Abstract
Translocation across intestinal epithelial cells is an established pathogenic feature of the zoonotic bacterial species Campylobacter jejuni. The number of C. jejuni virulence factors known to be involved in translocation is limited. In the present study, we investigated whether sialylation of C. jejuni lipooligosaccharide (LOS) structures, generating human nerve ganglioside mimics, is important for intestinal epithelial translocation. We here show that C. jejuni isolates expressing ganglioside-like LOS bound in larger numbers to the Caco-2 intestinal epithelial cells than C. jejuni isolates lacking such structures. Next, we found that ganglioside-like LOS facilitated endocytosis of bacteria into Caco-2 cells, as visualized by quantitative microscopy using the early and late endosomal markers early endosome-associated protein 1 (EEA1), Rab5, and lysosome-associated membrane protein 1 (LAMP-1). This increased endocytosis was associated with larger numbers of surviving and translocating bacteria. Next, we found that two different intestinal epithelial cell lines (Caco-2 and T84) responded with an elevated secretion of the T-cell attractant CXCL10 to infection by ganglioside-like LOS-expressing C. jejuni isolates. We conclude that C. jejuni translocation across Caco-2 cells is facilitated by ganglioside-like LOS, which is of clinical relevance since C. jejuni ganglioside-like LOS-expressing isolates are linked with severe gastroenteritis and bloody stools in C. jejuni-infected patients.
INTRODUCTION
Campylobacter jejuni, a zoonotic Gram-negative bacterial pathogen, is able to enter, survive in, and translocate across intestinal epithelial cells (22, 27, 42). Such gastroinvasive pathogens often employ common eukaryotic cellular pathways such as endocytosis (7, 22, 33, 42, 43). Endocytosis provides the general entry portal of eukaryotic cells for uptake of nutrients and for regulation of membrane-bound receptors and signaling (16). Endocytosis consists of early and later stages that can be conveniently distinguished using specific protein markers. The protein markers frequently used to study the different endocytic stages of intracellular pathogens are early endosome-associated protein 1 (EEA1), the GTPases Rab5 and Rab7, and lysosome-associated membrane protein 1 (LAMP-1) (16). EEA1 and Rab5 are involved in the early stages of endocytosis (40) and Rab7 marks later stages (31), whereas LAMP-1 marks the end stage, when late endosomes are fused with lysosomes (9, 10). At the final stages of endocytosis, endolysosomal vesicles, organelles in which large molecules and even intact bacteria can be degraded, are formed (16, 19, 23). Factors involved in the escape from the endosomal pathway have been studied well for intracellular bacteria such as Salmonella enterica, Listeria monocytogenes, Mycobacterium tuberculosis, and Brucella abortus (16). Studies to better define such factors in C. jejuni have recently been initiated (4).
Currently, for C. jejuni it is considered that the flagellum is the primary adherence factor that establishes contact between the eukaryotic cell membrane and specific bacterial-invasion factors (22). Proposed invasion factors are capsular polysaccharides, Peb1 and JlpA, or other still-unknown outer membrane-related structures (1, 24, 34–36). It is thought that these invasion factors activate host cell plasma membrane invaginations at sites where C. jejuni bacteria are present. These invaginations may then form an endosomal compartment that is transported from the apical to the basolateral surface, enabling C. jejuni translocation across the intestinal epithelial barrier (22, 27, 33). The effect of such an entrance mechanism on C. jejuni intracellular survival was noted by Watson and Galan, who showed that C. jejuni isolate 81176 invaded epithelial cells via unique small plasma membrane invaginations called caveolae. This specific manner of entrance was then found to contribute to the escape of 81176 from the canonical endocytic pathway (42). In other words, for this isolate it was demonstrated that it was able to escape lysosomal degradation, contributing to its intracellular survival (42). Recently, Buelow and coworkers showed that intracellular survival of C. jejuni isolate NCTC11168 is enhanced by the secreted Cia protein (Cj1450) that was found to block fusion of lysosomes with the C. jejuni-containing endosome (4). The studies by Watson and Galan (42) and Buelow et al. (4) did not show which specific bacterial invasion factor(s) facilitates endocytosis of C. jejuni into epithelial cells.
At present, there is increasing evidence that sialylated lipooligosaccharide (LOS) structures expressed on the C. jejuni outer surface (12) are potential internalization factors (18, 37). Interestingly, sialylated LOS structures are also present on the well-characterized C. jejuni 81176 isolate (17) that is able to induce severe colitis in humans and monkeys (2, 38). A more recent clinical study showed that sialylation of C. jejuni LOS structures, which mimic human peripheral nerve gangliosides (37), was strongly associated with severe gastroenteritis and bloody stools in C. jejuni-infected patients (32). Having noted the associations between expression of ganglioside-like LOS by different C. jejuni isolates and the induction of severe gastroenteritis and bloody stools in humans (2, 32), we set out to investigate a potential pathogenic mechanism that might explain this correlation. We hypothesized that severe gastroenteritis could be a result of increased numbers of endocytosed and translocating bacteria in the human intestine. The present work was therefore designed to explore the effect of ganglioside-like LOS on C. jejuni endocytosis, intracellular survival, and translocation across human intestinal epithelial cells.
MATERIALS AND METHODS
Bacterial strains.
Thirty-two clinical C. jejuni isolates and the 81176 reference isolate were used in this study (Table 1). To further analyze the role of ganglioside-like LOS, we used two previously generated Δcst-II mutants, GB11Δcst-II and GB19Δcst-II, and a cst-II complemented isolate, GB11Δcst-IIΔ, here named GB11 (C). The cst-II gene encodes a sialyltransferase that transfers sialic acid onto LOS structures (6). The specific protocols for generation of the Δcst-II mutants and the complemented GB11 (C) can be found elsewhere (11, 29). To minimize in vitro passaging, C. jejuni isolates were recovered from the original glycerol stock by culturing on Butzler agar plates (Becton, Dickinson, Breda, The Netherlands). The Δcst-II mutants were then grown on blood agar plates containing 7% sheep blood (Becton, Dickinson, Breda, The Netherlands) supplemented with vancomycin (Sigma-Aldrich, Zwijndrecht, The Netherlands) (10 μg/ml) and chloramphenicol (Sigma-Aldrich, Zwijndrecht, The Netherlands) (20 μg/ml); erythromycin (Sigma-Aldrich, Zwijndrecht, The Netherlands) (0.02 μg/ml) was used for selection of GB11 (C). A final passage on commercial Columbia blood agar plates (Becton, Dickinson, Breda, The Netherlands) for wild types, Δcst-II mutants, and GB11 (C) was allowed for optimal vitality and equal growth conditions before infection assays. All isolates and mutants and the complemented mutant were incubated at 37°C in an anaerobic jar under microaerophilic conditions using an Anoxomat gas mixer (Mart, Drachten, The Netherlands). Bacterial cells were harvested in Hanks balanced salt solution (HBSS) (Invitrogen, Breda, The Netherlands) at 37°C, and densities were set using optical density at 600 nm (OD600). The LOS class was determined by PCR, using protocols previously described (11).
Table 1.
C. jejuni isolates, presence of genes needed for sialic acid biosynthesis, and ganglioside-like LOS expression
| Isolatea | Presence of LBGESb | Ganglioside mimic(s)c |
|---|---|---|
| GB1 | + | None |
| GB3 | + | GM1, GD1a |
| GB4 | − | None |
| GB11 | + | GM1, GD1a |
| GB16 | + | GD1c |
| GB17 | + | GA1, GM1b, GD1c |
| GB18 | + | GM1, GD1a |
| GB19 | + | GD1c |
| GB22 | + | GM1a, GD1a |
| GB23 | + | GM2 |
| GB25 | + | GA1, GM1b, GD1c |
| GB29 | + | None |
| 81176 | + | GM2, GM3 |
| 11168 | + | GM1, GM2 |
| R3 | − | None |
| R33 | − | ND |
| R37 | − | None |
| R61 | − | ND |
| E9141 | − | ND |
| E9144 | − | ND |
| E9146 | − | ND |
| E98-623 | − | None |
| E98-624 | − | None |
| E98-706 | − | None |
| E98-1033 | + | GM1 |
| E98-652 | + | GM1a, GQ1b |
| E98-682 | + | GM1a, GQ1b |
| E98-1087 | + | GM1a |
| R65 | − | ND |
| R67 | − | ND |
| R95 | − | GA1 |
| R104 | − | ND |
| R109 | + | GM1a |
Isolates used to study colocalization of C. jejuni with LysoTracker DND-99, swarming, and chemokine secretion. GB, Guillain-Barré; R and E, enteritis-associated isolates.
PCR results, scoring the presence (+) or absence (−) of the LOS Biosynthesis Genes Enabling Sialylation (LBGES).
