Abstract
Clostridium perfringens type B causes enteritis and enterotoxemia in domestic animals. By definition, these bacteria must produce alpha toxin (CPA), beta toxin (CPB) and epsilon toxin (ETX) although most type B strains also produce perfringolysin O (PFO) and beta2 toxin (CPB2). A recently identified Agr-like quorum-sensing (QS) system in C. perfringens controls all toxin production by surveyed type A, C, and D strains, but whether this QS is involved in regulating toxin production by type B strains has not been explored. Therefore, the current study introduced agrB null mutations into type B strains CN1795 and CN1793. Both type B agrB null mutants exhibited reduced levels of CPB, PFO, and CPA in their culture supernatants, and this effect was reversible by complementation. The reduced presence of CPB in culture supernatant involved decreased cpb transcription. In contrast, the agrB null mutants of both type B strains retained wild-type production levels of ETX and CPB2. In a Caco-2 cell model of enteritis, culture supernatants of the type B agrB null mutants were less cytotoxic than supernatants of their wild-type parents. However, in an MDCK cell in vitro model for enterotoxemic effects, supernatants from the agrB null mutants or wild-type parents were equally cytotoxic after trypsin activation. Coupling these and previous results, it is now evident that strain-dependent variations exist in Agr-like QS system regulation of C. perfringens toxin production. The cell culture results further support a role for trypsin in determining which toxins contribute to disease involving type B strains.
INTRODUCTION
The Gram-positive, spore-forming, anaerobic bacterium Clostridium perfringens has a widespread environmental distribution, including soil, feces, foods, and a commensal presence in the gastrointestinal tract of both humans and animals (11). In addition, C. perfringens is a prolific toxin producer capable of expressing ≥17 different toxins (11). Variability in toxin production among strains helps to explain why this bacterium causes a broad array of enteric and histotoxic infections in humans and animals (11). It also provides the basis for a widely used classification system assigning C. perfringens strains to one of five types (A to E), depending upon their production of four typing toxins, i.e., alpha toxin (CPA), beta toxin (CPB), epsilon toxin (ETX), and iota toxin. In addition to producing at least one typing toxin, C. perfringens strains may produce additional toxins, such as perfringolysin O (PFO), enterotoxin (CPE), or beta2 toxin (CPB2).
C. perfringens type B strains cause disease in goats, calves, and foals that occurs when intestinal infection develops into enteritis or enterotoxaemia, a condition where type B bacteria growing in the intestines produce toxins that are absorbed into the circulation and then target internal organs (11). By definition, type B isolates must produce, at the minimum, CPA, CPB, and ETX. CPA, which is produced by all C. perfringens types, is a lethal 42-kDa protein with phospholipase C and sphingomyelinase activities (19). ETX, produced only by C. perfringens type B and D strains, is secreted as a prototoxin and then proteolytically activated to the mature ∼30-kDa pore-forming toxin in the gastrointestinal tract (14). ETX is listed as a class B CDC select toxin and ranks as the third-most-potent clostridial toxin after botulinum and tetanus toxins. CPB, a lethal 35-kDa pore-forming toxin, is produced by both type B and C strains (17, 18). Apart from CPA, ETX, and CPB, most type B strains also produce PFO and CPB2 (6). PFO is a lethal 55-kDa hemolytic pore-forming toxin belonging to the cholesterol-dependent cytolysin family (20). CPB2, a 28-kDa protein with no homology to CPB, can be produced by all types of C. perfringens bacteria although its action remains unknown (7).
The different pathologies comprising the C. perfringens disease spectrum involve, at least in part, variations in toxin gene carriage among strains. However, disease pathology and severity are also likely impacted by the amount of each toxin expressed by the infecting strain, so understanding the regulation of C. perfringens toxin production is important. While a detailed, comprehensive understanding of toxin synthesis regulation by C. perfringens is far from accomplished, several regulators of C. perfringens toxin production have now been identified. One such regulator is the Agr-like quorum-sensing (QS) system (13, 21). In C. perfringens, the agrD gene apparently encodes an autoinducer propeptide that is processed by the agrB-encoded protease to an active autoinducing peptide (3, 13, 21, 22). While the Agr QS system of Staphylococcus aureus also includes the AgrA/AgrC two-component regulatory system that responds to the AgrD-derived autoinducing peptide (12), C. perfringens apparently lacks agrA or agrC orthologues (3, 13, 21, 22), suggesting that it uses some other two-component regulatory system to mediate Agr-like QS effects.
The full scope of C. perfringens toxin production regulation by the Agr-like QS system has not yet been established. In particular, no information is currently available regarding whether this QS system is involved in regulating toxin production by type B strains. Therefore, the current study constructed isogenic agrB null mutants in two type B isolates, i.e., CN1793 and CN1795. These mutants were then used to evaluate whether the Agr-like QS plays a role in controlling toxin production by, and cytotoxic effects of, type B strains.
MATERIALS AND METHODS
Bacterial strains and culture media.
C. perfringens type B strains used in this work included CN1793 and CN1795, which were originally part of the Wellcome Collection. Bacterial culture media used in this study included fluid thioglycolate (FTG) medium (Difco Laboratories), TGY broth (3% tryptic soy broth [Becton, Dickinson], 2% glucose [Sigma-Aldrich], 1% yeast extract [Becton, Dickinson], 0.1% sodium thioglycolate [Sigma-Aldrich]), and brain heart infusion (BHI) agar (Becton, Dickinson). When indicated, chloramphenicol (15 μg/ml) was added to the culture medium.
Antibodies and reagents.
Mouse monoclonal antibodies against CPA, CPB, and ETX (6) were kindly provided by P. Hauer. Production of polyclonal antibodies against PFO and CPB2 has been described previously (9). Trypsin and trypsin inhibitor were purchased from Sigma-Aldrich. A lactate dehydrogenase (LDH) release assay kit was obtained from Invitrogen.
Construction of CN1793 and CN1795 agrB null mutants by insertional inactivation of target genes using a Clostridium-modified group II intron.
