus results from impaired absorption of CSF despite patent CSF pathways. Both communicating and noncommunicating hydrocephalus occur congenitally or are acquired secondary to trauma, tumor, hemorrhage, or infection (Guyot and Michael, 2000; Yoshioka et al., 2000). The development and progression of congenital hydrocephalus is not yet well understood. Only one hydrocephalus gene, L1CAM, has been identified in humans (Jouet et al., 1993). The mutations are distributed over the functional protein domains of L1CAM protein. The exact mechanisms by which these mutations cause a loss of L1CAM function remain unclear.
In the present study, we examined the role of PS1 using mice conditionally lacking PS1 in the neural crest cell lineages and found a hydrocephalic pathology in the mutant mice. Our findings suggest a possible role of PS1 in the regulation of CSF homeostasis.
2. Results
2.1 Congenital hydrocephalus in Wnt1-cre PS1-conditional knockout (cKO) mice
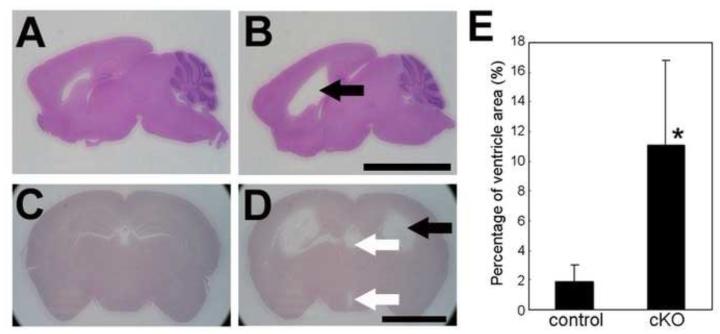
In the previous study, we crossed floxed PS1 and Wnt1-cre mice (Danialian et al., 1998; Yu et al., 2001; Saura et al., 2004) and generated mice lacking PS1 in the neural crest cell lineages (Nakajima et al., 2009). In contrast to PS1 null mice, which die perinatally (Shen et al., 1997; Koizumi et al., 2001; Nakajima et al., 2003, 2004), the Wnt1-cre PS1-cKO mice are viable with no obvious phenotypic abnormalities for several days after birth. Although 20% to 40% of mice exhibit reduced weight at 5 weeks of age, the remaining mice mature and do not show abnormal phenotypes in appearance (Nakajima et al., 2009). In the present study, we observed that nearly all mutant mice developed hydrocephalus at 5 weeks of age (Fig. 1), irrespective of their weight or sex. Histologic analyses revealed enlargement of the lateral and third ventricles in the mutant brains (Fig.1 B and D). Analyses of young mutant mice revealed enlarged ventricles in three of four brains at 4 days of age and three of three brains at 7 days of age (data not shown), indicating that the hydrocephalus of the mutants is congenital. No gross histologic abnormalities were detected, however, other than hydrocephalus.
Fig. 1.
Dilated ventricles in Wnt1-cre PS1-cKO brains.
Parasagittal (A, B) and coronal (C, D) brain sections of control (A, C) and Wnt1-cre PS1-cKO (B, D) mice, 5-weeks old, were stained with hematoxylin and eosin. Note the dilation of the lateral ventricles (black arrows in B and D) and third ventricles (while arrows in D). (E) Ventricle areas (lateral ventricles and third ventricles) and total brain areas of coronal sections from control (n=6) and Wnt1-cre PS1-cKO (n=8) mice were measured with Image J and the percentage of ventricle areas relative to the total brain areas was calculated. Values are mean ± SD. *: The percentages differed significantly between control and Wnt1-cre PS1-cKO brains (P < 0.005). Bar=5 mm (A, B), 3 mm (C, D).
