Abstract
Burkholderia pseudomallei is a Gram-negative soil bacterium and the causative agent of melioidosis, a disease of humans and animals. It is also listed as a category B bioterrorism threat agent by the U.S. Centers for Disease Control and Prevention, and there is currently no melioidosis vaccine available. Small modified nucleotides such as the hyperphosphorylated guanosine molecules ppGpp and pppGpp play an important role as signaling molecules in prokaryotes. They mediate a global stress response under starvation conditions and have been implicated in the regulation of virulence and survival factors in many bacterial species. In this study, we created a relA spoT double mutant in B. pseudomallei strain K96243, which lacks (p)ppGpp-synthesizing enzymes, and investigated its phenotype in vitro and in vivo. The B. pseudomallei ΔrelA ΔspoT mutant displayed a defect in stationary-phase survival and intracellular replication in murine macrophages. Moreover, the mutant was attenuated in the Galleria mellonella insect model and in both acute and chronic mouse models of melioidosis. Vaccination of mice with the ΔrelA ΔspoT mutant resulted in partial protection against infection with wild-type B. pseudomallei. In summary, (p)ppGpp signaling appears to represent an essential component of the regulatory network governing virulence gene expression and stress adaptation in B. pseudomallei, and the ΔrelA ΔspoT mutant may be a promising live-attenuated vaccine candidate.
INTRODUCTION
Burkholderia pseudomallei, a Gram-negative, motile, aerobic bacterium of the class betaproteobacteria, is widely distributed throughout the biosphere and is the causative agent of the life-threatening disease melioidosis (7). Infection with B. pseudomallei presents with a variety of nonspecific symptoms and can range from acute disease, which is rapidly fatal, to chronic or latent (i.e., asymptomatic) infections, which last for several decades (9). Despite antibiotic treatment, mortality rates for acute melioidosis can be 40 to 50% in areas where the disease is endemic, and relapse rates in surviving individuals are also high [for a review, see the work of Wiersinga et al. (48)]. Currently, there is no vaccine, and therefore, B. pseudomallei is listed as a category B select agent by the U.S. Centers for Disease Control and Prevention.
Guanosine tetra- and pentaphosphates, collectively referred to as ppGpp, are small signaling molecules that are produced in response to various stress conditions such as amino acid starvation [for reviews see the works of Cashel et al. (6), Magnusson et al. (27), Potrykus and Cashel (39), and Dalebroux et al. (12)]. ppGpp signaling is widespread across various genera of the bacterial kingdom but can also be found in plant chloroplasts (4). The ppGpp response has been extensively studied in Escherichia coli. In this organism, stress-induced ppGpp interacts with the RNA polymerase core enzyme and elicits a downregulation of ribosomal proteins and DNA replication and an upregulation of amino acid synthesis operons and genes involved in adaptation to stasis. This so-called “stringent response” mechanism causes the bacterial cell to stall growth and to redirect its resources to promote survival mechanisms until nutrient conditions improve.
In many gammaproteobacteria, ppGpp is synthesized from GTP and ATP by two enzymes, RelA and SpoT (see Fig. 1B). The latter is a bifunctional enzyme that has both synthetase and hydrolase activities. Deletion of the spoT gene is lethal due to toxic accumulation of ppGpp in the cell. On the other hand, deletion of both the relA and spoT genes has a dramatic impact on gene expression profiles: microarray analyses of strains lacking ppGpp-synthesizing enzymes have revealed deregulation of several hundred genes (18, 34, 46), thereby highlighting the role of ppGpp as a global signaling molecule.
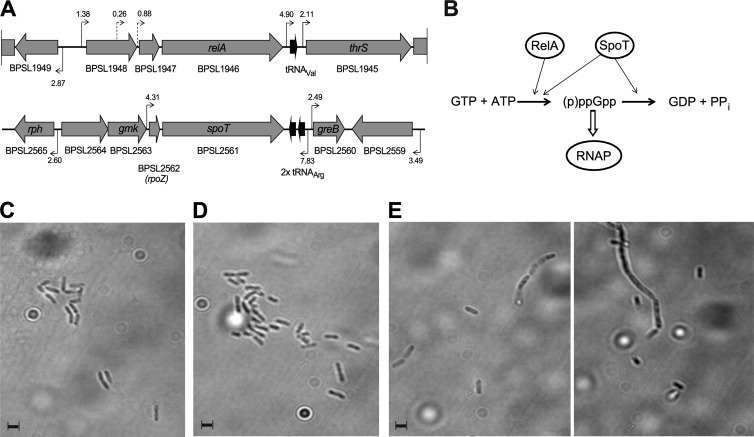
Fig 1.
Genetic organization and enzymatic activities of RelA-SpoT family proteins in B. pseudomallei and cell morphology of RelA-SpoT family mutants. (A) Schematic overview of the chromosomal regions surrounding the relA gene (BPSL1946 [top]) and the spoT gene (BPSL2561 [bottom]). Predicted promoters and their linear discriminant function (LDF) score are indicated. (B) Schematic of the classical synthesis pathway of (p)ppGpp. pppGpp is produced from GTP and ATP by either RelA- or SpoT-dependent mechanisms and is subsequently converted to ppGpp. ppGpp interacts directly with the RNA polymerase enzyme (RNAP) and thus affects gene expression. The effects of ppGpp induction are relieved by SpoT-mediated hydrolysis of ppGpp. (C to E) Cell morphology of B. pseudomallei wild-type strain K96243 (C), a K96243 ΔrelA single mutant (D), and a K96243 ΔrelA ΔspoT double mutant (E). All strains were grown to early stationary growth phase (24-h incubation at 37°C) in LB broth, and bacterial cells were visualized by bright-field microscopy at a magnification of ×100. The scale bars represent the average length of K96243 wild-type cells. In panel E, two images of K96243 ΔrelA ΔspoT mutant cells are presented, one focused on single cells (left) and one focused on a filament (right).
The diverse phenotypes of ppGpp-deficient strains indicate that this molecule affects various cellular processes, many involved in survival and adaptation to stress conditions but also others, such as sporulation, competence, quorum sensing, and virulence [reviewed by Magnusson et al. (27), Braeken et al. (5), and Dalebroux et al. (12)]. For example, in pathogenic bacteria such as Francisella tularensis, Brucella spp., Campylobacter jejuni, Legionella pneumophila, Helicobacter pylori, and Mycobacterium tuberculosis, the absence of ppGpp-synthesizing enzymes results in defects in stationary-phase survival, defects in intracellular replication, and attenuation in animal models of disease (10, 11, 16, 17, 21, 24, 41, 49).
In addition to their greatly reduced virulence, strains defective in ppGpp synthesis have been shown to confer protective immunity against challenge with wild-type bacteria. This is the case in Salmonella enterica serovar Typhimurium (33), Salmonella enterica serovar Gallinarum (37), Francisella tularensis (16), and Yersinia pestis (44). In Y. pestis, protection was conferred via a mixture of Th1 and Th2 immune responses, as measured by IgG1 and IgG2a levels (44). In S. Typhimurium, the ppGpp mutant elicited serum and mucosal antibody responses, measured by IgA and IgG titers, as well as cellular immune responses, measured by a delayed-type hypersensitivity (DTH) response (33). Similarly, in S. enterica, vaccination induced a B-cell response in blood measured by IgM, IgA, and IgG levels and induced CD4+ and CD8+ T-cell proliferation and gamma interferon (IFN-γ) and transforming growth factor (TGF)-β4 production in spleens (37). This may render ppGpp-deficient Salmonella strains promising candidates as live-attenuated vaccines against typhoid in chicken.
