Abstract
The apicomplexan parasite Toxoplasma gondii can cause severe disease in immunocompromised individuals. Previous studies in mice have focused largely on CD8+ T cells, and the role of CD4 T cells is relatively unexplored. Here, we show that immunization of the C57BL/6 strain of mice, in which the immunodominant CD8 T cell response to the parasite dense-granule protein GRA6 cannot be generated, leads to a prominent CD4 T cell response. To identify the CD4 T cell-stimulating antigens, we generated a T. gondii-specific, lacZ-inducible, CD4 T cell hybridoma and used it as a probe to screen a T. gondii cDNA library. We isolated a cDNA encoding a protein of unknown function that we call CD4Ag28m and identified the minimal peptide, AS15, which was presented by major histocompatibility complex (MHC) class II molecules to the CD4 T cells. Immunization of mice with the AS15 peptide provided significant protection against subsequent parasite challenge, resulting in a lower parasite burden in the brain. Our findings identify the first CD4 T cell-stimulating peptide that can confer protection against toxoplasmosis and provide an important tool for the study of CD4 T cell responses and the design of effective vaccines against the parasite.
INTRODUCTION
The intracellular protozoan parasite Toxoplasma gondii infects a wide range of warm-blooded hosts, including humans, leading to disease in immunocompromised individuals and congenital defects in developing fetuses. In immunocompetent hosts, a robust T cell response controls parasite growth via the protective cytokine gamma interferon (IFN-γ) (7, 14, 19, 44, 55, 58), although parasites can persist within cysts in the brain and muscle for the lifetime of the infected host (5, 9, 15, 29). The crucial role of T cells in controlling T. gondii infection is highlighted by the susceptibility of patients with T cell deficiencies to toxoplasmosis (23, 36).
An important approach to understanding the T cell response to infection is to define the peptide-major histocompatibility complex (MHC) ligands recognized by the T cell receptor (TCR) at a molecular level. This approach has been used to examine CD8 T cell responses to T. gondii, leading to the identification of a number of endogenous CD8 antigens (4, 7, 16, 40, 62). Most strikingly, mice possessing the MHC-I molecule Ld mount an immunodominant response to the HF10 decapeptide derived from the T. gondii protein GRA6 (4). This observation helps to explain the genetic resistance of BALB/c (H-2d) mice compared to the susceptible C57BL/6 (H-2b) strain (3, 6, 13, 56, 57, 61). However, the T cell response in H-2b mice to the parasite remains poorly understood.
While CD8 T cells play an important role in resistance to T. gondii, CD4 T cells also provide protection, especially when CD8 T cells are absent (2, 17, 37, 59). Moreover, CD4 T cells play an important immunoregulatory role during infection by producing the immunosuppressive cytokine interleukin-10 (IL-10) and can also contribute to immune-mediated pathology (24, 33, 43, 54). To date, our understanding of CD4 T cell responses during T. gondii infection has been based on analysis of polyclonal CD4 T cell populations of poorly defined specificity. Some CD4 Th1 clones reactive to T. gondii, and the parasite proteins that they respond to, have been described, although the identity of peptide epitopes and the relative magnitude of responding T cells are not known (47–49). In one recent study, CD4 T cells were raised using a lysed parasite extract and were found to respond predominantly to the parasite protein profilin. However, the contribution of this response during infection was not characterized and the antigenic peptide was not defined (65). Most importantly, whether the parasite-specific CD4 T cells identified so far can protect animals from toxoplasmosis remains unresolved. In most of these studies, protection was not examined (47–49, 65), and in one case, the relevant Th1 cells failed to protect (48). Thus, the question of how CD4 T cells detect and respond to Toxoplasma gondii remains largely unknown.
To address these issues, we have begun to characterize the CD4 T cell response in C57BL/6 (H-2b) mice. Surprisingly, we find that CD4 T cells, rather than CD8 cells, are the predominant IFN-γ-producing population observed in splenocytes isolated ex vivo from mice immunized with T. gondii. We isolated T. gondii-specific CD4 T cells from immunized mice, generated a T cell hybridoma, and showed that it recognizes a peptide derived from a previously unknown parasite protein, which we have named CD4Ag28m, presented by the Ab MHC class II molecule. Finally, we defined a 15-mer peptide from CD4Ag28m, AS15, and showed that immunization with the peptide prior to infection leads to lower parasite burden in the brain. The identification of a defined protective CD4 T cell response to T. gondii provides an important tool for further studies of T cell responses to the parasite and should facilitate the design of more effective vaccines.
MATERIALS AND METHODS
Mice and parasites.
C57BL/6J (B6) and the MHC class II-deficient B6.129S-H2dlAb1-Ea mice were obtained from the Jackson Laboratory. For all immunization and infection experiments, sex- and age-matched mice were used. Mice were used with the approval of the Animal Care and Use Committee of the University of California. The parental Prugniaud strain of T. gondii (PruΔhpt; hypoxanthine-xanthine-guanine phosphoribosyltransferase deficient) was a gift from J. Boothroyd (Stanford University). Tachyzoites (Tz) were maintained by passage in confluent monolayers of human foreskin fibroblasts grown in Dulbecco's modified Eagle medium (DMEM; Invitrogen) containing 10% fetal calf serum (FCS; HyClone) and 1% penicillin-streptomycin glutamine (Invitrogen).
In vivo infection and immunization.
Mice were immunized intraperitoneally (i.p.) with 1 × 106 to 5 × 106 tachyzoites that were irradiated (14,000 rads) and resuspended in 100 μl phosphate-buffered saline (PBS). For inducing protection, bone marrow-derived dendritic cells (BMDCs) were activated with lipopolysaccharide (LPS) for 24 h (100 ng/ml; Sigma), incubated for 90 min with 10 μM synthetic peptide, washed twice with PBS, and used for footpad immunization. Mice were immunized with 5 × 106 peptide-loaded BMDCs for 7 days and then infected with live tachyzoites (1 × 104) intraperitoneally.
Ex vivo analysis.
