Abstract
Thrombotic microangiopathies (TMAs) are a group of life-threatening disorders characterized by thrombocytopenia, fragmentation of erythrocytes, and ischemic organ damage. Genetic disorders, autoimmune disease, and cancer are risk factors for TMAs, but an additional, unknown trigger is needed to bring about acute disease. Recent studies suggest that DNA and histones are released during inflammation or infection and stimulate coagulation, thrombosis, thrombocytopenia, and organ damage in mice. We show that extracellular DNA and histones as well as markers of neutrophils are present in acute TMAs. Analysis of plasma from TMA patients of different clinical categories revealed elevated levels of DNA-histone complexes and myeloperoxidase (MPO) from neutrophil granules as well as S100A8/A9, a heterocomplex abundant in neutrophil cytosol. During therapy of thrombotic thrombocytopenic purpura, a subtype of TMAs often associated with severe ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motifs, member 13) deficiency, plasma DNA and MPO were inversely correlated with platelet counts, and their levels indicated amelioration or exacerbation of the disease. ADAMTS13 deficiency together with increased levels of plasma DNA and MPO were characteristic for acute thrombotic thrombocytopenic purpura. A minor infection often precedes acute TMA and extracellular DNA and histones released during the inflammatory response could provide the second hit, which precipitates acute TMA in patients with pre-existing risk factors.
Introduction
Thrombotic microangiopathies (TMAs) are a heterogeneous group of life-threatening disorders characterized by thrombocytopenia, fragmentation of erythrocytes, and ischemic organ damage that includes thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS).1–3 TTP is often associated with a severe deficiency in the von Willebrand factor–cleaving protease known as ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motifs, member 13). The deficiency can be because of autoantibodies (acquired TTP)4,5 or genetic mutations of ADAMTS13 (hereditary TTP).6–8 Typical HUS is associated with and follows enterohemorrhagic Escherichia coli infection with bloody diarrhea (D+HUS), occurs mainly in children, and is associated with acute renal failure.9,10 Atypical, diarrhea-negative (D−) HUS is very difficult to distinguish from TTP and has clearly overlapping clinical findings.1,3,11 In D−HUS patients, several hereditary traits were recently identified that result in a hyperactivity of the alternative complement pathway, either by defective regulation of complement activation or by hyperfunctional mutations of complement factors.3,11,12 Additional clinical conditions associated with TMAs are disseminated neoplasia; drugs including mitomycin C, quinine, and ticlopidine; hematopoietic stem cell transplantation; pregnancy; certain autoimmune diseases; and HIV infection.1–3,13
Whereas almost all patients with hereditary TTP have severe constitutional ADAMTS13 deficiency and ∼ 60% of patients clinically diagnosed with acquired TTP have severe autoantibody-induced ADAMTS13 deficiency, it is well known that some patients with severe hereditary deficiency may remain asymptomatic for many years.6,14 Furthermore, some patients with acute TTP and severe acquired ADAMTS13 deficiency go into clinical remission, although they remain severely deficient in ADAMTS13,15–17 suggesting a second hit is needed to bring about acute disease.
Extracellular DNA and histones have recently been shown to contribute to thrombosis. Extracellular DNA fibers provide scaffolding for platelet adhesion and promote fibrin deposition.18,19 Extracellular DNA is part of thrombi in animals and contributes to thrombus formation and stability.18–20 In vitro, histones induce platelet aggregation and enhance thrombin generation.21,22 When infused into mice, histones cause thrombocytopenia, promote thrombosis, and contribute to organ damage and death.18,20,22–25
Nucleosomes are a complex of DNA and histones and represent the basic building blocks of chromatin. Nucleosomes are released from necrotic tissue and are found in the circulation of patients with myocardial infarction, stroke, infections, trauma, cancer, or autoimmune diseases.26 Nucleosomes also can be liberated from inflammatory cells such as neutrophils. In response to infection or inflammatory stimuli, neutrophils release chromatin fibers, known as neutrophil extracellular traps (NETs).27 NETs bind microbes and contribute to host defense.27,28 NETs also can immobilize platelets and erythrocytes and stimulate thrombosis.18–20 Histones and neutrophil proteases in NETs are in part responsible for the antimicrobial and prothrombotic functions.18–20,27 NETs are released from neutrophils after a cell death program that proceeds from the dissolution of granular and nuclear membranes to chromatin decondensation and cytolysis.29 Other cell types known to release chromatin fibers are monocytes30 and mast cells,31 whereas eosinophils form extracellular traps from mitochondrial DNA.32
An infection often precedes acute TMA,33,34 and we speculated that liberation of DNA and histones during the inflammatory response could be implicated in the pathogenesis of certain forms of TMA. To test our hypothesis, we measured nucleosomes in the plasma of TMA patients of various clinical categories, at presentation with the acute disease episode, during plasma exchange therapy (PEX) and in clinical remission. In addition, we quantified markers of inflammation, namely, myeloperoxidase (MPO) from neutrophil granules35 and S100A8/A9, a heterocomplex abundantly stored in neutrophil cytoplasm.36 Our study reveals that nucleosomes and inflammatory markers are concomitantly elevated in acute TMA and reflect the disease state, also during periods of PEX. Inflammation in response to even a minor infection might result in the generation of extracellular DNA and histones and thus provide the trigger that brings about acute disease in patients at risk for TMA.