Here, we evaluated a ΔrelA ΔspoT double mutant of B. pseudomallei strain K96243 as a vaccine candidate. Live-attenuated vaccines offer the advantage of containing complex epitopes, thus stimulating both the antibody- and cell-mediated arms of the immune system. This is of particular importance with facultative intracellular pathogens such as B. pseudomallei. Moreover, live-attenuated vaccines usually confer strong and long-lasting immunity. Several live-attenuated vaccine strains of B. pseudomallei have been tested to date [reviewed by Sarkar-Tyson and Titball (42) and Patel et al. (38)]. Most of them are mutants with defects in the intracellular life stages, such as mutations in biosynthetic pathways or type III secretion systems. For example, the most successful B. pseudomallei vaccine strain characterized to date, strain 2D2, carries a mutation in the ilvI gene, which renders the strain auxotrophic for the synthesis of branched-chain amino acids (3). However, none of the live-attenuated vaccine candidates tested to date are able to confer sterilizing immunity or are able to protect against chronic disease (38). This demonstrates the need to assess whether different classes of mutants exhibit improved protective efficacy.
Here, we targeted an important signaling system, namely, synthesis of the alarmone ppGpp, for mutagenesis in B. pseudomallei. We found that the ΔrelA ΔspoT derivative was severely attenuated in both acute and chronic models of disease, and subsequently, we tested the ability of the mutant to confer protective immunity. Our results from protection studies in mice indicated that this mutant might be a medically relevant addition to the list of live-attenuated vaccines against melioidosis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains and plasmids used in this study are listed in Table 1. Unless stated otherwise, bacteria were grown with aeration in Luria broth (LB) at 37°C. When required, the antibiotics chloramphenicol (Sigma-Aldrich, Gillingham, United Kingdom), kanamycin (Sigma-Aldrich), and gentamicin (Sigma-Aldrich) were used at concentrations of 25 μg/ml, 50 μg/ml, and 50 μg/ml, respectively.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| B. pseudomallei strains | ||
| K96243 | Clinical isolate from Thailand | 23 |
| K96243 ΔrelA | Inactivation of BPSL1946 (relA) by complete deletion | This study |
| K96243 ΔrelA ΔspoT | Inactivation of BPSL1946 (relA) and BPSL2561 (spoT) by complete deletion | This study |
| 576 | Clinical isolate from Thailand | 2 |
| 2D2 | Auxotroph transposon mutant of B. pseudomallei 576 | 3 |
| E. coli strains | ||
| DH5 Δpir | recA1 gyrA (Nal) Δ(lacIZYA-argF) (ϕ80dlacΔ[lacZ]M15) pirRK6 | 43 |
| S17-1 Δpir | RPA-2 tra regulon; pirRK6 Smr Tpr | 43 |
| Plasmids | ||
| pDM4 | Suicide vector with R6K origin; Cmr | 29 |
| pDM4-ΔrelA | 600 bp up- and downstream of relA cloned into pDM4 via XhoI/SphI sites | This study |
| pDM4-ΔspoT | 600 bp up- and downstream of spoT cloned into pDM4 via SalI/XmaI sites | This study |
Mutant construction.
In-frame deletion mutants were constructed using a suicide plasmid containing regions homologous to up- and downstream regions of the target genes (26). Amplified DNA fragments used for constructing the suicide plasmid derivatives were generated by recombinant PCR. Briefly, 600 bp up- and downstream fragments of the relA (BPSL1946) and spoT (BPSL2561) genes of B. pseudomallei strain K96243 were PCR amplified using the primer combinations relA-1 and relA-2 (upstream), relA-3 and relA-4 (downstream), spoT-1 and spoT-2 (upstream), and spoT-3 and spoT-4 (downstream), respectively, and K96243 genomic DNA as a template. A second, recombinant PCR was performed using the outside primers (relA-1 plus relA-4 and spoT-1 plus spoT-4) and the respective up- and downstream PCR fragments from the first PCR as templates. The resulting 1.2-kb recombinant PCR products were cloned into the suicide vector pDM4 via its XhoI/SphI and SalI/XmaI sites, respectively. The presence of the recombinant PCR fragments in the resulting plasmids pDM4-ΔrelA and pDM4-ΔspoT was confirmed by PCR using primer combinations relA-up plus relA-down and spoT-up plus spoT-down, respectively. All plasmids were maintained in E. coli DH5 λpir cells. The sequences of all primers are listed in Table 2.
Table 2.
Oligonucleotides used in this study
| Name | Sequence (5′–3′)a | Purpose |
|---|---|---|
| relA-1-fw | CCCCTCGAGGAAGGCGCGCGGACAAGCGG | Mutagenesis |
| relA-2-rv | CGGCGCTCACTGCAGCATCGTCGGGTATGGCCAGTT | Mutagenesis |
| relA-3-fw | CCGACGATGCTGCAGTGAGCGCCGCCGGTGGGCCGC | Mutagenesis |
| relA-4-rv | CCCGCATGCCGTATCGACGAGTTCGCCGTC | Mutagenesis |
| spoT-1-fw | GCGGTCGACCTGCCGCCGTCGCTCGCGGC | Mutagenesis |
| spoT-2-rv | GCGGCGCTAACTAGTCATTTTCGCCTCCTGGGTTCG | Mutagenesis |
| spoT-3-fw | GCGAAAATGACTAGTTAGCGCCGCGCGGCGCCCGAC | Mutagenesis |
| spoT-4-rv | GCGCCCGGGGCCTGCGCGAAATCGAGGTCG | Mutagenesis |
| relA-up-fw | ACAACGGCACCGTCTATCTC | Confirmation of plasmids |
| relA-down-rv | GCGGTGTCATAACCGTATTC | Confirmation of plasmids |
| spoT-up-fw | CTCGCATTACCGTTGAAGAC | Confirmation of plasmids |
| spoT-down-rv | GCCCTTTCAGTTCGAAGTTG | Confirmation of plasmids |
| relA-3-fw | GCGTGCAGTCGGACCTGCTG | Confirmation of mutation |
| relA-4-rv | GTTCGGGAAAATTCGCAAGCC | Confirmation of mutation |
| relA-5-wt | GTGGAAGCGGAATCGGCTG | Confirmation of mutation |
| spoT-3-fw | GGTGAAGAAGCAGTTCCGCA | Confirmation of mutation |
| spoT-4-rv | CGCCCGAAACAAATGGCGG | Confirmation of mutation |
| spoT-5-wt | GCGGACGATGGTGTAGTGC | Confirmation of mutation |
| relA-RT-1 | AGATCCGCACGCAGGAAATG | RT-PCR analysis |
| relA-RT-2 | TTCGCTGTGCAGGTGGTAAG | RT-PCR analysis |
| spoT-RT-1 | GCGCATCAATGGCTCAAGTC | RT-PCR analysis |
| spoT-RT-2 | TTCAGGCGCATCGTCTTCAG | RT-PCR analysis |
| 16S-RT-1 | GCCAGTCACCAATGCAGTTC | RT-PCR normalization |
| 16S-RT-2 | ACCAAGGCGACGATCAGTAG | RT-PCR normalization |
Restriction sites are underlined. Complementary regions are in italics. Translational start and stop codons are in bold.