Mice were euthanized 4 to 6 weeks postinfection. Spleens and brains were collected and immediately processed or stored at −80°C for DNA extraction and further analysis. Spleens were dissociated into single-cell suspensions in complete RPMI medium (Invitrogen) supplemented with 10% FCS (HyClone). Erythrocytes were removed from the suspension using ammonium chloride potassium chloride lysis buffer (10 μM EDTA, 160 mM NH4Cl, and 10 mM NaHCO3). Brains were homogenized and digested for 1 h at 37°C with collagenase type IA (1 mg/ml; Sigma) and DNase I (100 μg/ml; Roche) in complete RPMI medium. Brains were further dissociated and filtered through 70-μm cell strainers and centrifuged for 20 min at 1,000 × g. Cells were resuspended in 60% (vol/vol) Percoll (GE Healthcare) and were overlaid on 30% (vol/vol) Percoll followed by centrifugation at 1,000 × g for 2 min. Infiltrating mononuclear cells were collected from the gradient interface, and red blood cells were removed via lysis with ammonium chloride potassium chloride lysis buffer. Cells were washed twice in complete RPMI medium before analysis. The proportion of T. gondii-specific or antigen-specific cells was monitored by intracellular cytokine staining (ICCS) for IFN-γ on CD4+ or CD8α+ cells. Antigen-presenting cells (APCs) were either infected the day before (as described below) or pulsed with antigenic peptide on the same day and were used for the ex vivo IFN-γ assay. Antigen-specific CD4+ cells were also detected via staining using MHC class II tetramers loaded with T. gondii antigenic peptide (as described below).
Parasite load analysis.
Genomic DNA was extracted from brain and spleen using the Wizard genomic DNA purification kit (Promega). Parasite burden in the spleen and brain was assessed by semiquantitative PCR as described previously (30). The number of cysts in the brain was determined by labeling a portion of the brain with fluorescein-conjugated Dolichos biflorus agglutinin (FL-1031; Vector Laboratories). This lectin stains the cyst wall, and the cysts were counted using an inverted fluorescence microscope.
Generation of T. gondii-specific T cell hybridomas.
C57BL/6 mice were immunized with 1 × 106 irradiated Pru tachyzoites (14,000 rads) intraperitoneally. Mice were euthanized 7 days postinfection, and spleens were harvested. Spleens were dissociated into single-cell suspensions in complete RPMI medium as described above. T. gondii-specific populations were expanded in vitro by weekly restimulations with irradiated syngeneic splenocytes (2,000 rads) that were infected with irradiated Pru tachyzoites the day before. The proportion of T. gondii-specific T cells was measured weekly by intracellular cytokine staining (ICCS) for IFN-γ. APCs were infected the day before (as described below). After 2 weeks of restimulation, responding T cells were fused to the TCRαβ-negative lacZ-inducible BWZ.36.CD8α fusion partner as described before (28, 38). The antigen specificity and MHC restriction of the hybridomas were assessed by overnight incubation with infected or uninfected splenocytes from wild-type (wt) C57BL/6 mice or MHC class II-deficient, B6.129S-H2dlAb1-Ea mice. The hybridoma response was quantitated by TCR-mediated induction of β-galactosidase upon the addition of chromogenic substrate CPRG (chlorophenol red-β-d-galactopyranoside; Roche) (52). The absorbance of the cleaved purple product was measured at 595 nm with a reference at 695 nm. The hybridomas were further subcloned to obtain monoclonal population of T cell hybridomas with a single TCR specificity.
Construction of cDNA library and recombinant constructs.
Poly(A)+ mRNA was isolated from about 10 × 108 Pru tachyzoites (which had been passed through a 3-μm filter) using the Oligotex Direct mRNA Midi/Maxi kit (Qiagen). This mRNA was used to generate a cDNA library using the Superscript cDNA synthesis kit and oligo(dT) primers (Invitrogen). The cDNA fragments were inserted unidirectionally into the prokaryotic expression vector pTRCHis between SalI and NotI and used to transform TOP10 electrocompetent bacteria (Invitrogen) to yield ∼109 CFU, with the range of the insert size being between 0.5 and 3 kbp. Various C-terminal and N-terminal deletion constructs of the antigenic cDNA (28.m00307) were generated via PCR using a high-fidelity Pfu Turbo polymerase system (Stratagene). The 5′ forward and 3′ reverse PCR primers that were used are listed in Table 1. All forward primers included the SalI site, and the reverse primers included NotI restriction sites. The PCR products were purified by agarose gel electrophoresis, digested with restriction enzymes SalI and NotI, subcloned into pTRCHis vector, and sequenced directly with gene-specific oligonucleotides.
Table 1.
Oligonucleotide primers
| Construct | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| DN-B | ACGGTAGTCGACGACGTTATGGTTGAGGCTTGGATG | AATCTGTATCAGGCTGAAAATC |
| ΔN-D | ACGGTAGTCGACGACGATGGTAGCTTTGCCGTCATC | AATCTGTATCAGGCTGAAAATC |
| ΔC-E | GAGGTATATATTAATGTATCG | CTTTTCCTTTTGCGGCCGCGAGCTGTTCCTTCAGGAC |
| ΔC-G | GAGGTATATATTAATGTATCG | CTTTTCCTTTTGCGGCCGCCTCCGTCTTCATGATTTG |
| ΔNΔC-J | ACGGTAGTCGACGACGATGGTAGCTTTGCCGTCATC | CTTTTCCTTTTGCGGCCGCGGCACGGTGTTCCCCCGC |
| ΔNΔC-K | TCGACGACCGAATGGCAGTCGAAATCCATCGTCCCGTCCCTGGGACAGCTCCTCCCTCGTTCTCCAGTGAGGATGTTGC | GGCCGCAACATCCTCACTGGAGAACGAGGGAGGAGCTGTCCCAGGGACGGGACGATGGATTTCGACTGCCATTCGGTCG |
Expression cloning.
Bacterial transformants were grown and induced to express the cDNA-encoded proteins according to the manufacturer's protocol (pTRCHis expression systems; Invitrogen). Briefly, transformed recombinant bacteria (10 CFU/well) were plated in 96-well U-bottom plates in a total volume of 180 μl and allowed to grow overnight at 37°C. The next day, the transformant density was determined by optical density at 600 nm (OD600) (an OD600 of 1.0 = 5 × 108 cells/ml), and approximately 2 × 107 to 3 × 107 transformants were plated into new 96-well U-bottom plates in a total volume of 180 μl. The bacteria were grown for 2 h at 37°C to attain log-phase growth, after which they were induced with the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and allowed further growth for 4 h at 37°C. The number of transformants was again estimated by OD600, and 1 × 106 to 2 × 106 cells/well were transferred into new 96-well plates. The original master plates of bacteria were stored at 4°C. BMDCs (5 × 104) from C57BL/6 mice resuspended in serum- and antibiotic-free medium were added to the plates containing the bacteria and incubated at 37°C for 1 h to allow phagocytosis. The plates were centrifuged for 2 min at 800 × g, and the supernatant was removed. BTg01Z hybridomas (1 × 105) were resuspended in complete medium containing 50 μg/ml gentamicin (to eliminate residual bacteria), added to the plates, and incubated overnight. T cell activation was measured as the lacZ response described above.
