Abstract
Salmonella enterica serovar Typhimurium is a Gram-negative member of the family Enterobacteriaceae and is a common cause of bacterial food poisoning in humans. The fimbrial appendages are found on the surface of many enteric bacteria and enable the bacteria to bind to eukaryotic cells. S. Typhimurium type 1 fimbriae are characterized by mannose-sensitive hemagglutination and are assembled via the chaperone/usher pathway. S. Typhimurium type 1 fimbrial proteins are encoded by the fim gene cluster (fimAICDHFZYW), with fimAICDHF expressed as a single transcriptional unit. The structural components of the fimbriae are FimA (major subunit), FimI, FimH (adhesin), and FimF (adaptor). In order to determine which components are required for fimbrial formation in S. Typhimurium, mutations in fimA, fimI, fimH, and fimF were constructed and examined for their ability to produce surface-assembled fimbriae. S. Typhimurium SL1344ΔfimA, -ΔfimH, and -ΔfimF mutants were unable to assemble fimbriae, indicating that these genes are necessary for fimbrial production in S. Typhimurium. However, SL1344ΔfimI was able to assemble fimbriae. In Escherichia coli type 1 and Pap fimbriae, at least two adaptors are expressed in addition to the adhesins. However, E. coli type 1 and Pap fimbriae have been reported to be able to assemble fimbriae in the absence of these proteins. These results suggest differences between the S. Typhimurium type 1 fimbrial system and the E. coli type 1 and Pap fimbrial systems.
INTRODUCTION
Salmonella enterica serovar Typhimurium (S. Typhimurium) has been shown to produce mannose-sensitive type 1 fimbriae on the bacterial surface (10, 31, 34). These fimbriae play a role in mediating bacterial adherence to eukaryotic cells, which is a critical step in successful colonization and pathogenesis of S. Typhimurium (3, 12, 29). The S. Typhimurium fimbrial gene (fim) cluster is proposed to be comprised of a six-gene operon encoding the structural and assembly components (fimAICDHF) and three independently transcribed regulatory genes, fimZ, -Y, and -W (7, 38, 39, 41, 45). The fimbrial appendage is primarily composed of multiple copies of the major fimbrial subunit FimA. FimC and FimD are the chaperone and usher proteins used to assemble fimbriae on the surface of the cell. The adhesive capability of the fimbriae is conferred by FimH, which is present at the tip of the fimbriae (19). Although functionally related to the Escherichia coli Fim proteins, the Salmonella fim genes and their products exhibit little relatedness at the nucleotide and amino acid level. The S. Typhimurium FimI protein also does not appear to be closely related to FimI of E. coli, and its function in Salmonella is unknown, although E. coli FimI mutants have been reported to be nonfimbriate (43). The S. Typhimurium FimF protein has little similarity to either of the E. coli adaptor proteins FimF and FimG but is presumed to function as an adaptor due to its location within the S. Typhimurium fim gene cluster and the presence of conserved amino acid domains found in both FimF and the Salmonella FimA subunit.
A number of well-characterized fimbrial systems are assembled using the chaperone/usher pathway (31, 40). These include E. coli type 1 fimbriae, which aid binding to a number of eukaryotic cell types, and Pap fimbriae, which are important for digalactoside-specific binding in uropathogenic E. coli (4, 21, 27). Both of these E. coli fimbrial systems include major subunits (FimA and PapA), an adhesin (FimH and PapG), and fimbrial adaptor proteins (FimF and FimG and PapE and PapF). Specific deletions in the genes encoding the adhesin proteins in both fimbrial systems result in bacteria that are able to produce surface-assembled fimbriae; however, these fimbriae are nonadhesive (24, 26). Additionally, it has been suggested that the E. coli fimbrial adaptor proteins are not absolutely required for the production of fimbriate bacteria. Mutations in the gene encoding the Pap fimbrial adaptor, papE, result in fimbriate and adhesive cells, while mutations in papF generate fimbriate but nonadhesive phenotypes (25). Similar results were observed in E. coli strains lacking the adaptors FimF and FimG in that adhesive fimbriae were produced on the bacterial surface. However, bacteria with a fimF mutation produce fewer fimbriae, and those lacking fimG produce longer fimbrial appendages. In both mutants, the fimbriae produced are functional and adhesive (37). As indicated above, the remaining E. coli type 1 structural gene, fimI, shares little homology with that of S. Typhimurium and is believed to be a minor subunit of the fimbriae. E. coli strains with a deleted fimI gene do not produce surface-assembled fimbriae (43).
