Abstract
Clostridium difficile can infect the large intestine and cause colitis when the normal intestinal microbiota is altered by antibiotic administration. Little is known about the innate immune signaling pathways that marshal inflammatory responses to C. difficile infection and whether protective and pathogenic inflammatory responses can be dissociated. Toll-like receptors predominantly signal via the MyD88 adaptor protein and are important mediators of innate immune signaling in the intestinal mucosa. Here, we demonstrate that MyD88-mediated signals trigger neutrophil and CCR2-dependent Ly6Chi monocyte recruitment to the colonic lamina propria (cLP) during infection, which prevent dissemination of bystander bacteria to deeper tissues. Mortality is markedly increased in MyD88-deficient mice following C. difficile infection, as are parameters of mucosal tissue damage and inflammation. Antibody-mediated depletion of neutrophils markedly increases mortality, while attenuated recruitment of Ly6Chi monocytes in CCR2-deficient mice does not alter the course of C. difficile infection. Expression of CXCL1, a neutrophil-recruiting chemokine, is impaired in the cLP of MyD88−/− mice. Our studies suggest that MyD88-mediated signals promote neutrophil recruitment by inducing expression of CXCL1, thereby providing critical early defense against C. difficile-mediated colitis.
INTRODUCTION
The incidence of Clostridium difficile infection is increasing worldwide (11). C. difficile, a Gram-positive rod, forms spores that are resistant to a variety of commonly used disinfectant agents (18). Orally acquired C. difficile spores can germinate and infect the large intestine following antibiotic-induced changes to the intestinal microbiota (18). Upon germination, C. difficile produces toxins A and B, which mediate the development of colitis (13). C. difficile colitis is the most common cause of diarrhea among hospitalized patients (18).
A single dose of clindamycin, an antibiotic that kills Gram-positive and obligate anaerobic bacteria, is sufficient to render mice susceptible to C. difficile infection and colitis (3). Challenge of antibiotic-treated mice with C. difficile spores results in diarrhea, weight loss, and mortality that peaks 2 to 4 days following inoculation (3, 9). Mice surviving the initial bout of colitis continue to shed C. difficile for many weeks but regain weight and resolve diarrhea beyond the fifth day of infection.
Antibiotics predispose to C. difficile infection by disrupting the composition and/or density of the microbiota. Clindamycin alters the intestine's microbial composition and diversity for up to 30 days (3, 14), and mice remain vulnerable to C. difficile infection for prolonged periods following antibiotic exposure. However, resolution of diarrhea and weight gain occur within 3 to 5 days, suggesting that innate immune defense mechanisms, and not restoration of the microbiota, mediate recovery from early, severe colitis.
Toll-like receptors (TLRs) respond to microbial molecules and drive the expression of antimicrobial proteins (16) and, in the setting of infection, trigger immune responses that lead to pathogen clearance (2, 15). The TLR adaptor molecule myeloid differentiation factor 88 (MyD88) is central to defense against intestinal pathogens (15). In mice infected with Citrobacter rodentium, MyD88 signaling promotes neutrophil recruitment to the colon, and MyD88-deficient mice succumb while wild-type mice recover (15). MyD88 deficiency also increases susceptibility to C. difficile infection (14, 19); however, the mechanism of MyD88-mediated protection in wild-type mice remains undefined. Stimulation of TLR5 by exogenously administered flagellin reduces morbidity and mortality following C. difficile infection, indicating that enhanced activation of TLRs can be protective (9).
Inflammatory cell infiltration of the colonic mucosa is a hallmark of C. difficile infection in mice and humans (4). It has been suggested that massive neutrophil infiltration may exacerbate colitis and delay recovery (10). A recent study, however, demonstrated that the innate immune receptor nucleotide-binding oligomerization domain-1 (NOD-1) contributes to neutrophil recruitment during C. difficile colitis (8) and NOD-1 deficiency increases mortality following C. difficile infection. Thus, neutrophil recruitment to the colonic lamina propria (cLP) may play an important role in the defense against C. difficile colitis (8).
In this study, we investigated the role of MyD88 signaling during C. difficile colitis. We find that MyD88 is critical for defense against early C. difficile infection and that recruitment of inflammatory monocytes and neutrophils to the cLP is markedly attenuated in mice lacking MyD88. Specific depletion of neutrophils with Ly6G-specific monoclonal antibodies increased susceptibility to C. difficile infection, while diminished inflammatory monocyte recruitment in CCR2-deficient mice did not alter the course of infection. These findings reveal an important role for neutrophils during the early stages of murine C. difficile colitis, contributing to a better understanding of the immune factors that play a role in defense against C. difficile colitis.
MATERIALS AND METHODS
Mice and infection.