In vitro differentiation and infection.
For all experiments, we used bone marrow-derived dendritic cells (BMDCs) as the antigen-presenting cells (APCs). Bone marrow cells were obtained from mouse femurs and tibias. Bone marrow cells were plated in complete medium containing granulocyte-macrophage colony-stimulating factor (GM-CSF) (10 ng/ml; Peprotech) for 6 days to allow for differentiation into DCs. After 6 days, BMDCs were harvested and washed once to remove any GM-CSF. The cells were then infected overnight with irradiated Pru tachyzoites (14,000 rads) at a various multiplicities of infection. The next day, cells were washed twice to remove any residual extracellular parasites and were used in the assays described above.
Flow cytometry.
Antibodies to mouse CD4 (RM4-5), anti-mouse CD8α (53-6.7), and anti-mouse IFN-γ (XMG1.2) were obtained from BD Biosciences. Surface staining with anti-mouse CD4 and CD8α antibodies was performed at 4°C for 30 min in flow buffer containing 3% (vol/vol) FCS and 1 mM EDTA in PBS. Intracellular cytokine staining for IFN-γ was performed using the Cytofix/Cytoperm kit (BD Pharmingen). Fluorescently labeled MHC class II tetramers bound to T. gondii antigenic peptide were obtained from the NIH tetramer facility. The concentration and time of staining of the tetramer were optimized, and cells were incubated with the tetramer at 37°C for 30 min. The cells were then washed and followed by surface staining at 4°C for 30 min with anti-mouse CD4 antibody. All flow cytometry data were acquired on an XL Analyzer or FC 500 (Coulter) and analyzed using FlowJo software (TreeStar).
Statistical analysis.
Prism software (GraphPad) was used for statistical analysis. P values were calculated using a two-tailed Mann-Whitney (nonparametric) test.
RESULTS
T. gondii-immunized C57BL/6 mice elicit a potent CD4 but weak CD8 T cell response.
To characterize the T cell response in the C57BL/6 (B6, H-2b) mice, we immunized the animals with irradiated tachyzoites from the type II T. gondii strain Prugniaud (Pru). Irradiated parasites can invade host cells, but they do not replicate, and intraperitoneal (i.p.) injection of irradiated parasites can induce robust T cell-mediated immunity that provides protection against challenge with live parasites (10, 17, 59). One week postimmunization, splenocytes harvested from immunized B6 mice were examined for T. gondii-specific CD4 and CD8 T cell responses ex vivo by measuring intracellular cytokine staining (ICCS) for IFN-γ. In contrast to the H-2d strain of mice, in which the CD8 T cell response predominates over the CD4 T cell response (4), B6 mice generated a robust CD4 T cell response but a far weaker CD8 T cell response toward T. gondii (Fig. 1A to C). This strong CD4 T cell response was observed ex vivo, as well as in cultures after in vitro restimulations with infected syngeneic APCs (Fig. 1A). Furthermore, during the in vitro restimulations, IFN-γ-producing CD4 T cells proliferated more vigorously than did their CD8 counterparts and thus accumulated to larger numbers (Fig. 1B versus Fig. 1C).
Fig 1.
T. gondii immunization of C57BL/6 mice elicits a potent CD4 but weak CD8 T cell response. C57BL/6 mice were immunized with irradiated T. gondii tachyzoites. Splenocytes were harvested from mice 1 week postimmunization, and T. gondii-specific CD4 and CD8 T cell responses were measured by intracellular cytokine staining for IFN-γ. Antigen-presenting cells with or without T. gondii were used as stimulators for ex vivo and in vitro restimulations. Ex vivo and in vitro restimulation results are shown as representative flow cytometry plots (A) and plots depicting the expansion of CD4 T cells (B) and CD8 T cells (C) over the course of two in vitro restimulations. Data are representative of three experiments.
Generation of T. gondii-specific CD4 T cell hybridomas.
To further characterize the CD4 T cell response at a clonal level, we generated lacZ-inducible T cell hybridomas from the responding CD4 T cells, as described previously (27, 52). After fusion, we successfully generated 14 T. gondii-specific CD4 T cell hybridomas and confirmed their specificity for T. gondii using uninfected versus infected B6 APCs. For example, the hybridoma BTg01Z.A produced lacZ specifically in response to T. gondii-infected wild-type cells but not in response to those lacking I-Ab (Fig. 2A and B). The T cell hybridoma did not recognize T. gondii-derived recombinant profilin (data not shown), earlier shown to be recognized by some CD4 T cells in B6 mice (65).
Fig 2.
lacZ response of subcloned T. gondii-specific CD4 hybridoma (BTg01Z.A). Wild-type APCs with or without T. gondii (A) or APCs lacking I-Ab MHC molecules with or without T. gondii (B) were tested for their ability to stimulate the BTg01Z.A hybridoma after an overnight culture. Data are representative of at least two independent experiments.
Identification of the cognate antigen recognized by BTg01Z.A hybridoma by expression cloning.
We have previously shown that recombinant Escherichia coli expressing a variety of proteins can serve as an exogenous antigen source for MHC class II presentation pathway in bone marrow-derived macrophages or DCs (BMDCs) and that detection of these antigens with the appropriate T cell hybridoma can be used to identify the relevant antigen (8, 50, 51). We used this approach to screen a T. gondii cDNA library (see Materials and Methods for details). We identified a positive pool, pTg778; further fractionated this pool into individual colonies; and rescreened the colonies for their ability to stimulate the BTg01Z.A hybrid. Six of 10 colonies tested scored positive (Fig. 3A). We further established that the T cell response to the recombinant E. coli was I-Ab restricted because BMDCs lacking I-Ab were unable to stimulate the BTg01Z.A hybrid (Fig. 3B). The E. coli colony pTg778.76 was selected for further characterization (Fig. 3C).
Fig 3.
BTg01Z.A response to the fractionated single clones is antigen specific and MHC restricted. (A) A positive pool, pTg778, from the initial screen was further fractionated until a single stimulatory clone was identified and sequenced. The BTg01Z.A response to individual bacterial colonies isolated from pool 778 was tested (10 representative clones are shown). Clone 778.76 was selected for further analysis. (B and C) BTg01Z.A response was antigen specific and MHC restricted.