Ganglioside-like LOS (GM) detected with mass spectrometry. GA1, ganglioside-like LOS without sialic acid; None, no ganglioside-like LOS was detected; ND, not determined. For the other isolates, ganglioside-like LOS structures were determined by rapid mass spectrometry screening (8) or by antibody probing (652, 682, 1033, 1087, R95, R109). Note that the presence of LBGES does not always mean ganglioside-like LOS expression due to frameshift mutations in the LBGES (12).
Motility assays.
C. jejuni suspensions were prepared to an OD600 of 1. A semisolid agar plate (Mueller-Hinton [MH] broth plus 0.4% agar) was inoculated with 1 μl of the bacterial suspension by stabbing. Plates were grown at 37°C under microaerophilic conditions, and the resulting circle diameters exemplifying bacterial swimming were measured after incubation for 24 h. The swimming analysis and diameter motility zone measurements were performed three times for wild types and a Δcst-II mutant strain.
Intestinal epithelial cell lines.
Two human intestinal epithelial cell lines, Caco-2 and T84, were used in this study. Caco-2 is a human colon carcinoma cell line, and T84 is a transplantable differentiating human carcinoma cell line; both harbor the characteristic of differentiating when maintained in culture for extended periods of time (5, 15, 21, 45). Caco-2 and T84 have been used earlier for C. jejuni studies (27, 45). We maintained the Caco-2 and T84 cell lines in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Breda, The Netherlands) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Breda, The Netherlands) and 1% nonessential amino acids (NEAA) (Invitrogen, Breda, The Netherlands). The cells were grown in a 75-cm2 flask (Greiner Bio-one, Alphen a/d Rijn, The Netherlands) at 37°C and 5% CO2 in a humidified air incubator.
Adhesion to intestinal epithelial cells.
Caco-2 cells were grown to 40% to 50% confluence on chamber slides (Greiner Bio-one, Alphen a/d Rijn, The Netherlands). C. jejuni was inoculated at a multiplicity of infection (MOI) of 100. Fifteen minutes after inoculation, the Caco-2 cells were washed three times with 37°C prewarmed HBSS (Invitrogen) and fixed with 4% formaldehyde (Sigma-Aldrich, Zwijndrecht, The Netherlands)–HBSS (Invitrogen) at room temperature for 2 h. Cells were washed three times with HBSS (Invitrogen) and blocked with 1% fetal bovine serum–HBSS (block buffer). To visualize C. jejuni, an anti-C. jejuni fluorescein isothiocyanate (FITC)-labeled antibody (Genway, San Diego, CA) was diluted 1:100 in block buffer and incubated for 1 h. The Caco-2 cells were washed three times with HBSS (Invitrogen) and dehydrated for 1 min with 70% ethanol and another minute with 100% ethanol (Sigma-Aldrich, Zwijndrecht, The Netherlands). The culture chamber on the slides was removed, and the slides with Caco-2 cells were air dried. Slides with Caco-2 cells were mounted with fluorescent mounting medium (Dako, Carpinteria, CA). A coverslip was placed on top, and slides were analyzed on an XI51 phase-contrast fluorescence microscope (Olympus, Leiden, The Netherlands). Photos were taken with an Olympus XM10 color camera at ×1,000 magnification and analyzed for adhesion with the Olympus software CellF̂ (Olympus). For each isolate, an average of 30 pictures were taken at random fields, showing binding of C. jejuni bacteria to the Caco-2 cells. Experiments were independently repeated three times.
Gentamicin exclusion invasion assay.
Epithelial cell invasion by C. jejuni was determined by growing Caco-2 cells on a cell culture multiwell plate (Greiner Bio-one, Alphen a/d Rijn, The Netherlands) (6 wells) into a monolayer until formation of domes was observed by phase-contrast microscopy. Formation of domes of the Caco-2 cells is a strong indication of differentiation (15, 30). The invasion protocol was performed as previously described (29). Briefly, Caco-2 monolayers with formation of domes were incubated together with C. jejuni at an MOI of 100 for 4 h at 37°C and 5% CO2. Following the invasion period, cells were washed with prewarmed DMEM (Invitrogen) and incubated for another 2 h in medium containing gentamicin (Sigma-Aldrich, Zwijndrecht, The Netherlands) (480 μg/ml) to kill extracellular bacteria. After the gentamicin treatment, the infected Caco-2 cells were washed three times with HBSS (Invitrogen) and lysed with 0.1% Triton X-100 (Sigma-Aldrich, Zwijndrecht, The Netherlands)–phosphate-buffered saline (PBS) (Invitrogen, Breda, The Netherlands) for 15 min at room temperature to release the intracellular bacteria. The number of viable bacteria released from the Caco-2 cells was assessed after serial (10-fold) dilutions of the lysates on Columbia blood agar plates (Becton, Dickinson).
Immunofluorescence.
Caco-2 cells were grown to 40% to 50% confluence on chamber slides (Greiner Bio-one), and C. jejuni was inoculated at an MOI of 100. A series of time course experiments were started, based on a prior study (42), to detect and confirm that the observed colocalization time points were optimal for analysis of colocalization of the endosomal primary antibody markers EEA-1 (Biosciences BD, Breda, The Netherlands), Rab5 (Santa Cruz, CA), Rab7 (Abcam, Cambridge, MA), and LAMP-1 (Abcam, Cambridge, MA) with intracellular C. jejuni cells. For EEA-1, the incubation time after inoculation of C. jejuni into the chambers of the chamber slides was 30 min; for Rab5, 40 min; for Rab7, 45 min; and for LAMP-1, 2 h. Prior to visualization, Caco-2 cells were washed three times with 37°C prewarmed HBSS (Invitrogen) and fixed with 4% formaldehyde (Sigma-Aldrich) in HBSS (Invitrogen) at room temperature for 2 h. Caco-2 cells were again washed three times with HBSS (Invitrogen). Afterward, the Caco-2 cells were permeabilized for 20 min with 0.1% HBSS–Triton X-100 solution and background antibody binding was blocked with block buffer (1% fetal bovine serum, 1% Tween 20, HBSS). Slides were then incubated for 1 h with the respective primary antibody (for EEA-1, Rab5, Rab7, or LAMP-1) at a 1:100 dilution in block buffer. The appropriate secondary antibodies from the IgG class (H+L), A594 labeled (Molecular Probes, Bleiswijk, The Netherlands), providing a red stain, were selected for EEA1, Rab5, Rab7, and LAMP-1. Further preparation and visualization occurred as described in Materials and Methods upon adherence of intestinal epithelial cells. Experiments were independently repeated three times. In order to obtain the mean percentage of colocalization of bacteria, the number of C. jejuni bacteria that colocalized with an endosomal marker was divided by the number of C. jejuni bacteria that did not colocalize with an endosomal marker but were instead bound on a Caco-2 cell and the result was multiplied by 100.
Lysotracker DND-99 staining.
To monitor the lysosomal development of vacuoles containing intracellular bacteria, we used the protocol described by the manufacturer (Invitrogen, Breda, The Netherlands) to test whether the C. jejuni-containing endosomal compartment was acidic. Briefly, the stock solution was diluted to a final working concentration of 1 mM in DMEM (Invitrogen) containing 10% FBS (Invitrogen) and 1% NEAA (Invitrogen). Caco-2 cells were preincubated with LysoTracker DND-99 (Invitrogen) for 30 min under the culture conditions described above. After preincubation, C. jejuni bacteria were added to a well in the chamber slide at an MOI of 100. After 2 h of incubation, Caco-2 cells were washed three times with prewarmed HBSS (Invitrogen) at 37°C to remove excess bacteria and LysoTracker DND-99 (Invitrogen). Fixation and visualization occurred as described above.
Intracellular survival.
To assay intracellular survival, an adapted gentamicin exclusion assay was used as described previously (42). Initially, the invasion protocol was followed, with the modification that after gentamicin treatment, the medium was replaced with complete DMEM (Invitrogen) still containing gentamicin (10 μg/ml) (Sigma-Aldrich) without penicillin and streptomycin for overnight incubation. Plating of the infection medium showed that no CFU were present after this treatment. After overnight incubation, three additional washes with HBSS (Invitrogen) were performed, after which the invasion protocol for plating was followed as previously described (42).
Translocation across differentiated Caco-2 cell monolayers.
Caco-2 cells were seeded onto Transwell filters at 4 × 10 5 cells/filter using 24-well plates (Costar, Corning Inc. Corning, NY) (5 μm pore size, 1.13 cm2). This pore size was large enough to enable further C. jejuni translocation after cell passage. The Caco-2 cells were allowed to differentiate for 19 days to form tight junctions (14, 15). The formation of tight junctions was established by transepithelial electric resistance measurements (TEER) (15). C. jejuni isolates were added at an MOI of 10 at the apical surface of the polarized Caco-2 monolayer on the Transwell filter (Costar Corning). After 72 h, 100-μl samples were taken from the apical and basolateral surfaces and serial dilutions (1:10) were plated on Columbia blood agar plates (Becton, Dickinson). Plates were incubated for 24 h at 37°C in an anaerobic jar under microaerobic conditions using an Anoxomat gas mixer (Mart). Colonies were counted, and the numbers of CFU per milliliter were calculated.