As previously described (3, 9, 22), the agrB-targeted intron donor plasmid pJIR750agrBi, which carries a chloramphenicol resistance marker, was used to inactivate the chromosomal agrB genes of CN1793 and CN1795. The pJIR750agrBi plasmid was electroporated into wild-type CN1793 or CN1795, and the transformation reaction culture was incubated in TGY broth for 4 h at 37°C. Transformants were then selected on BHI agar plates containing 15 μg/ml of chloramphenicol. Colonies were screened by PCR using primers AgrBKO-F and AgrBKO-R (9). When positive colonies were identified, a putative agrB null mutant was grown in FTG medium without antibiotics and subcultured daily for ∼10 days at 37°C to cure the intron-carrying donor plasmid pJIR750agrBi.
To complement the CN1793::agrB or CN1795::agrB null mutants, the previously constructed plasmid p3, which encodes the C. perfringens strain 13 agr operon (21), was transformed into the agrB null mutant strains by electroporation.
DNA extraction.
Genomic DNA from C. perfringens was purified from overnight cultures grown in TGY broth using a Master Pure Gram-Positive DNA purification kit (Epicentre Biotechnologies, WI) according to the manufacturer's instructions.
Southern blot assay.
Purified DNA samples were digested overnight with EcoRI at 37°C according to the manufacturer's instructions (New England BioLabs) and separated by electrophoresis on a 1% agarose gel. The separated DNA digestion products were transferred onto a positively charged nylon membrane (Roche) for hybridization with a digoxigenin (DIG)-labeled intron-specific probe, which was prepared as previously described using the primer pair AgrB-IBS and AgrB-EBS1d (9). CSPD substrate (Roche Applied Science) was used to detect probe hybridization according to the manufacturer's instructions.
RNA isolation.
RNA for reverse transcription-PCR (RT-PCR) analyses was extracted from the C. perfringens wild-type, agrB mutant, or agr complementing strain as described previously (3, 9, 21, 22). Briefly, 2 ml of a 4-h TGY C. perfringens culture was centrifuged at 4°C, and the pellet was resuspended in 200 μl of acetate solution (20 mM sodium acetate, pH 5, 1 mM EDTA, 0.5% sodium dodecyl sulfate [SDS; Bio-Rad]). This suspension received 200 μl of saturated phenol (Fisher Scientific), and the mixture was incubated at 60°C in a water bath with vigorous shaking for 5 min. After centrifugation at 4°C for 5 min, cold ethanol was added to the nucleic acid-containing supernatant, and the sample was mixed well. The mixed sample was centrifuged at 4°C for 5 min to obtain the RNA pellet, which was washed two times with cold 70% ethanol and finally resuspended in 100 μl of DNase-free, RNase-free water. All RNA samples were additionally treated with 2 U of DNase I (Promega) at 37°C for 30 min. To stop DNase I activity, DNase I inhibitor (Promega) was added to each reaction tube. RNA was quantified by absorbance at 260 nm using a NanoDrop 2000c spectrophotometer (Thermo scientific) and stored at −80°C.
RT-PCR.
RT-PCR was carried out using an AccesQuick RT-PCR system (Promega) according to the manufacturer's instructions. Briefly, 50 ng of each RNA sample was reverse transcribed to cDNA by adding 1 unit of reverse transcriptase at 45°C for 45 min. This cDNA was then used as the template for PCRs (30 s at 94°C, 30 s at 55°C, and 60 s at 72°C for extension). Primers used for RT-PCR included the following: (i) for cpb, cpbF (5′-GCGAATATGCTGAATCATCTA-3′) and cpbR (5′-GCAGGAACATTAGTATATCTTC-3′); (ii) for etx, etxF (5′-GCGGTGATATCCATCTATTC-3) and etxR (5′-CCACTTACTTGTCCTACTAAC-3′); (iii) for polC, polCF (5′-ACTTCCCTGCAAGCCTCTTCTCCT-3′) and polCR (5′-TGGTTCAGCTTG-TGAAGCAGGGC-3′); and (iv) for agrB, agrBF (5′-AAATATTCTGGAGGAGCACA-3′) and agrBR (5′-CTAAACTCCATCCTGAATTA-3′). A negative control lacking reverse transcriptase was always performed to address the possibility that PCR products arose from contaminating DNA present in the RNA sample. A positive control using genomic DNA was also included to demonstrate that the PCR was efficient under the assay conditions. C. perfringens DNA polymerase III (polC), a housekeeping gene, served as an internal control to normalize the expression levels between samples.
Sequencing of the agr locus in CN1793 and CN1795.
An ∼2.9-kb PCR product containing the agr locus was amplified from C. perfringens DNA using Taq Long-Range Master Mix (New England BioLabs) and primers agrF1 and agrR1 (21) in a Techne (Burkhardtsdorf, Germany) thermocycler. For this PCR, the following amplification conditions were used: 1 cycle of 95°C for 2 min; 35 cycles of 95°C for 30 s, 55°C for 30 s, and 65°C for 3 min; and a single extension of 65°C for 10 min. The PCR product was then cloned into the TOPO (Invitrogen) vector according to the manufacturer's instructions, and that insert was then sequenced by Genewiz, Inc. (South Plainfield, NJ).
Construction of a complementing plasmid carrying the CN1793 agr operon.
CN1793 chromosomal DNA was isolated using a Master Pure Gram-Positive DNA Purification Kit (Epicentre). The primers agrF1 and agrR1 were used to amplify the agr operon. The following PCR conditions were used: 1 cycle of 95°C for 2 min; 35 cycles of 95°C for 30 s, 55°C for 30 s, and 65°C for 3 min; and a single extension of 65°C for 10 min. The PCR product was then cloned into a TOPO vector (Invitrogen) and sequenced. The agr operon insert was removed from the TOPO vector using BamHI and EcoRI and ligated into E. coli-C. perfringens shuttle plasmid pJIR750, creating pCN1793(p3). The pCN1793(p3) plasmid was then introduced by electroporation into the agrB mutant, CN1795::agrB. Chloramphenicol-resistant (15 μg/ml chloramphenicol) transformants were selected, and the resultant agr complementing strain was named CN1795::agrB/pCN1793(p3).
Western immunoblotting. (i) Sample preparation.
TGY cultures were grown for the incubation times indicated in the figure legends or the text. Washed cell lysate samples were prepared by centrifugation of 16-h TGY cultures, followed by three washes of the centrifugation pellets with phosphate-buffered saline (PBS). The washed cells were then resuspended in 1 ml of lysis buffer (20 mM Tris-HCl, pH 8.0, 2 mM EDTA, 1.2% Triton X-100, with lysozyme added to 20 mg/ml immediately before use).