We determined the nucleotide sequence of pTg778.76. BLAST analysis with the T. gondii database (http://www.toxodb.org) yielded a perfect match with TGMe49-012300 (28.m00307), a gene located on chromosome X and encoding a hypothetical protein of 683 amino acids (Fig. 4). We call this protein CD4Ag28m to indicate its function as an antigen for CD4 T cells presented by Ab MHC class II molecules. This full-length protein sequence contains a putative signal peptide at its N terminus and is therefore likely to be secreted by T. gondii.
Fig 4.
The cognate antigen recognized by BTg01Z.A hybridoma is a previously unknown protein in the T. gondii database as identified by expression cloning. T. gondii database (www.toxodb.org) BLAST search results revealed that the antigenic protein encoded by clone pTg778.76 corresponded to a truncated version of the T. gondii protein TGMe49-012300 (28.m00307). Here, we call the protein CD4Ag28m for “CD4 antigen 28m.” pTg778.76 cDNA is missing the 5′ UTR and the codons for the first 96 amino acids of the open reading frame. Alignment of the amino acids encoded by the truncated cDNA clone compared to the full-length protein in the database is depicted. Dashes represent missing amino acids in the sequence, and dots represent alignment between the two sequences. The final antigenic peptide in the pTg778.76 open reading frame is boxed.
Identification of the minimal antigenic peptide within clone 778.76 recognized by BTg01Z.A hybridoma.
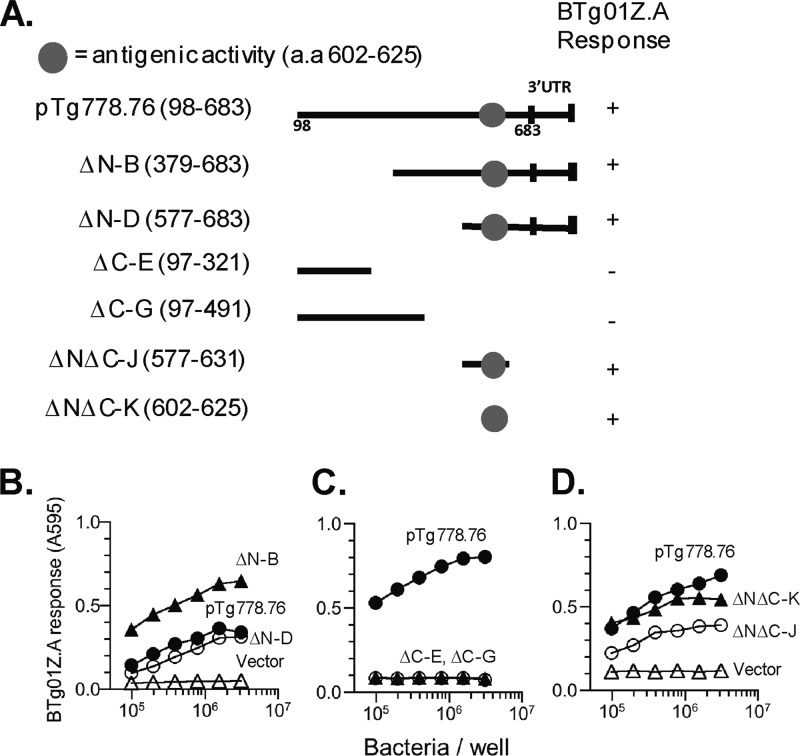
Compared to the 28.m00307 sequence in the T. gondii database (toxodb.org), plasmid pTg778.76 was missing the sequences corresponding to the 5′ untranslated region (UTR) and the first 96 amino acids from the N terminus of the putative CD4Ag28m protein (Fig. 4). Using PCR mutagenesis with the appropriate primers (Table 1), we generated various N-terminal and C-terminal deletion constructs of pTg778.76 (Fig. 5A) and tested their ability to stimulate BTg01Z.A (Fig. 5B to D). These analyses allowed us to narrow down the antigenic activity to a region of 24 amino acids, 602 to 625 (Fig. 5D).
Fig 5.
Identification of the antigenic epitope within CD4Ag28m that stimulates the BTg01Z.A hybridoma. (A) Schematic representation of the N-terminal and C-terminal deletion constructs generated to identify the antigenic epitope within clone pTg778.76. The clone itself was truncated and missing the 5′ UTR and the first 96 amino acids. The specific amino acids tested in each construct are shown in parentheses, and the region with the antigenic activity is indicated by the circle. (B to D) BTg01Z.A lacZ response against different deletion constructs expressed in E. coli that were incubated with wild-type BMDCs for presentation after an overnight stimulation.
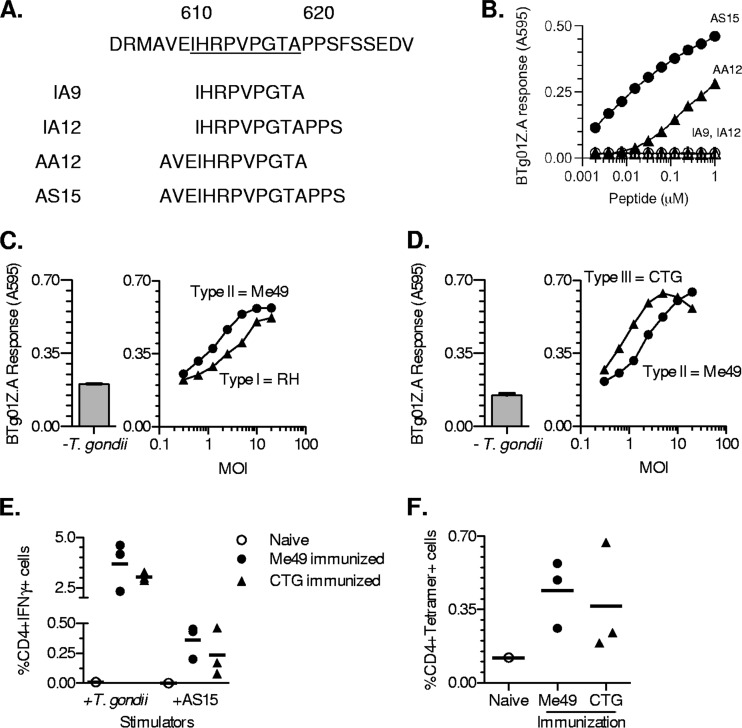
To independently confirm the assignment of antigenic activity, we tested overlapping synthetic peptides within the 24-mer region 602 to 625 (Fig. 6A). BMDCs pulsed with peptides IA9 and IA12 failed to stimulate the BTg01Z.A hybridoma (Fig. 6B), while BMDCs pulsed with AA12 stimulated a BTg01Z.A response. However, the response was substantially higher to BMDCs pulsed with the 15-mer peptide, AS15 (Fig. 6B). We therefore concluded that AS15 is the optimal peptide contained within CD4Ag28m and is presented by Ab and recognized by the BTg01Z.A T cell.