C57BL/6 female mice were purchased from The Jackson Laboratory. MyD88−/− mice (1) were obtained from Shizuo Akira (Osaka, Japan) and were bred to C57BL/6J mice in our laboratory for more than 10 generations prior to their use in this study. CCR2.GFP mice (22) were generated and maintained in the Memorial Sloan-Kettering Cancer Center vivarium. For C. difficile infections, mice received a single dose of clindamycin (200 μg per dose) by intraperitoneal injection 1 day prior to challenge with 103 CFU of C. difficile spores (strain VPI 10463). CD196 (Fig. 1E) was administered at 104 CFU/mouse. C. difficile spores were obtained as previously described (9) and administered by gavage. To equilibrate the microbiota, MyD88−/− and CCR2−/− mice were cohoused with C57BL/6 mice for 2 to 4 weeks (at a ratio of 1:1) prior to antibiotic administration. All mice were maintained in a specific-pathogen-free facility at the Memorial Sloan-Kettering Cancer Center Animal Resource Center. Age- and gender-matched animals were used for all experiments. Experiments were approved by institutional guidelines.
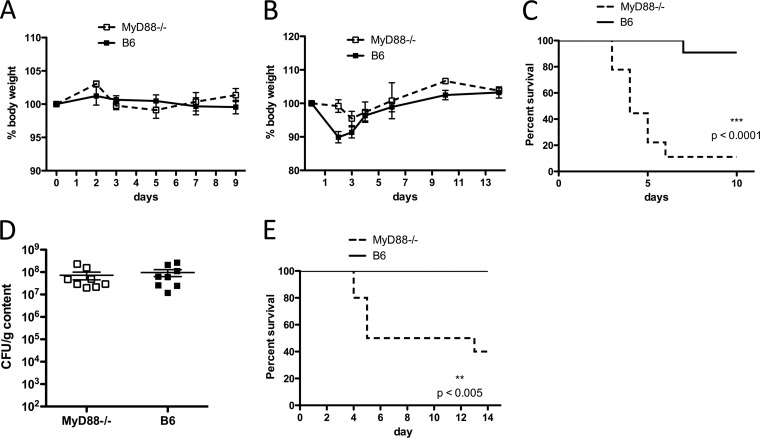
Fig 1.
Increased mortality of MyD88-deficient mice during C. difficile colitis. (A) MyD88−/− and C57BL/6 (B6) mice without prior antibiotic treatment were challenged with a high dose of C. difficile VPI10463 spores (105) and followed for weight loss. Results are representative of two independent experiments (both with n = 4 or 5/group). (B to D) Mice received clindamycin on day −1 and were infected with C. difficile VPI10463 spores on day 0. Mice (n = 10/group) were followed for weight loss (B) and survival (C). The log-rank (Mantel-Cox) test was used for statistical analysis of survival. Results are representative of 2 independent experiments. (D) C. difficile burden in the cecum was assessed 2 days postchallenge in MyD88−/− and wild-type mice. Results are pooled from two independent experiments (B to E). (E) Mice received a single dose of clindamycin and were challenged with 104 spores of C. difficile strain CD196.
Quantitative culture of C. difficile.
For quantitation of C. difficile, cecal contents were suspended in deoxygenated phosphate-buffered saline (PBS), and 10-fold dilutions of the suspension were plated anaerobically on beef heart infusion supplement (BHIS) plates containing taurocholate, d-cycloserine, and cefoxitin for specific selection of C. difficile. Plates were placed in a 37°C incubator within the anaerobic chamber (Coy labs) overnight. Bacterial burden in the mesenteric lymph nodes (MLN) was determined by mashing the lymph nodes between frosted glass slides and plating onto Columbia 5% sheep blood agar (Gibco), followed by anaerobic incubation at 37°C for 48 h.
Histology and immunofluorescence.
Mice were euthanized, the cecum and colon were obtained from naïve or infected mice, and the bulk of the content was gently removed. For histology, tissues were fixed in 4% paraformaldehyde overnight, washed, and dehydrated in ethanol prior to paraffin embedding. Sections were cut and stained for hematoxylin and eosin. The histological score was determined by examination by a single blinded histopathologist. The severity of tissue damage was quantified in each section, taking into account the following parameters: epithelial cell loss, cellular infiltration, and edema. A score of 0 to 3, denoting increasingly severe abnormality, was assigned for each of these parameters (10). For immunofluorescence studies, tissues were fixed for 6 h in fresh 4% paraformaldehyde and incubated in 30% sucrose overnight prior to snap-freezing and sectioning. Slides were blocked with undiluted donkey serum and stained with 15 μg/ml 1A8 antibody, followed by Texas Red-conjugated anti-rat IgG. Images were obtained in an inverted Leica microscope.
Flow cytometry and antibodies.