2.2 Hydrocephalus is associated with abnormalities of the subcommissural organ (SCO) and the Sylvian aqueduct in Wnt1-cre PS1-cKO mice
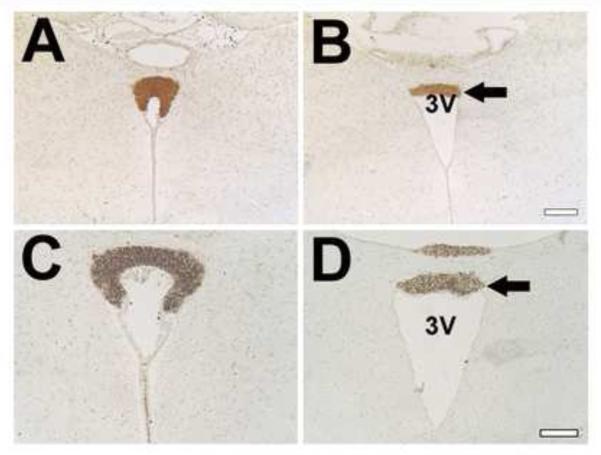
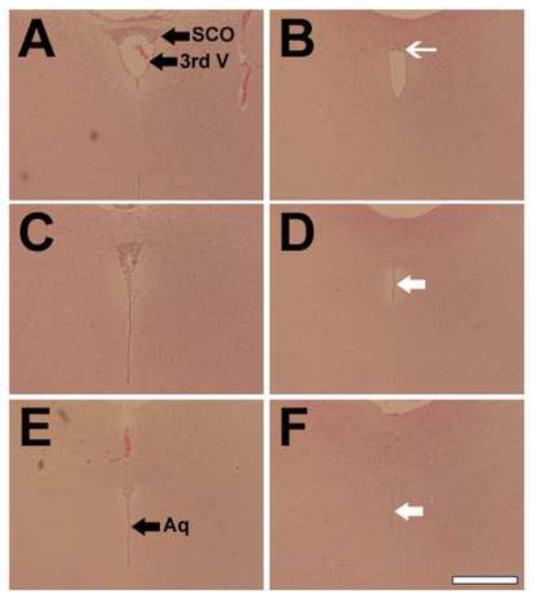
Because the SCO and the Sylvian aqueduct are often affected in mice exhibiting hydrocephalus, we examined the structures in Wnt1-cre PS1-cKO mice. The SCO is located in the roof of the third ventricle at the entrance of the Sylvian aqueduct, and spans the rostral part of the aqueduct (Rodríguez et al., 1998; Meiniel, 2007). Analyses of coronal sections obtained from mutant mice revealed deformations of the SCO and dilation of the third ventricle (Fig.2 B, D, and F). In mutant brains, the SCO in the caudal part of the aqueduct was obviously reduced in size and the aqueduct exhibited intensive stenosis (Fig.3).
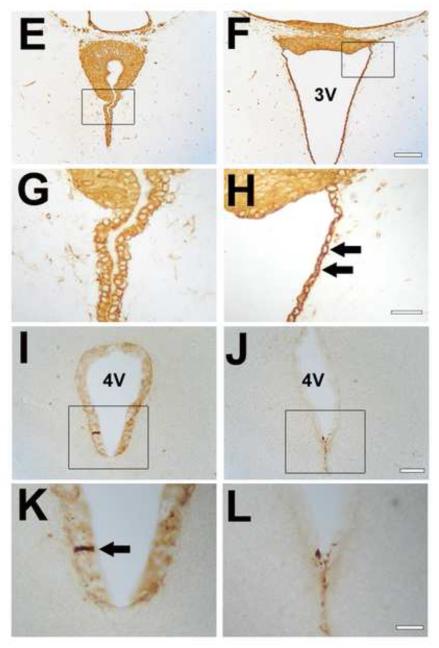
Fig. 2.
Abnormalities of the SCO and ependymal cells in Wnt1-cre PS1-cKO mice.
Four-μm-thick coronal paraffin sections through the SCO of control (A, C, E, G) and Wnt1-cre PS1-cKO (B, D, F, H) mouse brains, 5-weeks old, were stained immunohistochemically with anti-SCO-glycoproteins (AFRU for A, B; Mab4A6 for C, D) or anti-vimentin (E-H) antibody. (G) and (H) show the views at a higher magnification of the areas marked by the boxes in (E) and (F), respectively. Note the deformed SCOs (B, D, F) in the mutants. Immunoreactivity for SCO-glycoproteins is reduced in the mutant SCOs (arrows in B and D). The ependymal cells in the mutants are an unusual shape and vimentin expression in the cells is increased (arrows in H). Thirty-μm-thick coronal cryostat sections through the rostral part of the 4th ventricle of control (I, K) and Wnt1-cre PS1-cKO (J, L) mouse brains, 5-weeks old, were stained immunohistochemically with anti-SCO-glycoproteins antibody (Mab4A6). Note the lack of Reissner’s fiber in the mutants (J, L), compared with the control (Reissner’s fiber is indicated by an arrow in K). 3V=3rd ventricle. 4V=4th ventricle. Bar=0.2 mm (A, B), 0.1 mm (C, D), 0.1 mm (E, F), 0.03 mm (G, H), 0.05 mm (I, J), 0.02 mm (K, L).