The S. Typhimurium fim gene cluster possesses only one adaptor protein, while both the E. coli fim and pap systems possess genes encoding at least two adaptors. Additionally, E. coli fimbrial genes are unable to complement S. Typhimurium Fim mutants and similarly Salmonella Fim proteins cannot be used to assemble E. coli fimbriae (8, 14). The necessity for the presence of adhesin and adaptor proteins for production of surface-assembled fimbriae had not been examined in S. Typhimurium. Noting the genetic and functional differences between E. coli type 1 and Pap fimbrial gene clusters and the S. Typhimurium Fim gene cluster, we examined what components of S. Typhimurium fimbriae are required for fimbrial assembly.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Table 1 lists the bacterial strains, plasmids, and oligonucleotides used in this study. All strains were grown on Luria-Bertani (LB) media at 37°C, unless otherwise stated. Media were supplemented with ampicillin (100 μg/ml), kanamycin (25 μg/ml), or chloramphenicol (25 μg/ml) as necessary. Manipulation of DNA and construction of plasmids were carried out using standard techniques. Nucleotide sequencing was performed by the University of Iowa DNA sequencing facility.
Table 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Description or sequence (5′–3′) | Reference or source |
|---|---|---|
| Strains | ||
| SL1344 | Wild-type S. Typhimurium, fimbriate | 17 |
| SL1344ΔfimA | SL1344 fimA deletion mutant, nonfimbriate | This study |
| SL1344ΔfimA | SL1344 fimA partial deletion mutant, C-terminal half of gene intact, nonfimbriate | This study |
| SL1344fimA::TT | SL1344 fimA insertion mutant, nonfimbriate | This study |
| SL1344fimA::TT::fimA | SL1344 fimA insertion mutant, fimA integrated in single copy, fimbriate | This study |
| SL1344ΔfimI | SL1344 fimI deletion mutant, fimbriate | This study |
| SL1344ΔfimH | SL1344 fimH deletion mutant, nonfimbriate | This study |
| SL1344ΔfimH::fimH | SL1344 fimH deletion mutant, fimH integrated in single copy, fimbriate | This study |
| SL1344ΔfimF | SL1344 fimF deletion mutant, nonfimbriate | This study |
| SL1344ΔfimHF | SL1344 fimHF double deletion mutant, nonfimbriate | This study |
| NEB 5-α | E. coli subcloning strain | NEB (Ipswich, MA) |
| Plasmids | ||
| pGEM-T Easy | Ampr subcloning vector | Promega (Madison, WI) |
| pfimA | Ampr vector carrying fimA | This study |
| pfimAI | Ampr vector carrying fimAI | This study |
| pfimAIC | Ampr vector carrying fimAIC | This study |
| pfimAICDHF | Ampr vector carrying fimAICDHF | This study |
| pISF101 | Camr vector carrying 12.8-kbp S. Typhimurium fim gene cluster | 8 |
| pfimH | Ampr vector carrying fimH | This study |
| pfimF | Ampr vector carrying fimF | This study |
| pfimHF | Ampr vector carrying fimHF | This study |
| pLA2 | Kanr CRIM integration vector | 16 |
| pINT | Ampr CRIM integration helper plasmid | 16 |
| pLA2fimA | Kanr CRIM integration vector carrying fimA inserted at SphI/KpnI | 16 |
| pKD3 | Camr Red recombinase template plasmid | 9 |
| pKD46 | Ampr Red recombinase expression plasmid | 9 |
| Oligonucleotides | ||
| SAZ12 | ATGATCCTTCGGCGCGTTTTCATCGCTATCGGTTGTGTTTTGTTCAGCCCTGTGTAGGCTGGAGCTGCTTC | |