(ii) Western blot analysis.
Each sample was mixed with 5× loading buffer and electrophoresed on an SDS-containing, 12% polyacrylamide gel. The separated proteins were transferred onto a nitrocellulose membrane, and the blot was then incubated for 1 h with blot washing buffer (20 mM Tris-HCl [pH 8.0], 0.3 M NaCl, 0.1% [vol/vol], Tween 20) containing 5% (wt/vol) nonfat dry milk before incubation with primary antibody overnight at 4°C. Blots were washed three times with Tris buffer (containing 0.1% Tween 20) and incubated with goat anti-mouse horseradish peroxidase (HRP) or anti-rabbit HRP secondary antibodies for 1 h at room temperature. After three more washes, the blots were treated with an ECL (enhanced chemiluminescence) Western blotting detection kit (Amersham) and exposed to X-ray film (Life Science Products) to detect the immunoreactive protein bands.
Cell culture.
Human-derived, enterocyte-like Caco-2 cells were routinely maintained in minimal essential Eagle's medium (MEM) (Sigma) supplemented with 10% fetal bovine serum (FBS; Life Technologies), 1% nonessential amino acids (Sigma), 1% glutamine (Sigma), streptomycin (100 U/ml), and penicillin (100 μg/ml). Madin-Darby canine kidney (MDCK) cells were cultured in a 50:50 mixture of Dulbecco's modified Eagle's medium (DMEM) (Sigma) and Ham's F12 (Sigma) supplemented with heat-inactivated 3% FCS, 1% nonessential amino acids, 1% glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Each cell line was normally harvested with 0.25% trypsin (Gibco), resuspended in the cell culture medium, and maintained at 37°C in a 5% CO2 humidified atmosphere.
Morphological damage assays.
MDCK or Caco-2 cells were used for morphological damage assays. The cells were seeded in six-well plates and incubated until confluent. For cell culture infections, each C. perfringens strain (wild-type, agrB mutant, or complementing strain) was inoculated into TGY broth and grown at 37°C overnight. The culture supernatants were then removed and filter sterilized using a 0.45-um-pore-size filter (Millipore). The sterile supernatants were dialyzed overnight against PBS (pH 7.4) at 4°C and then directly applied to Caco-2 or MDCK cells. To help determine whether toxicity involved secreted ETX, some aliquots of the sterile, dialyzed supernatants were treated with trypsin at a concentration of 12.5 μg/ml at 37°C for 1 h. The trypsin was then inactivated by the addition of an equal volume of trypsin inhibitor at room temperature for 30 min. After centrifugation, the activated supernatants were applied to Caco-2 or MDCK cells.
The development of morphological damage was checked every hour using a Zeiss Axiovert 25 inverted microscope. After a 3-h treatment, the cytopathic effects caused by supernatants from different strains were analyzed and photographed using a Canon Powershot G5 fitted to a Zeiss Axiovert 25 microscope. Images were then processed using Adobe Photoshop CS4.
Neutralization assays.
To assess whether any observed MDCK cell damage was specifically caused by ETX present in trypsin-treated supernatants of different strains, a seroneutralization approach was applied. Briefly, 10 μl of a neutralizing monoclonal antibody (MAb) against ETX toxin was added to the trypsin-activated supernatants (prepared as described above), and the mixtures were incubated at room temperature for 15 min. The ETX-neutralized supernatants were then applied to MDCK cells.
LDH release cytotoxicity assay.
A lactate dehydrogenase (LDH) release assay kit for mammalian cell death (Invitrogen) was used to quantify Agr-like QS involvement in type B strain-induced cytotoxicity for Caco-2 cells or MDCK cells. The assay was performed, as described by the supplier, using Caco-2 or MDCK monolayers treated for 3 h at 37°C with different supernatants prepared as described above. The absorbance of each sample was measured at 490 nm with an iMark microplate reader (Bio-Rad). As described in the kit instructions, cells treated with 1% Triton X-100 were used to determine maximal LDH release. The results are expressed as the percentage of LDH release versus the total LDH present in cells.
Statistical analysis.
All values are expressed as means ± standard deviations (SD). For statistical evaluations, Student's t test was performed, and P values of <0.05 were considered significant.
Nucleotide sequence accession numbers.
The nucleotide sequences of C. perfringens agr locus sequences CN1793 and CN1795 were deposited in the GenBank database under accession numbers JQ966304 and JQ966305.
RESULTS
In silico analysis of the agr locus in type B isolates CN1793 and CN1795.
The current study first amplified the agr operon from type B strains CN1793 and CN1795 and then sequenced the PCR products. These analyses revealed that both isolates possess an agr locus of ∼2.9 kb, which matches the size of the agr locus product amplified from other C. perfringens strains using the same PCR primers (3, 9, 21, 22). However, compared against the CN1795 agr locus, the agr locus present in CN1793 contained an extra 28 nucleotides inserted 156 bp upstream of the agr operon (Fig. 1A). To evaluate whether these extra 28 nucleotides are also present in other C. perfringens strains, an in silico analysis was performed for the previously sequenced agr loci of strain 13 (type A, GenBank accession number NC_003366.1), ATCC 3626 (type B, GenBank accession number ABDV01000004.1), JGS1495 (type C, GenBank accession number ABDU01000023.2), CN3718 (type D, GenBank accession number JN543538), and JGS1987 (type E, GenBank accession number ABDW01000022.1). While the agr loci of all seven strains showed >97% sequence identity at the nucleotide level (Fig. 1B), the 28-nucleotide insertion upstream of the agr operon was present only in CN1793 (Fig. 1A).
Fig 1.
Comparison of sequences upstream of the first ORF in the agr operon of different C. perfringens strains. (A) Alignment of sequences upstream of the first ORF of the agr operon in five C. perfringens strains. Note the 28-nucleotide insertion present 156 bp upstream of the first ORF in the agr operon of CN1793. (B) DNA identity of seven agr operons, including C. perfringens type A to E strains. (C) Complementation of the CN1795 agrB null mutant with the CN1793 agr operon restores agr-regulated toxin production. Shown is Western blot analysis of CPB, ETX, CPB2, and PFO production by overnight cultures of CN1795, CN1795::agrB, and the complementing strain CN1795::agrB/pCN1793(p3).