To characterize infiltration of the cLP by neutrophils and monocytes during C. difficile infection, cLP leukocytes were isolated using a modification of a previously published protocol (26). Briefly, the colon was placed on ice immediately after isolation, opened longitudinally, and cut into 1- to 2-cm sections. The tissue was washed in cold PBS and placed in 30 mM EDTA in Hank's balanced salt solution (HBSS) at 37°C for 30 min with gentle shaking. Tissues were then cut into small fragments with a razor blade and placed in 0.5 mg/ml collagenase IV at 37°C for 30 min with gentle shaking, and cells were recovered and placed in cold Dulbecco's modified Eagle medium containing 10% fetal calf serum. Tissue fragments were incubated a second time in collagenase for 15 min, and cells were pooled with those recovered earlier. Leukocytes were separated by Percoll gradient. For flow cytometry, inflammatory monocytes were defined as CD45+Ly6ChiCD11b+ and neutrophils as CD45+Ly6GhiCD11b+ (see Fig. 3A). For antibody depletion studies, RB6-8C5 and 1A8 were purified from hybridoma cultures in our laboratory. Three doses of antibody (0.3 mg/ml per dose) were administered intraperitoneally every other day beginning on the day prior to C. difficile challenge.
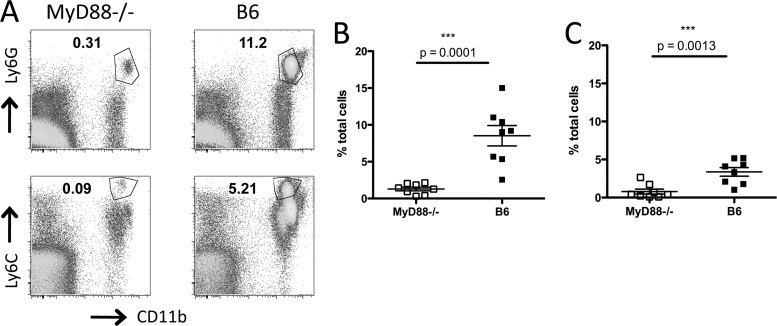
Fig 3.
MyD88 signaling is required for neutrophil and monocyte recruitment to the cLP during C. difficile colitis. MyD88−/− and wild-type C57BL/6 (B6) mice received a single dose of clindamycin on day −1 and were challenged with C. difficile the following day. Two days postinfection, mice were sacrificed, and the percentage of neutrophils (A and B) and monocytes (A and C) in the cLP was assessed by flow cytometry. The gating strategy is shown in panel A, and results are shown as percentages of total cells in the cLP. Results are pooled from two independent experiments. Student's t test (Mann-Whitney) was used for statistical analysis.
Real-time PCR.
To determine expression levels of CXCL1 in colon, tissue was obtained and homogenized in a bead beater. RNA was isolated by TRIzol separation (Invitrogen). Primers for CXCL1 and GAPDH were obtained from Qiagen and used in SYBR green real-time PCR.
RESULTS
MyD88−/− mice rapidly succumb to C. difficile infection.
To determine the susceptibility of MyD88−/− mice in our colony to C. difficile infection, we first cohoused MyD88−/− and C57BL/6 mice for at least 2 weeks to equilibrate the microbiota. To determine whether impaired innate immune signaling renders mice susceptible to C. difficile colitis even without prior antibiotic treatment, we challenged untreated wild-type and MyD88−/− mice with a high dose of C. difficile spores (105). In the absence of prior antibiotic treatment, MyD88−/− mice are resistant to C. difficile colitis (Fig. 1A). Next, we treated wild-type and MyD88−/− mice with clindamycin prior to C. difficile challenge (103 CFU) and found that MyD88 deficiency markedly increases mortality (Fig. 1C). However, the burdens of C. difficile in the ceca of MyD88−/− and wild-type mice were similar (Fig. 1D). Although C. difficile strain VPI 10463 has been widely studied and is commonly used in mouse models of C. difficile colitis (4, 9, 17, 21), we wanted to confirm the importance of MyD88 in defense against the disease by using another toxin-producing strain of C. difficile, CD196 (25). As shown in Fig. 1E, C57BL/6 mice infected with CD196 do not succumb to C. difficile colitis; however, 50% of MyD88−/− mice die of the disease by day 5 postinfection. These results demonstrate that MyD88 signaling makes a major contribution to defense against C. difficile colitis. Interestingly, while MyD88−/− mice have higher mortality following C. difficile challenge, weight loss is less pronounced than in C57BL/6 mice (Fig. 1B).