| SAZ13 | CTAATTGTAATTGATCAGGAAGGTCGCATCCGCGTTAGCAAGTCCGGGCTCATATGAATATCCTCCTTAG | |
| SAZ41 | ATGATAAGGAAAGGCGCGGCGCTAGCGGGGCTTGTTTTGATGTCGCCCGTTGTGTAGGCTGGAGCTGCTTC | |
| SAZ42 | TCATGGGTAAACCAGCGTAAACCACACTTCTGAATGAATTCGTCCCGGCGCATATGAATATCCTCCTTAG | |
| SAZ43 | GTTGCGGTAGTGCTATTGTCCGCAGAGGAGACAGCCAGCAAATTAGGGTTCATATGAATATCCTCCTTAG | |
| SAZ44 | ACAGGATGCCGAAACCGGG | |
| SAZ45 | CCCCGATAGCCTCTTCCGT | |
| SAZ56 | ATGACCTCTACTATTGCGAGTCTGATGTTTGTCGCTGGCGCAGCGGTTGCG | |
| SAZ83 | GTAATTCAAGGGAAATCCATGAAACATAAATTAATGACCTCTACTATTGCTGTGTAGGCTGGAGCTGCTTC | |
| SAZ84 | CCGGAGTAGGATCAGCCGCAACCGCTGCGCCAGCGACAAACATCAGACTCCATATGAATATCCTCCTTAG | |
| SAZ90 | GGCTGTCTCCTCTGCGGAC | |
| SAZ91 | TAAACGCATTCGTGCGGT | |
| SAZ92 | ATTTGATGACCAGCAGC | |
| SAZ93 | ATACGCAGCGTGTTTTC | |
| SAZ94 | GCCGGAAGACGCGCCGAC | |
| SAZ95 | CACCCGGTAAATACCGG | |
| SAZ96 | ACGTCCGTTACCGTTTG | |
| SAZ97 | CCAACCTGATTTTTCCGGCAGCG | |
| SAZ98 | AGGCCTCAGGGCAGGCG | |
| SAZ99 | GGCGGGCAACCTTCAAGTT | |
| SAZ108 | GCGTTGCTCTCGAGGGGAGTACATTTACAATAA | |
| BD4 | CGGAGGATAGCCTGAAGCAGCCGATTA | |
| BD5 | CTTCGCCCAGAGATGAGTTGGCCTGA | |
| BD11 | TATTCGCGCCTGGCCAATCA | |
| BD12 | GGCCTTCACTCTATCGTTGAGCT | |
| pLA2fimAfwd | CTGGCATGCCATTCAGGCCGTAGGTATCA | |
| pLA2fimArev | ACAGGTACCGATAGCCTCTTTCCGTTGAG | |
| fimHdelfwd | ATGAAAATATACTCAGCGCTATTGCTGGCGGGGACCGCGCTCTTTTTCACTGTGTAGGCTGGAGCTGCTTC | |
| fimHdelrev | TTAATCATAATCGACTCGTAGATAGCCGCGCGCAGTAAACGGCCCTTCCGCATATGAATATCCTCCTTAG | |
| CRIM P1 | GGCATAATAGCAATGTAC | |
| CRIM P2 | ACTTAACGGCTGACATGG | |
| CRIM P3 | ACGAGTATCGAGATGGCA | |
| CRIM P4 | AAATATGCCCTTCGCGCA |
Construction and characterization of the S.
Typhimurium fim mutants. Nonpolar Fim deletion mutants were constructed in S. Typhimurium SL1344 using λ Red recombinase and PCR products as described in detail by Datsenko and Wanner (9). Briefly, oligonucleotides were designed to amplify regions of the gene to be deleted, as well as an antibiotic resistance marker and an FLP recognition target (FRT). The resulting linear PCR product was then directly transformed into the desired strain previously transformed with a Red recombinase-producing plasmid, and selection for the appropriate antibiotic was performed. Antibiotic resistance was subsequently eliminated using a helper plasmid able to express the FLP recombinase. Finally, helper plasmids were cured from the deletion mutants by growth at 42°C.
Primer pair SAZ43-SAZ56 was used to delete 285 nucleotides (codons 16 to 111) from the 557-nucleotide fimA gene for the construction of SL1334ΔfimA. Primers SAZ83 and SAZ84 were used for SL1344fimA::TT, in which 85 nucleotides were inserted into the fimA gene such that a translation termination codon would be introduced following the fifth codon of fimA. Deletions in the remaining fim genes were constructed using primer pair SAZ41 and SAZ42 for SL1344ΔfimI, SAZ12 and SAZ13 for SL1344ΔfimF, fimHdelfwd and fimHdelrev for SL1344ΔfimH, and fimHdelfwd and SAZ13 for SL1344ΔfimHF. For all of these mutations, primers were constructed to remove the complete reading frame of the genes without removal of coding sequences from adjacent genes. All mutations were confirmed by sequencing the appropriate regions of DNA.