CXCL8 and CXCL10 measurement.
For CXCL8 and CXCL10 measurements, 96-well flat-bottomed plates (Nunc, Roskilde, Denmark) were coated with 3 ng of rabbit anti-human CXCL8 or CXCL10 (Endogen, Cambridge, MA) per well. CXCL8 is interleukin-8 (IL-8) and is a neutrophil recruiter, and CXCL10, also known as gamma interferon-induced protein 10 (IP-10) as opposed to IL-10, is a T-cell recruiter. Overnight culture supernatants from Caco-2 and T84 cells grown to confluence in 6-well plates that had been infected at an MOI of 100 with wild-type isolates (Table 1) the previous day were diluted 1 to 1 in PBS (Invitrogen) and supplemented with 0.1% Tween 20 and bovine serum albumin (3 mg/ml). CXCL8 and CXCL10 were detected and quantified against a calibration line with a biotin-coupled detection antibody (0.5 mg/ml). The assay was developed with avidin-peroxidase (Gibco-BRL, Breda, The Netherlands) (500 mg/ml) and TMB (3,3′,5,5′-tetramethylbenzidine; Sigma) according to the manufacturers' instructions.
Statistical analysis.
Statistical analysis was performed using Instat software (version 2.05a; GraphPad Software, San Diego, CA). Log transformation was used to equalize variances. Differences between GM+ and GM− isolates were tested for significance with a nonparametric Mann-Whitney U test, since column statistics had shown that the Gaussian distributions were unequal for the isolates. A paired t test was used to test for significant differences between the wild type and Δcst-II mutants. For comparisons of the wild-type GB11, knockout mutant GB11Δcst-II, and GB11 (C), an analysis of variance (ANOVA) test was used. A P value < 0.05 indicates statistical significance.
RESULTS
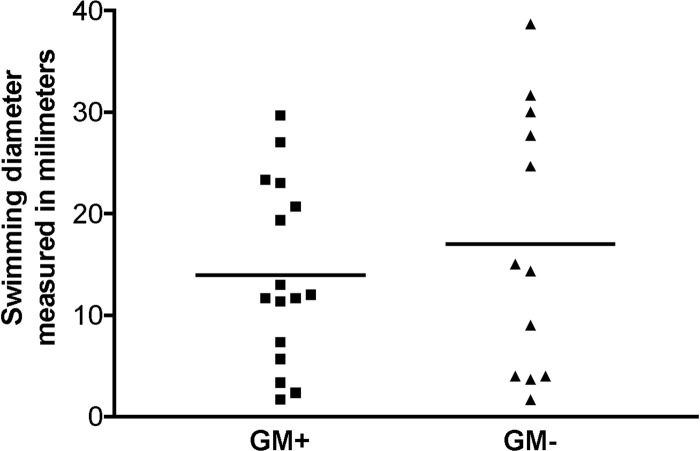
Ganglioside-like LOS expression by cst-II does not affect C. jejuni swimming behavior.
C. jejuni motility is suggested to be of importance for adherence, invasion, and epithelial translocation (13, 44). We analyzed whether differences in motility existed between ganglioside-like LOS-expressing C. jejuni isolates (GM+) and isolates that do not express such mimics (GM−). Swimming behavior was compared for 16 (GM+) and 12 (GM−) isolates in low-percentage agar culture plates. No significant differences in swimming were found between these two groups of isolates (P = 0.67) (Fig. 1). Knockout mutagenesis of a gene essential for the generation of ganglioside-like LOS, sialyltransferase cst-II, confirmed that motility in low-percentage agar culture plates was not dependent on the presence or absence of ganglioside-like LOS, since the wild-type (GM+) isolate GB11 and its cst-II mutant, GB11Δcst-II (GM−), displayed equal swimming diameters, both with a mean of 11 mm.
Fig 1.
Ganglioside-like LOS expression by cst-II does not affect C. jejuni swimming behavior. The plots show the mean swimming diameters in millimeters for 16 GM+ isolates and 12 GM− isolates (three independent experiments). No significant differences in swimming were found between GM+ and GM− isolates (P = 0.67; Mann-Whitney U test).
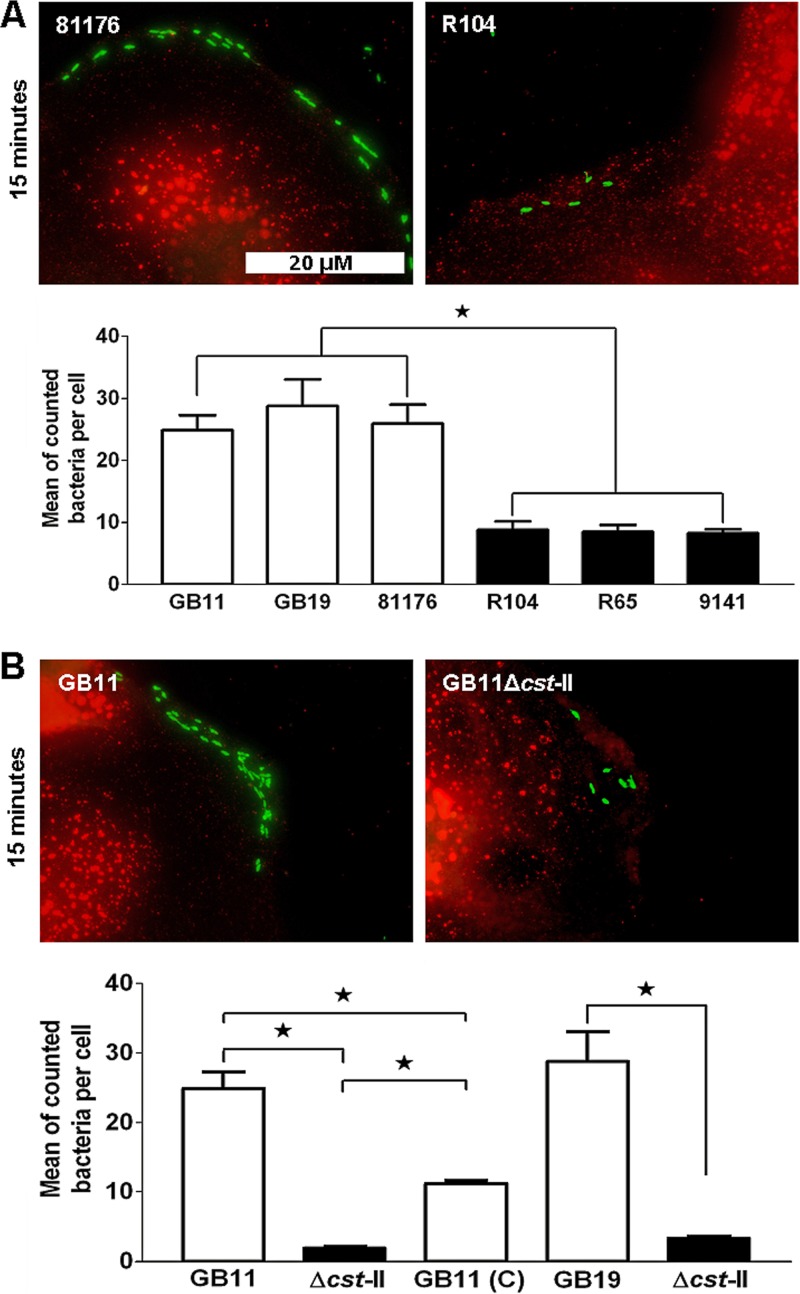
Ganglioside-like LOS increases the number of C. jejuni bacteria bound to Caco-2 cells.
We determined whether C. jejuni ganglioside-like LOS expression had an effect on binding of bacteria to the Caco-2 cells. Microscopic analysis of Caco-2 cells during the first 15 min of infection revealed that isolate 81176 (GM+) bound more efficiently to the Caco-2 cells than isolate R104 (GM−) (Fig. 2). Next, quantitative microscopic analysis showed a 3-fold-increased binding capacity of the GM+ GB11, GB19, and 81176 isolates to the Caco-2 cells in comparison with the GM− isolates R104, R65, and 9141, which all lack ganglioside-like LOS (P < 0.0001) (Fig. 2A). The effect of C. jejuni ganglioside-like LOS expression on binding to the Caco-2 cells was further determined by using Δcst-II mutants. Previously, we showed that loss of the cst-II gene lead to loss of ganglioside-like LOS production (11). Microscopic analysis revealed that the wild-type isolate GB11 (GM+) bound more efficiently to the Caco-2 cells than its Δcst-II mutant (GM−) (Fig. 2). Quantitative microscopic analysis established that the capacity of the GB11Δcst-II and GB19Δcst-II mutants to bind the Caco-2 cells was strongly reduced compared to that of the corresponding wild-type isolates (P < 0.0001) (Fig. 2B). We also found that the cellular binding levels of GB11 (C) were significantly elevated compared to those of the GB11Δcst-II mutant but were still lower than those of the original GB11 wild-type isolate (Fig. 2B), likely because of the absence of GD1 structures in GB11 (C) (see Fig. S1 in the supplemental material). These results show that ganglioside-like LOS contributes strongly to C. jejuni intestinal epithelial cell binding during the first 15 min of infection.