Similar to the sequencing results previously obtained for other C. perfringens strains (see above), the agr operons of CN1793 and CN1795 contained four open reading frames (ORFs) encoding the hypothetical proteins CPE1562 and CPE1563, as well as AgrB and AgrD. Translation of these ORFs showed that the agr loci of CN1793 and CN1795 encode a putative AgrB protein, the CPE1562 hypothetical protein, and the CPE1563 hypothetical protein, each sharing ≥99% identity with the corresponding proteins encoded by the sequenced agr loci of other C. perfringens strains. In addition, the deduced amino acid sequences of the AgrD proteins encoded by both CN1793 and CN1795 are 100% identical to the AgrD proteins of other examined strains.
Construction and characterization of CN1793 and CN1795 agrB null mutants.
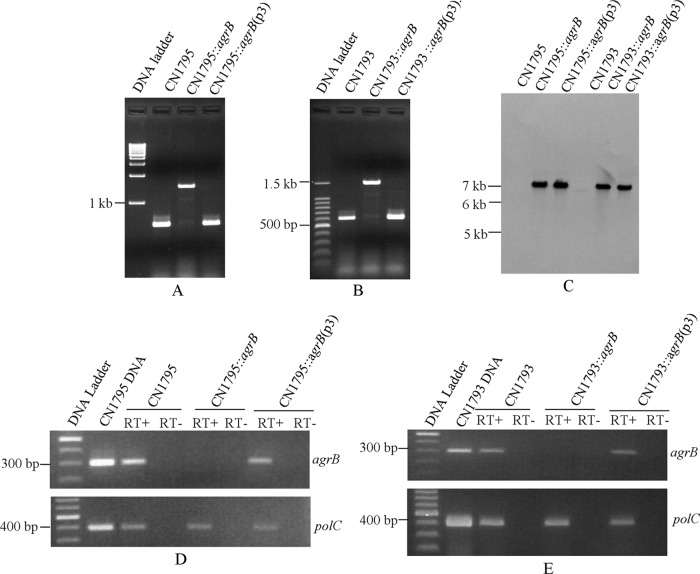
To evaluate whether the agr operon is important for toxin regulation in C. perfringens type B strains, the agrB genes in strains CN1793 and CN1795 were insertionally inactivated using a Clostridium-modified Targetron insertional mutagenesis system (4). Construction of the agrB isogenic null mutants of CN1793 and CN1795 was first verified by PCR analyses using primers specific to agrB sequences located upstream and downstream of the intron insertion site. Using DNA from wild-type CN1793 and CN1795, the internal agrB primers specifically amplified a PCR product of ∼650 bp (Fig. 2A and B). However, consistent with the insertion of an ∼900-bp intron into the agrB gene, the same primers amplified a PCR product of ∼1.6 kb using DNA from the putative agrB null mutant (Fig. 2A and B). Southern blot analyses using an intron-specific probe demonstrated the presence of a single intron insertion in both agrB mutants (Fig. 2C). No intron signal was detected using wild-type DNA from CN1793 or CN1795, as expected.
Fig 2.
Construction of agrB null mutants in C. perfringens type B strains CN1793 and CN1795 by intron-based insertional mutagenesis. PCR analyses using primers to internal agrB ORF sequences and DNA from wild-type CN1795, the agrB null mutant (CN1795::agrB), or the complemented strain CN1795::agrB(p3) (A) or from wild-type CN1793, the agrB null mutant (CN1793::agrB), or the complemented strain CN1793::agrB(p3) (B). (C) Southern blot hybridization analysis for the presence of a group II intron insertion in wild-type CN1793 or CN1795, the agrB null mutants of those strains, and complementing strains. DNA from each strain was digested with EcoRI, electrophoresed on a 0.8% agarose gel, transferred onto a nylon membrane, and hybridized with a DIG-labeled intron-specific probe. Sizes of DNA fragments in kilobases (kb) are shown to the left. (D) RT-PCR analyses for agrB mRNA expression by wild-type CN1795, CN1795::agrB, or CN1795::agrB(p3). Sample RNA was collected from 4-h TGY cultures. As indicated, reverse transcriptase (RT) was (+) or was not (−) added to the reaction tubes. DNA polymerase III (polC) served as an internal control. (E) Similar RT-PCR analyses for agrB mRNA expression by wild-type CN1793, CN1793::agrB, or CN1793::agrB(p3).
To establish whether any phenotypic differences that might be observed in later experiments between wild-type CN1793 and CN1795 versus their isogenic agrB null mutants were specifically attributable to agrB inactivation, the complementation plasmid p3 containing the intact agr operon of type A strain 13 was transformed into each of the agrB null mutants by electroporation. PCR demonstrated the presence of the wild-type agrB gene in the two complemented strains, which were named CN1793::agrB(p3) and CN1795::agrB(p3) (Fig. 2A and B).
To evaluate whether agrB expression had been silenced in the putative agrB null mutant and restored in the complementing strains, an RT-PCR assay was performed. RNA from wild-type CN1793 and CN1795 or from the complementing strains of their agrB mutants was able to support RT-PCR amplification of an ∼300-bp product from agrB mRNA. In contrast, no PCR product from agrB mRNA was detectable using RNA from either agrB null mutant (Fig. 2D and E), confirming intron disruption of agrB gene expression in both CN1793::agrB and CN1795::agrB.
Inactivation of agrB gene expression in C. perfringens type B strains reduces supernatant levels of CPA, CPB, and PFO during in vitro culture.
To begin assessing whether the agr operon regulates in vitro toxin production by type B isolates, colony CPA activity was first evaluated using egg yolk agar plates, and colony PFO activity was examined on horse blood agar plates. When grown on egg yolk agar plates (data not shown), colonies of the wild-type or agrB complemented strains were surrounded by a zone of insoluble diacylglycerol precipitation, which is indicative of lecithin breakdown due to the phospholipase C activity of CPA (1, 2, 17). However, both the CN1793::agrB and CN1795::agrB null mutants failed to produce this characteristic precipitation zone around their colonies, indicating that the agr operon is needed for the CPA activity surrounding colonies of these two type B strains. When grown on horse blood agar plates (data not shown), colonies of the wild-type or complemented strains were surrounded by a zone of β-hemolysis indicative of C. perfringens PFO activity (2). In contrast, colonies of the agrB null mutants were not surrounded by this β-hemolytic zone, supporting their loss of PFO activity.