Biparental mating.
The pDM4 derivatives were introduced into E. coli strain S17 λpir by electroporation. The resulting donor strains and the B. pseudomallei recipient strains were grown overnight in LB medium at 37°C with aeration. One milliliter of the cultures was pelleted by centrifugation, and the cells were resuspended in 0.5 ml fresh LB medium. Ten microliters of these suspensions was transferred onto 1-cm by 1-cm squares of nitrocellulose membrane (Hybond-ECL; GE Healthcare, Little Chalfont, United Kingdom) placed on brain heart infusion (BHI; Fluka Sigma-Aldrich) agar plates, either individually for control plates or with donor and recipient together for the conjugations, and the plates were incubated overnight at 37°C. The cells were scraped off the membranes using sterile inoculation loops and transferred to tubes containing 1 ml sterile PBS. One hundred microliters of these suspensions was plated onto LB agar plates supplemented with gentamicin to select against the donor strain and chloramphenicol to select for B. pseudomallei transconjugants carrying the pDM4 constructs integrated into the chromosome.
Allelic exchange.
The generation of clean in-frame deletion mutants relied on a second recombination event, where the integrated pDM4 plasmid constructs were deleted from the chromosome by using sacB-mediated counterselection. Overnight cultures of sacB+ transconjugants were serially diluted in sterile PBS and plated onto salt-free LB agar plates containing 10% (wt/vol) sucrose, which were incubated at 24°C for 2 to 3 days. Colonies obtained on these plates were tested for sensitivity to chloramphenicol by patching them onto LB agar plates with and without chloramphenicol. Chloramphenicol-sensitive colonies were analyzed for carrying a deletion of the target gene. Confirmation of the mutants was performed by PCR using a primer that binds upstream of the fragment used for mutagenesis (primer series 3) combined either with a reverse primer that binds within the coding region of the target gene (primer series 5), resulting in a PCR product only with wild-type cells, or with a reverse primer that binds within the downstream region used for mutagenesis (primer series 4), resulting in different-sized fragments with the wild type and deletion mutants. Mutants were also confirmed by performing RT-PCR using the primer combinations relA-RT-1 plus relA-RT-2 and spoT-RT-1 plus spoT-RT-2, which specifically amplify the relA and spoT transcripts, respectively, as described below.
The relA spoT double mutant was obtained by first deleting the relA gene, followed by a second round of mutagenesis deleting the spoT gene. A ΔspoT single mutant could not be obtained, despite several attempts.
RNA extractions.
For transcriptional studies, bacteria were grown aerobically at 37°C in LB broth overnight. Total RNA was extracted from 4.5 ml of these stationary-phase cultures using TRIzol reagent (Invitrogen, through Life Technologies, Paisley, United Kingdom) according to the manufacturer's recommendations. In brief, cells were harvested by centrifugation at 12,000 rpm for 5 min. Each cell pellet from 1.5 ml culture was resuspended in 1 ml TRIzol reagent. Cells were lysed by pipetting, and samples were stored at −80°C until they had passed a sterility check. Total RNA was extracted after the addition of chloroform and precipitated in isopropanol overnight at −20°C. The RNA was pelleted by centrifugation, washed with 70% ethanol, air dried for 5 min at room temperature, and resuspended in nuclease-free water. Contaminating DNA was removed by DNase I (Ambion, through Life Technologies, Paisley, United Kingdom) digestion for 45 min at 37°C, followed by phenol-chloroform extractions, isopropanol precipitation, and resuspension of the total RNA in nuclease-free water as described above.
Semiquantitative RT-PCR.
For cDNA synthesis, 4 μg of total RNA was mixed with 3 μl of random primers at 3 μg/μl (Invitrogen) and 1 μl of a deoxynucleoside triphosphate (dNTP) mixture at 10 mM each (Promega, Southampton, United Kingdom). After primer annealing at 65°C for 5 min, a mix of first-strand buffer, dithiothreitol (DTT), 40 U RNase Out recombinant RNase inhibitor (Invitrogen), and 200 U Superscript III reverse transcriptase (Invitrogen) was added according to the manufacturer's recommendations. cDNA synthesis was performed at 50°C for 60 min, followed by heat inactivation at 70°C for 15 min. cDNA samples were 10× diluted in water and directly used for PCR amplification. For the adjustment of cDNA amounts, 16S rRNA was used as an internal standard, using primers 16S-RT-1 and 16S-RT-2. The sequences of all primers are listed in Table 2.
Macrophage uptake and intracellular survival assays.
Bacterial uptake and survival inside macrophages were quantified using a modified kanamycin protection assay as described previously (47). In summary, J774A.1 murine macrophages were infected at a multiplicity of infection (MOI) of 10 and incubated at 37°C for 2 h to allow bacterial internalization to occur. The medium was then replaced with fresh medium containing 250 μg/ml kanamycin in order to suppress growth of extracellular bacteria. At appropriate time points, cells were washed and lysed in 0.1% (vol/vol) Triton X-100, and serial dilutions of the lysis mixture were plated on LB agar plates for the determination of intracellular bacterial numbers.
Galleria mellonella killing assays.
Bacterial survival and virulence for G. mellonella larvae were assessed by killing assays as described previously (47). Briefly, 1 × 104 CFU of bacteria in a volume of 10 μl were injected into the hemocoels of 10 larvae per bacterial strain. Larvae were incubated statically at 37°C inside petri dishes, and the number of dead larvae was scored periodically. Intracellular bacterial numbers were determined 20 h postinfection by draining the hemocoels of three larvae per strain and plating serial dilutions onto LB agar plates.
Animal infection studies.
Female C57BL/6 mice (6 to 8 weeks old; Harlan Laboratories, Bicester, Oxon, United Kingdom) were used throughout the studies. All animal experiments were performed in accordance with the guidelines of the Animals (Scientific Procedures) Act of 1986 and were approved by the local ethical review committee at the London School of Hygiene and Tropical Medicine. For each infection, aliquots were thawed from frozen bacteria stocks and diluted in pyrogen-free saline (PFS). Prior to intranasal infection, mice were anesthetized intraperitoneally with ketamine (50 mg/kg; Ketaset; Fort Dodge Animal Health, IA) and xylazine (10 mg/kg; Rompum; Bayer, Leverkusen, Germany) diluted in PFS. Infection was performed by intranasally (i.n.) administering a total volume of 50 μl containing 2,500 CFU (acute model) or 100 CFU (chronic model) of wild-type B. pseudomallei K96243 or the isogenic ΔrelA ΔspoT mutant. The infection dose was confirmed as described elsewhere (22). Control uninfected mice received 50 μl of PFS i.n.