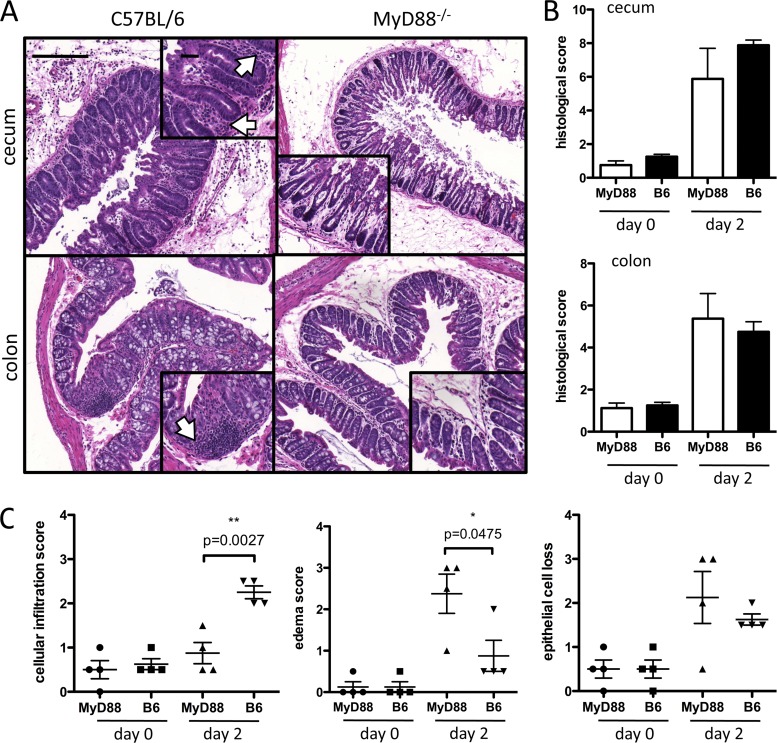
To investigate the possible mechanism by which MyD88 deficiency results in increased mortality following C. difficile infection, we performed histological analyses of the lower intestine (Fig. 2). The histology of the colonic and cecal mucosa of infected mice was evaluated for tissue damage by scoring for edema, inflammatory cell infiltration, and epithelial destruction. Although the aggregate scores for these parameters were similar in wild-type and in MyD88−/− mice infected with C. difficile (Fig. 2A and B), we noticed that the cecum and colon of MyD88−/− mice had fewer inflammatory cells infiltrating the lamina propria, and quantification of this parameter revealed a marked difference in cellular infiltration in the colon of MyD88−/− and wild-type mice (Fig. 2A and C).
Fig 2.
Intestinal damage in MyD88−/− and C57BL/6 (B6) mice during C. difficile colitis. Mice were challenged with C. difficile 1 day following clindamycin administration. Two days postinfection, the cecum and colon were removed, fixed, and stained with hematoxylin and eosin. (A) Representative images of cecum and colon from infected mice. (B) Blind scoring of three parameters of intestinal damage (epithelial cell loss, edema, and cellular infiltration) revealed a similar overall degree of tissue inflammation and destruction in the cecum and colon of MyD88−/− mice. (C) Histological scores for each of the parameters graded in panel B are shown. Scale bars represent 200 μm for large images and 50 μm for insets. Arrows point to areas of cellular infiltration.
MyD88 signaling is required for recruitment of inflammatory cells during C. difficile infection.
Our histologic observations suggested that MyD88 signaling may be involved in recruitment of inflammatory cells to the cLP. To further address this, we treated MyD88−/− and C57BL/6 mice with a 200-μg dose of clindamycin on day −1 and challenged them with 103 CFU of C. difficile VPI 10463 spores the following day. Two days later, we assessed cellular infiltration in the cLP by flow cytometry. As shown in Fig. 3A to C, there is marked infiltration of the cLP by neutrophils and Ly6Chi monocytes in C. difficile-infected mice. In contrast, recruitment of neutrophils and monocytes to the cLP was reduced in MyD88−/− mice. These results indicate that MyD88 signaling is required for monocyte and neutrophil recruitment to the cLP during C. difficile colitis.
Monocyte and neutrophil recruitment to the cLP during C. difficile colitis.
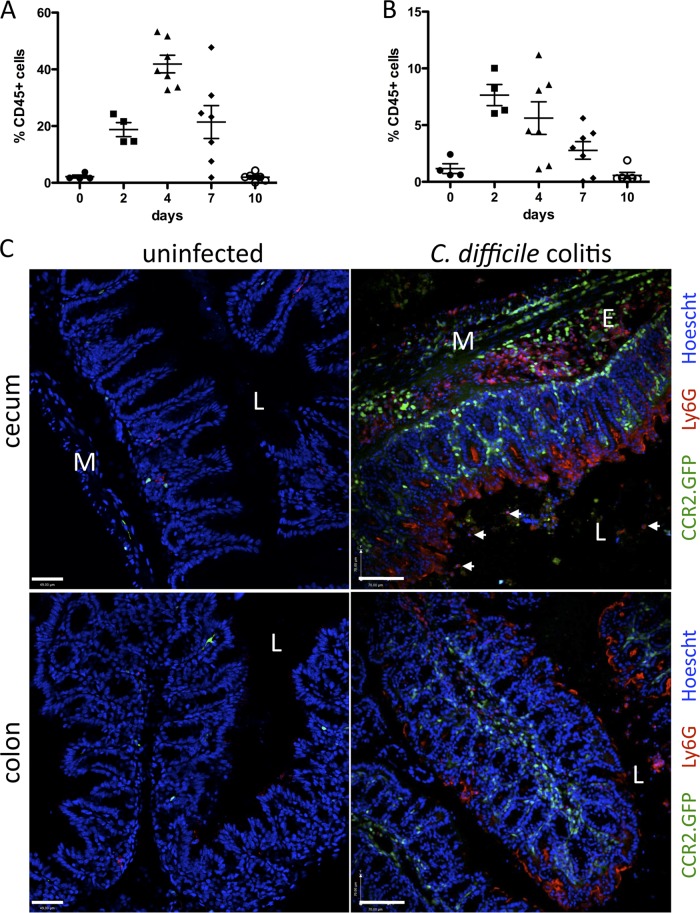
We next investigated the kinetics of neutrophil and monocyte recruitment to the colon during C. difficile colitis. Mice were infected with C. difficile following clindamycin treatment, and cellular populations infiltrating the cLP were characterized by flow cytometry 2, 4, 7, and 10 days later. As shown in Fig. 4A, the neutrophil population markedly increases 4 days following infection, reaching peak frequencies of 40 to 50% of all leukocytes in the cLP. Monocytes reach the highest levels on day 2, constituting up to 10% of leukocytes in the cLP (Fig. 4B). By day 10, monocyte and neutrophil frequencies are restored to preinfection levels (Fig. 4A and B).