Methods
Patient plasma samples
Plasma samples were selected from patients referred for ADAMTS13 activity testing for diagnostic purposes to the Hemostasis Research Laboratory, Department of Hematology, Bern University Hospital, and the University of Bern (Bern, Switzerland) over a period of ∼ 10 years. Some patients had been treated at Bern University Hospital and serial plasma samples were obtained during the disease course. All patients had received a diagnosis of TMA by their referring physicians defined by microangiopathic hemolytic anemia with schistocytes on the blood smear and thrombocytopenia with or without clinically apparent ischemic organ dysfunction. A selection of citrated plasma samples was prepared by one of us (J.A.K.H.) and included (1) samples from patients with acute acquired TTP displaying autoantibody-mediated severe ADAMTS13 deficiency (ADAMTS13 activity < 5% of normal), (2) samples from patients with D+HUS and a proven preceding infection with enterohemorrhagic E coli, (3) samples from patients with a tumor-associated thrombotic microangiopathy; and (4) samples from patients with a TMA of unknown etiology (designated as not otherwise specified). All patients in categories 2 to 4 had ADAMTS13 activity levels above 30% of normal. Remission is defined as absence of clinical and laboratory abnormalities without PEX for at least 30 days.16,37 Exacerbation signifies worsening clinical or laboratory signs of TMA after (partial) normalization while on (daily) PEX or within 30 days after stopping plasma therapy.16,37 Disease relapse is defined as recurrence of thrombocytopenia and microangiopathic hemolytic anemia after achievement of a remission.16 All investigators except J.A.K.H., who prepared the learning and investigation cohort samples, were blinded concerning diagnosis, clinical course, and ADAMTS13 activity values until completion of nucleosome and DNA analyses. The study was approved by the responsible Ethics Committee (Kantonale Ethikkommission, Bern, Switzerland).
Determination of platelet counts, ADAMTS13 activity, and ADAMTS13 functional inhibitors
Platelet counts were determined at the referring centers' or Bern University Hospital's routine laboratories. ADAMTS13 activity and functional inhibitors were determined by the quantitative immunoblotting assay16,38 and the FRETS-VWF73 assay,39 slightly modified as described previously.40 The detection limit of these assays is at 3% to 5% (immunoblotting) and 1% (FRETS-VWF73) of normal ADAMTS13 activity, respectively.
Quantification of nucleosomes
Nucleosomes were quantified by ELISA (Cell death detection kit; Roche) according to manufacturer's instructions. One unit of nucleosomes refers to the average amount of nucleosomes quantified in plasma from healthy controls.
Quantification of plasma DNA
Plasma was diluted in phosphate-buffered saline (PBS; Invitrogen). Fifty microliters of diluted plasma was mixed with 50 μL of PBS containing SytoxGreen (final concentration 2μM; Invitrogen) to label DNA. Fluorescence was recorded in a fluorometer (Fluoroskan; Thermo Fisher Scientific). Autofluorescence was considered as background and determined in samples mixed with PBS without SytoxGreen. DNA concentrations were calculated based on a standard curve of known concentrations of DNA (Invitrogen).
Quantification of lactate dehydrogenase activity
Fifty microliters of plasma diluted in PBS was mixed with 50 μL of a chromogenic lactate dehydrogenase (LDH) substrate (Promega). The kinetics of the change in optical density at 490 nm was measured at room temperature with an ELISA reader (SpectraMax190; Molecular Devices).
Quantification of S100A8/S100A9
S100A8/S100A9 (S100A8/A9) heterocomplexes (also known as calprotectin) were quantified by ELISA (Human Calprotectin ELISA kit; Hycultbiotech) according to manufacturer's instructions. One unit of S100A8/A9 refers to the average amount of S100A8/A9 quantified in plasma from healthy controls.
Quantification of MPO
MPO in plasma was quantified by a commercially available kit according to manufacturer's instructions (Zen MPO ELISA; Invitrogen). One unit of MPO refers to the average amount of MPO quantified in plasma from healthy controls.
Statistical evaluation
Statistical analysis was performed using Prism Version 4.0b software (GraphPad Software) and included Mann-Whitney test and Kruskal-Wallis test with Dunn posthoc test. Results were considered significant at P less than .05. Spearman rank correlation coefficients were calculated with Bonferroni-adjusted significance levels and considered significant at P less than .005.
Results
Acute TMAs are associated with increased levels of plasma DNA and nucleosomes
We compiled a learning cohort of plasma samples from 29 patients with acute TMAs and 10 healthy controls (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). TMA was characterized as acquired TTP with severe ADAMTS13 deficiency in 6 cases. Four patients were diagnosed with D+HUS; 8 with tumor-associated acute TMA; and in 11 cases, the etiology was not otherwise specified.