Immunization studies.
Female C57BL/6 mice were immunized i.n. with 1 × 105 CFU of B. pseudomallei 2D2 (3) or the ΔrelA ΔspoT mutant in a volume of 50 μl. Sham-immunized mice received 50 μl PFS. Five weeks later they were challenged i.n. with 1 × 103 CFU of B. pseudomallei strain 576 in a volume of 50 μl, and survival was monitored. On day 55 postinfection, survivors were culled and lungs and spleens aseptically removed into cold PBS. Organs were homogenized through a 100-μm cell strainer (BD Falcon, CA), serial 10-fold dilutions of tissue homogenates were plated onto tryptic soy agar plates (Sigma-Aldrich), and bacterial colonies were counted after incubation for 24 to 48 h at 37°C. The limit of detection was 50 CFU/organ.
Bright-field microscopy.
Ten microliters of bacterial cultures was applied to coverslips placed inside the wells of a 24-well plate, and cells were fixed using paraformaldehyde (4% in PBS) for 30 min. The coverslips were washed three times in phosphate-buffered saline and inverted onto a drop of Vectashield mounting medium (Vector Laboratories) on top of a glass slide. The microscope slides were fumigated overnight before they could be removed from the BSL3 suite. Samples were imaged at a magnification of ×100 using a Zeiss Axiophot microscope equipped with VisiView software (Visitron Systems GmbH).
In silico analyses.
Homologies between amino acid sequences were determined using NCBI's BLASTP algorithm. Conserved domains were analyzed using NCBI's conserved domain database (CDD). Promoter predictions were performed using the Bprom software (Softberry).
Statistical analyses.
Differences between average values were tested for significance by performing an unpaired, two-sided Student's t test. Log rank tests of survival data were performed using the GraphPad Prism software, version 5.01 (GraphPad Software, San Diego, CA). P values of ≤0.05 were considered significant.
RESULTS
RelA and SpoT proteins in B. pseudomallei.
RelA and SpoT play key roles in the generation of ppGpp in bacteria. The genome of B. pseudomallei strain K96243 was searched for homologues of the RelA and SpoT proteins of Escherichia coli, which produced two matches. BPSL1946 (predicted molecular mass = 82.3 kDa) had 40% overall identity to RelAE.coli, whereas BPSL2561 (predicted molecular mass = 88 kDa) had 45% identity to SpoTE.coli. Interestingly, no significant similarity was detected in the first 40 amino acid residues of the N terminus of RelA of B. pseudomallei (RelABps), whereas the start codon of SpoTBps seemed to be misannotated by 59 amino acid residues. The identity between the two paralogs was 34%, and both proteins were conserved in the closely related species B. mallei and B. thailandensis. The B. pseudomallei proteins possess typical conserved domains for RelA-SpoT-family proteins (28). No other RelA-SpoT-family proteins were found in the genome by BLAST and PFAM searches. The organization of the chromosomal regions surrounding the relA and spoT genes, respectively, is depicted in Fig. 1A.
To further characterize the two proteins and the contribution of ppGpp synthesis in B. pseudomallei, a ΔrelA single mutant and a ΔrelA ΔspoT double mutant were created in B. pseudomallei strain K96243. The correct genotypes of the mutants were confirmed by PCR, and the absence of relA and/or spoT transcripts was confirmed by reverse transcription-PCR (data not shown). Since both genes are located at the end of putative transcriptional units, no polar effects on downstream genes is expected (Fig. 1A). A ΔspoT single mutant could not be obtained despite several attempts.
Stationary-phase survival is decreased in a ΔrelA ΔspoT double mutant of B. pseudomallei.
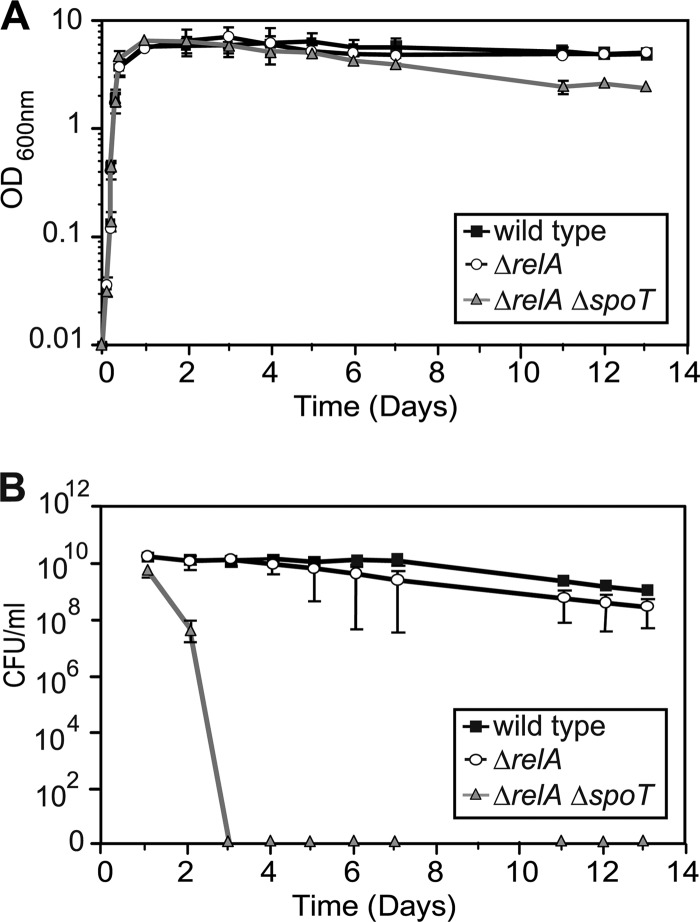
As a first phenotypic test, the growth of the mutants in axenic culture was analyzed. Both the ΔrelA single mutant and a ΔrelA ΔspoT double mutant grew at rates similar to that of the wild type during exponential growth in LB medium (Fig. 2A). Cultures of the ΔrelA ΔspoT mutant reached higher optical densities than cultures of the wild type and the ΔrelA mutant at the transition into stationary phase between 12 and 24 h postinoculation, but the optical density decreased steadily during the remainder of the experiment. The optical densities of wild-type and ΔrelA mutant cultures remained at a constant level throughout stationary growth phase and were found to be twice as high as the values observed with the ΔrelA ΔspoT mutant on day 13. Despite the elevated optical density after 24 h incubation, the number of CFU in cultures of the ΔrelA ΔspoT mutant was lower than in cultures of the wild type and the ΔrelA single mutant (Fig. 2B), and numbers further decreased over time until no viable bacteria could be detected at day 3 after inoculation (Fig. 2B). In contrast, the number of viable cells remained constant in cultures of the wild type for up to 1 week postinoculation and decreased only marginally during the second week. The survival of the ΔrelA single mutant was more variable, with two of four cultures exhibiting a decrease in the number of CFU over time. However, on average, there was no significant difference relative to the wild type (Fig. 2B). This indicates a severe defect in stationary-phase survival only in the absence of both ppGpp-synthesizing enzymes, similar to what has been described for other organisms (21, 31, 41).