Fig 6.
BTg01Z.A recognizes the 15-mer epitope, AS15, from the parasite protein CD4Ag28m, present in the type I, II, and III strains of T. gondii. (A) Sequences of overlapping peptides within the 24-mer antigenic region were used for fine mapping of the BTg01Z.A epitope. The core sequence that binds the I-Ab MHC class II molecule is underlined. (B) BTg01Z.A lacZ response to wild-type BMDCs that were pulsed with various concentrations of indicated peptides. (C and D) Wild-type BMDCs were infected in vitro with an irradiated type I (RH), II (Me49), or III (CTG) strain of T. gondii. BTg01Z.A lacZ response after an overnight stimulation with type I and II (C)- or type II and III (D)-infected BMDCs. The left panels depict background response against uninfected BMDCs. MOI, multiplicity of infection. (E and F) C57BL/6 mice were immunized with 5 × 106 irradiated T. gondii tachyzoites from type II (Me49) and III (CTG) strains. Splenocytes were harvested 2 weeks postimmunization, and T. gondii- or AS15-specific CD4 T cell responses were measured by intracellular cytokine staining for IFN-γ or by staining with tetramers. (E) Compiled data showing CD4 T cell responses from immunized or naïve mice toward T. gondii-infected or AS15 peptide-pulsed APCs. Data are corrected for background based on CD4 T cell responses toward uninfected or irrelevant peptide-pulsed APCs. (F) Compiled data showing MHC class II I-Ab–AS15 tetramer staining on splenocytes from immunized or naïve animals. Each dot represents an individual mouse. Cells were also costained with CD4 antibody. Data are representative of three independent experiments.
Interestingly, although the full-length protein is polymorphic between the type I, II, and III strains of T. gondii, the AS15 epitope is present and conserved in all CD4Ag28m alleles of these strains (http://www.toxodb.org) (Fig. 6C and D). To test for the antigenicity of type I and type III strains, we infected APCs in vitro with irradiated tachyzoites. As expected, both type I- and type III-infected APCs were able to stimulate BTg01Z.A hybridoma, to levels similar to that seen with the type II strain (Fig. 6C and D). To assess the response in vivo, we immunized B6 mice with irradiated tachyzoites from the type II or III strain, harvested splenocytes, and performed an ex vivo restimulation in the presence of T. gondii-infected or AS15 peptide-pulsed APCs. The assay showed that immunization with either strain of parasite generated a response comparable to that of T. gondii-infected and AS15-pulsed APCs (Fig. 6E). Furthermore, staining with MHC class II I-Ab AS15 tetramers revealed comparable percentages of tetramer-positive cells among splenocytes from mice immunized with either parasite strain (Fig. 6F). Although CD4Ag28m is polymorphic between the different strains of T. gondii, the AS15 epitope is conserved and immunogenic in B6 mice.
Detection of AS15-specific CD4 T cells in T. gondii-infected or -immunized mice.
To determine the fraction of the total T. gondii-specific CD4 T cell response directed toward the AS15 peptide and to examine the kinetics of antigen-specific T cell expansion throughout the course of infection, we infected B6 mice orally with 25 to 40 type II cysts and harvested splenocytes, lymphocytes from the mesenteric lymph node (MLN), or brain leukocytes 1 to 4 weeks postinfection. Ex vivo restimulation of CD4 T cells from infected mice followed by intracellular cytokine staining (ICCS) for IFN-γ revealed that 2% of splenic CD4 T cells at 2 weeks postinfection were AS15 specific. Based on the average that 18% of CD4 T cells in the spleen responded to intact T. gondii-infected APCs, we estimate that 11% of the T. gondii-specific CD4 T cell response is specific for the AS15 peptide at this time point. The AS15-specific T cell percentage decreased after 2 weeks, dropping to 0.6% at 4 weeks postinfection (Fig. 7A and B). In the MLN, the percentage of AS15-specific cells at 2 weeks was 1.7% (Fig. 7C), whereas in the brain AS15-specific T cells comprised 3.8% and 1.5% of CD4 T cells at 3 and 4 weeks postinfection, respectively (Fig. 7D). No significant response above background was observed in naive mice or when T cells from T. gondii-infected mice were restimulated in vitro with an irrelevant Ab MHC-binding peptide (OVAp). Quantification of AS15-specific T cells by MHC class II tetramers gave comparable but slightly higher values (Fig. 7E to G).
Fig 7.
A fraction of the T. gondii-specific CD4 T cell response is AS15 specific during acute and chronic infection. (A to G) C57BL/6 mice were orally infected with 25 to 40 T. gondii cysts. Splenocytes, lymphocytes from mesenteric lymph node (MLN), and brain leukocytes were harvested from mice 1 to 4 weeks, 1 to 2 weeks, and 3 to 4 weeks postinfection, respectively. T. gondii-specific CD4 T cell responses were measured by intracellular cytokine staining for IFN-γ or by staining with peptide-MHC tetramers. (A) Representative flow cytometry plots of splenocytes from naïve and chronically infected mice. Left panels show intracellular IFN-γ staining after in vitro restimulation with antigen-presenting cells either with or without T. gondii or 10 μM AS15 peptide. Right panels show MHC class II I-Ab–AS15 tetramer staining. (B to D) Compiled data showing T. gondii- and AS15-specific responses from spleens of infected or naïve mice over the course of infection (B), lymphocytes from MLN during the acute phase of infection (C), and brain during chronic infection (D). (E to G) Compiled data showing flow cytometry analysis of MHC class II I-Ab–AS15 tetramer staining on splenocytes from infected or naïve animals over the course of infection (E), lymphocytes from MLN during the acute phase of infection (F), and brain during chronic infection (G). Each circle or bar represents an average of four mice. Data are representative of at least two independent experiments. (H to J) C57BL/6 mice were immunized with 5 × 106 irradiated T. gondii tachyzoites. Splenocytes were harvested from mice 2 weeks postimmunization, and T. gondii-specific CD4 T cell responses were measured by intracellular cytokine staining for IFN-γ or by staining with tetramers. (H) Representative flow cytometry plots from naïve and immunized mice depicting ex vivo IFN-γ staining and tetramer results. (I) Compiled data showing splenic T. gondii-specific and AS15-specific responses from immunized mice. Each circle represents an individual mouse. (J) Compiled data showing MHC class II I-Ab–AS15 tetramer staining on splenocytes from immunized mice. All data showing intracellular cytokine staining for IFN-γ are corrected for background based on responses by CD4 T cells toward uninfected APCs or APCs pulsed with irrelevant peptide. Cells were also costained with CD4 antibody. Data are representative of at least three independent experiments.