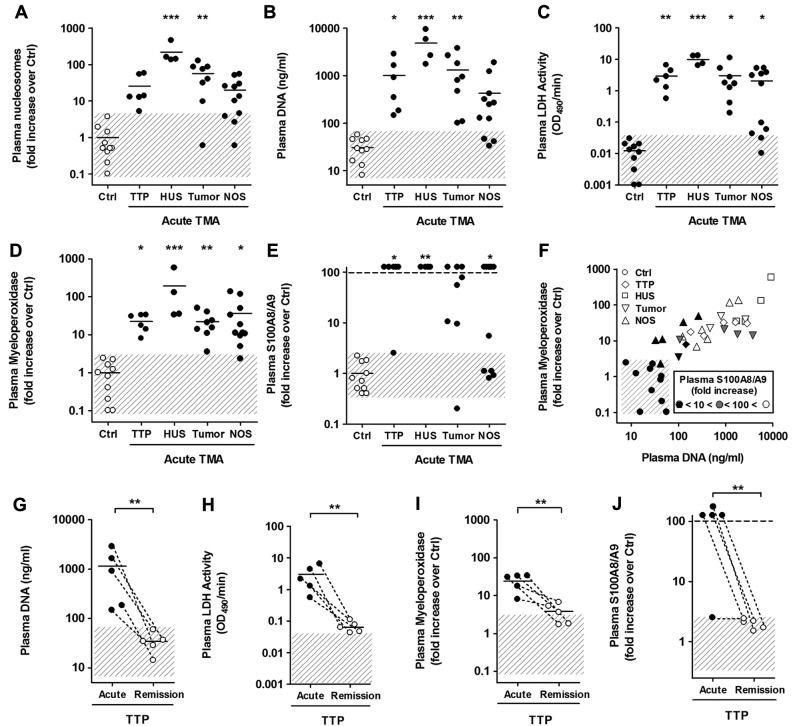
We first questioned whether circulating nucleosomes are elevated in patients with acute TMA compared with healthy controls. A nucleosome comprises 147 base pairs (bp) of DNA wrapped around a core of double represented histone proteins H2A, H2B, H3, and H4. A linker DNA of 10 to 100 bp connects nucleosomes. Histone H1 binds to the linker DNA and is important for chromatin organization. Nucleosomes were quantified by ELISA using a capturing antibody against an epitope shared by all histones and a detecting antibody against DNA (Figure 1A). As an alternative approach, we quantified the amount of circulating double-stranded DNA using a fluorescent DNA probe (Figure 1B). We observed comparable results with both assays (nucleosomes vs DNA: Spearman rank correlation coefficient R = 0.8955, n = 39, P < .0001) and nucleosomes as well as DNA were increased in all patients with TTP or HUS, 7 of 8 tumor-associated TMAs, and 7 of 11 patients with TMAs of unknown etiology. The analysis was conducted in a blinded manner, and we conclude that plasma nucleosome and DNA elevation is a shared marker of acute TMA of different clinical categories.
Figure 1.
Plasma nucleosomes, DNA, LDH, MPO, and S100A8/A9 are markers of acute TMAs. (A-F) Analysis of plasma samples of the learning cohort including healthy controls (Ctrl; n = 10) and from patients with acquired TTP (n = 6), D+HUS (n = 4), tumor-associated TMA (Tumor; n = 8), and TMAs that are not otherwise specified ([NOS]; n = 11). (A) Quantification of nucleosomes by ELISA. Quantification of DNA by a fluorescent DNA probe (B) and LDH activity using a chromogenic substrate (C). (D) Quantification of myeloperoxidase by ELISA, a marker of granules in neutrophils and monocytes. (E) Quantification of S100A8/A9, a cytosolic protein complex present in neutrophils. (F) Correlation of DNA with MPO and S100A8/A9 in plasma from TMA patients and healthy controls. The symbol color indicates the level of S100A8/A9 compared with healthy controls. In most patients with acute TMA, all 3 markers were elevated, indicating that inflammation, and neutrophils, monocytes, or both contributed to the release of nucleosomes in acute TMA. (G-J) Comparison of DNA (G), LDH (H), MPO (I), and S100A8/A9 (J) in plasma from 5 patients with acquired TTP collected at presentation with acute disease (Acute) or in remission (Remission). All 4 markers were elevated in acute disease and normalized in remission, indicating that they reflect disease activity in TMA patients. Shaded area indicates the range of nucleosomes, DNA, LDH, MPO, or S100A8/A9 in plasma from healthy controls (*P < .05, **P < .01,***P < .001).
We further characterized circulating nucleosomes of TMA patients. Immunodetection of histone H3 revealed the 16-kDa native protein and 1 or 2 histone H3 fragments in plasma from 17 of 29 patients with acute TMA (supplemental Figure 1A; supplemental Table 1). Analysis of isolated plasma DNA by gel electrophoresis showed that the length of circulating DNA was ∼ 180 bp (supplemental Figure 1B). The length corresponds to DNA of mononucleosomes with residual linker DNA. Indeed, plasma nucleosomes are reported to be predominantly degraded to single units.26 These findings also indicated that circulating DNA in TMA patients is predominantly of nuclear rather than mitochondrial origin.