Complementation of the SL1344 Fim mutants was performed using plasmids possessing an intact and functional copy of appropriate fim genes. All genes were cloned from strain SL1344 using conventional techniques and the cloning vector pGEM-T Easy (Promega, Madison, WI). In addition, complementation by reintroduction of fim genes onto the chromosome of SL1344 mutants was accomplished by integrating a plasmid containing the gene into the λ integration site on the SL1344 chromosome using the conditional-replication, integration, excision, and retrieval (CRIM) plasmid-host system devised by Haldimann and Wanner (16). Briefly, primers were used to clone fim genes downstream of heterologous promoters into a Pir-dependent CRIM plasmid. When this plasmid is transformed into a non-Pir host containing a helper plasmid synthesizing Int, the entire CRIM plasmid is integrated onto the chromosome of the non-Pir host. Complementation vectors were constructed using primer pair SAZ44 and SAZ45 for pfimA, BD4 and BD5 for pfimH, and BD11 and BD12 for pfimF.
fim gene expression.
Total RNA was extracted from Salmonella strains after growth under conditions favoring fimbrial production using a method previously described by Chouika et al. (6). After isolation of RNA, any residual DNA was removed using a commercially available DNA-free kit (Ambion, Austin, TX). The absence of DNA was verified by conventional PCRs in the absence of reverse transcriptase. Copy DNA (cDNA) was synthesized from isolated RNA using a Superscript III reverse transcriptase kit (Invitrogen, Carlsbad, CA) as directed by the manufacturer. PCRs were carried out on cDNA using nucleotide primers specific for the amplification of target regions. Primer pair SAZ90-SAZ91 was used to amplify the intergenic region between fimA and fimI, SAZ92-SAZ93 was used for fimI to fimC, SAZ94-SAZ95 was used for fimC to fimD, SAZ96-SAZ97 was used for fimD to fimH, and SAZ98-SAZ99 was used for fimH to fimF. PCR products were analyzed by agarose gel electrophoresis. Primers, immediately internal to the predicted translation and termination codons, within appropriate fim genes were used to detect gene transcription in mutants. The location of transcription initiation sites upstream of individual fim genes was also investigated using the 5′ RACE (rapid amplification of cDNA ends) techniques (13). RNA was isolated as described above, and the detection of transcription initiation was performed according to the manufacturer's instructions (Invitrogen, Carlsbad, CA).
Detection of surface-associated type 1 fimbriae.
Bacterial cultures were serially grown in static 10-ml broth cultures at 37°C and were subsequently harvested by centrifugation and resuspended in a small volume (100 μl) of phosphate-buffered saline (PBS) as previously described (11, 32). Fifty microliters of this suspension was mixed with 2 μl of S. Typhimurium type 1-specific fimbrial antiserum. Fimbrial appendages present on the surface of bacterial cells were detected by agglutination of cells in this mixture. Serological agglutination titers of strains were also determined as previously described (10, 34, 44). Additionally, mannose-sensitive hemagglutination was assayed using a 3% suspension of guinea pig erythrocytes in the presence or absence of mannose (11, 32). Samples for transmission electron microscopy were prepared by placing aliquots of bacteria grown statically in broth for 48 h at 37°C prior to placement on carbon-coated copper grids for 1 min. Grids were stained for 30 s with 2% phosphotungstic acid or uranyl acetate, and images were visualized using a JEOL JEM-1230 transmission electron microscope.
Total FimA production by bacteria was determined from bacterial lysates by Western blot analyses (42). Bacterial suspensions (approximately 109 bacteria in 20-μl volumes) were sonicated and boiled in SDS lysis buffer prior to being spotted onto nitrocellulose membranes. FimA subunits were detected using FimA-specific serum following development with peroxidase-labeled goat anti-rabbit serum. Immunoblotting of bacterial lysates was performed as previously described by our group (18). Also, Western blot analyses were performed on lysates following SDS gel electrophoresis and subsequent transfer to membranes using standard procedures. Commercially available anti-GroEL (Sigma, St. Louis, MO) was used to detect intracellular GroEL in all bacterial lysates.