Ex vivo analysis of splenocytes from mice that had been immunized with irradiated parasites gave similar results (Fig. 7H to J). Although the overall parasite-specific CD4 T cell responses were lower in immunized than in infected mice (5% in immunized mice versus 8 to 18% in infected mice), the AS15-specific responses, as measured by ICCS or MHC class II tetramer staining, were well above background. The AS15-specific response by ICCS averaged 0.2% of the total CD4 T cells, or 4% out of the total T. gondii-specific CD4 T cell response (Fig. 7H to J). Overall, we conclude that AS15-specific CD4 T cells represent a substantial fraction of the total CD4 T cell response to T. gondii in C57BL/6 mice.
Immunization with AS15 peptide protects B6 mice against T. gondii challenge.
Given previous indications that CD4 T cells can play a protective role during T. gondii infection (2, 37, 59), we asked whether immunization with the AS15 peptide alone could elicit immunity to live parasites. We immunized B6 mice in the footpad with LPS-activated BMDCs pulsed with AS15 or an irrelevant peptide (OVAp). We then challenged mice 7 days postimmunization with the type II strain of T. gondii (10,000 Pru tachyzoites [Tz] i.p.) and measured the CD4 T cell responses, cyst numbers, and parasite loads in the brains of infected mice. Compared to control immunized mice, AS15-immunized mice produced a greater IFN-γ+ CD4 T cell response to AS15-pulsed BMDCs (Fig. 8A), confirming that the peptide immunization elicited CD4 T cells specific for AS15 peptide. Staining with MHC class II tetramers confirmed that AS15-specific CD4 T cells were present in the brains of both groups of mice, but with a higher frequency in AS15-immunized mice (Fig. 8B). Cyst burden (Fig. 8C) was significantly lower in the AS15-immunized group than in the control group (Fig. 8C). This reduction in cyst load was observed from 4 to 11 weeks after challenge and was also observed following immunization of mice with AS15 peptide in complete Freund's adjuvant (data not shown). In addition, the total parasite load as measured by semiquantitative PCR was reduced in AS15-immunized mice, an effect that was most striking at 4 weeks after challenge (Fig. 8D and data not shown). Together, these data indicate that AS15-specific T cell responses can protect mice against T. gondii infection and that protection is enhanced by AS15 peptide immunization.
Fig 8.
Immunization with AS15 peptide lowers the cyst burden and parasite load in the brains of infected mice. C57BL/6 mice were immunized with LPS-activated BMDCs pulsed with AS15 or control peptide (OVAp). Seven days postimmunization, these mice were infected with 1 × 104 live T. gondii tachyzoites, intraperitoneally. Panels show analysis of CD4 responses and parasite loads from infected mice. (A) IFN-γ response by brain CD4 T cells as measured by ICCS for IFN-γ using flow cytometry after ex vivo restimulation with AS15-pulsed APCs. Data are background corrected based on the values from APCs pulsed with irrelevant peptide. (B) MHC class II I-Ab–AS15 tetramer staining on brain leukocytes. Cells were also costained with CD4 antibody. (C) Number of cysts in the brain as measured by staining a portion of the brain with fluorescent lectin to detect the cysts. (D) The parasite load in the brain measured using semiquantitative PCR on genomic DNA extracted from the tissue. The data in panels A through D are compiled from three experimental groups analyzed at days 28 and 30 after challenge with at least 4 mice per condition in each experiment. *, P < 0.05; **, P < 0.01.
DISCUSSION
CD4 T cells are an important component of the immune response to Toxoplasma gondii, but the nature of the parasite antigens recognized by CD4 T cells is largely unknown. Here, we examine the CD4 T cell response in T. gondii-infected C57BL/6 (B6) mice, a strain that is widely used by immunologists and is relatively susceptible to infection. We observe that CD4 T cell responses predominate over the CD8 T cell response in B6 mice, and we define a parasite antigen, CD4Ag28m, which accounts for a substantial fraction of the CD4 T cell response in immunized or infected mice. We show that a 15-mer AS15 peptide derived from the CD4Ag28m protein is presented by an I-Ab MHC molecule and that this antigenic peptide is conserved in all three strains of T. gondii tested here. Finally, we demonstrate that immunization of mice with the AS15 peptide can protect the animals from subsequent infection, resulting in lower parasite burden in the brain.
Importantly, identification of the antigen, CD4Ag28m, allowed us to better understand the role of the T. gondii-specific CD4 T cell response in B6 mice. Since the peptide-MHC class II ligands recognized by CD4 T cells are generated in specialized APCs using unique antigen-processing pathways, identifying pathogen antigens that elicit CD4 T cell responses is a challenging undertaking. Even when the genome sequences are known, candidate peptides are difficult to predict due to poor definition of MHC class II binding consensus motifs. Direct purification of the processed peptides from infected cells is also difficult because the peptides not only are present in small amounts but also represent a heterogeneous mixture with ragged N and C termini. Thus, unlike MHC class I binding homogenous peptides that elute in a single peak on high-performance liquid chromatography, the active MHC II binding peptides elute in multiple peaks that effectively reduce recovery. Genetic approaches are also difficult, because MHC class II molecules generally present peptides obtained from exogenous sources; endogenous expression of transfected cDNAs in APCs does not yield appropriate pMHC II ligand. We overcame these limitations by developing a unique expression cloning strategy for identifying CD4 T cell-stimulating antigens as described previously (8, 41, 50, 51). Currently, CD4 epitopes for pathogens such as Salmonella spp. (12, 42), Mycobacterium spp. (1, 20, 63), Trypanosoma cruzi (26), Leishmania spp. (25, 53, 64), and Plasmodium spp. (18, 22, 46, 60) have all been identified as previously known conserved surface, abundant, or secreted proteins. In contrast, the cDNA expression library provides an unbiased and immunologically relevant approach that can be applied to identify any CD4 T cell-stimulating antigen.