Fig. 3.
Reduced SCO and stenosis of the Sylvian aqueduct in Wnt1-cre PS1-cKO mice.
Rostral to caudal coronal sections stained with hematoxylin and eosin from control (A, C, E) and Wnt1-cre PS1-cKO (B, D, F) mouse brains, 5-weeks old. Note the reduced formation of the SCO (white thin arrow in B) and the stenosis of the Sylvian aqueduct (white arrows in D and F) in the mutants. 3rd V=third ventricle, Aq=Sylvian aqueduct. Bar=0.4 mm
The major function of the SCO is thought to be the secretion of high molecular weight glycoproteins that facilitate CSF flow. To examine whether the SCO was properly differentiated in Wnt1-cre PS1-cKO mice, we performed immunohistochemical analyses. Reduced expression of the SCO-glycoproteins was revealed by immunostaining using AFRU and Mab4A6 antibodies, both of which react with the SCO-glycoproteins (Fernández-Llebrez et al., 2001; Rodríguez et al., 1998) (Fig.2 B and D). Relative levels of the SCO-immunostaining density with Mab4A6 antibodies were 100 ± 0.7 (mean ± SEM, n=3) in controls versus 88.4 ± 2.2 (n=3) in the mutants (p=0.007). To evaluate the presence of Reissner’s fiber, we prepared 30-μm thick cryosections and stained them with Mab4A6 antibody. Reissner’s fiber was detected in the rostral part of the 4th ventricle in the control mice, but not in the mutant mice (Fig. 2 I-L). In the mutant mice, only small aggregates were detected in the rostral part of the 4th ventricle, indicating the substantial loss of Reissner’s fiber in the Sylvian aqueduct of the mutant mice.
To examine the state of the ependymal cells, we performed immunohistochemistry for vimentin, an ependymal marker protein. We found that vimentin immunoreactivity was increased in the ependymal cells, but not in the SCO region, of the mutant mice. The shape of the ependymal cells along the 3rd ventricle was also abnormal in the mutant mice (Fig. 2 E-H).
2.3 Restricted deficiency of PS1 in Wnt1-cre PS1-cKO brains
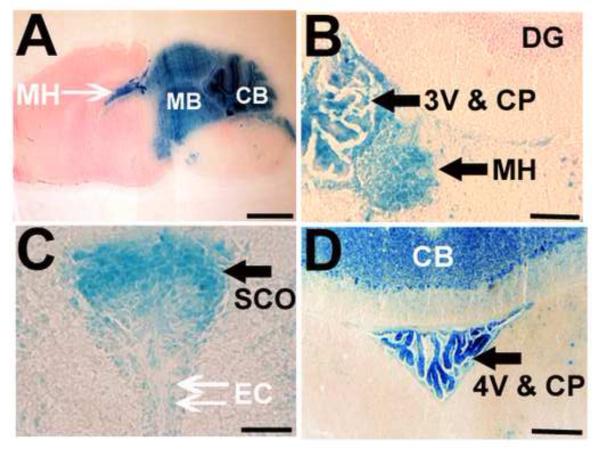
To identify the areas lacking the PS1 gene in Wnt1-cre PS1-cKO brains, X-gal analysis was performed using ROSA26 reporter mice (Mao et al., 1999) crossed with the Wnt1-cre mice. X-gal staining suggested a defect of the floxed PS1 gene in the cerebellum, midbrain, and medial habenula (Fig.4 A). PS1 gene defect was also suggested in the SCO, ependymal cells, and choroid plexus of the third and fourth ventricles (Fig.4 B, C and D).
Fig. 4.
Restricted Wnt1-cre mediated recombination in the brains.