Fig 2.
Ganglioside-like LOS increases the number of C. jejuni bacteria bound to the Caco-2 cells. Visualization of C. jejuni bacteria that were bound to the Caco-2 cells after 15 min of inoculation is shown. C. jejuni was detected using FITC-labeled (green) antibodies. Red-labeled antibodies detected EEA1 in Caco-2 cells. White bars indicate numbers of GM+ bacteria that did bind the Caco-2 cells; black bars indicate binding by GM− bacteria. The differences in binding between GM+ isolates GB11, GB19, and 81176 and GM− isolates R104, R65, and 9141 were significant (Mann-Whitney U test; *, P < 0.0001). The differences in cellular binding between wild-type GB11 (GM+), GB11Δcst-II (GM−), and GB11 (C) (GM+) (one-way ANOVA; *, P < 0.0001) and GB19 and the GB19Δcst-II mutant (paired t test; *, P < 0.0001) were also significant.
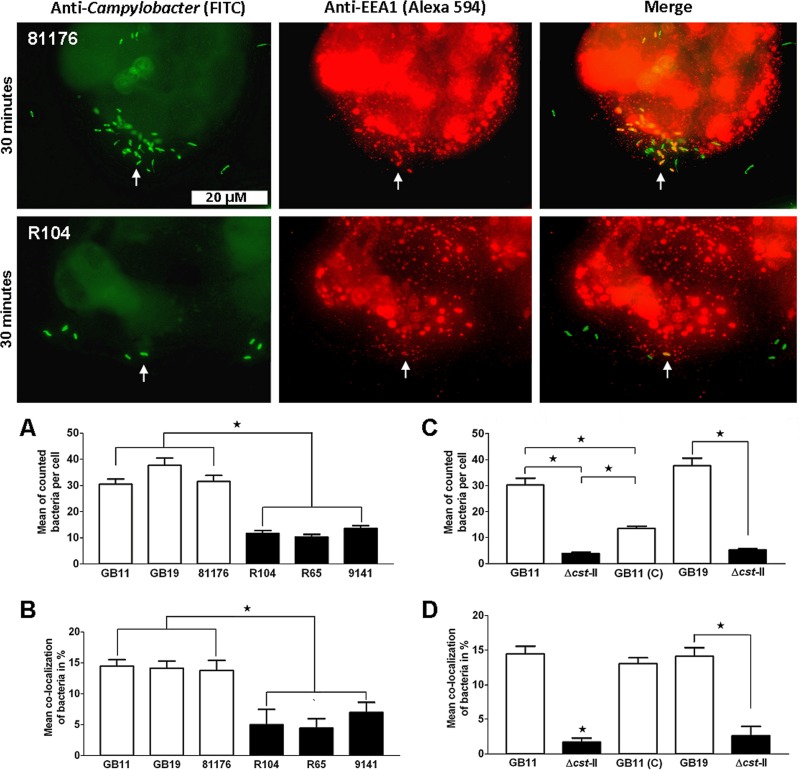
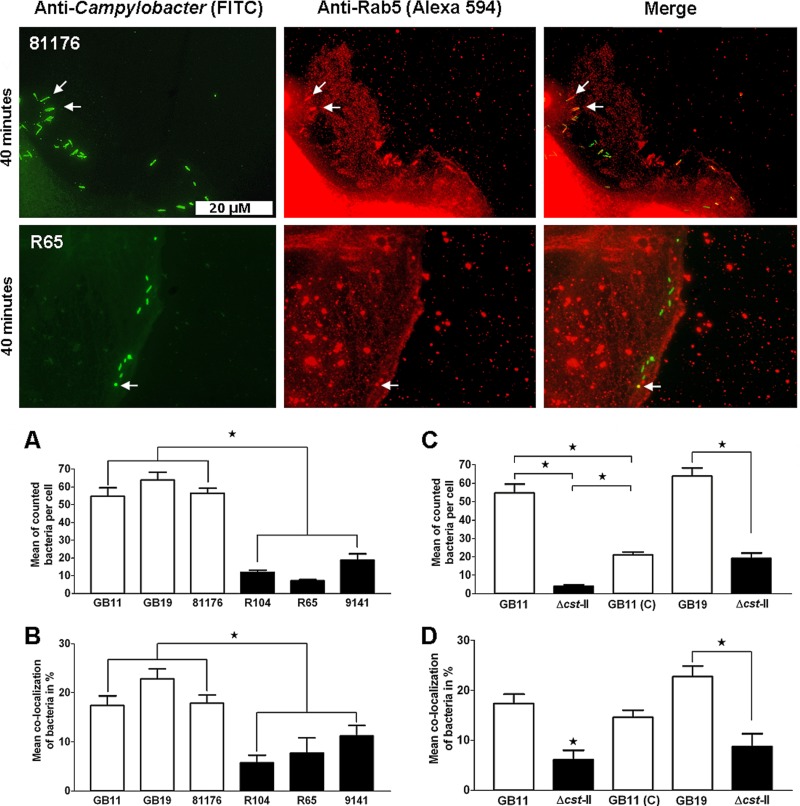
Ganglioside-like LOS enhances C. jejuni endocytosis.
To further explore the involvement of ganglioside-like LOS during the early stages of infection, we determined the colocalization of our isolates with the early endosomal markers EEA1 and Rab5. Microscopic analysis revealed that isolate 81176 (GM+) colocalized in larger numbers with the early endosomal markers EEA1 and Rab5 than isolates R104 and R65 (GM−), respectively (Fig. 3 and Fig. 4). In the time frame used to study EEA1 and Rab5 colocalization with the C. jejuni isolates, we continued to observe that C. jejuni ganglioside-like LOS contributed strongly to Caco-2 cell binding (Fig. 3A and C and 4A and C). Next, all three of GM+ isolates 81176, GB11, and GB19 showed increased colocalization with the early endosomal markers EEA1 and Rab5 in comparison to the GM− isolates R104, R65, and 9141 (P < 0.0001; Fig. 3B and 4B). Comparing the wild-type isolates (GB11 and GB19) and the corresponding Δcst-II mutants (GB11Δcst-II and GB19Δcst-II) and GB11 (C) unambiguously established that the observed differences in colocalization with EEA1 and Rab5 were dependent on ganglioside-like LOS expression by C. jejuni (Fig. 3D and 4D).
Fig 3.
C. jejuni bacteria colocalize in enhanced numbers with EEA1 when they express ganglioside-like LOS. Visualization of C. jejuni bacteria that colocalized with the endosomal marker EEA1 in the Caco-2 cells after 30 min of inoculation is shown. The white arrows indicate examples of colocalization; colocalizing bacteria and EEA1 signals appear yellow in the merged pictures (right). Significant differences in cellular binding and colocalization with EEA1 were observed (A and B) between GM+ isolates GB11, GB19, and 81176 and GM− isolates R104, R65, and 9141 (Mann-Whitney U test; *, P < 0.0001) and (C and D) between wild-type GB11, GB11Δcst-II, and GB11 (C) (one-way ANOVA; *, P < 0.0001) and GB19 and the GB19Δcst-II mutant (paired t test; *, P < 0.0001).
Fig 4.
C. jejuni bacteria colocalize in enhanced numbers with Rab5 when they express ganglioside-like LOS. Visualization of C. jejuni bacteria that colocalized with the endosomal marker Rab5 in the Caco-2 cells after 40 min of inoculation is shown. Significant differences in cellular binding and colocalization with Rab5 were observed (A and B) between GM+ isolates GB11, GB19, and 81176 and GM− isolates R104, R65, and 9141 (Mann-Whitney U test; *, P < 0.0001) and (C and D) between wild-type GB11, GB11Δcst-II, and GB11 (C) (one-way ANOVA; *, P < 0.0001) and GB19 and the GB19Δcst-II mutant (paired t test; *, P < 0.0001).
Rab7 is not involved in C. jejuni endocytosis.
The Rab7 protein is a marker for later stages of endosomal trafficking (41) but is not essential for endosome maturation preceding fusion with lysosomes (3). All GM+ and GM− isolates used in this study were tested at different time points between 15 min and 2 h postinfection (hpi) for colocalization with Rab7. We detected a significant increase in colocalization with Rab7 only for isolate R65 (GM−) after 45 min (results not shown). For the other isolates, no significant colocalization with Rab7 was observed at any point in time used in this study. In summary, Rab7 appeared not to be a good marker to study later stages of endosomal trafficking of C. jejuni in the Caco-2 cells.