Our screen for CD4 antigens identified CD4Ag28m, previously a “hypothetical” protein in the T. gondii database whose location and function are currently unknown. Nonetheless, CD4Ag28m contains a predicted signal sequence, suggesting that it is a secretory protein. Secretion of parasite antigens into the host cell is known to be important for presentation via the MHC class I pathway, which samples the host cytosol and stimulates CD8 T cell responses to intracellular pathogens. This is in line with evidence that secretion into the host cell promotes recognition by CD8 T cells (31) and the fact that all of the T. gondii CD8 epitopes identified to date are derived from secreted parasite proteins (4, 16, 40, 62). On the other hand, the impact of secretion on MHC class II presentation of potential parasite antigens is less clear, since both secreted and nonsecreted parasite antigens should have ready access to the class II MHC pathway via phagocytosis of intact parasites and debris. Indeed, we observed robust AS15-specific T cell response when APCs were provided heat-killed parasites (unpublished data). However, while both secreted and nonsecreted parasite proteins may be presented by bystander (noninvaded) APCs, secreted proteins may be preferentially presented by invaded APCs and this pathway may be particularly important in vivo where antigen concentration is often limiting. Indeed, enhanced recognition by CD4 T cells of a secreted version of the model antigen OVA has been reported elsewhere (45), and CD4 responses to Salmonella are enhanced by secretion into host cells (21). Manipulation of MHC class II antigen presentation by T. gondii (32, 34, 35, 39) may represent a way for the parasite to evade the most effective CD4 responses directed toward secreted antigens on invaded host cells.
A CD4 T cell response directed toward a single parasite peptide, AS15, is sufficient to mediate immune protection as indicated by the decreased parasite load in vaccinated mice. This raises the question of how AS15-specific CD4 T cells contribute to the control of infection. One possibility is that these CD4 cells may contribute to protection by activating macrophages via expression of the protective cytokine IFN-γ (55, 58). Alternatively, AS15-specific CD4 cells may provide helper activity for antibody or CD8 T cell responses, as suggested by the impairment of intracerebral CD8 T cells in CD4 T cell-depleted mice (37). A third possibility is that immunization with the peptide may alter the balance between different types of T helper cells, such as between Tregs and Th1 cells, as suggested by the plasticity of Tregs and Th1 function during lethal T. gondii infection (43). The ability to track AS15-specific T cells in vivo should help to dissect the mechanism by which vaccination generates protective T cell responses and how these responses provide protection.
Lastly, the identification of an immunogenic peptide which is capable of generating protection in mice may aid in the development of more effective vaccines against toxoplasmosis. In this regard, it is encouraging that, although CD4Ag28m is polymorphic, the AS15 epitope is conserved between the three North American and European strains of T. gondii. It will be important to determine if this protein or peptide is also immunogenic and protective in other species, including humans. Inclusion of CD4 epitopes such as AS15 can potentially be used to boost responses to CD8 T cell epitopes (11) and thus improve the efficacy of vaccination.
ACKNOWLEDGMENTS
We are grateful to the NIH tetramer facility for producing the AS15-Ab tetramer and Hamlet Chu (Robey laboratory) and James Moon and Marion Pepper from the Marc Jenkins laboratory (University of Minnesota) for further assistance in generating MHC class II tetramers.
This research was supported by a grant from the National Institutes of Health (PO1AI065831) to Ellen A. Robey and Nilabh Shastri. Nicolas Blanchard was supported by a fellowship from the International Human Frontier Science Program. Harshita Satija Grover was supported in part by a Cancer Research Coordinating Committee fellowship.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Andersen P, Andersen AB, Sorensen AL, Nagai S. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359–3372 [PubMed] [Google Scholar]
- 2. Araujo FG. 1991. Depletion of L3T4+ (CD4+) T lymphocytes prevents development of resistance to Toxoplasma gondii in mice. Infect. Immun. 59:1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blackwell JM, Roberts CW, Alexander J. 1993. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 15:317–324 [DOI] [PubMed] [Google Scholar]
- 4. Blanchard N, et al. 2008. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat. Immunol. 9:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boothroyd JC. 2009. Toxoplasma gondii: 25 years and 25 major advances for the field. Int. J. Parasitol. 39:935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown CR, et al. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419–428 [PMC free article] [PubMed] [Google Scholar]
- 7. Brown CR, McLeod R. 1990. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 145:3438–3441 [PubMed] [Google Scholar]
- 8. Campbell DJ, Shastri N. 1998. Bacterial surface proteins recognized by CD4+ T cells during murine infection with Listeria monocytogenes. J. Immunol. 161:2339–2347 [PubMed] [Google Scholar]
- 9. Carruthers VB. 2002. Host cell invasion by the opportunistic pathogen Toxoplasma gondii. Acta Trop. 81:111–122 [DOI] [PubMed] [Google Scholar]
- 10. Chtanova T, et al. 2009. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity 31:342–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cong H, et al. 2011. Towards an immunosense vaccine to prevent toxoplasmosis: protective Toxoplasma gondii epitopes restricted by HLA-A*0201. Vaccine 29:754–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cookson BT, Bevan MJ. 1997. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J. Immunol. 158:4310–4319 [PubMed] [Google Scholar]
- 13. Deckert-Schluter M, et al. 1994. Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect. Immun. 62:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Denkers EY. 1999. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect. 1:699–708 [DOI] [PubMed] [Google Scholar]
- 15. Denkers EY, Gazzinelli RT. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frickel EM, et al. 2008. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J. Infect. Dis. 198:1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286–292 [PubMed] [Google Scholar]
- 18. Grillot D, et al. 1990. Immune responses to defined epitopes of the circumsporozoite protein of the murine malaria parasite, Plasmodium yoelii. Eur. J. Immunol. 20:1215–1222 [DOI] [PubMed] [Google Scholar]
- 19. Hakim FT, et al. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310–2316 [PubMed] [Google Scholar]
- 20. Harris DP, et al. 1995. Cross-recognition by T cells of an epitope shared by two unrelated mycobacterial antigens. Eur. J. Immunol. 25:3173–3179 [DOI] [PubMed] [Google Scholar]
- 21. Hess J, Ladel C, Miko D, Kaufmann SH. 1996. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J. Immunol. 156:3321–3326 [PubMed] [Google Scholar]
- 22. Hirunpetcharat C, et al. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP1[19]) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400–3411 [PubMed] [Google Scholar]