Sagittal (A) and coronal (B, C, D) brain sections of Wnt1-cre (Tg/+); Rosa26-LacZ (floxed/+) mice, 5-weeks old, were stained with X-gal reagent. The presence of a conditional Rosa26-LacZ allele allowed for X-gal staining of cells in which loxP sites were recombined with the Wnt1-cre allele. Note that the X-gal staining is restricted to the cerebellum (A, D), midbrain (A), and medial habenula (A, B). Strong β-galactosidase staining is also present in cells forming the SCO (C), ependymal cells (C), and choroid plexuses in the 3rd (B) and 4th ventricles (D). 3V=third ventricle, 4V=fourth ventricle, CB=cerebellum, CP=choroid plexus, DG=dentate gyrus, EC=ependymal cell, MB=midbrain, MH=medial habenula, SCO=subcommissural organ. Bar= 2 mm (A), 0.1 mm (B), 0.05 mm (C), 0.2 mm (D).
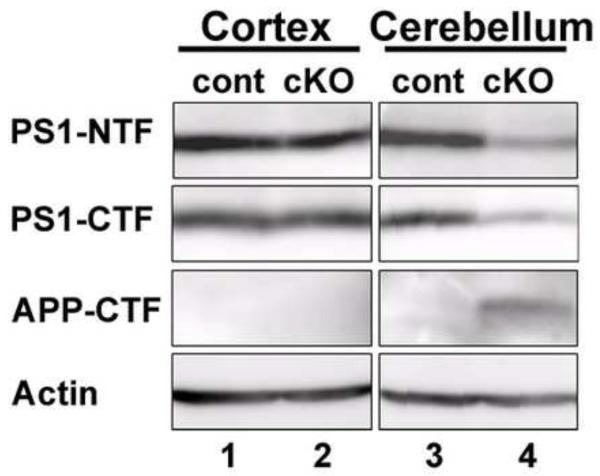
Reduced PS1 protein levels in the mutant mice were confirmed by Western blot analyses. PS1 protein levels in the mutant mouse cerebellum were obviously reduced, although those in the mutant mouse cortex were comparable to those in control mice (Fig.5). It is well established that β-secretases process APP to generate membrane-tethered APP-CTF (APP-stub) and that PS1 is required for further cleavage of the APP-stub at the γ-secretase site to produce Aβ peptides (Price and Sisodia, 1998). Deficiency of PS1 protein function was revealed by the appearance of the APP-stub in the mutant mouse cerebellum (Fig.5).
Fig. 5.
Western blotting for PS1.
Western blot analyses of protein lysates from the cortex (lanes 1 and 2) and cerebellum (lanes 3 and 4) of the control (lanes 1 and 3) and Wnt1-cre PS1-cKO (lanes 2 and 4) mice. Anti-PS1-NTF, anti-PS1-CTF, anti-APP-CTF, or anti-actin antibody was used to stain the blotted proteins. Note the reduction of PS1-NTF and -CTF band intensities and the appearance of the APP-stub in the mutant cerebellum (lane 4).
3. Discussion
To examine the possible role of PS1 in the neural crest cell lineage, we developed Wnt1-cre-induced PS1-cKO mice lacking PS1 in tissues comprising the neural crest-derived cells and analyzed the pathology of the mutant brains in detail. The pathologic analyses revealed a consistent abnormality of enlarged ventricles in the mutant brains. The sites of mutant brain parenchyma showing pathologic alterations included the SCO, ependymal cells, and the Sylvian aqueduct. These findings suggest a possible role of PS1 in the regulation of CSF homeostasis.
Congenital hydrocephalus is a human disease with a high mortality rate and the incidence of the disease is approximately 1 in 1500 births (Schurr et al., 1953). Hydrocephalus can result from an overproduction of CSF by the choroid plexus, failure to drain the CSF at the subarachnoid space, and blockage of the CSF flow through the narrow Sylvian aqueduct. Indeed, stenosis of the Sylvian aqueduct is considered to be the primary cause of congenital hydrocephalus, occurring with a high probability (Pérez-Fígares et al., 2001). Here, we demonstrated that the Wnt1-cre PS1-cKO mice showed stenosis of the Sylvian aqueduct (Fig.3), suggesting that the stenosis is the direct cause of hydrocephalus in the mutants.