Fig 4.
Monocyte and neutrophil infiltration of the cLP in C. difficile-infected mice. C57BL/6 mice were infected with 103 CFU C. difficile following a single dose of clindamycin the day before. Leukocytes were isolated from the cLP and analyzed by flow cytometry to determine neutrophil (A) and monocyte (B) infiltration during the course of infection. Results are expressed as the percentage of CD45+ cells in the cLP and are pooled from two independent experiments. (C) CCR2.GFP mice were challenged with C. difficile following clindamycin administration and sacrificed 3 days later. Frozen sections of cecum and colon were stained with 1A8 antibody (red, anti-Ly6G) to identify neutrophils. Hoechst (blue) was used for nuclear staining. L, lumen; E, edema; M, muscle layer. White arrows point to neutrophils within the luminal content. Scale bars represent 50 μm. Images are representative of 3 independent experiments with 2 to 5 mice each.
To determine the localization of infiltrating monocytes in the cLP during C. difficile infection, we infected CCR2.GFP mice (22). We identified inflammatory monocytes in the cLP by green fluorescent protein (GFP) fluorescence and infiltrating neutrophils by staining with a Ly6G-specific monoclonal antibody. Immunohistologic analyses of the colon and cecum are shown in Fig. 4C. Monocytes and neutrophils can be readily identified in the cLP, and their localization is distinct. While both cell types are found in areas of edema, particularly in the cecum, monocytes are distributed throughout the lamina propria and do not enter the intestinal lumen. Neutrophils, in contrast, are closely associated with the intestinal epithelium, which, as shown in Fig. 2, is discontinuous secondary to toxin-mediated damage.
Gr-1+ cells are critical for protection against C. difficile colitis.
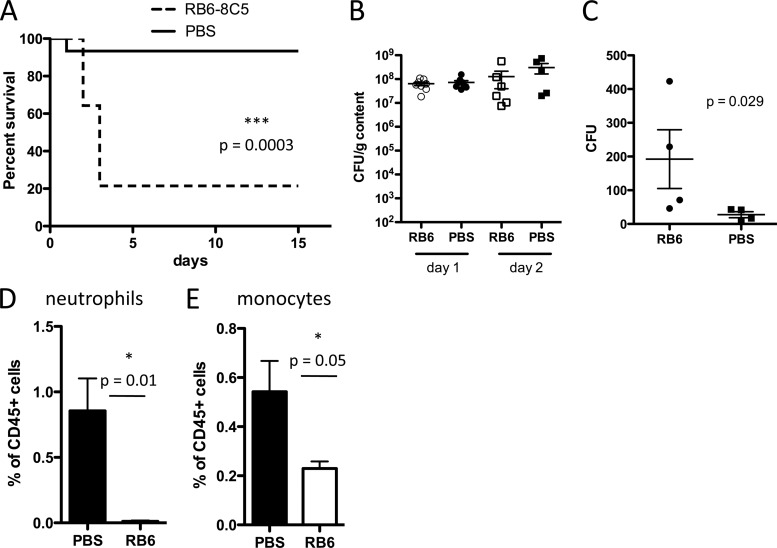
Given the apparent role of MyD88 signaling for inflammatory cell recruitment to the cLP during C. difficile infection, we hypothesized that depletion of inflammatory cells would recapitulate the effects of MyD88 deficiency. We depleted Gr-1+ cells by administering RB6-8C5 antibody every other day, beginning on the day prior to C. difficile challenge. RB6-8C5-treated mice had markedly higher mortality following C. difficile infection, with approximately 80% mortality within 3 days of infection (Fig. 5A). Increased mortality, however, was not associated with increased growth or expansion of C. difficile in the large intestine on days 1 or 2 postinfection (Fig. 5B). To determine if RB6-8C5 depletion renders mice susceptible to systemic dissemination of commensal bacteria from the gut, we quantified viable bacteria in mesenteric lymph nodes 2 days following infection. RB6-8C5 treatment resulted in higher bacterial counts (Fig. 5C), suggesting that Gr1+ cells play a role in containing luminal bacteria and preventing systemic bacterial dissemination during C. difficile colitis.
Fig 5.