RESULTS
The fimAICDHF genes of S. Typhimurium SL1344 are expressed as a single transcriptional unit.
The S. Typhimurium fim genes are comprised of a gene cluster containing six genes encoding structural and assembly components as well as three convergently transcribed regulatory genes (fimZYW) as shown in Fig. 1. In order to determine if the former group of genes (fimAICDHF) can be transcribed from the fimA promoter, intergenic regions were examined for the presence of RNA using primers homologous to the coding regions flanking these intergenic sites. Reverse transcriptase PCR (RT-PCR) was performed using RNA prepared from strains grown under conditions favoring type 1 fimbria production and strongly fimbriate bacteria. As shown in Fig. 2A, PCR-generated products were obtained for the intergenic regions between fimA and fimI, fimI and fimC, fimC and fimD, fimD and fimH, and fimH and fimF using SL1344 as the source of RNA. All primers were designed to amplify regions that were approximately 500 nucleotides in length and possessed the complete predicted region between the translation termination site of one gene and the translation initiation site of the adjacent gene. These results indicate that the intergenic regions of the fimAICDHF gene cluster are transcribed consistent with fimAICDHF being a single transcriptional unit. In addition, no transcriptional initiation sites between these fim genes could be detected using the 5′ RACE technique (data not shown). The transcription initiation site of fimA has previously been reported by our group (45).
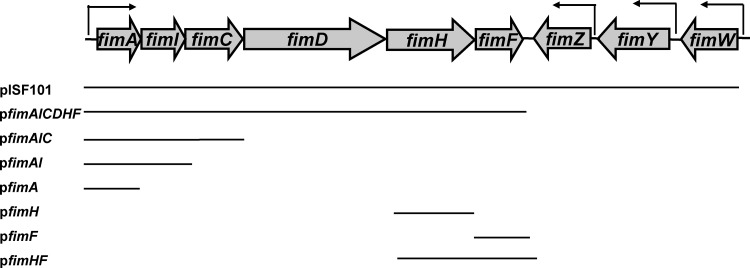
Fig 1.
Genetic organization of the S. Typhimurium fim gene cluster and plasmids used in this study. The arrows indicate promoter regions and directions of transcription. Solid lines indicate DNA carrying the fim genes retained on recombinant plasmids used in this study.
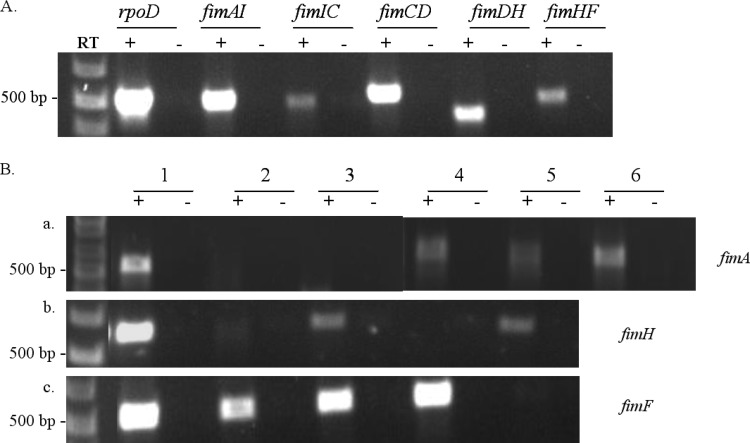
Fig 2.
(A) Transcriptional analysis of the intergenic regions of the fim gene cluster. The intergenic regions within fimAICDHF are linked on one transcript. RT-PCRs were carried out on RNA isolated from wild-type SL1344. The intergenic regions amplified are indicated by the underlined genes. Primers amplifying rpoD were included as a control. RT +/− indicates the presence or absence, respectively, of reverse transcriptase in reaction mixtures. (B) Transcription of fim genes in S. Typhimurium SL1344 mutants. RT-PCRs were carried out on RNA isolated from wild-type S. Typhimurium SL1344 and Fim mutants. The numbers indicate the strains from which RNA was prepared, and RT-PCRs were performed with (+) or without (−) reverse transcriptase: 1, S. Typhimurium SL1344; 2, SL1344ΔfimA; 3, SL1344fimA::TT; 4, SL1344ΔfimH; 5, SL1344ΔfimF; 6, SL1344ΔfimA+ (pfimA). RT-PCRs were performed with fimA-specific primers (a), fimH primers (b), or fimF primers (c).