Fig 2.
Growth and stationary-phase survival of wild-type K96243 and isogenic mutants. All strains were inoculated into LB broth at an optical density of 0.01, and cultures were incubated at 37°C with aeration. (A) Growth curve representing the optical densities of the cultures at the indicated time points. (B) Stationary-phase survival of the strains determined by plating samples onto LB agar plates at the indicated time points. All experiments were performed in at least three independent experiments, and data are plotted on a logarithmic scale as means with standard deviations (A) or standard errors of the means (B) for those experiments.
In keeping with this finding, early-stationary-growth-phase cells of the ΔrelA single mutant did not appear to differ in size and shape from wild-type cells (Fig. 1C and D). In contrast, cells of the ΔrelA ΔspoT mutant appeared slightly bigger on average (Fig. 1E, left) or had formed filaments of various lengths (Fig. 1E, right), thereby indicating stress-related changes in cell morphology in the absence of both ppGpp-synthesizing enzymes.
A ΔrelA ΔspoT mutant of B. pseudomallei is unable to replicate inside macrophages.
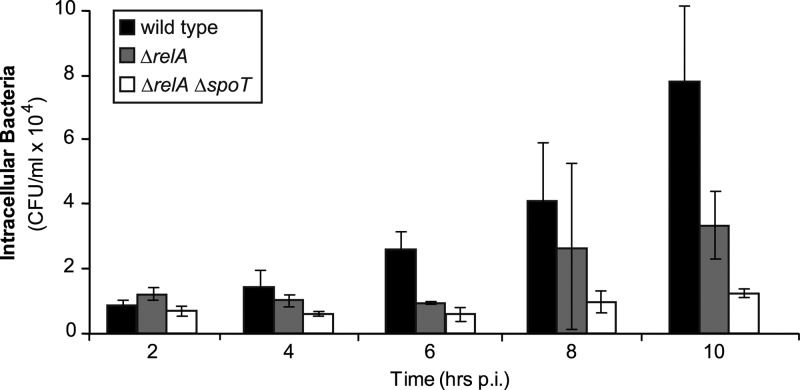
B. pseudomallei is a facultative intracellular organism that can survive inside macrophages. In order to assess the intracellular survival ability of the ΔrelA and ΔrelA ΔspoT mutants, murine macrophages were infected with B. pseudomallei K96243 or isogenic mutants and the numbers of intracellular bacteria were determined up to 10 h postinfection. While the wild type exhibited intracellular replication over the course of the experiment, the numbers of the ΔrelA ΔspoT mutant did not increase compared to the input (P = 0.024 at 10 h postinfection [p.i.]) (Fig. 3). The extent of bacterial uptake into phagocytic cells, represented by the number of bacteria at 2 h after initial infection, was not altered between the mutant and the wild type. The ΔrelA single mutant exhibited an intermediate replication rate and no significant difference in initial bacterial numbers. This suggests that the presence of RelA-SpoT-family proteins is crucial for intracellular survival in phagocytic cells.
Fig 3.
Intracellular survival of wild-type K96243 and isogenic mutants. J774A.1 murine macrophages were infected with wild-type K96243 and isogenic mutants at an MOI of 10, and intracellular bacterial numbers were determined at the indicated time points after initial infection by plating onto LB agar plates. Bars and error bars represent the means and standard deviations of a representative experiment performed in three technical replicates for each time point.
A ΔrelA ΔspoT mutant of B. pseudomallei is attenuated in an insect model of infection.
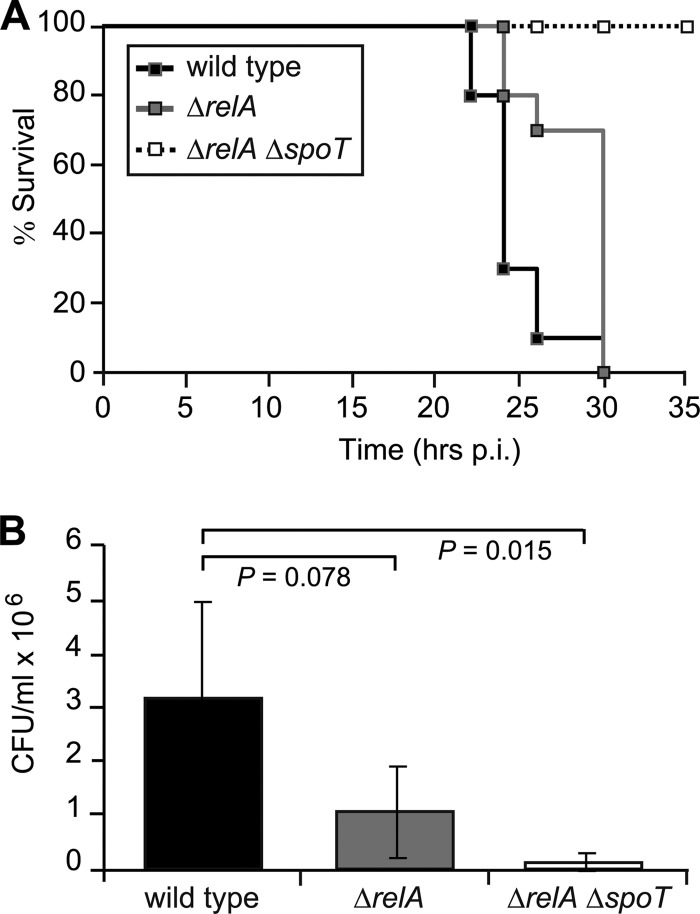
Previously, we demonstrated the value of wax moth (G. mellonella) larvae as a model system to assess virulence of B. pseudomallei isolates (47). Here, G. mellonella larvae were infected with 1 × 104 CFU of B. pseudomallei K96243 or isogenic mutants, and survival of the larvae was monitored (Fig. 4A). The median survival time of larvae infected with the wild type was 24 h, whereas infection with the ΔrelA mutant resulted in a median survival time of 30 h (log rank test, P = 0.004). No larvae infected with the ΔrelA ΔspoT double mutant had died by the end of 35 h (P = 0.002). When bacteria inside the larvae were enumerated prior to the onset of paralysis at 20 h postinfection, a >2,000-fold increase in bacterial numbers compared to the input was observed with the wild type (Fig. 4B). The growth rates of the ΔrelA and ΔrelA ΔspoT mutants were 2 times and 11 times lower than that of wild type, respectively. This indicates a severe intracellular growth defect and a significant attenuation in insect virulence in the absence of RelA-SpoT-family proteins.
Fig 4.
Killing of G. mellonella larvae by wild-type K96243 and isogenic mutants. (A) Groups of 10 larvae per strain were injected with 1 × 104 CFU of bacteria and maintained at 37°C. Dead and live larvae were scored at indicated time points and plotted as Kaplan-Meier survival curves. All curves were significantly different from each other (log rank test; P < 0.01 in all cases). (B) The number of bacteria present inside the larvae at 20 h postinfection was determined by aseptically removing the bottom 2 mm of five larvae per strain, draining the hemocoel, and plating serial dilutions onto LB agar plates. Results are means and standard deviations from two independent experiments.