Plasma LDH, MPO, S100A8/A9, and nucleosomes are concomitantly elevated in acute TMAs
Nucleosomes can originate from dying tissue including tumors23,26 or inflammatory leukocytes.27 LDH is a cytoplasmic enzyme and its release into plasma reflects the degree of tissue damage. Strongly elevated levels of plasma LDH are characteristic for acute TMA, and as expected we measured increased levels of LDH activity in the different groups of patients with acute TMA (Figure 1C). LDH also is released from dying neutrophils during NET formation in vitro.29 To elucidate whether inflammation and cell death of leukocytes are associated with LDH and DNA release in acute TMA, we quantified MPO and S100A8/A9. These proteins are expressed in neutrophils and released together with DNA and LDH when NETs are formed. MPO is a heme protein stored in granules of neutrophils and monocytes35 known to produce reactive oxygen species. MPO is particularly abundant in neutrophils and accounts for 25% of granular proteins and for 5% of all proteins. We measured MPO in plasma by ELISA and detected significantly elevated levels in all clinical groups of acute TMA compared with healthy controls (Figure 1D). In addition, MPO correlated with DNA in patient plasma samples (Figure 1F; supplemental Figure 2). We next quantified S100A8/A9 complexes in plasma. The complex belongs to a group of calcium-binding S100 proteins and is expressed in different cell types, but it is mainly ascribed to circulating neutrophils and monocytes, in which it accounts for 50% of the cytosolic proteins.36 S100A8/A9 is also known as myeloid-related protein 8/14 or calgranulin A/B, and it is used as a diagnostic marker of inflammation in human plasma. We measured S100A8/A9 in plasma by ELISA and detected elevated levels in 5 of 6 patients with TTP, in all 4 patients with D+HUS, 7 of 8 patients with tumor-associated TMAs, and 7 of 11 patients with TMA of unknown etiology (Figure 1E). Importantly, S100A8/A9, MPO, and DNA were concomitantly elevated in 5 of 6 TTP patients, all 4 patients with D+HUS, 7 of 8 patients with tumor-associated TMA, and 7 of 11 patients with TMA of unknown etiology (Figure 1F). Correlation of plasma LDH, S100A8/A9, and MPO with plasma DNA strongly indicates an association of neutrophils, monocytes, or both, with extracellular DNA and histones released in acute TMA (supplemental Figure 2).
Plasma DNA, LDH, MPO, and S100A8/A9 reflect disease state in TTP patients before and after therapy
In the following experiments, we analyzed plasma DNA, LDH, MPO, and S100A8/A9 levels in TTP patients who achieved remission after plasma therapy. TTP patients are routinely treated by daily PEX in combination with corticosteroids.1,2,13,16 Our learning cohort included plasma samples from 5 patients with acquired TTP collected both at presentation with acute disease before any plasmatherapy and when the patients had achieved remission (supplemental Table 1). Levels of plasma DNA (Figure 1G) and S100A8/A9 (Figure 1J) were normalized in all 5 patients in clinical remission, whereas LDH and MPO tended to normalize but remained slightly above control values in all 5 and in 3 patients, respectively (Figure 1H-I). This indicated that DNA, MPO, and S100A8/A9 might reflect the disease activity in TTP patients, similarly to LDH.
To test this idea, we compiled a second, investigational cohort with available plasma samples from 11 TTP patients collected at the day of admission, during therapy, and in remission (Table 1; supplemental Table 2). First, we analyzed series of plasma samples collected during daily PEX and corticosteroid therapy of 5 patients with acute TTP because of a severe acquired ADAMTS13 deficiency (Table 1; supplemental Table 2 patients A-E). We compared plasma DNA, MPO, ADAMTS13 activity, and platelet counts during the disease course. Patients A (Figures 2A and 3A) and B (Figures 2B and 3B) represent examples of a good outcome during PEX and corticosteroid therapy. Patient A had at admission 35 000 platelets/μL, 457 ng/mL DNA, 23.8-fold elevated levels of MPO, and ADAMTS13 activity below 5% of normal with a circulating inhibitor (supplemental Table 2 sample 45). Within 10 days of daily PEX, platelet counts recovered to 204 000/μL, DNA levels reached 29 ng/mL, MPO levels became normal, and ADAMTS13 activity was 100% (supplemental Table 2 samples 45-54). Patient B was treated with 3 daily PEX after admission. Platelet counts increased from 29 000 to 302 000/μL at day 8. DNA levels decreased from 729 ng/mL to 14 ng/mL. Plasma MPO at admission was 20.6-fold and at day 8 1.9-fold higher than in control samples. ADAMTS13 activity increased from less than 5% to 44% (supplemental Table 2 samples 60-63). Patient C (Figures 2C and 3C) did not achieve remission, progressively deteriorated, and died during the course of therapy. The patient remained thrombocytopenic with platelet counts below 35 000/μL throughout the 10-day disease course (supplemental Table 2 samples 65-76). At admission, the DNA levels were ∼ 4-fold higher than in control samples (132 ng/mL; supplemental Table 2 sample 65). During daily PEX and corticosteroid therapy, DNA levels progressively increased to extremely high levels and were ∼ 530-fold higher compared with controls at day 10 (16 404 ng/mL; supplemental Table 2 sample 76). The kinetic of plasma MPO was similar, and MPO reached levels 220-fold higher than controls at day 10 (Figure 3C; supplemental Table 2 sample 76). At this point, the patient died from multiorgan failure and concomitant sepsis with Staphylococcus aureus, presumably acquired from an indwelling central venous catheter. At autopsy, widespread microthrombi were found in the brain, heart, spleen, and colon consistent with TTP. The concomitant sepsis might be largely responsible for the extremely high values of DNA because nucleosomes are strongly elevated in sepsis and correlate with severity of disease and mortality.41
Table 1.