Also, individual Fim mutants of SL1344 were examined for their ability to produce fim-specific mRNA from nonmutated genes. The results of these assays are shown in Fig. 2B. SL1344ΔfimA and SL1344fimA::TT are predicted not to produce full-length fimA transcript compared to the parental strain, and no fimA-specific mRNA was observed to be produced by these strains (Fig. 2B). However, full-length fimH- and fimF-specific mRNA was produced by these strains, although fimH gene expression appeared to be decreased in the FimA mutant. This result would suggest that mutations within fimA could have an effect on fimH gene expression. Complementation of fimA gene expression in the FimA mutants was achieved following transformation with a cloned fimA gene alone (see below).
As observed for the FimA mutant, the FimH and FimF mutants of SL1344 did not produce detectable levels of specific mRNA, but expression of the other fim genes was detected (Fig. 2B). Consequently, for all mutants examined, deletion (or insertion for one FimA mutant) of a single fim gene resulted in elimination of detectable transcription of that gene. Regardless of primer design, the levels of fimH-specific transcript were consistently lower than those observed for fimA and fimF (Fig. 2), but detectable levels of RNA were detected in all but the FimH mutant using these primers. However, as indicated above, we cannot rule out the possibility that fimH transcription was altered in FimA mutants. Because all of our experiments to investigate fim-specific transcript production were performed using in vitro techniques, we also cannot eliminate the possibility that in vivo some of the fim genes utilize internal promoters. Also, our in vitro analyses do not definitively prove the absence of internal promoters within the fim gene cluster but do indicate that intragenic transcription within the cluster does occur.
fimA, fimH, and fimF are required for surface-assembled fimbrial formation in SL1344.
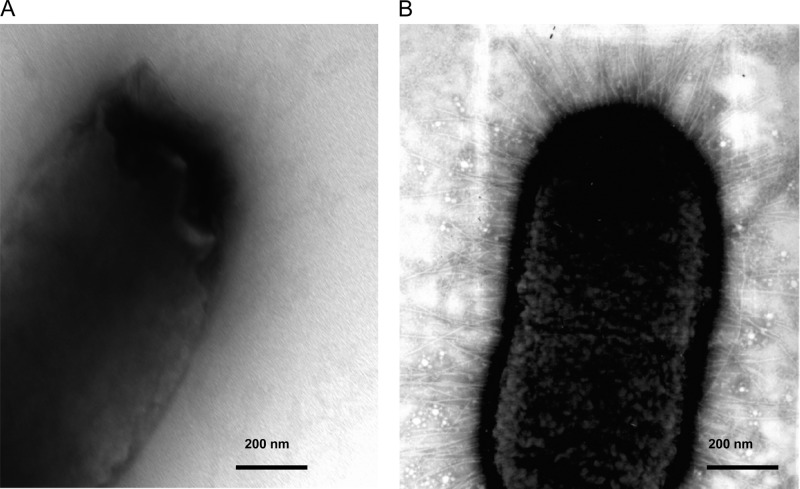
The specific Fim mutants of strain SL1344 were also examined for their ability to produce surface-assembled type 1 fimbriae. The mutants were examined for their ability to produce these fimbriae using serum raised against purified fimbriae isolated from a recombinant E. coli strain. The results are summarized in Table 2. The absence of the genes encoding the major fimbrial subunit (FimA), the FimF adaptor, or the fimbrial adhesin (FimH) resulted in a nonfimbriate phenotype with no fimbriae detected by reactivity with antibodies or by direct observation by electron microscopy (Fig. 3). However, the FimI deletion mutant was strongly fimbriate and exhibited a titer 8 times higher than that produced by fimbriae assembled on the parental strain (Table 2). Electron microscopic observation of the FimI mutant did not, however, suggest that the fimbriae produced by this strain were morphologically different from those produced by the parental strain SL1344.
Table 2.