K96243 ΔrelA ΔspoT is attenuated in murine models of infection and is able to induce a protective immune response.
To further investigate the role of RelA and SpoT in virulence, C57BL/6 mice were infected i.n. with B. pseudomallei K96243 or the ΔrelA ΔspoT double mutant at two different doses. A high dose of 2,500 CFU of B. pseudomallei K96243 has been shown to result in acute disease, with death of the mice occurring within 10 days postinfection, whereas a lower dose of <1,000 CFU results in a chronic infection (8). The ΔrelA ΔspoT double mutant was attenuated in both the acute and the chronic model of infection, and all mice survived for at least up to 70 days postinfection (Fig. 5A and B). None of the mice infected with the B. pseudomallei ΔrelA ΔspoT mutant exhibited any outward signs of disease or weight loss.
Fig 5.
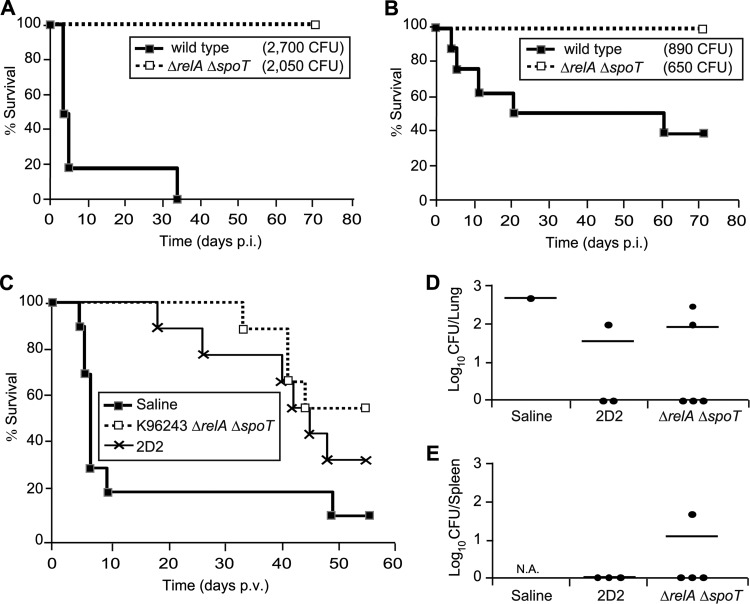
Survival and protection of mice infected with wild-type K96243 and its isogenic ΔrelA ΔspoT mutant. (A and B) C57BL/6 mice were challenged intranasally with an intended dose of 2,500 CFU (acute challenge [A]) or 500 CFU (chronic challenge [B]). Actual infection doses for each experiment are given in parentheses. Log rank tests confirmed a significant difference in survival between the wild type and the ΔrelA ΔspoT mutant in both cases (P < 0.001 and P < 0.05, respectively). (C to E) C57BL/6 mice were immunized intranasally with 1 × 105 CFU of strain 2D2 or K96243 ΔrelA ΔspoT. After 5 weeks of incubation, mice were challenged intranasally with 1,000 CFU of B. pseudomallei strain 576. Mice were monitored for survival, and numbers are plotted as Kaplan-Meier survival curves (C). Log rank tests confirmed a significant difference in survival of the K96243 ΔrelA ΔspoT and the 2D2 cohorts compared to the saline control (P < 0.01 and P < 0.05, respectively). At day 55 after infection with the wild type, bacterial burdens were assessed in lungs (D) and spleens (E) of survivors. Note that only one mouse had survived up to this time point in the saline-treated sample group. The spleen of this mouse and that of one survivor in the K96243 ΔrelA ΔspoT sample group had extremely large abscesses and could not be harvested. N.A., not available.
To test whether the ΔrelA ΔspoT mutant is able to protect mice from subsequent infection, C57BL/6 mice were immunized i.n. with 1 × 105 CFU of K96243 ΔrelA ΔspoT or B. pseudomallei strain 2D2 as a positive control and challenged 5 weeks later with 1 × 103 CFU of B. pseudomallei strain 576 given i.n., which usually results in an acute infection in naïve mice, with a time to death of <10 days postinfection. Immunization of mice with K96243 ΔrelA ΔspoT resulted in significantly increased survival of mice compared to PFS control mice, with 100% survivors up to 30 days postchallenge (P = 0.0061) (Fig. 5C). The pattern of survival of the immunized animals was comparable to the pattern of survival of mice that had been immunized with strain 2D2. Colonization data of lungs and spleens of surviving animals that had been vaccinated with the K96243 ΔrelA ΔspoT mutant showed undetectable CFU levels in three of five mice at day 55 after infection with the wild type (Fig. 5D and E). However, bacteria could be detected in the remaining mice, and one spleen sample exhibited extremely large abscesses. This indicates that vaccination with a ΔrelA ΔspoT mutant of B. pseudomallei induces high levels of protective immunity against B. pseudomallei infection, but as with all other live-attenuated vaccines tested to date, sterile immunity was not achieved.
DISCUSSION
In this study, we characterized the phenotype of a ΔrelA ΔspoT derivative of B. pseudomallei and have assessed its potential to confer protective immunity on mice. Our results demonstrate that RelA-SpoT-family proteins play an important role in survival and virulence of B. pseudomallei and that a ΔrelA ΔspoT mutant may be a promising live-attenuated vaccine candidate.
The ppGpp-mediated stringent response is not well characterized in members of the betaproteobacteria. In phylogenetic analyses, it has been shown that RelA and SpoT proteins of betaproteobacteria such as Bordetella spp. and Neisseria spp. cluster next to the gammaproteobacterial branch (30). ppGpp production has been described for Neisseria gonorrhoeae (20). In contrast to E. coli, the gonococcal SpoT protein does not seem to contribute to ppGpp production, and a relA single mutant had a growth defect on rich solid medium. The B. pseudomallei proteins share 45% and 44% identity with the RelA and SpoT proteins of N. gonorrhoeae, respectively.
From our data, we propose that the stringent response in B. pseudomallei may be similar to the one observed in E. coli. First, we were unable to create a spoT single mutant, which indicates that SpoT is the only enzyme that can hydrolyze ppGpp in B. pseudomallei and thus prevent toxic accumulation. Moreover, the intermediate phenotype of the relA single mutant and its normal growth rate indicate that SpoTBps also contributes to ppGpp synthesis. Interestingly, as seen in Fig. 1A, SpoTBps is encoded upstream of the transcription elongation factor GreB, which also interacts with the RNA polymerase through the secondary channel (25), similarly to ppGpp but with different effects (1, 40). More detailed studies on the stringent response in B. pseudomallei could provide a better understanding of survival and adaptability capacities of this organism.
Here, we characterized the phenotype of a B. pseudomallei mutant that is defective in RelA and SpoT enzymes, which are usually involved in ppGpp synthesis and degradation. In other bacteria, it has been shown that lack of ppGpp-synthesizing enzymes results in defects in stationary-phase survival (21, 31, 41). Our results show that this is also the case in B. pseudomallei. The ΔrelA ΔspoT mutant had lost all viability after 3 days of incubation (Fig. 2B). The viability of the ΔrelA single mutant seemed to be variable, with some cultures having lost viability after 1 week, some exhibiting an initial decrease in CFU followed by a plateau at intermediate levels, and others showing no difference relative to the wild type (data not shown), which might indicate the involvement of suppressor mutants or other stochastic effects, which remain to be elucidated.