Although Wnt1-cre-induced PS1-cKO mice showed hydrocephalus with stenosis of the Sylvian aqueduct, the severity of the symptoms was moderate compared to those of other hydrocephalus models such as hyh mutant mice (Jiménez et al., 2001; Wagner et al., 2003) or mice with conditional knockout of the Pten or β-catenin gene (Ohtoshi, 2008). The mice in these other models show severe hydrocephalus with total obstruction of the CSF flow, referred to as the noncommunicating hydrocephalic subtype. The partial obstruction of the CSF circulation in the Wnt1-cre-induced PS1-cKO mice with stenosis of the Sylvian aqueduct is referred to a the communicating hydrocephalic subtype, and might cause only moderate hydrocephalic symptoms.
In addition to the stenosis of the aqueduct, we uncovered developmental defects of the SCO and the ventricular ependymal cells in the mutant mice (Fig.2). The SCO and the ventricular ependymal cells are thought to facilitate the flow of CSF through the confining canals of the ventricular system (Rodríguez et al., 1998; Meiniel, 2007). We found the reduced immunostaining with the antibodies against the SCO-glycoproteins (Fig.2). The secretion of the SCO-glycoproteins from the SCO is thought to be required to maintain the patency of the Sylvian aqueduct (Huh et al., 2009), suggesting that the reduced secretion of the SCO-glycoproteins is the cause of the aqueduct stenosis.
In the present study, we clearly demonstrated the lack of Reissner’s fiber in the 4th ventricle of the mutants, that is the structure formed by polymerization of the glycoproteins produced by the SCO. This finding is consistent with the hypothesis that Reissner’s fiber is indispensable to maintain the patency of the Sylvian aqueduct (Pérez-Fígares et al., 1998; Vio et al., 2000; Pérez-Fígares et al., 2001).
Expression of SCO-glycoproteins and vimentin was altered in the SCOs and the ventricular ependymal cells, respectively, where PS1 is suggested to be lacking in the mutant brains (Figs. 2 and 4). These changes in the protein expression might result from the PS1 deficiency: PS1 affects the activities of Notch and β-catenin and these PS1-regulated proteins have crucial roles in developmental gene expression (Gottardi and Gumbiner, 2004; Koo and Kopan, 2004). The defects in the SCO might cause the hydrocephalus with stenosis induced by the lack of Reissner’s fiber, or the defects in the ventricular ependymal cells might lead to the disease. Taking into account the possibility that the SCO derives from the ependymal layer (Rodríguez et al., 1998; Meiniel, 2007), abnormalities in the ependymal cells in a broad sense could induce the hydrocephalus.
Abnormal SCO development is a common feature in rodents that display congenital hydrocephalus (Meiniel, 2007; Huh et al., 2009). In particular, the consistent SCO defects in mice carrying mutations in Wnt1 cell lineages is noteworthy. Ectopic engrailed 1 expression and deficiency of huntingtin as well as PS1 in the dorsal midline cell-lineages all lead to hydrocephalus and SCO defects in mice (Louvi and Wassef, 2000; Dietrich et al., 2009), suggesting essential roles of the SCO in CSF homeostasis.
4. Experimental procedures
4.1 Animals
All mice were maintained under a controlled temperature and photoperiod (23°C, 12h light and 12h dark) with food and water provided ad libitum. All experimental procedures followed the Guideline for Animals Experimentation prepared by the Animal Care and Use Committee of Matsuyama University.
PS1 floxed mice were described previously (Yu et al., 2001). Wnt1-cre transgenic mice (Danielian et al., 1998) were obtained from Jackson Laboratory (#003829; Bar Harbor, ME, USA). The Wnt1-cre transgene has been used extensively to inactivate floxed genes in almost all neural crest cell lineages (Chai et al., 2000; Jiang et al., 2000; Brewer et al., 2004). Rosa-cre-reporter mice (Rosa26-LacZ floxed mice; Mao et al., 1999) were also obtained from Jackson Laboratory (#003504). Mice with a C57BL/6J and 129 hybrid background were used.
4.2 Histology, immunohistochemistry, and detection of β-galactosidase (lacZ) activity
For hematoxylin and eosin staining, brains were fixed in 4% paraformaldehyde in Mg2+-, Ca2+-free phosphate buffered saline [PBS(−)] at 4°C, embedded in paraffin, and sectioned at a thickness of 4 μm.