Type 1 fimbrial production by SL1344 deletion mutants
| Strain | Agglutinationa | MHSAb |
|---|---|---|
| WTc SL3144 | +++ (800) | +++ |
| SL3144ΔfimA | − | N/D |
| SL1344fimA::TT | − | N/D |
| SL3144ΔfimI | +++ (6,400) | +++ |
| SL3144ΔfimH | − | − |
| SL3144ΔfimF | − | − |
| SL3144ΔfimHF | − | − |
+++, visible agglutination after 60 s; −, no detectable agglutination after 5 min. Numbers in parentheses are titers of reactivity with anti-FimA serum.
MSHA, mannose-sensitive agglutination of guinea pig erythrocytes; N/D, not done.
WT, wild type.
Fig 3.
Nonfimbriate and fimbriate strains of S. Typhimurium. (A) S. Typhimurium SL1344ΔfimH; (B) S. Typhimurium SL1344.
Complementation of the FimH- or FimF-negative mutants, with restoration of the fimbriate phenotype, could be achieved only using the homologous cloned genes (Table 3). Also, complementation of the FimHF double mutant was observed only with a plasmid possessing both fim genes but not with fimH or fimF alone. In all cases, restoration of the fimbriate phenotype was associated with mannose-sensitive hemagglutinating activity. These results indicate that the constructed deletion mutants did not have polar effects on adjacent genes that would have resulted in lack of gene expression.
Table 3.
Complementation of SL1344 Fim mutants
| Strain (plasmid) | Type 1 fimbriaeb |
|---|---|
| SL1344fimA::TT | − |
| SL1344fimA::TT::fimA | +++ |
| SL1344fimA::TT (pISF101) | +++ |
| SL1344fimA::TT (pfimA) | − |
| SL1344fimA::TT (pfimAI) | − |
| SL1344fimA::TT (pfimAIC) | − |
| SL1344fimA::TT (pfimAICDHF) | +++ |
| SL3144ΔfimH | − |
| SL3144ΔfimH (vca) | − |
| SL3144ΔfimH (pfimH) | +++ |
| SL3144ΔfimH::fimH | +++ |
| SL3144ΔfimH (pfimF) | − |
| SL3144ΔfimH (pfimHF) | +++ |
| SL3144ΔfimF | − |
| SL3144ΔfimF (vc) | − |
| SL3144ΔfimF (pfimH) | − |
| SL3144ΔfimF (pfimF) | +++ |
| SL3144Δfim (pfimHF) | +++ |
| SL3144ΔfimHF | − |
| SL3144ΔfimHF (vc) | − |
| SL3144ΔfimHF (pfimH) | − |
| SL3144ΔfimHF (pfimF) | − |
| SL3144ΔfimHF (pfimHF) | +++ |
vc, strain transformed with cloning vector alone.
+++ and −, restoration or not of fimbriate phenotype, respectively.
Complementation of a FimA mutant with the cloned fimA determinant carried on a multicopy plasmid did not restore the ability of transformants to produce type 1 fimbriae. However, this plasmid was shown to produce FimA subunits (see below). These transformants were nonfimbriate as determined by seroreactivity and electron microscopy. However, if this mutant was transformed with a plasmid, pISF101, carrying the complete fim gene cluster, the transformants were fully fimbriate (8). Deletion derivatives of this plasmid expressing fimA alone, fimAI, or fimAIC were not able to complement the FimA mutant to restore fimbrial production (Fig. 1; Table 3). Because pISF101 possesses the regulatory genes fimZ, -Y, and -W, a plasmid possessing only the genes encoding the structural and assembly components (fimAICDHF) was constructed (Fig. 1). Transformation of the FimA mutant with this plasmid did facilitate the production of type 1 fimbriae by transformants (Table 3).
In order to eliminate any effects of gene dosage on the phenotype of transformed mutants, we reintroduced individual fim genes back onto the chromosome of the FimA and FimH mutants. The FimH mutant carrying a reintroduced and intact fimH on its chromosome was fully fimbriate and exhibited a phenotype identical to that of the FimH mutant transformed with a plasmid-borne cloned fimH gene. Interestingly, when the fimA gene was carried on the chromosome of the FimA mutant, type 1 fimbriae were produced by this strain, unlike the phenotype observed using a cloned fimA gene on a cloning vector (Table 3). All strains carrying the homologous gene as an integrated determinant exhibited phenotypic Salmonella fimbrial phase variation as originally described by Old and Duguid (32, 33) and optimally produced surface-assembled fimbriae following serial subculture in static liquid media.
Fimbrial subunits are not accumulated by Fim mutants.