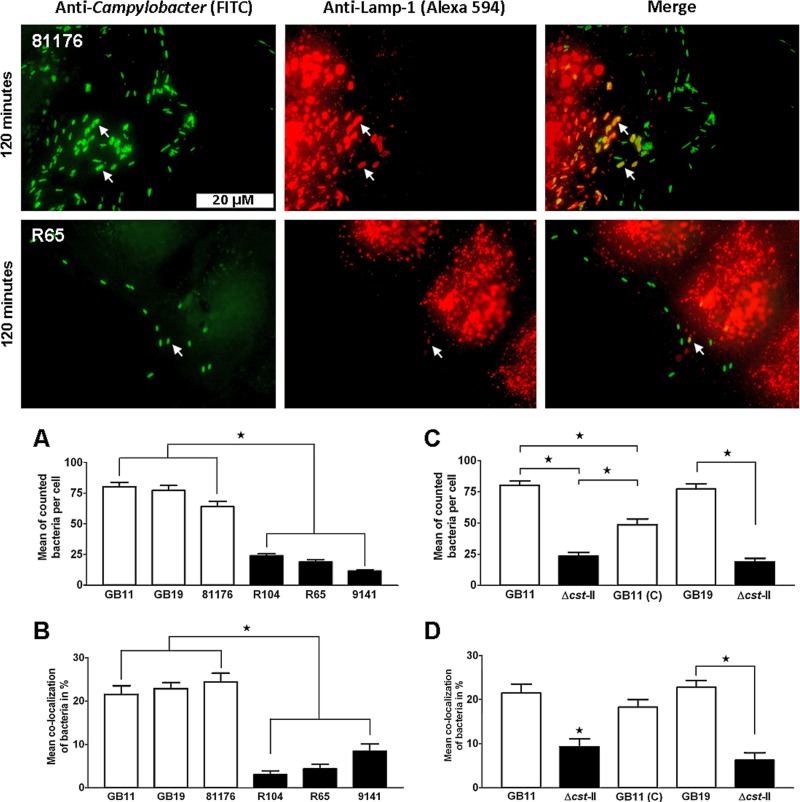
Ganglioside-like LOS-expressing C. jejuni bacteria colocalize in larger numbers with LAMP-1.
We then determined colocalization with LAMP-1, a marker associated with the fusion between late endosomes and lysosomes (39). We observed at 2 hpi that both the 81176 and R65 isolates colocalized with LAMP-1 (Fig. 5). At 2 hpi, we still found that C. jejuni ganglioside-like LOS expression promoted cellular binding during the ongoing C. jejuni infection of the Caco-2 cells (Fig. 5A and C). We screened all isolates at 2 hpi and found that the GM+ GB11, GB19, and 81176 isolates colocalized with LAMP-1 in larger numbers than the GM− R104, R65, and 9141 isolates (Fig. 5B). We also observed that GM+ bacteria tended to cluster in large compartments that colocalized with LAMP-1, a characteristic that was not observed for GM− isolates (Fig. 5). Comparing the wild-type isolates (GB11 and GB19), the Δcst-II mutants (GB11Δcst-II and GB19Δcst-II), and GB11 (C) established that the observed differences in colocalization with LAMP-1 were determined by the expression of ganglioside-like LOS (Fig. 5D).
Fig 5.
C. jejuni bacteria colocalize in enhanced numbers with LAMP-1 when they express ganglioside-like LOS. Visualization of C. jejuni bacteria that associated with the endosomal marker LAMP-1 in the Caco-2 cells after 120 min of inoculation is shown. Significant differences in cellular binding and colocalization with LAMP-1 were observed (A and B) between GM+ isolates GB11, GB19, and 81176 and GM− isolates R104, R65, and 9141 (Mann-Whitney U test; *, P < 0.0001) and (C and D) between wild-type GB11, GB11Δcst-II, and GB11 (C) (one-way ANOVA; *, P < 0.0001) and GB19 and the GB19Δcst-II mutant (paired t test; *, P < 0.0001).
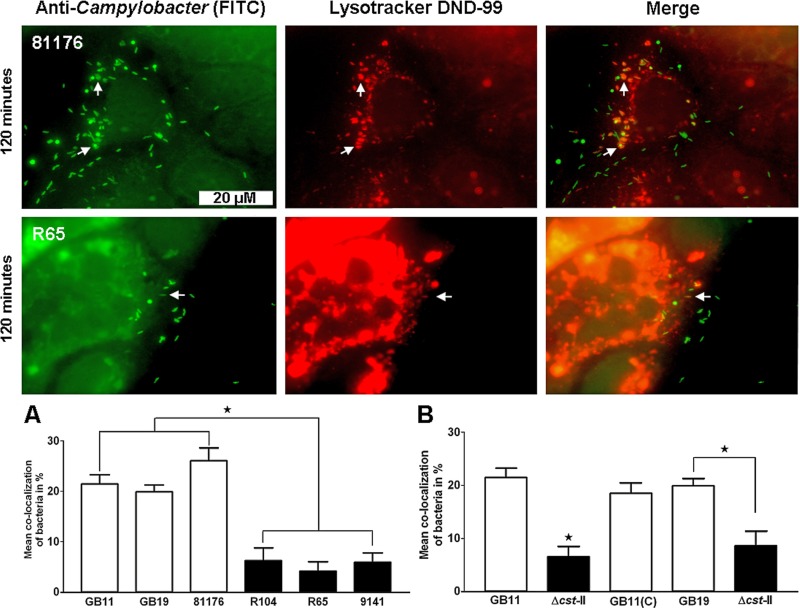
C. jejuni-containing vesicles are acidic.
We explored the physical nature of the C. jejuni-containing vesicles that colocalized with LAMP-1 by using LysoTracker DND-99, a stain specific for acidic (endo-)lysosomes (28). At 2 hpi, we observed that the 81176 (GM+) isolate colocalized in larger numbers with LysoTracker DND-99 in comparison to isolate R65 (GM−) (Fig. 6). To confirm this observation, we tested the GM+ GB11, GB19, and 81176 isolates and the GM− R104, R65, and 9141 isolates for colocalization with LysoTracker DND-99. We found that the GM+ isolates colocalized in larger numbers with LysoTracker DND-99 than the GM− isolates (P < 0.0001) (Fig. 6A). As was observed at 2 hpi for LAMP-1 staining, LysoTracker DND-99 colocalization also revealed that GM+ isolates clustered in large intracellular compartments. As for LAMP-1, we were able to establish by using wild-type isolates (GB11 and GB19), Δcst-II mutants (GB11Δcst-II and GB19Δcst-II), and GB11 (C) that the observed differences in colocalization with Lysotracker DND-99 were dependent on C. jejuni ganglioside-like LOS expression (Fig. 6B).
Fig 6.
C. jejuni-containing vesicles are acidic. Visualization of C. jejuni bacteria that associated with Lysotracker DND-99 in the Caco-2 cells after 120 min of inoculation is shown. Significant differences in colocalization with Lysotracker DND-99 were observed between (A) GM+ isolates (GB11, GB19 and 81176) and GM− isolates (R104, R65, and 9141) (Mann-Whitney U test; *, P < 0.0001) and (B) wild-type GB11, GB11Δcst-II, and GB11 (C) (one-way ANOVA; *, P < 0.0001) and GB19 and the GB19Δcst-II mutant (paired t test; *, P < 0.0001).
To investigate if there was any isolate effect on the acidic nature of the C. jejuni-containing vacuole, we extended the LysoTracker DND-99 study with 14 additional C. jejuni isolates (5 GM+ and 9 GM− isolates) (see Fig. S2 in the supplemental material). We established that the GM+ isolates colocalized in larger numbers with LysoTracker DND-99 than the GM− isolates (P < 0.0001) (see Fig. S2 in the supplemental material). These results confirm that both the GM+ and GM− isolates at 2 hpi were localized in acidic, presumably destructive endolysosomal vesicles in the Caco-2 cells.
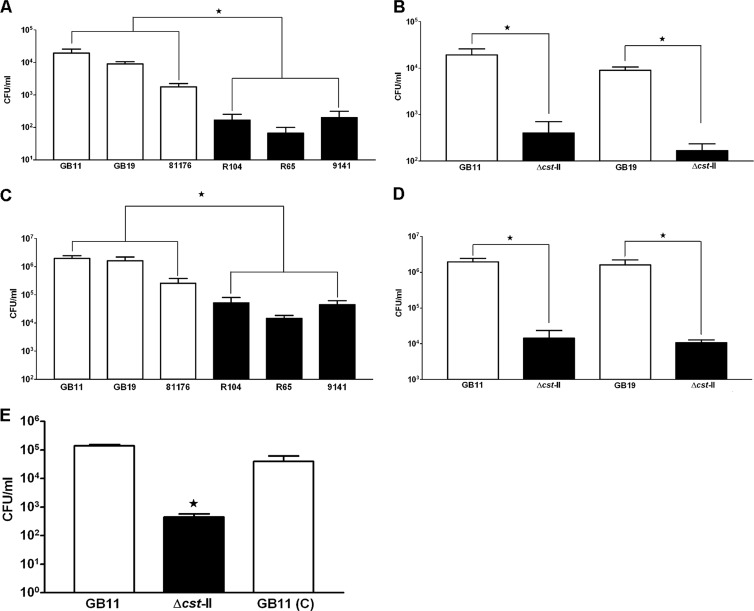
Significant differences in survival and invasion between GM+ and GM− C. jejuni isolates.