Investigational cohort: demographic data, clinical course, and treatment modalities in 11 TTP patients who were studied during acute disease episodes, under treatment and in remission
| Patient | Figure | Age, y | Sex | Diagnosis | Treatment | Course and outcome |
|---|---|---|---|---|---|---|
| A* | 2A | 38 | F | Acute, acquired TTP | PEX, corticosteroids | Single episode, in remission since 9.5 y |
| B | 2B | 69 | M | Acute, acquired TTP | PEX, corticosteroids | Single episode, in remission since 1.5 y |
| C | 2C | 57 | M | Acute, acquired TTP | PEX, corticosteroids | Death of disease day 11, presumably due to catheter sepsis, blood cultures positive for S aureus |
| D | 2D | 29 | M | Acute, acquired TTP | PEX, corticosteroids, rituximab | Single episode, because of plasma-refractory disease additional treatment with 4 weekly doses of rituximab, starting on day 20, in remission since 1 y |
| E† | 2E | 17 | M | Acute, acquired TTP | PEX, corticosteroids, splenectomy | Single episode, splenectomy performed because of plasma-refractory disease on day 29, in remission since 10.5 y despite the reappearance of severe ADAMTS13 deficiency and ADAMTS13 inhibitor 3.5 y after splenectomy |
| F | 37 | M | Recurrent, acquired TTP | PEX, steroids, rituximab | First acute episode and 2 relapses over a follow-up period of 5 y, treatment of both relapses included 4 weekly doses of rituximab | |
| G | 36 | M | Recurrent, acquired TTP | PEX, corticosteroids | First seen in remission 5 mo after second acute episode, relapsed 3 times over a follow-up period of 2.5 y | |
| H | 59 | M | Recurrent, acquired TTP | PEX, corticosteroids | First seen in remission 1 y after third acute episode and 3 wks before fourth acute episode | |
| I | 51 | M | Hereditary TTP | PEX, FFP infusions | Second acute TTP episode, leading to a diagnosis of severe congenital ADAMTS13 deficiency, in remission on regular FFP infusions | |
| J | 61 | M | Hereditary TTP | PEX | First acute TTP episode and diagnosis of severe congenital ADAMTS13 deficiency and hereditary TTP, no prophylactic FFP infusions so far | |
| K | 49 | F | Hereditary TTP | FFP infusion | Following the fifth acute TTP episode diagnosis of severe congenital ADAMTS13 deficiency, no prophylactic FFP infusions so far |
Patients (M indicates male; and F, female) with acute idiopathic TTP with severe acquired ADAMTS13 deficiency were treated with daily PEX with fresh frozen plasma (FFP) replacement, and corticosteroids. Patients A through E had their first acute TTP episode. Patients A and B responded well to therapy. Patient C died at day 11 of therapy, presumably due to catheter sepsis. Patients D and E developed exacerbations during daily PEX therapy that led to the treatment with rituximab (patient D) or splenectomy (patient E). Patients F through H had a history of recurrent acute TMA bouts over several years. Patients I through K were diagnosed with hereditary TTP because of severe constitutional ADAMTS13 deficiency. Plasma samples were available from acute disease bouts and from periods of clinical remission. Only patient I was receiving regular prophylactic FFP transfusions.
Patient A has been previously published as patient 4 in Fontana et al.52
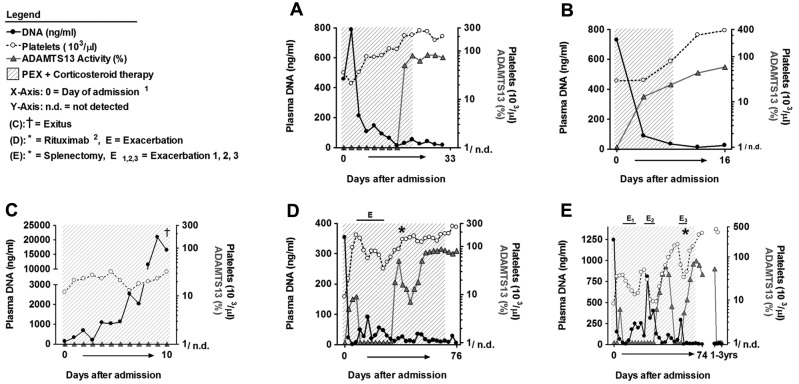
Figure 2.
Plasma DNA levels indicate the disease state of TTP patients during therapy. Analysis of serial plasma samples from patients of the investigational cohort. Time course of plasma DNA (black circles), ADAMTS13 activity (gray triangles), and platelet counts (open circles) of patients A through E with acute acquired TTP during PEX and corticosteroid therapy. The shaded area marks the time of PEX therapy. Samples with ADAMTS13 activity below 5% were plotted as 1/n.d. (not detected). Day 0 is the time of admission and start of therapy. Patient A (A) and patient B (B) represent a good outcome of PEX therapy. Platelet counts, ADAMTS13 activity, and plasma DNA levels normalized during therapy. (C) Patient C developed a catheter sepsis during therapy. Platelet counts and ADAMTS13 activity did not improve, and DNA levels increased during therapy. The patient died 10 days after admission. Patient D (D) and patient E (E) were refractory to PEX and developed 1 (E in panel D) and 3 (E1-E3 in panel E) exacerbations during therapy, respectively. Each exacerbation was associated with a decrease in platelet counts concomitant with an increase in circulating DNA. Both patients finally achieved remission either after additional therapy with rituximab (D) or after splenectomy (E). Reappearance of ADAMTS13 deficiency in patient E (E) 3 years after remission was not associated with thrombocytopenia or increases in plasma DNA. (1The x-axis not linearly formatted. Data shown are from consecutive plasma samples and the time between plasma sample collections varies. 2Rituximab was given 4 times at weekly intervals.)