A role for the agr operon in regulating toxin production by type B strains was then definitively demonstrated by Western blotting (Fig. 3A) to compare toxin levels in supernatants removed from 4-h (exponential phase) or 8-h (stationary phase) TGY cultures of wild-type CN1793 and CN1795 versus the isogenic agrB null mutants. Western blot analyses showed the presence of CPB and PFO in both the 4-h and 8-h TGY culture supernatants and of CPA in overnight TGY cultures of wild-type CN1793 and CN1795. However, culture supernatants from the agrB null mutants CN1793::agrB and CN1795::agrB contained no detectable levels of these three toxins at similar time points. The absence of CPA, CPB, and PFO in supernatants of these mutants was specifically attributable to disruption of the agr operon since wild-type toxin levels were detected in supernatants after the mutants had been complemented with a plasmid carrying the wild-type agr operon (Fig. 3A).
Fig 3.
Western blot analysis of the presence of CPA, CPB, and PFO in supernatants removed from cultures of CN1793 and CN1795 and their derivatives grown in TGY broth. Western blot analyses of the presence of CPB or PFO in supernatants removed from cultures of wild-type CN1793 and CN1795, their isogenic agrB null mutants (CN1793::agrB and CN1795::agrB, respectively), and the complementing strains [CN1793::agrB(p3) and CN1793::agrB(p3), respectively] grown in TGY broth at 4 h or 8 h. For CPA detection, the samples of overnight TGY culture supernatants were concentrated 10 times and then subjected to Western blot analysis.
To assess whether the decreased toxin levels present in supernatants of agrB mutant cultures were attributable to impaired growth, comparative growth studies were performed by measuring culture optical density, at regular time points, for cultures of the wild-type parents, agrB mutants, and complementing strains. These analyses detected similar growth of all strains in TGY broth (data not shown). Consistent with these results, SDS-PAGE analysis of culture supernatants demonstrated similar levels of secreted proteins for the wild-type parents, agrB mutants, and complementing strains of both CN1793 and CN1795 (data not shown).
Inactivation of agrB expression in type B strains does not affect in vitro supernatant levels of ETX or CPB2.
In previous studies, the agr-like quorum-sensing system was shown to positively regulate ETX production by C. perfringens type D strain CN3718 (3) and CPB2 production by C. perfringens type A non-food-borne human gastrointestinal disease strain F5603 (9). To evaluate whether production of ETX or CPB2 by type B strains CN1793 and CN1795 is also regulated by the agr operon, Western blot analyses were performed using the same TGY supernatant samples employed in the experiment shown in Fig. 3. Surprisingly, silencing of agrB gene expression in CN1793 or CN1795 had no effect on ETX and CPB2 supernatant levels compared to levels in the isogenic wild-type or complementing strains (Fig. 4).
Fig 4.
Western blot analysis of the presence of ETX and CPB2 in supernatants removed from cultures of CN1793 (top) and CN1795 (bottom) and their derivatives grown in TGY broth for 4 h or 8 h.
The reduced CPB levels detected in culture supernatants of agrB null mutants involve decreased CPB production due to less cpb transcription.
To distinguish whether the reduced levels of some toxins present in culture supernatants of the type B agrB null mutants involved lower toxin production or impaired toxin secretion, CN1795, CN1795::agrB, and the complementing strain CN1795::agrB(p3) were each grown in 10 ml of TGY broth for 16 h. After the bacteria in these cultures were washed to remove secreted proteins, Western blotting was performed on lysates of the washed bacterial cells to detect cytoplasmic CPB (representing a toxin whose presence is reduced in culture supernatants of the type B agrB mutants) or cytoplasmic ETX (representing a toxin whose presence is not reduced in supernatants of the type B agrB mutants). Both ETX and CPB were detectable in all samples during these Western blot studies (Fig. 5A); however, the CPB signal was much weaker in cell lysates of CN1795::agrB than in cell lysates of wild-type CN1795 or CN1795::agrB(p3). In contrast, ETX signal intensity was similar using cell lysates of wild-type CN1795, CN1795::agrB, or CN1795::agrB(p3). In addition, the size of CPB or ETX was noticeably larger in cell lysates than in TGY supernatants, probably because the cell lysates contained the precursor forms of ETX or CPB proteins that still possessed an intact signal peptide, which could later be cleaved from the toxins by signal peptidase during secretion. These results demonstrated that the Agr-like QS regulatory system regulates CPB production, but not ETX production, by C. perfringens type B strains.
Fig 5.

The Agr QS system regulates production of CPB, but not ETX, by C. perfringens type B strain CN1795. (A) The presence of ETX and CPB in washed vegetative cells of wild-type CN1795 and its isogenic derivatives was analyzed by Western blotting. The CN1795 TGY culture supernatant was also used as a control in this analysis. (B) RT-PCR analysis for cpb, etx, and polC transcription of 4-h TGY cultures of the wild-type strains, agr mutants, or complementing strains. As indicated, reverse transcriptase (RT) was (+) or was not (−) added to the reaction tubes.
To evaluate if the decreased CPB production noted for the agrB mutant in the results shown in Fig. 5A was due to less cpb transcription, RT-PCR was performed (Fig. 5B). These studies detected cpb mRNA in cultures of wild-type CN1795 but not in CN1795::agrB cultures. The loss of cpb transcription for CN1795::agrB was specifically due to inactivation of the agr operon since cpb mRNA was detected in cultures of the complementing strain. Consistent with the Western blot results shown in Fig. 5A indicating that the Agr-like QS system does not regulate ETX production, etx mRNA was detected in cultures of wild-type CN1795, CN1795::agrB, and CN1795::agrB(p3).
Complementation of the CN1795 agrB null mutant with the CN1793 agr operon restores agr-regulated toxin production.