A previously published adapted gentamicin killing assay (42) was used to study whether enhanced cellular endocytosis by GM+ isolates correlated with increased intracellular survival. All isolates (GM+ and GM−) showed a 50-to-100-fold reduction in CFU per milliliter at 24 hpi (Fig. 7A and B) compared to the initially retrieved numbers for invasion at 4 hpi of Caco-2 cells (Fig. 7C and D). Intracellular survival rates calculated in percentages revealed no significant differences between GM+ and GM− isolates; only 1% to 3% of the initially invaded GM+ and GM− isolates detected at 4 hpi survived after 24 hpi. In contrast, the total number of intracellular recoverable GM+ bacteria was significantly higher than the number seen with the GM− isolates (P < 0.0001) (Fig. 7A). This finding was confirmed using two wild-type isolates, GB11 and GB19 (GM+), and the corresponding Δcst-II mutants (GM−) (Fig. 7B). Comparing the intracellular survival of GB11 (C) with that of the GB11Δcst-II mutant (GM−) and GB11 wild type (GM+) showed restoration of survival to near-wild-type levels in the survival assays (Fig. 7E). These assays demonstrated that the more C. jejuni bacteria enter the Caco-2 cells, the more survive, a process found to be facilitated by C. jejuni ganglioside-like LOS.
Fig 7.
Significant differences in survival and invasion between GM+ and GM− C. jejuni isolates. The bar graphs show survival of C. jejuni isolates in Caco-2 cells after 24 h for (A) wild-type isolates only and (B) the wild type and Δcst-II mutants and invasion capacity after 4 h for (C) wild-type isolates only and (D) wild-type isolates and Δcst-II mutants. Survival and intracellular recovery are shown as CFU per milliliter (CFU/ml). Data are shown as the standard errors of the means of the results of at least three independent experiments. Differences in survival and invasion between GM+ and GM− (Mann-Whitney U test; *, P < 0.0001) and wild types (GM+) and Δcst-II mutants (GM−) (paired t test; *, P = 0.0039) were significant. (E) Differences in survival between wild-type GB11, GB11Δcst-II, and GB11 (C) after 24 h were significant (one-way ANOVA; *, P = 0.0001).
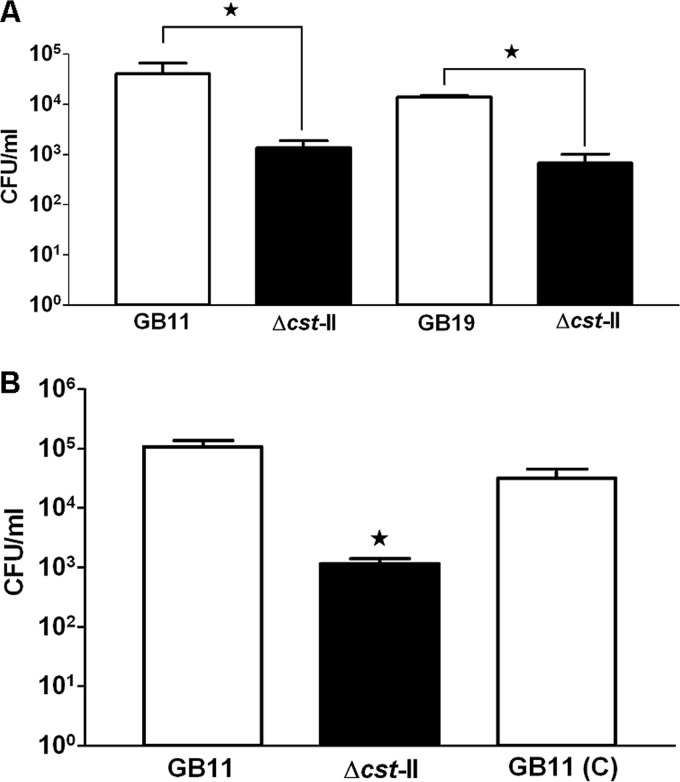
C. jejuni translocation across Caco-2 cells is facilitated by ganglioside-like LOS.
At 72 hpi, C. jejuni isolates GB11 and GB19 (GM+) had translocated more efficiently across differentiated Caco-2 cells than the corresponding Δcst-II mutants (GM−), without disrupting the transepithelial electric resistance (TEER) (see Fig. S3 in the supplemental material). On average, we sampled 4.9 ×104 CFU/ml and 4.4 ×104 CFU/ml translocating bacteria from the basolateral side for GB11 and GB19 (GM+), respectively (Fig. 8A). For the two corresponding Δcst-II mutant strains (GM−), 8.6 ×102 CFU/ml and 4.3 ×102 bacteria, respectively, were sampled, representing results that were lower by factors of 50 to 100 (Fig. 8A). Comparing the translocation efficiency of GB11 (C) (GM+) with that of the GB11Δcst-II mutant (GM−) and GB11 wild-type (GM+) isolate in translocation assays confirmed that C. jejuni ganglioside-like LOS indeed facilitated intestinal epithelial cell translocation (Fig. 8B). In addition, the total numbers of bacteria of the C. jejuni wild-type isolates, the corresponding Δcst-II mutants, and GB11 (C) that had translocated after 72 hpi (Fig. 8A and B) correlated well with the numbers of C. jejuni bacteria that were recovered for each isolate after completion of the survival assays (Fig. 7B and E).
Fig 8.
C. jejuni translocation across Caco-2 cells is facilitated by ganglioside-like LOS. (A) Translocation of C. jejuni isolates across Caco-2 cells was measured as CFU per milliliter after 72 h, using wild-type isolates GB11 and GB19 (GM+) in comparison with their Δcst-II mutants, GB11Δcst-II and GB19Δcst-II (GM−), respectively. Differences in translocation between the wild type (GM+) and the Δcst-II mutant (GM−) were significant (paired t test; *, P < 0.005). (B) Translocation efficiency restored to near-wild-type levels was observed for GB11 (C). Data are shown as the standard errors of the means of the results of at least three independent experiments performed in duplicate. Differences in translocation between GB11, GB11Δcst-II, and GB11 (C) were significant (one-way ANOVA; *, P = 0.0001).
Ganglioside-like LOS-expressing C. jejuni bacteria elevate CXCL10 release from intestinal epithelial cells.
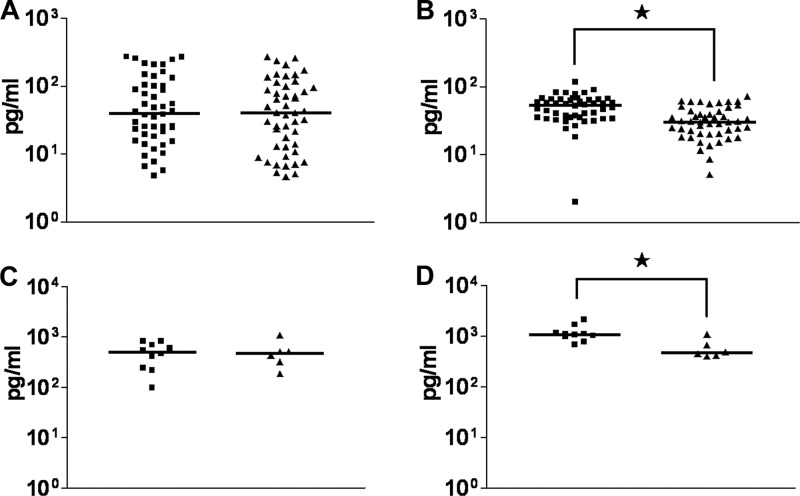
Levels of CXCL8 secretion by Caco-2 cells following infection by GM+ or GM− isolates were not significantly different (Fig. 9A). In contrast, CXCL10 secretion was induced significantly more upon infection by isolates expressing ganglioside-like LOS in comparison with GM− isolates (P < 0.0001) (Fig. 9B). To confirm the generality of this finding, a subset of 10 GM+ and 6 GM− isolates was used to test their capacity to induce secretion of CXCL8 and CXCL10 using a different intestinal epithelial cell line, T84. Using this cell line established, as for the Caco-2 cells, that the levels of CXCL8 secretion were not significantly different upon infection by GM+ or GM− isolates, whereas for CXCL10 the secretion was significantly more elevated upon infection by GM+ isolates compared to GM− isolates (P = 0.0047) (Fig. 9C and D).
Fig 9.