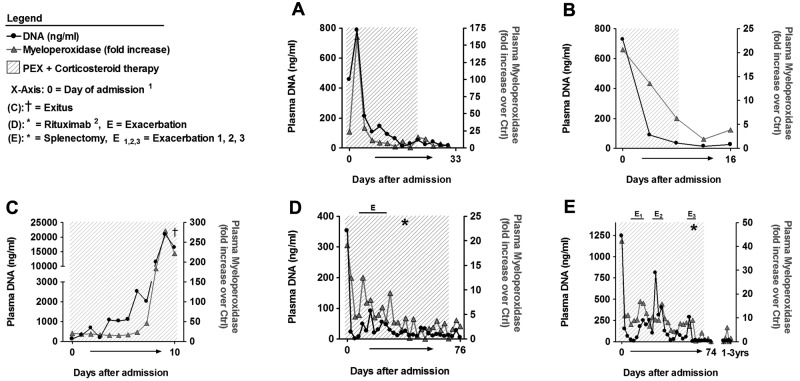
Figure 3.
Plasma MPO correlated with DNA levels in TTP patients during therapy. Patient samples and DNA levels are identical to those in Figure 2. MPO levels were quantified, plotted as fold increase over MPO levels of healthy control samples, and compared with plasma DNA. DNA and MPO levels follow similar kinetics in all patients. (1The x-axis not linearly formatted. Data shown are from consecutive plasma samples and the time between plasma sample collections varies. 2Rituximab was given 4 times at weekly intervals.)
Patients D (Figures 2D and 3D; supplemental Table 2 samples 77-106) and E (Figures 2E and 3E; supplemental Table 2 samples 107-141) were diagnosed with plasma-refractory acute acquired TTP with exacerbations during prolonged PEX and corticosteroid therapy. At admission, both patients were severely thrombocytopenic (< 10 000 platelets/μL), ADAMTS13 deficient (< 5% of normal) and plasma DNA (patient D, 353 ng/mL; patient E, 1243 ng/mL) and MPO levels (patient D, 19.1-fold; patient E, 42.3-fold) were increased. Both patients only transiently responded to therapy, with exacerbations leading to the application of rituximab (patient D) and splenectomy (patient E). Each exacerbation with worsening platelet counts was associated with an increase in plasma DNA (Figure 2D-E) and MPO (Figure 3D-E). After rituximab or splenectomy, respectively, the patients achieved remission and have not developed further TTP episodes at the time of this writing. These data indicate that plasma DNA and MPO closely parallel amelioration and exacerbation during the course of PEX therapy of acute TTP.
Approximately 3 years after achieving remission, patient E again developed severe ADAMTS13 deficiency because of inhibitory autoantibodies (Figure 2E; supplemental Table 2 samples 138-141). This episode of recurring and persisting severe ADAMTS13 deficiency was not associated with thrombocytopenia or increased DNA and MPO levels. This supports our hypothesis that a second hit is needed to precipitate acute TMA in patients with preexisting risk factors such as severe ADAMTS13 deficiency. Release of DNA and histones, even during moderate inflammation, could provide such a trigger.
We further tested the hypothesis and analyzed patients with recurrent acquired TTP (patients F-H; Table 1; supplemental Table 2). We analyzed plasma from patient F obtained at the first acute episode, in remission and during 2 relapses (supplemental Table 2 samples 142-155). Plasma from patient G was first obtained in remission 5 months after the second acute TTP episode (supplemental Table 2 samples 156-160). Thereafter, the patient relapsed 3 times and developed acute TTP episodes. Patient H was first seen in remission 1 year after the third acute episode and had 1 acute disease flare-up (supplemental Table 2 samples 161-163). All acute TTP episodes in patients F-H were associated with severe ADAMTS13 deficiency and increases in plasma DNA and MPO (available only for patients G and H). Importantly, patients G and H showed persisting severe acquired ADAMTS13 deficiency also during clinical remission, whereas DNA and MPO were elevated only during disease flare-ups.
We also analyzed plasma from 3 patients with hereditary TTP because of homozygous or compound heterozygous ADAMTS13 mutations, leading to severe constitutional deficiency of ADAMTS13 activity (patients I-K; Table 1; supplemental Table 2 samples 164-171). Plasma DNA and MPO were elevated in acute disease bouts in 2 of 3 patients. In all remission samples of the 3 patients, DNA levels were normal, but plasma MPO remained slightly elevated, suggesting that clearance of circulating MPO could be altered in congenitally ADAMTS13 deficient patients. In summary, the data indicate that severe acquired and hereditary ADAMTS13 deficiency per se do not lead to an increase in extracellular DNA, whereas MPO was slighty elevated in hereditary TTP patients even in apparent clinical remission.
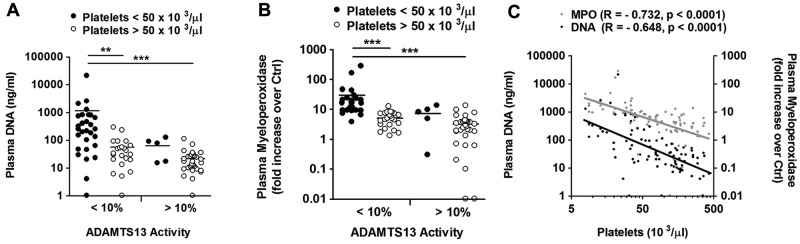
Next, we analyzed 100 samples from patients with acquired TTP from the investigational cohort of which ADAMTS13 activity, platelet counts, DNA as well as MPO levels were available (supplemental Table 2). Consistent with our hypothesis, thrombocytopenia (platelet count < 50 000/μL) was characterized by the combination of ADAMTS13 deficiency, increased levels of plasma DNA (Figure 4 A), and MPO (Figure 4B). Moreover, the amount of circulating DNA and MPO was inversely correlated with the number of circulating platelets (Figure 4C).
Figure 4.