For immunohistochemistry with paraffin sections, brains were fixed in 4% paraformaldehyde in PBS(−) at 4°C, embedded in paraffin, and sectioned at a thickness of 4 μm. Antigen retrieval was performed after deparaffinization and rehydration by treating the slides with boiling Target Retrieval Solution (DAKO no. S1700, Carpinteria, CA, USA) for 40 min. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in distilled water for 10 min. The sections were incubated overnight with rabbit antiserum (AFRU; Rodríguez et al., 1998) or mouse monoclonal antibody (Mab4A6; Fernández-Llebrez et al., 2001) against SCO-glycoproteins or rabbit monoclonal anti-vimentin antibody (Abcam, Tokyo, Japan, ab92547). After washing in PBS(−), immunoreactivity was detected with the ENVISION+ system HRP Rabbit (DAKO, #K4003) or M.O.M. Kit (Vector, Burlingame, CA, USA) for detecting mouse primary antibody and diaminobenzidine.
For immunohistochemistry with cryosections, brains were perfused with 4% paraformaldehyde in PBS(−), postfixed with the 4% paraformaldehyde solution for 2 days, and rinsed with PBS(−). The brains were serially immersed into 10%, 20%, and 30% sucrose in PBS(−), embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan), and sectioned at a 30-μm thickness using a cryostat. The sections were stored at −80°C until use. The sections were thawed and refixed with the 4% paraformaldehyde solution for 30 min. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in PBS(−) for 5 min. The sections were incubated overnight with mouse monoclonal Mab4A6 antibody. Immunoreactivity was detected using a M.O.M. Kit (Vector, Burlingame, CA, USA) and diaminobenzidine.
Mouse brains were stained for β-galactosidase activity according to standard procedures. The brains were embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan) without fixation and sectioned at a thickness of 30 μm. After slight drying, the brain sections on glass slides were fixed in 4% paraformaldehyde in PBS(−) for 5 min, and washed with PBS(−) three times. The sections were stained for 24 to 48 h with X-gal staining solution containing 0.2% X-gal, 2 mM MgCl2, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide in 100 mM sodium phosphate (pH8.0). After staining, the tissues were counterstained with eosin.
For measurement of the total brain and ventricle areas on the brain sections, and of the immunostaining densities with Mab4A6, we used Image J, an image processing and analysis program (Dr. Wayne Rasband, NIH, Bethesda, MD, USA).
4.3 Western blot analysis
Brain tissues were homogenized with a glass Dounce homogenizer in RIPA buffer containing 50 mM Tris-HCl (pH8.0), 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% NP40, 0.5% NaDOC, and Complete protease inhibitor cocktail (Boehringer Mannheim GmbH, Mannheim, Germany) and centrifuged for 30 min at 20,000xg. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% polyacrylamide), the extracted proteins (50 μg) were transferred to a polyvinylidene difluoride membrane (BioRad Laboratories, Hercules, CA, USA). The blots were probed with the appropriate antibodies, which were detected using the ECL Plus Western Blotting Detection System (GE Healthcare UK Limited, Little Chalfont Buckinghamshire, UK). Rabbit anti-PS1-NTF antibody and anti-PS1-CTF antibody were described in Koizumi et al. (2000). Anti-APP-CTF antibody was purchased from Sigma (Sigma, St. Louis, MO, USA), which was developed in rabbit using a synthetic peptide corresponding to the C-terminal of human APP-695 (amino acids 676-695) conjugated to keyhole limpet hemocyanin as the immunogen. Rabbit anti-actin antibody was also obtained from Sigma.
4.4 Statistics
Statistical significance was determined by a two-tailed paired Student’s t-test. A p value of less than 0.05 was considered statistically significant.