Depletion of Gr1+ cells increases mortality during C. difficile colitis. Mice received a single dose of clindamycin on day −1, followed by infection with C. difficile VPI10463 the next day. Mice received three doses of RB6-8C5 antibody (anti-Gr1, 0.3 mg/dose) or PBS every other day starting on day −1. (A) Mice (n = 10/group) were followed for survival. The log-rank (Mantel-Cox) statistical test was used. (B) The burden of C. difficile in the cecum of animals treated with RB6-8C5 or PBS was quantified on days 1 and 2. In panels A and B, results are pooled from at least two independent experiments. (C) Mice received two doses of RB6-8C5 or PBS on days −1 and 1. On day 2 postinfection, bacterial counts in the mesenteric lymph nodes were determined. (D and E) Mice were infected with C. difficile 1 day following clindamycin and either RB6-8C5 or PBS administration. On day 1 postinfection, the percentages of neutrophils and monocytes were assessed in the cLP of mice treated with RB6-8C5 or PBS and found to be significantly reduced.
Neutrophil recruitment to the cLP is required for defense against C. difficile.
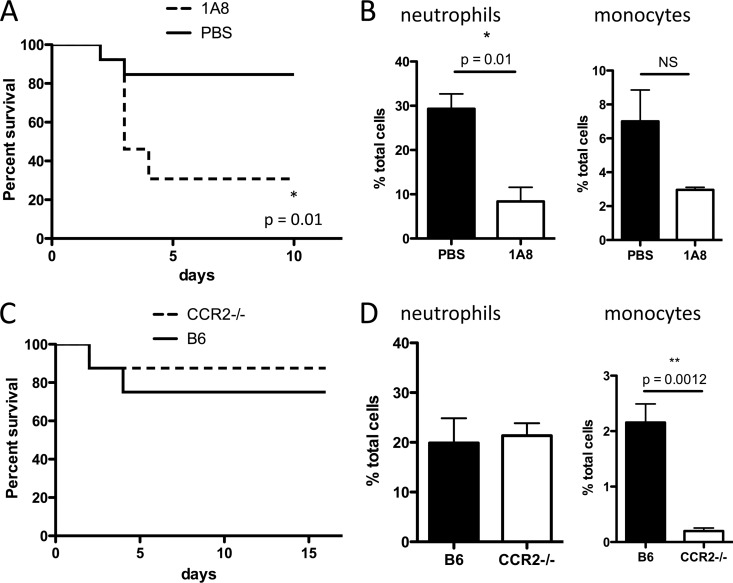
Because Gr-1+ cells include both Ly6G+ neutrophils and Ly6C+ monocytes (7) and both are depleted by administration of RB6-8C5 antibody (Fig. 5D and E), we aimed to determine which of these cell populations promotes survival following C. difficile infection. In a strategy similar to that used for depletion of Gr-1+ cells, we used 1A8 (anti-Ly6G) antibody to deplete neutrophils and thus prevent their recruitment to the cLP. Administration of 1A8 antibody resulted in approximately 3-fold reduction in neutrophils (Fig. 6B), which was sufficient to markedly increase mortality during C. difficile colitis (Fig. 6A). However, this finding does not exclude a potentially important role for monocytes in defense. The role of CCR2 in monocyte recruitment from the bone marrow is well established (23). We corroborated that CCR2−/− mice have normal neutrophil but impaired monocyte recruitment to the cLP during C. difficile infection (Fig. 6D). We treated these mice with clindamycin and challenged them with C. difficile spores. As shown in Fig. 6C, impaired monocyte recruitment to the cLP does not render CCR2−/− mice more susceptible to severe C. difficile colitis. These results demonstrate that neutrophils are key players in defense against C. difficile, such that a 60% reduction in neutrophil recruitment renders mice highly susceptible to the disease. However, attenuation of monocyte recruitment did not have an effect on survival during C. difficile colitis.
Fig 6.
Neutrophil deficiency increases mortality during C. difficile colitis. (A and B) Mice received clindamycin on day −1 and were challenged with C. difficile spores the following day. Three doses of 1A8 antibody (anti-Ly6G, 0.3 mg/dose) or PBS were administered every other day starting on day −1. (A) Mice (n = 8/group) were followed for survival. (B and D) Mice were sacrificed on day 2 postinfection, and neutrophil and monocyte infiltration was assessed by flow cytometry. CCR2−/− mice are not more susceptible to C. difficile colitis than their wild-type C57BL/6 counterparts (B6) (C). Results are representative of two independent experiments. Log-rank and Student's t test were used for statistical analysis.
MyD88-mediated signals promote neutrophil recruitment to the colon.