Elevated plasma DNA and MPO levels together with ADAMTS13 deficiency characterize the acute disease state in patients with acquired TTP. Analysis of 100 samples of patients with acquired TTP from the investigational cohort of which DNA, MPO, platelet counts, and ADAMTS13 activity were available. Comparison of ADAMTS13 activity to platelet count and plasma DNA (A) or MPO (B). Plasma DNA and MPO were elevated predominantly in ADAMTS13-deficient patients (< 10%) with thrombocytopenia (< 50 000 platelets/μL). (C) Correlation of plasma DNA and MPO with platelet counts. Both plasma DNA and MPO negatively correlated with the number of circulating platelets (*P < .05, **P < .01, ***P < .001).
Discussion
Our results indicate that DNA-histone complexes are released during acute TMA and correlate with disease activity during therapy. DNA and histones stimulate thrombosis and cause cytotoxicity in mice.19,20,25 It is conceivable that they are implicated in clinical characteristics such as thrombocytopenia, microthrombosis, organ damage, and potentially mortality in patients with TMA.
Circulating DNA and histones in patients with TMA were fragmented. DNA is likely to be cleaved by endogenous DNase1 in plasma.42 Activated protein C may be responsible for the cleavage of histones,25 but other proteases, such as neutrophil elastase, may cleave histones before they are released as part of NETs.43 Whether fragmented DNA or histones in plasma are functional and can still stimulate thrombosis or tissue damage is not clear. Histone fragments have been shown to exhibit potent antimicrobial activity.44 However, histones loose their toxicity in vitro and in vivo after treatment with activated protein C.25 DNase 1 cleaves DNA and neutralizes its procoagulant activity in vitro,45 suggesting that the degraded circulating DNA is inactive. We observed previously that in baboon deep vein thrombosis plasma DNA appearance follows a similar kinetics as the fibrin degradation product D-Dimer.18,46 It is plausible that circulating nucleosomes reflect the degradation of extracellular chromatin within a thrombus and thus ongoing thrombolysis.
Circulating DNA and histones in patients with TMA may originate from NETs. We found that levels of S100A8/A9 and MPO are strongly correlated with levels of plasma DNA in patients during acute disease. The triad of cytoplasmic, granular, and nuclear components that are released during NET formation in vitro29,47 and found in plasma during acute TMA supports the idea that NETs or their degradation products, respectively, contribute to the pool of circulating DNA and histones. Interestingly, MPO can be liberated from activated neutrophils by degranulation only in moderate levels in vitro,47 and S100A8/A9 lacks transmembrane regions and it is not clear how the complex passes the plasma membrane.36 Cytolysis of neutrophils in the course of NET formation could provide a release mechanism29,47 and explain the high levels of MPO and S100A8/A9 in acute TMA. However, it is likely that plasma DNA originates also from other cell types,23,26 including tumor cells in the case of neoplasia-associated TMA. Furthermore, DNA and histones may be liberated from necrotic tissue after ischemic damage. Under pathologic conditions, extracellular DNA and histones may be part of a feedback-loop between inflammation and tissue damage. Necrosis attracts neutrophils48 that in turn exacerbate tissue damage and in vitro experiments suggest that NETs49 or extracellular histones25 contribute to the cytotoxicity. Histones and DNA isolated from different sources have similar activity in vitro18,25 and it is likely that the exposure of these highly conserved molecules represents an evolutionarily maintained mechanism to fight infections and stimulate thrombus formation.50,51
A minor infection often precedes acute TMA. DNA and histones may be released at infected sites to control invading microbes but also may provide a second hit that precipitates acute disease in patients at risk for TMA, such as those with severe ADAMTS13 deficiency.
Supplementary Material
Acknowledgments
The authors thank Lesley Cowan for help preparing the manuscript.
This work was supported in part by Swiss National Science Foundation grant 32003B-124892 (J.A.K.H., B.L.); the ISTH 2007 Presidential Fund (J.A.K.H.); the National Institutes of Health National Heart, Lung, and Blood Institute grant R01 HL102101 (D.D.W.); and a fellowship from the Deutsche Forschungsgemeinschaft, Germany (FU 742/1-1; T.A.F.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.A.F. designed and performed experiments, analyzed data, and wrote the manuscript; J.A.K.H. provided plasma samples, analyzed data, and wrote the manuscript; D.S. performed experiments; D.D.W. designed experiments, analyzed data, wrote the manuscript, and oversaw the study; and B.L. designed experiments, provided plasma samples and clinical and laboratory data of patients with TMA, analyzed data, wrote the manuscript, and oversaw the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Lämmle, Department of Hematology and Central Hematology Laboratory, Inselspital, Bern University Hospital and University of Bern, CH-3010 Bern, Switzerland; e-mail: bernhard.laemmle@insel.ch; or Denisa D. Wagner, Immune Disease Institute, 3 Blackfan Circle, Boston, MA 02115; e-mail: wagner@idi.harvard.edu.