As mentioned earlier, in silico analyses detected an extra 28-nucleotide insertion in the upstream, nonencoding region of the agr operon in CN1793 (Fig. 1A). To evaluate whether this agr operon with the extra 28-nucleotide insertion can complement toxin production by agr null mutants of other C. perfringens strains, a plasmid [pCN1793(p3)] carrying this operon was transformed into the CN1795 agrB null mutant CN1795::agrB, creating the complementing strain CN1795::agrB/pCN1793(p3). When 16-h TGY culture supernatants of CN1795, CN1795::agrB, or CN1795::agrB/pCN1793(p3) were collected and subjected to Western blotting using CPB or PFO antibodies (Fig. 1C), the agr operon p3 from CN1793 was able to recover wild-type CPB and PFO production levels for the CN1795::agrB mutant.
Comparison of Caco-2 cell cytotoxicity induced by culture supernatants of type B wild-type parents versus their isogenic agrB null mutants.
In a previous study, the Agr-like QS system was shown to be necessary for C. perfringens type C stain CN3685, which produces CPA, PFO, and CPB but not ETX, to induce cytotoxicity in enterocyte-like Caco-2 cells (23). Therefore, the present study investigated whether toxin regulation by the Agr-like QS system of type B strains also has cytotoxic consequences for Caco-2 cells.
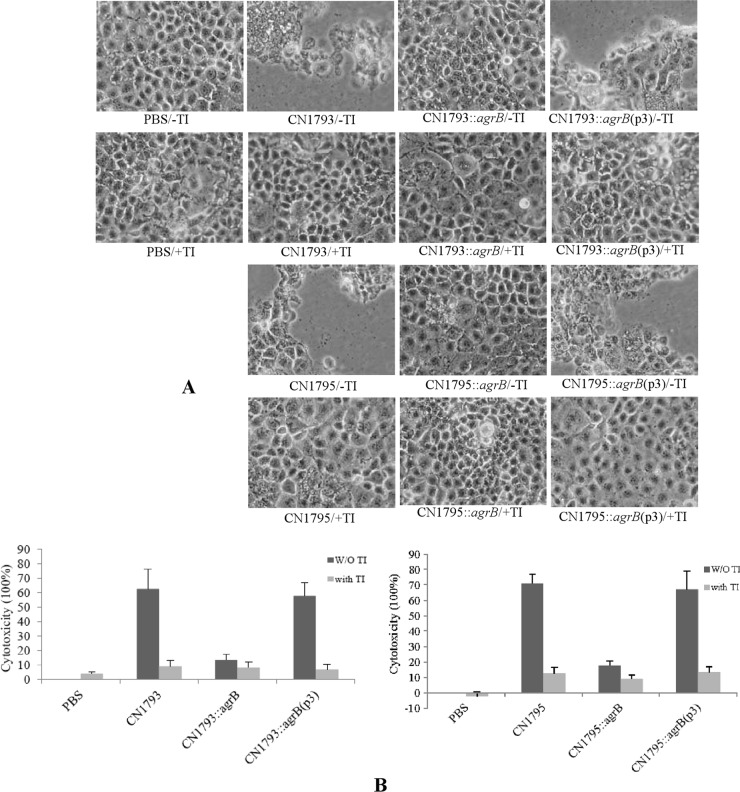
When filter-sterilized 16-h TGY culture supernatants from the wild-type parents, their agrB null mutants, or complementing strains were applied to Caco-2 cells for 3 h (Fig. 6A), the culture supernatants of wild-type CN1793 or CN1795 caused significant Caco-2 cell rounding and detachment from confluent monolayers. However, the morphological cell damage to Caco-2 cells was comparatively weaker and no cell detachment was observed using similarly prepared culture supernatants of the agrB null mutants. Complementation of the CN1793 or CN1795 agrB null mutants to restore agr operon expression fully reversed this attenuated morphological damage (Fig. 6A), confirming that the reduced cell damage caused by supernatants of these mutants was specifically attributable to inactivation of their agr operons.
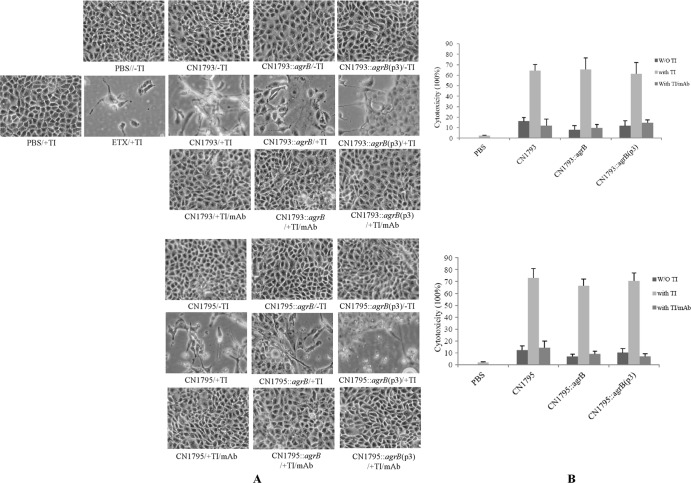
Fig 6.
Cytotoxic activities on Caco-2 cells of supernatants removed from cultures of two C. perfringens type B strains, their agrB null mutants, or complementing strains. (A) Morphological damage to Caco-2 cells. Culture supernatants from wild-type CN1793 and CN1795 and their isogenic derivatives were (+TI) or were not (−TI) pretreated with trypsin, followed by the addition of trypsin inhibitor. (B) Lactate dehydrogenase (LDH) release by Caco-2 cells after treatment with type B culture supernatants. LDH release was measured as an indicator of Caco-2 cytotoxicity caused by the supernatants removed from cultures of C. perfringens type B strains CN1793 and CN1795, their agrB null mutants, or the complementing strains. The experiment was repeated three times, and results shown are the mean values. Error bars represent SD. W/O, without.
In some animals with type B disease, toxins produced in the intestines could encounter intestinal proteases prior to their absorption into the circulation. To mimic this effect, the type B supernatants were pretreated with trypsin and then incubated with trypsin inhibitor. When the trypsin-pretreated supernatants were applied to Caco-2 cells, no morphological damage developed, even using trypsin-treated supernatants from the wild-type parent.
LDH release from damaged or destroyed cells was determined in order to quantitatively compare cytotoxicity induced by these supernatants. In the absence of trypsin pretreatment, cell death rates caused by culture supernatants of the wild-type and complementing strains were similarly high. However, cytotoxicity caused by the supernatant of the agrB null mutant was significantly reduced (Fig. 6B). Trypsin pretreatment virtually eliminated the ability of all tested type B culture supernatants to induce LDH release (Fig. 6B).