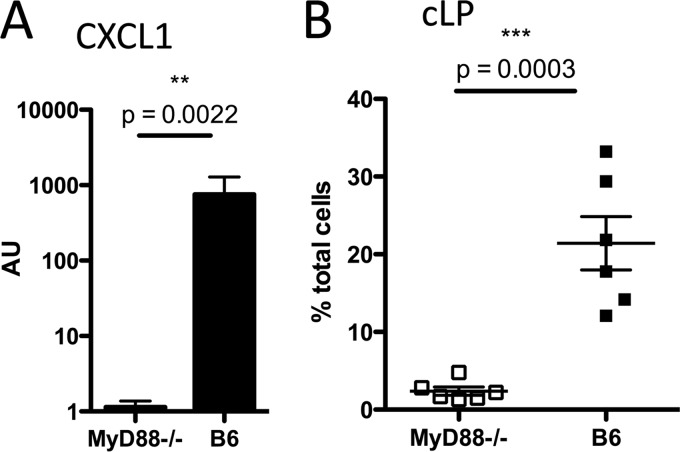
Our findings suggest that a mechanism contributing to the susceptibility of MyD88−/− mice to C. difficile colitis may involve impaired neutrophil recruitment. We next wanted to determine whether MyD88-mediated signals are required for the expression of CXCL1, a potent neutrophil-recruiting chemokine, in the colon. As shown in Fig. 7A, there is an approximately 1,000-fold reduction in CXCL1 expression in the colon of MyD88−/− mice during C. difficile infection. We corroborated that in these mice there was impaired neutrophil recruitment, and similar to the results shown in Fig. 3, there was a striking reduction in neutrophil infiltration into the cLP (Fig. 7B). Our studies suggest that MyD88-dependent expression of CXCL1 in the colon is required for neutrophil recruitment to the cLP during C. difficile infection.
Fig 7.
MyD88 deficiency results in impaired neutrophil recruitment from the bone marrow during C. difficile infection. (A) Mice were sacrificed 2 days postinfection, and the level of expression of the neutrophil-recruiting chemokine CXCL1 (KC) was assessed by quantitative PCR of colonic tissue. (B) The percentage of neutrophils in cLP was assessed by flow cytometry in the same mice as for panel A. Results are pooled from two independent experiments. AU, arbitrary units; B6, C57BL/6 mice.
DISCUSSION
In this study, we investigated the role of MyD88-mediated signaling in C. difficile colitis. We find that mortality following C. difficile infection is markedly increased in antibiotic-treated MyD88−/− mice. While our demonstration of increased susceptibility in MyD88−/− mice is consistent with two previous studies (14, 19), we extend previous work by demonstrating that MyD88-mediated signals are essential for recruitment of neutrophils and monocytes to the cLP during C. difficile infection. We have used specific depletion strategies to demonstrate that neutrophils provide a barrier in the aftermath of intestinal epithelial destruction during C. difficile infection, thereby preventing systemic dissemination of intestinal bacteria.
MyD88-mediated signaling is essential for intestinal homeostasis and defense against a range of infections. Our experiments show that induction of the chemokine CXCL1 (KC) in the colon during C. difficile infection is dependent on MyD88 signaling. CXCR2 is a chemokine receptor that binds CXCL1 and is expressed by neutrophils. Upon stimulation by CXCL1, CXCR2-mediated signals counter constitutive retention signals in the bone marrow that are mediated by CXCR4, thereby enabling neutrophils to traffic out of the bone marrow and to circulate in the bloodstream. CXCR2 is also believed to enable neutrophils to respond to CXCL1 in peripheral tissues, enabling neutrophil infiltration into sites of infection (20). Interestingly, the innate immune receptor NOD-1 also plays a role in CXCL1 expression, as demonstrated by low serum levels of the chemokine in NOD-1-deficient mice that were infected with C. difficile intraperitoneally (8).
Using CCR2−/− mice, we demonstrated that a 90% reduction in monocyte recruitment to the cLP does not affect mortality during C. difficile colitis. Monocytes mediate clearance of many infectious pathogens, including intestinal pathogens such as C. rodentium (12) and Toxoplasma gondii (6). During C. rodentium infection, monocytes promote Th1 differentiation of responding T cells and enhance T cell-dependent IgG production, which contribute to recovery from infection (12). However, the role of monocytes in protection against these intestinal infections appears to be crucial at later stages of infection. Neutrophil-depleted mice infected with C. difficile succumb to colitis 2 to 4 days following infection, and it is possible, therefore, that monocytes are essential for defense against later stages of C. difficile infection. While impaired monocyte recruitment in CCR2−/− mice does not result in higher susceptibility to C. difficile colitis up to 2 weeks postinoculation, it remains possible that CCR2-independent trafficking of Ly6Chi monocytes may contribute to immune defense against C. difficile infection.
Neutrophils provide early innate immune defense in a broad range of infections. In the gut, neutrophils are required for protection against the murine intestinal pathogen C. rodentium (15). In C. difficile infection, a role for neutrophils in protection has been suggested by RB6-8C5 depletion (8). However, the Gr-1 epitope targeted by RB6-8C5 is also highly expressed on monocytes, which are also depleted with RB6-8C5 treatment (5). Our experiments using the Ly6G-specific 1A8 antibody therefore demonstrate more specifically that neutrophils are critical for defense against C. difficile infection. Neutrophils, however, do not appear to mediate defense by decreasing the density of intestinal infection with C. difficile or reducing the concentration of toxin in the intestinal lumen. The burden of C. difficile in the cecum is not increased in neutrophil-depleted mice, and C. difficile is not disseminating systemically (our unpublished observations). However, dissemination of commensal intestinal bacteria to mesenteric lymph nodes is increased in C. difficile-infected mice that have undergone neutrophil depletion, suggesting a role for neutrophils in preventing systemic dissemination of intestinal bacteria, consistent with the findings of Hasegawa et al. (8).