Ganglioside-like LOS-expressing C. jejuni bacteria elevate the CXCL10 release from intestinal epithelial cells. (A) CXCL8 and (B) CXCL10 secretion from the Caco-2 cells was analyzed after infection with 32 C. jejuni isolates (Table 1). Scatter plots show the measurements of the results of 3 independent experiments. (C) CXCL8 and (D) CXCL10 secretion from the T84 cells. Blocks represent the GM+ C. jejuni isolates; triangles represent the GM− C. jejuni isolates. An asterisk (*) indicates a significant difference in released secretion of the corresponding chemokine from the Caco-2 cells (Mann-Whitney U test; P < 0.0001) or the T84 cells (Mann-Whitney U test; P < 0.0047). For both of the untreated cell lines (Caco-2 and T84), CXCL8 secretion and CXCL10 secretion in the culture supernatants were nondetectable.
DISCUSSION
Ganglioside-like LOS expression by C. jejuni has been shown to correlate with disease complications in humans in different studies (11, 32, 37), but the pathogenic mechanism(s) behind this process remains obscure. In the present study, we extended our previous findings (11, 18, 20) aimed at characterizing the role of ganglioside-like LOS expression in C. jejuni pathogenesis and induction of disease complications. Some intracellular bacteria have developed mechanisms to avoid delivery to lysosomes in intestinal epithelial cells to be able to survive, replicate, and translocate (3, 16). Earlier, it was shown that C. jejuni isolate 81176 survives in the intracellular environment by lysosomal escape (42). In this study, we were able to link ganglioside-like LOS expression with increased endocytosis into and translocation across Caco-2 cells but not with lysosomal escape.
So far, C. jejuni lysosomal escape has been visualized only with endosomal protein markers in nonintestinal epithelial cells (4, 42). In our study, using Caco-2 cells we could clearly demonstrate that GM+ and GM− C. jejuni bacteria are located in an acidic intracellular compartment with endolysosome characteristics wherein most C. jejuni bacteria are killed. Our finding that only a small percentage (1% to 3%) of the C. jejuni bacteria (GM+ and GM−) survived at 24 hpi suggests that C. jejuni survival in the Caco-2 cells depends on the number of endocytosed bacteria rather than on differential intracellular survival of GM+ versus GM− isolates. A simple explanation for this finding could be that Caco-2 cells do not have enough “endosomal capacity” to clear all infecting bacteria. Data showing that C. jejuni bacteria are completely eradicated by professional phagocytic cells such as macrophages (42) give support to this notion.
In the present study, we corroborated with our endosomal analysis the findings of Hu et al., who observed that less than 4% of the Caco-2 cells in their study became infected by C. jejuni isolate 81176, depending on the MOI used (22). Next, those authors observed by electron microscopy that the number of internalized C. jejuni bacteria ranged between 1 and 20 bacteria per infected Caco-2 cell (22), a variation we also observed for our 81176 isolate in our colocalization experiments. The low number of infected Caco-2 cells could form an explanation in the present study for the large number of GM+ C. jejuni bacteria bound to the Caco-2 cells with respect to the MOI used. Earlier, Hu et al. had already speculated that this low number of infected Caco-2 cells might be related to the expression of a specific surface receptor, making some of the Caco-2 cells hypersusceptible for C. jejuni (22).
Our Transwell experiments clearly showed that the differentiated Caco-2 cells are an efficient barrier blocking C. jejuni translocation, because after 72 hpi, only a small percentage of the inoculum used translocated across differentiated Caco-2 monolayers. In line with previous studies (22, 26, 45), our data also provide support for a transcytosis-mediated model for C. jejuni translocation across host epithelia, including apical endocytosis and basolateral exocytosis.
First, in the time line of our translocation assay, we did not observe any significant change in the TEER, a strong indication that the tight junctions of the differentiated Caco-2 cells remained intact (15) in the presence of C. jejuni. Second, visualization of the C. jejuni endocytosis process with the endosomal markers EEA1, Rab5, LAMP-1, and Lysotracker DND-99 showed that C. jejuni bacteria are transported via the cellular endosomal pathway. Finally, the numbers of intracellular surviving C. jejuni bacteria cultured from dome-forming Caco-2 cell monolayers in a multiwell culture plate corresponded with the numbers of C. jejuni bacteria translocating across differentiated Caco-2 cells in a Transwell system. Of course, we have to be careful in comparing such experiments concerning the potential differences in the Caco-2 cell culture methods and differentiation status. On the other hand, in our opinion, the fact that similar numbers for survival and translocation were observed suggests that the mechanism of C. jejuni translocation across Caco-2 cells is favorable for cellular transcytosis, a process we here show to be facilitated by ganglioside-like LOS.
Next, we were able to show that enhanced endocytosis of ganglioside-like LOS-expressing C. jejuni bacteria was linked with increased secretion of CXCL10 by intestinal epithelial cells. Our findings on CXCL10 corroborate a previous study showing that the intestinal epithelial internalization capacity of the C. jejuni isolates correlated with the amount of CXCL10 release (25). Future in vivo studies are necessary to investigate if this link between the expression of ganglioside-like LOS, the enhanced internalization capacity of C. jejuni, and the amount of CXCL10 secretion is of clinical relevance.
In our experiments, phase variation could have influenced ganglioside-like LOS expression. Earlier, interaction studies of comparisons between C. jejuni and sialic acid binding immunoglobulin-like lectins by Heikema et al. established that the strains, some of which were used in the present study, still express functional membrane-bound ganglioside-like LOS (20). Furthermore, mass spectrometry using exactly the same bacterial glycerol stocks as used in the present study had demonstrated the GM+ and GM− LOS structures of the isolates (8, 12) used in this study. Most importantly, the results obtained with the wild types, cst-II mutants, and complemented cst-II mutant show that any effect of phase variation on the study results has been negligible.
Based on our results, we hypothesize that the effect of C. jejuni ganglioside-like LOS on cellular endocytosis, increased translocation, and elevated CXCL10 secretion could form a mechanistic basis for the severe gastroenteritis phenotype that has been observed in patients infected with GM+ C. jejuni isolates (32).
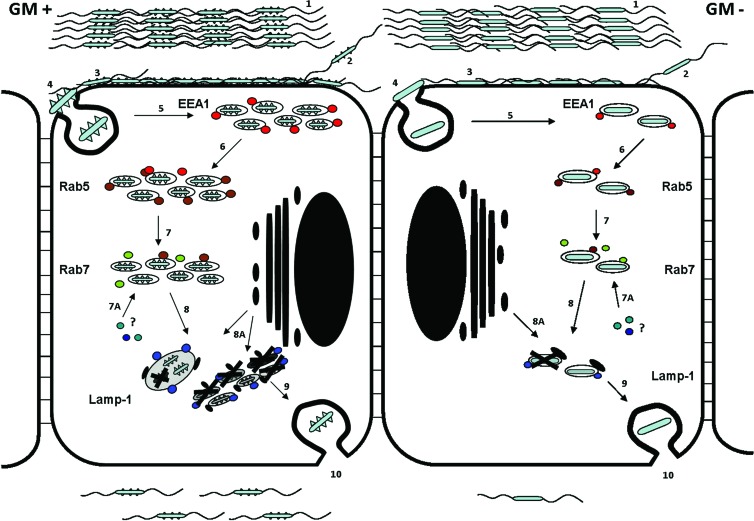
We conclude that C. jejuni translocation across intestinal epithelial cells is facilitated by ganglioside-like LOS, as depicted in a graphical model (Fig. 10), and propose that ganglioside-like LOS belongs to the repertoire of virulence factors that are used by a subset of C. jejuni bacteria to interact with the host epithelium.
Fig 10.
Schematic model for C. jejuni ganglioside-like LOS-dependent cellular endocytosis, survival, and translocation. 1, equal amounts of C. jejuni bacteria express ganglioside-like LOS (GM+; left panel) or do not express ganglioside-like LOS (GM−. right panel) before adherence to an intestinal epithelial cell. 2, the translocation process starts by C. jejuni bacteria attaching to the Caco-2 cell at the apical surface. 3, C. jejuni ganglioside-like LOS is used to bind onto the cell membrane of the Caco-2 cell. 3, GM+ C. jejuni adhere 2-to-3-fold more efficiently to epithelial cells. 4, GM+ LOS enhances the cellular entry process (endocytosis) based on colocalization studies using the early endosomal markers EEA1 and Rab5. 5, early endosomal antigen 1 (EEA1; red dots) colocalizes with C. jejuni-containing endosomes 30 min postinfection. 6, Rab5 (brown dots) colocalizes C. jejuni-containing endosomes 40 min postinfection. 5 and 6, higher numbers of GM+ bacteria colocalize with the endosomal markers EEA1 and Rab5, reflecting increased endocytosis, compared to GM− isolates. 7, 45 min postinfection, Rab7 (green dots) colocalizes to some GM− bacteria, but most of the GM+ or GM− C. jejuni isolates do not colocalize with Rab7 within the first 2 h of infection. 7A, maturation of the endosome does not depend on Rab7; other factors (Rab GTPases) may associate with the C. jejuni-containing endosome to enable fusion with lysosomes. 8 and 8A, at 2 hpi, LAMP-1 (blue dots) colocalizes with the C. jejuni-containing vesicle and lysosomes (black dots) fuse with the C. jejuni-containing endosome. At 2 hpi, C. jejuni-containing endosomes show fusion when the contained bacteria are GM+, leading to formation of larger endosomes containing multiple bacteria. LAMP-1 and LysoTracker DND-99 colocalization studies reveal that the C. jejuni-containing vesicles have strong characteristics of an endolysosome. 9, only 1% to 3% of the invading bacteria survive and translocate at the basolateral surface at 72 hpi. 10, at 72 hpi, higher absolute numbers of GM+ C. jejuni isolates translocate compared to GM− isolates.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Human Frontier Science Program (RGP 38/2003).