Comparison of MDCK cell cytotoxicity induced by culture supernatants of wild-type strains and their agrB null mutants.
As described earlier in this study, silencing the agrB gene did not affect ETX production by type B isolates CN1793 and CN1795. Since Caco-2 cells are not affected by ETX (data not shown), we evaluated whether inactivation of the agrB gene in these type B strains affects culture supernatant cytotoxicity for MDCK cells, which are sensitive to ETX (16). After 3 h of incubation, MDCK cells treated with sterile supernatants from cultures of the parent type B strains or their derivatives remained healthy (Fig. 7A). Therefore, aliquots of the same type B supernatants were pretreated with trypsin, followed by the addition of trypsin inhibitor, and these samples were applied to MDCK cells for 2 h. When morphological changes were evaluated, the trypsin-pretreated supernatants from the wild-type, agrB mutant, or complementing strains damaged MDCK cells equally well (Fig. 7A). This cytotoxic effect involved ETX since (i) MDCK cells treated with a mock sample consisting of PBS (instead of type B supernatant) containing trypsin and trypsin inhibitor did not develop cytotoxicity (Fig. 7A) and (ii) preincubating any trypsin-treated culture supernatant with an ETX-neutralizing MAb blocked the development of morphological damage (Fig. 7A).
Fig 7.
Cytotoxic activities on MDCK cells of supernatants removed from cultures of two C. perfringens type B strains, their agrB null mutants, or the complementing strains. (A) Effects of CN1793 or CN1795 supernatants on MDCK cells. Supernatants removed from cultures of wild-type CN1793 and CN1795 and their isogenic derivatives were (+TI) or were not (−TI) treated with trypsin, followed by the addition of trypsin inhibitor. As indicated, some supernatants were incubated with ETX-neutralizing MAb. (B) Quantitative analysis of MDCK cell cytotoxicity caused by supernatants removed from wild-type CN1793 and CN1795 and their isogenic derivatives measured by LDH release. The error bars shown represent the standard error of the mean calculated from three independent experiments.
An LDH release assay quantitatively confirmed that, in the absence of trypsin pretreatment, MDCK cytotoxicity was essentially absent using any tested type B culture supernatant. However, similarly high levels of cytotoxicity were observed using the trypsin-pretreated supernatants from cultures of the type B parents, agrB mutants, or complementing strains (Fig. 7B). The LDH release from damaged MDCK cells caused by the trypsin-pretreated supernatants involved ETX since this effect was blocked by preincubating any of these supernatants with an ETX neutralizing MAb (Fig. 7B).
DISCUSSION
Agr QS systems have become established as important virulence regulators for some Gram-positive pathogens, including Staphylococcus aureus and Listeria monocytogenes (12, 15). Agr-like QS systems were also recently identified in several pathogenic clostridial species, where they are similarly becoming implicated in virulence (3, 5, 13, 21, 22). For example, it was demonstrated that the Agr-like QS system is necessary for C. perfringens type C strain CN3685 to cause either necrotic enteritis or enterotoxemia in animal models (22).
Mechanistically, the Agr-like QS system has been shown to control several processes important for clostridial pathogenicity. First, this QS system was found to be necessary for both C. botulinum and C. perfringens to sporulate at wild-type levels (5, 9), which is important since spores often contribute to the transmission of enteric and histotoxic C. perfringens infections as well as botulism. Second, Agr-like QS systems were demonstrated to positively regulate the production of several clostridial toxins. For example, this QS system was found to be important to obtain wild-type production levels of botulinum neurotoxins (5), which cause the flaccid paralysis of botulism. With respect to C. perfringens, inactivation of the agrB gene in type A strain F5603 caused a loss of enterotoxin production (9), which could be attributable to CPE production being strictly sporulation dependent (8, 10).
However, the Agr-like QS system can also positively regulate production of C. perfringens toxins that (unlike CPE) are expressed during vegetative growth. For example, studies using agrB mutants and complementing strains established the importance of the Agr-like QS for positively regulating CPB production during the vegetative growth of type C strain CN3685 (22), which is significant since CPB is necessary and sufficient for this strain to cause either necrotic enteritis or lethal enterotoxemia in animal models (17). In addition, inactivation of the agrB gene in type A strain 13 or F5603, type C strain CN3685, or type D strain CN3718 reduced their PFO and alpha toxin production (9, 13, 21, 22), which is important since these two toxins are involved when C. perfringens causes gas gangrene (1, 2). Notably, the results obtained in the current studies using agrB null mutants and complementing strains of type B strains CN1793 and CN1795 are consistent with the earlier results. Specifically, this study showed that agrB expression positively regulates CPA, CPB, and PFO production by these two type B strains. Collating these new type B strain results with earlier findings, it increasingly appears that the Agr-like QS system regulates CPA, CPB, and PFO production by most (or all) C. perfringens strains, regardless of their type.
It was also recently reported that the Agr-like QS system controls ETX production by type D strain CN3718 (3). Thus, prior to the current study, it was conceivable that the Agr-like QS system would be a global regulator that universally controls all toxin production by every C. perfringens strain. In this regard, the single most significant finding of the current study is the first evidence refuting this possibility. Specifically, the current results demonstrated that inactivating the agrB gene did not affect the ability of two type B strains to produce wild-type levels of ETX. Furthermore, in this study, the agrB null mutants of both type B strains also produced wild-type levels of CPB2, in contrast to earlier results indicating that CPB2 production by type A strain F5603 is positively regulated by the Agr-like QS system (9).
The reason for the regulatory difference in ETX (and apparently CPB2) production between C. perfringens strains is not yet clear. The demonstrated involvement of the Agr-like QS system in regulating production of three other toxins by CN1793 and CN1795 rules out the possibility that the Agr-like QS system is not functional in these two type B strains. In addition, the observation that two different type B strains do not require the Agr-like QS system for producing wild-type levels of ETX or CPB2 could suggest that this trait is common among many type B strains although this possibility requires further study. To further assess whether these differences in etx regulation are type related, it should be determined whether ETX production (and possibly CPB2 production, if present) by type D strains other than CN3718 is also regulated by the Agr-like QS system. Regardless, the current type B results add new complexity to our still emerging, and as yet very incomplete, understanding of toxin production regulation by the Agr-like QS system of C. perfringens. Many unanswered questions about this regulatory system are now coming under study in this and other laboratories.