We demonstrate that neutrophils aggregate near the intestinal lumen in areas where C. difficile infection has damaged the intestinal epithelial barrier. Neutrophils appear to create a barrier to prevent bacterial dissemination into underlying tissues and the bloodstream. Our immunohistologic studies demonstrate that neutrophils invade the cecal and colonic lumen while Ly6Chi monocytes remain within the cLP. Thus, while MyD88-mediated signals are required for the recruitment of neutrophils and monocytes to the cLP, once they enter the cLP these two cell populations presumably respond to different cues for further localization. This may result from distinct chemokine receptor expression by neutrophils and monocytes, or it may result from distinct responsiveness to adhesion molecules. For example, inflammatory monocytes recruited to hepatic foci of Listeria monocytogenes infection localize in a peripheral ring upon binding ICAM1, while neutrophils directly invade the site of bacterial infection (24). It is possible, therefore, that Ly6Chi monocytes bind adhesion molecules and become lodged in the lamina propria while neutrophils traffic toward bacterial populations in the intestinal lumen. Further studies will be required to identify the signals that explain the distinct trafficking of neutrophils and inflammatory monocytes during C. difficile infection. Defining the relative contributions of neutrophils and monocytes in defense against C. difficile infection and in pathogenesis has important potential clinical implications, particularly in immunocompromised patient populations that are at particularly high risk of infection.
ACKNOWLEDGMENTS
This work was supported by grants R01AI042135 and R37AI039031 to E.G.P. and a Career Development Award from the Northeast Biodefense Center to I.J.
We declare no conflict of interest.
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Adachi O, et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 2. Brandl K, Plitas G, Schnabl B, Dematteo RP, Pamer EG. 2007. MyD88-mediated signals induce the bactericidal lectin RegIII{gamma} and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 204:1891–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buffie CG, et al. 2012. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect. Immun. 80:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen X, et al. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 [DOI] [PubMed] [Google Scholar]
- 5. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70 [DOI] [PubMed] [Google Scholar]
- 6. Dunay IR, et al. 2008. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29:306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleming TJ, O'Huigin C, Malek TR. 1993. Characterization of two novel Ly-6 genes. Protein sequence and potential structural similarity to alpha-bungarotoxin and other neurotoxins. J. Immunol. 150:5379–5390 [PubMed] [Google Scholar]
- 8. Hasegawa M, et al. 2011. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J. Immunol. 186:4872–4880 [DOI] [PubMed] [Google Scholar]
- 9. Jarchum I, Liu M, Lipuma L, Pamer EG. 2011. Toll-like receptor-5 stimulation protects mice from acute Clostridium difficile colitis. Infect. Immun. 79:1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly CP, et al. 1994. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J. Clin. Invest. 93:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly CP, LaMont JT. 2008. Clostridium difficile—more difficult than ever. N. Engl. J. Med. 359:1932–1940 [DOI] [PubMed] [Google Scholar]
- 12. Kim YG, et al. 2011. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34:769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuehne SA, et al. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713 [DOI] [PubMed] [Google Scholar]
- 14. Lawley TD, et al. 2009. Antibiotic treatment of Clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect. Immun. 77:3661–3669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. 2007. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179:566–577 [DOI] [PubMed] [Google Scholar]
- 16. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118:229–241 [DOI] [PubMed] [Google Scholar]
- 17. Reeves AE, et al. 2011. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes 2:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7:526–536 [DOI] [PubMed] [Google Scholar]
- 19. Ryan A, et al. 2011. A role for TLR4 in Clostridium difficile infection and the recognition of surface layer proteins. PLoS Pathog. 7:e1002076 doi:10.1371/journal.ppat.1002076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sadik CD, Kim ND, Luster AD. 2011. Neutrophils cascading their way to inflammation. Trends Immunol. 32:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Savidge TC, et al. 2011. Host S-nitrosylation inhibits clostridial small molecule-activated glucosylating toxins. Nat. Med. 17:1136–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Serbina NV, Hohl TM, Cherny M, Pamer EG. 2009. Selective expansion of the monocytic lineage directed by bacterial infection. J. Immunol. 183:1900–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serbina NV, Pamer EG. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7:311–317 [DOI] [PubMed] [Google Scholar]
- 24. Shi C, et al. 2010. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J. Immunol. 184:6266–6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stabler RA, et al. 2010. In-depth genetic analysis of Clostridium difficile PCR-ribotype 027 strains reveals high genome fluidity including point mutations and inversions. Gut Microbes 1:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weigmann B, et al. 2007. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2:2307–2311 [DOI] [PubMed] [Google Scholar]