We thank Michel Gilbert, Dennis Brochu, and Jianjun Li (NRC, Ottawa, Canada) for the mass spectrometry analysis of the LOS.
Footnotes
Published ahead of print 9 July 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Bacon DJ, et al. 2001. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81176. Mol. Microbiol. 40:769–777 [DOI] [PubMed] [Google Scholar]
- 2. Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472–479 [DOI] [PubMed] [Google Scholar]
- 3. Brumell JH, Scidmore MA. 2007. Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol. Mol. Biol. Rev. 71:636–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buelow DR, Christensen JE, Neal-McKinney JM, Konkel ME. 2011. Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol. Microbiol. 80:1296–1312 doi:10.1111/j.1365-2958.2011.07645.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen ML, Ge Z, Fox JG, Schauer DB. 2006. Disruption of tight junctions and induction of pro-inflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect. Immun. 74:6581–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu CP, et al. 2004. Structural analysis of the sialyltransferase Cst-II from Campylobacter jejuni in complex with a substrate analogue. Nat. Struct. Mol. Biol. 11:163–170 [DOI] [PubMed] [Google Scholar]
- 7. De Melo MA, Gabbiani G, Pechere JC. 1989. Cellular events and intracellular survival of Campylobacter jejuni during infection of HEp-2 cells. Infect. Immun. 57:2214–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dzieciatkowska M, et al. 2008. Rapid method for sensitive screening of oligosaccharide epitopes in the lipooligosaccharide from Campylobacter jejuni strains isolated from Guillain-Barré syndrome and Miller Fisher syndrome patients. J. Clin. Microbiol. 46:3429–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eskelinen EL. 2006. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Aspects Med. 27:495–502 doi:10.1016/j.mam.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 10. Eskelinen EL, Tanaka Y, Saftig P. 2003. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13:137–145 doi:10.1016/S0962-8924(03)00005-9 [DOI] [PubMed] [Google Scholar]
- 11. Godschalk PC, et al. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J. Clin. Invest. 114:1659–1665 doi:10.1172/JCI200415707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Godschalk PC, et al. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect. Immun. 75:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grant CC, Konkel ME, Cieplak W, Jr, Tompkins LS. 1993. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in non-polarized and polarized epithelial cell cultures. Infect. Immun. 61:1764–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grasset E, Bernabeu J, Pinto M. 1985. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am. J. Physiol. 248:C410–C418 [DOI] [PubMed] [Google Scholar]
- 15. Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. 1984. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am. J. Physiol. 247:C260–C267 [DOI] [PubMed] [Google Scholar]
- 16. Gruenberg J, van der Goot FG. 2006. Mechanisms of pathogen entry through the endosomal compartments. Nat. Rev. Mol. Cell Biol. 7:495–504 [DOI] [PubMed] [Google Scholar]
- 17. Guerry P, et al. 2002. Phase variation of Campylobacter jejuni 81176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect. Immun. 70:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Habib I, et al. 2009. Correlation between genotypic diversity, lipooligosaccharide gene locus class variation, and Caco-2 cell invasion potential of Campylobacter jejuni isolates from chicken meat and humans: contribution to virulotyping. Appl. Environ. Microbiol. 75:4277–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamasaki M, Yoshimori T. 2010. Where do they come from? Insights into autophagosome formation. FEBS Lett. 584:1296–1301 doi:10.1016/j.febslet.2010.02.061 [DOI] [PubMed] [Google Scholar]
- 20. Heikema AP, et al. 2010. Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect. Immun. 78:3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hidalgo IJ, Raub TJ, Borchardt RT. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749 [PubMed] [Google Scholar]
- 22. Hu L, Tall BD, Curtis SK, Kopecko DJ. 2008. Enhanced microscopic definition of Campylobacter jejuni 81176 adherence to, invasion of, translocation across, and exocytosis from polarized human intestinal Caco-2 cells. Infect. Immun. 76:5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang J, Brumell JH. 2009. Autophagy in immunity against intracellular bacteria. Curr. Top. Microbiol. Immunol. 335:189–215 [DOI] [PubMed] [Google Scholar]
- 24. Jin S, et al. 2001. JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol. Microbiol. 39:1225–1236 [DOI] [PubMed] [Google Scholar]
- 25. Johanesen PA, Dwinell MB. 2006. Flagellin-independent regulation of chemokine host defense in Campylobacter jejuni-infected intestinal epithelium. Infect. Immun. 74:3437–3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalischuk LD, Leggett F, Inglis GD. 2010. Campylobacter jejuni induces transcytosis of commensal bacteria across the intestinal epithelium through M-like cells. Gut Pathog. 2:14 doi:10.1186/1757-4749-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Konkel ME, Mead DJ, Hayes SF, Cieplak W., Jr 1992. Translocation of Campylobacter jejuni across human polarized epithelial cell monolayer cultures. J. Infect. Dis. 166:308–315 [DOI] [PubMed] [Google Scholar]
- 28. Lemieux B, Percival MD, Falgueyret JP. 2004. Quantitation of the lysosomotropic character of cationic amphiphilic drugs using the fluorescent basic amine Red DND-99. Anal. Biochem. 327:247–251 [DOI] [PubMed] [Google Scholar]
- 29. Louwen R, et al. 2008. The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect. Immun. 76:4431–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MacCallum A, Hardy SP, Everest PH. 2005. Campylobacter jejuni inhibits the absorptive transport functions of Caco-2 cells and disrupts cellular tight junctions. Microbiology 151:2451–2458 doi:10.1099/mic.0.27950-0 [DOI] [PubMed] [Google Scholar]
- 31. Miaczynska M, Zerial M. 2002. Mosaic organization of the endocytic pathway. Exp. Cell Res. 272:8–14 doi:10.1006/excr.2001.5401 [DOI] [PubMed] [Google Scholar]
- 32. Mortensen NP, et al. 2009. Sialylation of Campylobacter jejuni lipooligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 11:988–994 doi:10.1016/j.micinf.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 33. Oelschlaeger TA, Guerry P, Kopecko DJ. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. U. S. A. 90:6884–6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pei Z, Blaser MJ. 1993. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homologue of the binding component in gram-negative nutrient transport systems. J. Biol. Chem. 268:18717–18725 [PubMed] [Google Scholar]
- 35. Pei Z, et al. 1998. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect. Immun. 66:938–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pei ZH, Ellison RT, III, Blaser MJ. 1991. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J. Biol. Chem. 266:16363–16369 [PubMed] [Google Scholar]
- 37. Perera VN, et al. 2007. Molecular mimicry in Campylobacter jejuni: role of the lipooligosaccharide core oligosaccharide in inducing anti-ganglioside antibodies. FEMS Immunol. Med. Microbiol. 50:27–36 [DOI] [PubMed] [Google Scholar]
- 38. Russell RG, Blaser MJ, Sarmiento JI, Fox J. 1989. Experimental Campylobacter jejuni infection in Macaca nemestrina. Infect. Immun. 57:1438–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saftig P, Schroder B, Blanz J. 2010. Lysosomal membrane proteins: life between acid and neutral conditions. Biochem. Soc. Trans. 38:1420–1423 [DOI] [PubMed] [Google Scholar]
- 40. Simonsen A, et al. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394:494–498 doi:10.1038/28879 [DOI] [PubMed] [Google Scholar]
- 41. Vonderheit A, Helenius A. 2005. Rab7 associates with early endosomes to mediate sorting and transport of Semliki Forest virus to late endosomes. PLoS Biol. 3:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watson RO, Galan JE. 2008. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wooldridge KG, Williams PH, Ketley JM. 1996. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 21:299–305 [DOI] [PubMed] [Google Scholar]
- 44. Yao R, et al. 1994. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol. Microbiol. 14:883–893 [DOI] [PubMed] [Google Scholar]
- 45. Zheng J, Meng J, Zhao S, Singh R, Song W. 2008. Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB. Infect. Immun. 76:4498–4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.