- 23. Israelski DM, Remington JS. 1993. Toxoplasmosis in patients with cancer. Clin. Infect. Dis. 17(Suppl. 2):S423–S435 [DOI] [PubMed] [Google Scholar]
- 24. Jankovic D, et al. 2007. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jardim A, Alexander J, Teh HS, Ou D, Olafson RW. 1990. Immunoprotective Leishmania major synthetic T cell epitopes. J. Exp. Med. 172:645–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kahn SJ, Wleklinski M. 1997. The surface glycoproteins of Trypanosoma cruzi encode a superfamily of variant T cell epitopes. J. Immunol. 159:4444–4451 [PubMed] [Google Scholar]
- 27. Karttunen J, Sanderson S, Shastri N. 1992. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl. Acad. Sci. U. S. A. 89:6020–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karttunen J, Shastri N. 1991. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc. Natl. Acad. Sci. U. S. A. 88:3972–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim K, Weiss LM. 2004. Toxoplasma gondii: the model apicomplexan. Int. J. Parasitol. 34:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirisits MJ, Mui E, McLeod R. 2000. Measurement of the efficacy of vaccines and antimicrobial therapy against infection with Toxoplasma gondii. Int. J. Parasitol. 30:149–155 [DOI] [PubMed] [Google Scholar]
- 31. Kwok LY, et al. 2003. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J. Immunol. 170:1949–1957 [DOI] [PubMed] [Google Scholar]
- 32. Lang C, Gross U, Luder CG. 2007. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol. Res. 100:191–203 [DOI] [PubMed] [Google Scholar]
- 33. Liesenfeld O, Kosek J, Remington JS, Suzuki Y. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luder CG, et al. 2003. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J. Neuroimmunol. 134:12–24 [DOI] [PubMed] [Google Scholar]
- 35. Luder CG, Lang T, Beuerle B, Gross U. 1998. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin. Exp. Immunol. 112:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luft BJ, Remington JS. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211–222 [DOI] [PubMed] [Google Scholar]
- 37. Lutjen S, Soltek S, Virna S, Deckert M, Schluter D. 2006. Organ- and disease-stage-specific regulation of Toxoplasma gondii-specific CD8-T-cell responses by CD4 T cells. Infect. Immun. 74:5790–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malarkannan S, Mendoza LM, Shastri N. 2001. Generation of antigen-specific, lacZ-inducible T-cell hybrids. Methods Mol. Biol. 156:265–272 [DOI] [PubMed] [Google Scholar]
- 39. McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. 2004. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. Immunol. 173:2632–2640 [DOI] [PubMed] [Google Scholar]
- 40. Mendes EA, Caetano BC, Penido ML, Bruna-Romero O, Gazzinelli RT. 2011. MyD88-dependent protective immunity elicited by adenovirus 5 expressing the surface antigen 1 from Toxoplasma gondii is mediated by CD8(+) T lymphocytes. Vaccine 29:4476–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mougneau E, et al. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563–566 [DOI] [PubMed] [Google Scholar]
- 42. Musson JA, et al. 2002. Processing of viable Salmonella typhimurium for presentation of a CD4 T cell epitope from the Salmonella invasion protein C (SipC). Eur. J. Immunol. 32:2664–2671 [DOI] [PubMed] [Google Scholar]
- 43. Oldenhove G, et al. 2009. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31:772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parker SJ, Roberts CW, Alexander J. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84:207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. 2004. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect. Immun. 72:7240–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reece WH, et al. 2004. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat. Med. 10:406–410 [DOI] [PubMed] [Google Scholar]
- 47. Reichmann G, Dlugonska H, Fischer HG. 1997. T cell receptor specificities of Toxoplasma gondii-reactive mouse CD4+ T lymphocytes and Th1 clones. Med. Microbiol. Immunol. 186:25–30 [DOI] [PubMed] [Google Scholar]
- 48. Reichmann G, Dlugonska H, Hiszczynska-Sawicka E, Fischer H. 2001. Tachyzoite-specific isoform of Toxoplasma gondii lactate dehydrogenase is the target antigen of a murine CD4(+) T-cell clone. Microbes Infect. 3:779–787 [DOI] [PubMed] [Google Scholar]
- 49. Reichmann G, et al. 1997. Detection of a novel 40,000 MW excretory Toxoplasma gondii antigen by murine Th1 clone which induces toxoplasmacidal activity when exposed to infected macrophages. Immunology 92:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sahara H, Shastri N. 2003. Second class minors: molecular identification of the autosomal H46 histocompatibility locus as a peptide presented by major histocompatibility complex class II molecules. J. Exp. Med. 197:375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanderson S, Campbell DJ, Shastri N. 1995. Identification of a CD4+ T cell-stimulating antigen of pathogenic bacteria by expression cloning. J. Exp. Med. 182:1751–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanderson S, Shastri N. 1994. LacZ inducible, antigen/MHC-specific T cell hybrids. Int. Immunol. 6:369–376 [DOI] [PubMed] [Google Scholar]
- 53. Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stumhofer JS, et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7:937–945 [DOI] [PubMed] [Google Scholar]
- 55. Suzuki Y, Conley FK, Remington JS. 1989. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045–2050 [PubMed] [Google Scholar]
- 56. Suzuki Y, et al. 1994. MHC class I gene(s) in the D/L region but not the TNF-alpha gene determines development of toxoplasmic encephalitis in mice. J. Immunol. 153:4649–4654 [PubMed] [Google Scholar]
- 57. Suzuki Y, Joh K, Orellana MA, Conley FK, Remington JS. 1991. A gene (s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732–739 [PMC free article] [PubMed] [Google Scholar]
- 58. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki Y, Remington JS. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946 [PubMed] [Google Scholar]
- 60. Takita-Sonoda Y, et al. 1996. Plasmodium yoelii: peptide immunization induces protective CD4+ T cells against a previously unrecognized cryptic epitope of the circumsporozoite protein. Exp. Parasitol. 84:223–230 [DOI] [PubMed] [Google Scholar]
- 61. Williams DM, Grumet FC, Remington JS. 1978. Genetic control of murine resistance to Toxoplasma gondii. Infect. Immun. 19:416–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilson DC, et al. 2010. Differential regulation of effector- and central-memory responses to Toxoplasma gondii Infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog. 6:e1000815 doi:10.1371/journal.ppat.1000815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yanagisawa S, Koike M, Kariyone A, Nagai S, Takatsu K. 1997. Mapping of V beta 11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. Int. Immunol. 9:227–237 [DOI] [PubMed] [Google Scholar]
- 64. Yang DM, Rogers MV, Liew FY. 1991. Identification and characterization of host-protective T-cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology 72:3–9 [PMC free article] [PubMed] [Google Scholar]
- 65. Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. 2006. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity 25:655–664 [DOI] [PubMed] [Google Scholar]