Finally, the cytotoxicity results obtained in this study could offer potential insights into type B disease. Specifically, they establish that both examined type B strains produce toxins, whose production is regulated by the Agr-like QS system, that are capable of killing enterocyte-like Caco-2 cells. However, the cytotoxic effects of these Caco-2 cell-active toxins are inactivated by trypsin. In contrast, these type B strains can also kill MDCK cells, but this effect is Agr-like QS independent and requires trypsin activation of their ETX. This variability of trypsin pretreatment effects in ameliorating or activating type B supernatant cytotoxicity for various cell lines is consistent with some toxins (e.g., CPB) produced by type B strains being trypsin sensitive while others (e.g., ETX) are trypsin activated (6).
Previous studies using a mouse model with intravenous injection of type B culture supernatants also observed differences in lethality between type B culture supernatants that were pretreated with trypsin or left untreated (6). Those findings, coupled with the current results, support the complexity of type B pathogenesis, where at least five different toxins are typically produced (6; also the present study). Furthermore, these results suggest that, as for type C strains, the pathogenicity of type B strains can be influenced by host defenses, such as trypsin activity in the intestines. In this regard, it is notable that type B infections often affect very young animals (11) where the antitrypsin activity of colostrum may inhibit ETX activation but preserve activity of trypsin-sensitive toxins. The relative contributions of different toxins to type B pathogenicity is difficult to definitively evaluate using cell culture models or intravenous injection models, so studies are planned to address the importance of various toxins for type B pathogenesis using a molecular version of Koch's postulates for analysis, where each toxin gene will be inactivated and the resulting toxin null mutants will then be tested for virulence in animal models.
ACKNOWLEDGMENTS
This research was generously supported by grant R01 AI056177 from the National Institute of Allergy and Infectious Diseases (B.A.M., principal investigator) and by the Middle Atlantic Regional Center of Excellence, via funding from grant 2U54AI057168-09 (Myron Levine, principal investigator).
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Awad MM, Bryant AE, Stevens DL, Rood JI. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191–202 [DOI] [PubMed] [Google Scholar]
- 2. Awad MM, Ellemor DM, Boyd RL, Emmins JJ, Rood JI. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904–7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J, Rood JI, McClane BA. 2011. Epsilon toxin production by Clostridium perfringens type D strain CN3718 is dependent upon the agr operon but not the VirS/VirR two component regulatory system. mBio. 2(6):e00275–11 doi:10.1128/mBio.00275-11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4. Chen Y, McClane BA, Fisher DJ, Rood JI, Gupta P. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl. Environ. Microbiol. 71:7542–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooksley C, et al. 2010. Regulation of neurotoxin production and sporulation by a putative agrBD signaling system in proteolytic Clostridium botulinum. Appl. Environ. Microbiol. 76:4448–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernandez-Miyakawa ME, et al. 2007. Both epsilon-toxin and beta-toxin are important for the lethal properties of Clostridium perfringens type B isolates in the mouse intravenous injection model. Infect. Immun. 75:1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gibert M, Jolivet-Reynaud C, Popoff MR. 1997. Beta2 toxin, a novel toxin produced by Clostridium perfringens. Gene 203:65–73 [DOI] [PubMed] [Google Scholar]
- 8. Harry KH, Zhou R, Kroos L, Melville SB. 2009. Sporulation and enterotoxin (CPE) synthesis are controlled by the sporulation-specific factors SigE and SigK in Clostridium perfringens. J. Bacteriol. 191:2728–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Chen J, Vidal JE, McClane BA. 2011. The Agr-like quorum-sensing system regulates sporulation and production of enterotoxin and beta2 toxin by Clostridium perfringens type A non-food-borne human gastrointestinal disease strain F5603. Infect. Immun. 79:2451–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, McClane BA. 2010. Evaluating the involvement of alternative sigma factors SigF and SigG in Clostridium perfringens sporulation and enterotoxin synthesis. Infect. Immun. 78:4286–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins T. 2006. The enterotoxic clostridia, p 688–752 In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E. (ed), The prokaryotes, 3rd ed Springer, New York, NY [Google Scholar]
- 12. Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu. Rev. Genet. 42:541–564 [DOI] [PubMed] [Google Scholar]
- 13. Ohtani K, et al. 2009. Virulence gene regulation by the agr system in Clostridium perfringens. J. Bacteriol. 191:3919–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popoff MR. 2011. Epsilon toxin: a fascinating pore-forming toxin. FEBS J. 278:4602–4615 [DOI] [PubMed] [Google Scholar]
- 15. Riedel CU, et al. 2009. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol. Microbiol. 71:1177–1189 [DOI] [PubMed] [Google Scholar]
- 16. Robertson SL, Li J, Uzal FA, McClane BA. 2011. Evidence for a prepore stage in the action of Clostridium perfringens epsilon toxin. PLoS One 6:e22053 doi:10.1371/journal.pone.0022053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sayeed S, et al. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol. Microbiol. 67:15–30 [DOI] [PubMed] [Google Scholar]
- 18. Smedley JG, III, Fisher DJ, Sayeed S, Chakrabarti G, McClane BA. 2004. The enteric toxins of Clostridium perfringens. Rev. Physiol. Biochem. Pharmacol. 152:183–204 [DOI] [PubMed] [Google Scholar]
- 19. Titball RW, Naylor CE, Basak AK. 1999. The Clostridium perfringens alpha-toxin. Anaerobe 5:51–64 [DOI] [PubMed] [Google Scholar]
- 20. Tweten RK. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vidal JE, Chen J, Li J, McClane BA. 2009. Use of an EZ-Tn5-based random mutagenesis system to identify a novel toxin regulatory locus in Clostridium perfringens strain 13. PLoS One 4:e6232 doi:10.1371/journal.pone.0006232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vidal JE, et al. 2012. Evidence that the Agr-like quorum sensing system regulates the toxin production, cytotoxicity and pathogenicity of Clostridium perfringens type C isolate CN3685. Mol. Microbiol. 83:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vidal JE, Ohtani K, Shimizu T, McClane BA. 2009. Contact with enterocyte-like Caco-2 cells induces rapid upregulation of toxin production by Clostridium perfringens type C isolates. Cell Microbiol. 11:1306–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]