The assumed role of the ppGpp-mediated stringent response is to reduce growth when cells encounter unfavorable growth conditions, including nutrient deprivation upon entry into stationary phase, and to redirect resources into adaptation and survival mechanisms (27). In general, ppGpp mutants seem to be unable to modulate their growth rates and prepare for stationary-phase survival. Stationary-phase adaptation includes morphological changes such as a reduced cell size and rounding of cells (36). The morphology of ΔrelA ΔspoT mutant cells differed from that of wild-type cells, as the mutant cells appeared slightly bigger on average (compare Fig. 1C and E). This suggests that the ΔrelA ΔspoT mutant is unable to adopt a different morphology in response to stationary-phase growth, whereas the filamentation indicates that the bacteria experience stress, and we speculate that these two effects might contribute to the reduced survival.
In this study, we assessed the virulence of relA and relA spoT mutants in three different infection models: a macrophage in vitro model, an insect model, and mouse models of acute and chronic melioidosis. The ΔrelA ΔspoT double mutant was found to be severely attenuated in all of the models, whereas the ΔrelA single mutant exhibited intermediate levels of attenuation in the two systems. Previously, we demonstrated the usefulness of the macrophage model and the Galleria mellonella insect model for comparing the virulence of different Burkholderia isolates (47). The results presented here demonstrate that either model can also be used to assess the virulence of mutants, and in our case, the virulence in the nonmammalian models reflected the virulence in mice. Both model systems also provided further insights into the possible mode of attenuation: mutants lacking ppGpp-synthesizing enzymes are attenuated not because of reduced bacterial uptake or due to enhanced killing and clearance by macrophages and insect hemocytes but rather because of a defect in intracellular replication (see bacterial numbers in Fig. 3 and 4B). The macrophage model may also prove useful to elucidate the role of ppGpp in the intracellular lifestyle of B. pseudomallei in future studies.
In addition to being severely attenuated in mice, in both an acute and a chronic model of disease, the K96243 ΔrelA ΔspoT double mutant also partially protected immunized animals from intranasal challenge with virulent wild-type bacteria (Fig. 5C). Pioneering studies by Dannenberg and Scott (13–15) had already demonstrated the feasibility of immunization against experimental melioidosis using attenuated B. pseudomallei. However, the genetic basis of attenuation of these strains is not known. More recently, other workers assessed the feasibility of immunization against experimental melioidosis using rationally attenuated B. pseudomallei mutants. Immunization by the i.p. route, with a range of mutants, has been shown to protect against acute disease following a subsequent i.p. challenge with wild-type B. pseudomallei (35, 42). However, disease following an i.n. or inhalational challenge is more severe than that seen following i.p. challenge (45), and there are few studies which have demonstrated protection against an i.n. or inhalational challenge. In part, this might reflect the finding that protection against an i.n. challenge appears to be dependent on immunization by the same route (19). Immunization of BALB/c mice i.n. with two doses of an Δasd mutant has been shown to provide protection against acute disease following a low-dose challenge (four 50% lethal doses), but deaths of challenged mice were recorded from day 15 postchallenge onwards (35). A similar pattern of protection was observed after i.n. immunization of BALB/c mice with an ilvI mutant (2D2), after i.n. challenge with 1 × 102 CFU of wild-type B. pseudomallei (19). However, these studies were carried out in BALB/c mice, and we recently reported that the C57BL/6 infection model reflects the spectrum of melioidosis seen in humans (8). Therefore, we considered that the C57BL/6 infection model would allow a more meaningful assessment of the potential for live-attenuated mutants to induce protective immunity in humans. In this study, we report that C57BL/6 mice immunized i.n. with either 2D2 or the ΔrelA ΔspoT mutant of B. pseudomallei were protected against acute disease following an i.n. challenge (1 × 103 CFU) of B. pseudomallei strain 576, which has proven to provide a robust measure of protection in many other vaccination studies. However, as in previous studies, mice eventually succumbed to disease.
In addition to incomplete protection by B. pseudomallei live-attenuated vaccines, concerns exist over their safety. The vaccine strain, even though unable to cause overt disease, might persist in the host and cause disease later in life. All our data indicate a defect of the B. pseudomallei ΔrelA ΔspoT mutant in long-term survival in broth culture and in macrophages, but the strain has not been tested for latency in vivo. Moreover, suppressor mutations of ppGpp-deficient strains have been reported in E. coli, which abolish their auxotrophy on minimal medium plates (6, 32, 50). Therefore, more studies on the proneness to acquire suppressor mutations and on the molecular mechanism of the intracellular replication defect, including subcellular localization experiments, have to be performed on the B. pseudomallei ΔrelA ΔspoT mutant before it can be considered safe. Nevertheless, identification of different classes of protective vaccine strains may be useful for the creation of a live-attenuated vaccine strain carrying a combination of mutations, which could increase both protection and safety.
ACKNOWLEDGMENTS
This work was supported by Wellcome Trust award WT085162AIA.