References
- 1.Lämmle B, Kremer Hovinga JA, Alberio L. Thrombotic thrombocytopenic purpura. J Thromb Haemost. 2005;3(8):1663–1675. doi: 10.1111/j.1538-7836.2005.01425.x. [DOI] [PubMed] [Google Scholar]
- 2.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347(8):589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–277. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furlan M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 5.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimura Y, Matsumoto M, Isonishi A, et al. Natural history of Upshaw-Schulman syndrome based on ADAMTS13 gene analysis in Japan. J Thromb Haemost. 2011;9(Suppl 1):283–301. doi: 10.1111/j.1538-7836.2011.04341.x. [DOI] [PubMed] [Google Scholar]
- 7.Kokame K, Matsumoto M, Soejima K, et al. Mutations and common polymorphisms in ADAMTS13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A. 2002;99(18):11902–11907. doi: 10.1073/pnas.172277399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 9.Scheiring J, Andreoli SP, Zimmerhackl LB. Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS). Pediatr Nephrol. 2008;23(10):1749–1760. doi: 10.1007/s00467-008-0935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073–1086. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 11.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361(17):1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 12.Richards A, Goodship JA, Goodship TH. The genetics and pathogenesis of haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura. Curr Opin Nephrol Hypertens. 2002;11(4):431–435. doi: 10.1097/00041552-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 13.George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116(20):4060–4069. doi: 10.1182/blood-2010-07-271445. [DOI] [PubMed] [Google Scholar]
- 14.Furlan M, Lämmle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol. 2001;14(2):437–454. doi: 10.1053/beha.2001.0142. [DOI] [PubMed] [Google Scholar]
- 15.Kremer Hovinga JA, Studt JD, Demarmels Biasiutti F, et al. Splenectomy in relapsing and plasma-refractory acquired thrombotic thrombocytopenic purpura. Haematologica. 2004;89(3):320–324. [PubMed] [Google Scholar]
- 16.Kremer Hovinga JA, Vesely SK, Terrell DR, Lämmle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(8):1500–1511. doi: 10.1182/blood-2009-09-243790. [DOI] [PubMed] [Google Scholar]
- 17.Peyvandi F, Lavoretano S, Palla R, et al. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;93(2):232–239. doi: 10.3324/haematol.11739. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 20.Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10(1):136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9(9):1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118(13):3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, Evankovich J, Yan W, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54(3):999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol. 2011;187(5):2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Clin Lab Sci. 2009;46(1):1–24. doi: 10.1080/10408360802485875. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 28.Buchanan JT, Simpson AJ, Aziz RK, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow OA, von Kockritz-Blickwede M, Bright AT, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010;8(5):445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Köckritz-Blickwede M, Goldmann O, Thulin P, et al. Phagocytosis-independent antimicrobial activity of mast cells by extracellular trap formation. Blood. 2008;111(6):3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 32.Yousefi S, Gold JA, Andina N, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 33.Douglas KW, Pollock KG, Young D, Catlow J, Green R. Infection frequently triggers thrombotic microangiopathy in patients with preexisting risk factors: a single-institution experience. J Clin Apher. 2010;25(2):47–53. doi: 10.1002/jca.20226. [DOI] [PubMed] [Google Scholar]
- 34.Park YA, Schultz EF, Hay SN, Brecher ME. Thrombotic thrombocytopenic purpura and urinary tract infections: is there a connection? Am J Clin Pathol. 2011;135(1):85–88. doi: 10.1309/AJCPGM0SC6KOTCAN. [DOI] [PubMed] [Google Scholar]
- 35.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foell D, Frosch M, Sorg C, Roth J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin Chim Acta. 2004;344(1-2):37–51. doi: 10.1016/j.cccn.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 37.Vesely SK, George JN, Lämmle B, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102(1):60–68. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 38.Studt JD, Böhm M, Budde U, Girma JP, Varadi K, Lämmle B. Measurement of von Willebrand factor-cleaving protease (ADAMTS-13) activity in plasma: a multicenter comparison of different assay methods. J Thromb Haemost. 2003;1(9):1882–1887. doi: 10.1046/j.1538-7836.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 39.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 40.Kremer Hovinga JA, Mottini M, Lämmle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost. 2006;4(5):1146–1148. doi: 10.1111/j.1538-7836.2006.01904.x. [DOI] [PubMed] [Google Scholar]
- 41.Zeerleder S, Zwart B, Wuillemin WA, et al. Elevated nucleosome levels in systemic inflammation and sepsis. Crit Care Med. 2003;31(7):1947–1951. doi: 10.1097/01.CCM.0000074719.40109.95. [DOI] [PubMed] [Google Scholar]
- 42.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawasaki H, Iwamuro S. Potential roles of histones in host defense as antimicrobial agents. Infect Disord Drug Targets. 2008;8(3):195–205. doi: 10.2174/1871526510808030195. [DOI] [PubMed] [Google Scholar]
- 45.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104(15):6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier TR, Myers DD, Jr, Wrobleski SK, et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99(2):343–351. doi: 10.1160/TH07-10-0608. [DOI] [PubMed] [Google Scholar]
- 47.Urban CF, Ermert D, Schmid M, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 49.Gupta AK, Joshi MB, Philippova M, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584(14):3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Altincicek B, Stotzel S, Wygrecka M, Preissner KT, Vilcinskas A. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol. 2008;181(4):2705–2712. doi: 10.4049/jimmunol.181.4.2705. [DOI] [PubMed] [Google Scholar]
- 51.Wen F, White GJ, VanEtten HD, Xiong Z, Hawes MC. Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol. 2009;151(2):820–829. doi: 10.1104/pp.109.142067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontana S, Kremer Hovinga JA, Studt JD, Alberio L, Lämmle B, Taleghani BM. Plasma therapy in thrombotic thrombocytopenic purpura: review of the literature and the Bern experience in a subgroup of patients with severe acquired ADAMTS-13 deficiency. Semin Hematol. 2004;41(1):48–59. doi: 10.1053/j.seminhematol.2003.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.