Acknowledgements
This work was supported in part by Grant-in-Aid for Scientific Research (C) to M. Nakajima, Ministerio de Ciencia e Innovación, Madrid, Spain SAF2010-19087, and Junta de Andalucía, SAS 08-0029 and P07-CVI-03079, Spain
Footnotes
Author Contributions:
M.N. provided the experimental design, performed histologic experiments, and wrote the paper. K.M. performed the AFRU and GFAP immunohistochemistry. N.M. performed the MAB4A6 immunohistochemistry and Western blot analysis. Y. Fukunaga performed X-gal staining analysis and mouse genotyping. S.W. and S.O. performed histologic experiments, mouse genotyping, and mouse husbandry. J.P. and P.F. provided antibodies, information for antibody use, and interpretation of the results. J.S. provided mutant mice, information for mouse genotyping, and interpretation of the results. Y. Furukawa provided interpretation of the results, wrote the paper, and guarantees the integrity of the results.
References
- Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev. Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr., Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Molecular genetics of Alzheimer’s disease. Annals of medicine. 1998a;30:560–565. doi: 10.3109/07853899809002605. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Presenilin mutations in Alzheimer’s disease. Human Mutation. 1998b;11:183–190. doi: 10.1002/(SICI)1098-1004(1998)11:3<183::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Current Biology. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dietrich P, Shanmugasundaram R, Shuyu E, Dragatsis I. Congenital hydrocephalus associated with abnormal subcommissural organ in mice lacking huntingtin in Wnt1 cell lineages. Hum. Mol. Genet. 2009;18:142–150. doi: 10.1093/hmg/ddn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Llebrez P, Miranda E, Estivill-Torrús G, Cifuentes M, Grondona JM, López-Avalos MD, Pérez-Martín M, Pérez J. Analysis and quantification of the secretory products of the subcommissural organ by use of monoclonal antibodies. Microsc. Res. Tech. 2001;52:510–519. doi: 10.1002/1097-0029(20010301)52:5<510::AID-JEMT1036>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Fishman RA. Cerebrospinal fluid in diseases of the nervous system. 2nd ed WB Saunders; Philadelphia: 1992. [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. Journal of Cell Biology. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot LL, Michael DB. Post-traumatic hydrocephalus. Neurol. Res. 2000;22:25–28. doi: 10.1080/01616412.2000.11741034. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Huh MS, Todd MA, Picketts DJ. SCO-ping out the mechanisms underlying the etiology of hydrocephalus. Physiology (Bethesda) 2009;24:117–126. doi: 10.1152/physiol.00039.2008. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiménez AJ, Tomé M, Páez P, Wagner C, Rodríguez S, Fernández-Llebrez P, Rodríguez EM, Pérez-Fígares JM. A programmed ependymal denudation precedes congenital hydrocephalus in the hyh mutant mouse. J. Neuropathol. Exp. Neurol. 2001;60:1105–1119. doi: 10.1093/jnen/60.11.1105. [DOI] [PubMed] [Google Scholar]
- Jouet M, Feldman E, Yates J, Donnai D, Paterson J, Siggers D, Kenwrick S. Refining the genetic location of the gene for X linked hydrocephalus within Xq28. J. Med. Genet. 1993;30:214–217. doi: 10.1136/jmg.30.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Nakajima M, Yuasa S, Saga Y, Sakai T, Kuriyama T, Shirasawa T, Koseki H. The role of presenilin 1 during somite segmentation. Development. 2001;128:1391–1402. doi: 10.1242/dev.128.8.1391. [DOI] [PubMed] [Google Scholar]
- Koo EH, Kopan R. Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nature Medicine. 2004:S26–33. doi: 10.1038/nm1065. [DOI] [PubMed] [Google Scholar]