We thank Muthita Vanaporn for her help in constructing the mutants and Sok Lau for her help with the microscopy. We thank Robert Gilbert and all of the members of the London School of Hygiene and Tropical Medicine Biological Services Facility for animal husbandry. We also thank Carlos Balsalobre for critically reading the manuscript and his helpful comments.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Åberg A, Shingler V, Balsalobre C. 2008. Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol. Microbiol. 67:1223–1241 [DOI] [PubMed] [Google Scholar]
- 2. Atkins T, et al. 2002. Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. J. Med. Microbiol. 51:539–553 [DOI] [PubMed] [Google Scholar]
- 3. Atkins T, et al. 2002. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect. Immun. 70:5290–5294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atkinson GC, Tenson T, Hauryliuk V. 2011. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One 6:e23479 doi:10.1371/journal.pone.0023479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Braeken K, Moris M, Daniels R, Vanderleyden J, Michiels J. 2006. New horizons for (p)ppGpp in bacterial and plant physiology. Trends Microbiol. 14:45–54 [DOI] [PubMed] [Google Scholar]
- 6. Cashel M, Gentry D, Hernandez VJ, Vinella D. 1996. The stringent response, p 1458–1496 In Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. (ed), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 7. Cheng AC, Currie BJ. 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18:383–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conejero L, et al. 2011. Low-dose exposure of C57BL/6 mice to Burkholderia pseudomallei mimics chronic human melioidosis. Am. J. Pathol. 179:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Currie BJ, Fisher DA, Anstey NM, Jacups SP. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R. Soc. Trop. Med. Hyg. 94:301–304 [DOI] [PubMed] [Google Scholar]
- 10. Dahl JL, et al. 2003. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. U. S. A. 100:10026–10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalebroux ZD, Edwards RL, Swanson MS. 2009. SpoT governs Legionella pneumophila differentiation in host macrophages. Mol. Microbiol. 71:640–658 [DOI] [PubMed] [Google Scholar]
- 12. Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. 2010. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74:171–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dannenberg AM, Jr, Scott EM. 1958. Melioidosis: pathogenesis and immunity in mice and hamsters. I. Studies with virulent strains of Malleomyces pseudomallei. J. Exp. Med. 107:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dannenberg AM, Jr, Scott EM. 1958. Melioidosis: pathogenesis and immunity in mice and hamsters. II. Studies with avirulent strains of Malleomyces pseudomallei. Am. J. Pathol. 34:1099–1121 [PMC free article] [PubMed] [Google Scholar]
- 15. Dannenberg AM, Jr, Scott EM. 1960. Melioidosis: pathogenesis and immunity in mice and hamsters. III. Effect of vaccination with avirulent strains of Pseudomonas pseudomallei on the resistance to the establishment and the resistance to the progress of respiratory melioidosis caused by virulent strains; all-or-none aspects of this disease. J. Immunol. 84:233–246 [PubMed] [Google Scholar]
- 16. Dean RE, Ireland PM, Jordan JE, Titball RW, Oyston PCF. 2009. RelA regulates virulence and intracellular survival of Francisella novicida. Microbiology 155:4104–4113 [DOI] [PubMed] [Google Scholar]
- 17. Dozot M, et al. 2006. The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell. Microbiol. 8:1791–1802 [DOI] [PubMed] [Google Scholar]
- 18. Durfee T, Hansen A-M, Zhi H, Blattner FR, Jin DJ. 2008. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 190:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Easton A, et al. 2011. Combining vaccination and postexposure CpG therapy provides optimal protection against lethal sepsis in a biodefense model of human melioidosis. J. Infect. Dis. 204:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher SD, Reger AD, Baum A, Hill SA. 2005. RelA alone appears essential for (p)ppGpp production when Neisseria gonorrhoeae encounters nutritional stress. FEMS Microbiol. Lett. 248:1–8 [DOI] [PubMed] [Google Scholar]
- 21. Gaynor EC, Wells DH, MacKichan JK, Falkow S. 2005. The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol. Microbiol. 56:8–27 [DOI] [PubMed] [Google Scholar]
- 22. Haque A, et al. 2006. Role of T cells in innate and adaptive immunity against murine Burkholderia pseudomallei infection. J. Infect. Dis. 193:370–379 [DOI] [PubMed] [Google Scholar]
- 23. Holden MTG, et al. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. U. S. A. 101:14240–14245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim S, Watanabe K, Suzuki H, Watarai M. 2005. Roles of Brucella abortus SpoT in morphological differentiation and intramacrophagic replication. Microbiology 151:1607–1617 [DOI] [PubMed] [Google Scholar]
- 25. Koulich D, et al. 1997. Domain organization of Escherichia coli transcript cleavage factors GreA and GreB. J. Biol. Chem. 272:7201–7210 [DOI] [PubMed] [Google Scholar]
- 26. Logue C-A, Peak IRA, Beacham IR. 2009. Facile construction of unmarked deletion mutants in Burkholderia pseudomallei using sacB counter-selection in sucrose-resistant and sucrose-sensitive isolates. J. Microbiol. Methods 76:320–323 [DOI] [PubMed] [Google Scholar]
- 27. Magnusson LU, Farewell A, Nyström T. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 13:236–242 [DOI] [PubMed] [Google Scholar]
- 28. Marchler-Bauer A, et al. 2011. CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res. 39:D225–D229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milton DL, O'Toole R, Horstedt P, Wolf-Watz H. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mittenhuber G. 2001. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol. 3:585–600 [PubMed] [Google Scholar]
- 31. Mouery K, Rader BA, Gaynor EC, Guillemin K. 2006. The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid, and aerobic shock. J. Bacteriol. 188:5494–5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy H, Cashel M. 2003. Isolation of RNA polymerase suppressors of a (p)ppGpp deficiency. Methods Enzymol. 371:596–601 [DOI] [PubMed] [Google Scholar]
- 33. Na HS, et al. 2006. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24:2027–2034 [DOI] [PubMed] [Google Scholar]
- 34. Nascimento MM, Lemos JA, Abranches J, Lin VK, Burne RA. 2008. Role of RelA of Streptococcus mutans in global control of gene expression. J. Bacteriol. 190:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norris MH, et al. 2011. The Burkholderia pseudomallei asd mutant exhibits attenuated intracellular infectivity and imparts protection against acute inhalation melioidosis in mice. Infect. Immun. 79:4010–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nyström T. 2004. Stationary-phase physiology. Annu. Rev. Microbiol. 58:161–181 [DOI] [PubMed] [Google Scholar]
- 37. Park S-I, et al. 2010. Immune response induced by ppGpp-defective Salmonella enterica serovar Gallinarum in chickens. J. Microbiol. 48:674–681 [DOI] [PubMed] [Google Scholar]
- 38. Patel N, et al. 2011. Development of vaccines against Burkholderia pseudomallei. Front. Microbiol. 2:198 doi:10.3389/fmicb.2011.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Potrykus K, Cashel M. 2008. (p) ppGpp: still magical? Annu. Rev. Microbiol. 62:35–51 [DOI] [PubMed] [Google Scholar]
- 40. Potrykus K, et al. 2006. Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA. J. Biol. Chem. 281:15238–15248 [DOI] [PubMed] [Google Scholar]
- 41. Primm TP, et al. 2000. The stringent response of Mycobacterium tuberculosis is required for long-term survival. J. Bacteriol. 182:4889–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sarkar-Tyson M, Titball RW. 2010. Progress toward development of vaccines against melioidosis: a review. Clin. Ther. 32:1437–1445 [DOI] [PubMed] [Google Scholar]
- 43. Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 44. Sun W, Roland KL, Branger CG, Kuang X, Curtiss RIII. 2009. The role of relA and spoT in Yersinia pestis KIM5+ pathogenicity. PLoS One 4:e6720 doi:10.1371/journal.pone.0006720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Titball RW, et al. 2008. Burkholderia pseudomallei: animal models of infection. Trans. R. Soc. Trop. Med. Hyg. 102:S111–S116 [DOI] [PubMed] [Google Scholar]
- 46. Traxler MF, Chang D-E, Conway T. 2006. Guanosine 3′,5′-bispyrophosphate coordinates global gene expression during glucose-lactose diauxie in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 103:2374–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wand M, Müller C, Titball R, Michell S. 2010. Macrophage and Galleria mellonella infection models reflect the virulence of naturally occurring isolates of B. pseudomallei, B. thailandensis and B. oklahomensis. BMC Microbiol. 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. 2006. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat. Rev. Microbiol. 4:272–282 [DOI] [PubMed] [Google Scholar]
- 49. Zhou YN, et al. 2008. Regulation of cell growth during serum starvation and bacterial survival in macrophages by the bifunctional enzyme SpoT in Helicobacter pylori. J. Bacteriol. 190:8025–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou YN, Jin DJ. 1998. The rpoB mutants destabilizing initiation complexes at stringently controlled promoters behave like “stringent” RNA polymerases in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 95:2908–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]