- Lee MK, Slunt HH, Martin LJ, Thinakaran G, Kim G, Gandy SE, Seeger M, Koo E, Price DL, Sisodia SS. Expression of presenilin 1 and 2 (PS1 and PS2) in human and murine tissues. J. Neurosci. 1996;16:7513–7525. doi: 10.1523/JNEUROSCI.16-23-07513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Wassef M. Ectopic engrailed 1 expression in the dorsal midline causes cell death, abnormal differentiation of circumventricular organs and errors in axonal pathfinding. Development. 2000;127:4061–4071. doi: 10.1242/dev.127.18.4061. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proceedings of the National Academy of Sciences of the US A. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Meiniel A. The secretory ependymal cells of the subcommissural organ: which role in hydrocephalus? Int. J. Biochem. Cell Biol. 2007;39:463–468. doi: 10.1016/j.biocel.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Moriizumi E, Koseki H, Shirasawa T. Presenilin 1 is essential for cardiac morphogenesis. Developmental Dynamics. 2004;230:795–799. doi: 10.1002/dvdy.20098. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Watanabe S, Okuyama S, Shen J, Furukawa Y. Restricted growth and insulin-like growth factor-1 deficiency in mice lacking presenilin-1 in the neural crest cell lineage. Int. J. Dev. Neurosci. 2009;27:837–843. doi: 10.1016/j.ijdevneu.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Yuasa S, Ueno M, Takakura N, Koseki H, Shirasawa T. Abnormal blood vessel development in mice lacking presenilin-1. Mechanisms of Development. 2003;120:657–667. doi: 10.1016/s0925-4773(03)00064-9. [DOI] [PubMed] [Google Scholar]
- Ohtoshi A. Hydrocephalus caused by conditional ablation of the Pten or β-catenin gene. Cerebrospinal Fluid Res. 2008;5:16. doi: 10.1186/1743-8454-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Fígares JM, Jiménez AJ, Pérez-Martín M, Fernández-Llebrez P, Cifuentes M, Riera P, Rodríguez S, Rodríguez EM. Spontaneous congenital hydrocephalus in the mutant mouse hyh. Changes in the ventricular system and the subcommissural organ. J. Neuropathol. Exp. Neurol. 1998;57:188–202. doi: 10.1097/00005072-199802000-00010. [DOI] [PubMed] [Google Scholar]
- Pérez-Fígares JM, Jimenez AJ, Rodríguez EM. Subcommissural organ, cerebrospinal fluid circulation, and hydrocephalus. Microsc. Res. Tech. 2001;52:591–607. doi: 10.1002/1097-0029(20010301)52:5<591::AID-JEMT1043>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu. Rev. Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- Rodríguez EM, Rodríguez S, Hein S. The subcommissural organ. Microsc. Res. Tech. 1998;41:98–123. doi: 10.1002/(SICI)1097-0029(19980415)41:2<98::AID-JEMT2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, Malkani S, Zhang D, Shankaranarayana Rao BS, Chattarji S, Kelleher RJ, 3rd, Kandel ER, Duff K, Kirkwood A, Shen J. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. 2004 doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Schurr PH, McLaurin RL, Ingraham FD. Experimental studies on the circulation of the cerebrospinal fluid and methods of producing communicating hydrocephalus in the dog. J. Neurosurg. 1953;10:515–525. doi: 10.3171/jns.1953.10.5.0515. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Vio K, Rodríguez S, Navarrete EH, Pérez-Fígares JM, Jiménez AJ, Rodríguez EM. Hydrocephalus induced by immunological blockage of the subcommissural organ-Reissner’s fiber (RF) complex by maternal transfer of anti-RF antibodies. Exp. Brain Res. 2000;135:41–52. doi: 10.1007/s002210000474. [DOI] [PubMed] [Google Scholar]
- Wagner C, Batiz LF, Rodríguez S, Jiménez AJ, Páez P, Tomé M, Pérez-Fígares JM, Rodríguez EM. Cellular mechanisms involved in the stenosis and obliteration of the cerebral aqueduct of hyh mutant mice developing congenital hydrocephalus. J. Neuropathol. Exp. Neurol. 2003;62:1019–1040. doi: 10.1093/jnen/62.10.1019. [DOI] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg L.h., Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Inagawa T, Tokuda Y, Inokuchi F. Chronic hydrocephalus in elderly patients following subarachnoid hemorrhage. Surg. Neurol. 2000;53:119–124. doi: 10.1016/s0090-3019(99)00185-8. discussion 124-5. [DOI] [PubMed] [Google Scholar]
- Yu H, Saura CA, Choi SY, Sun LD, Yang X, Handler M, Kawarabayashi T, Younkin L, Fedeles B, Wilson MA, Younkin S, Kandel ER, Kirkwood A, Shen J. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Nakajima M, Aizawa H, Sahara N, Koizumi K, Sakai T, Usami M, Kobayashi S, Kuroyanagi H, Mori H, Koseki H, Shirasawa T. Impaired cell cycle control of neuronal precursor cells in the neocortical primordium of presenilin-1-deficient mice. Journal of Neuroscience Research. 2002;70:501–513. doi: 10.1002/jnr.10430. [DOI] [PubMed] [Google Scholar]