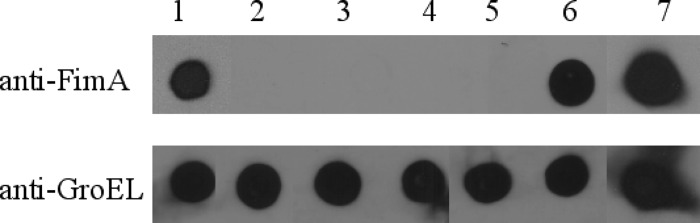
Since the FimH, FimF, and FimHF mutants were phenotypically nonfimbriate but did express fimA-specific transcripts, the presence of FimA subunits from whole-cell lysates was determined. Lysates of both sonicated and nonsonicated bacterial suspensions were used in Western blot assays to detect both intracellular and surface-assembled FimA subunits. None of the mutants produced detectable levels of fimbrial subunits. A fimbrial phenotype was invariably associated with detection of FimA subunit by the immune serum (Fig. 4). Therefore, only the parental strain and FimA mutants complemented with an integrated fimA gene produced detectable amounts of FimA. The antiserum used in these assays had been raised against purified recombinant FimA produced in E. coli lysates. Although these E. coli transformants do not assemble Salmonella type 1 fimbriae, FimA subunits can be detected in boiled extracts (Fig. 4). To ensure that S. Typhimurium cultures had been lysed in preparation for the immunoblot assays, all lysates were tested for the presence of the intracellular chaperone GroEL. This intracellular protein could be detected in all strains (Fig. 4).
Fig 4.
Production of FimA by S. Typhimurium SL1344 mutants. Immunoblot assays were carried out on lysates from wild-type S. Typhimurium SL1344 and various mutants with antibody raised against SL1344 FimA or bacterial GroEL. Strains are indicated by numbers above the blots: 1, S. Typhimurium SL1344; 2, SL1344ΔfimA; 3, SL1344ΔfimH; 4, SL1344ΔfimF; 5, SL1344ΔfimA + pfimA; 6, SL1344fimA::TT::fimA; 7, E. coli + pfimA.
DISCUSSION
Type 1 fimbriae are produced by most strains and species of enterobacteria and play a role in facilitating adherence to host cells and tissues (5, 15, 29). These fimbriae are characterized by their ability to mediate adherence that is subject to inhibition in vitro by soluble mannose-containing compounds and are therefore also commonly referred to as mannose-sensitive fimbriae or pili (10). The ability to mediate mannose-sensitive binding to target cells is due to the production of an adhesin protein (FimH) that is distinct from the major fimbrial subunit (FimA). In enterobacteria, the gene cluster encoding the products necessary for fimbrial assembly is the fim cluster, and these genes have been cloned and extensively analyzed in three bacterial species: E. coli, Salmonella serovars, and Klebsiella pneumoniae (8, 14, 26). In each, the fim gene cluster is comprised of genes encoding structural components of the assembled appendages as well as genes necessary for the ordered assembly of the fimbriae. The genes and their products in E. coli and K. pneumoniae are closely related, and the fimbriae of the two species have been shown to be immunologically cross-reactive (1, 10, 34). However, strains of K. pneumoniae possess a gene, fimK, that is absent from the E. coli fim gene cluster and has been proposed to play a role in fim gene regulation (36). The S. Typhimurium fim gene cluster exhibits a genetic organization, with respect to genes encoding the structural and assembly components, similar to that of E. coli. However, the gene products exhibit limited amino acid sequence similarity and cannot be functionally interchanged between the two systems, and assembled type 1 fimbriae of Salmonella are antigenically distinct from those of E. coli (8, 34). In addition, the E. coli gene cluster possesses one additional gene that is proposed to encode an adaptor protein (26, 37). Individual deletions in the E. coli genes encoding the adaptors (FimF and FimG) as well as the adhesin (FimH) have been reported to result in changes in the ordered assembly of type 1 fimbriae but retention of fimbrial production as detected by electron microscopy and the ability of whole bacteria to react with fimbria-specific antisera (26, 37). This indicates that each of these proteins is not absolutely required for initiation of fimbrial assembly but may play a role in the numbers and morphology of the appendages produced. In order to determine whether the structural components of the S. Typhimurium fimbriae (FimA, FimF, and FimH) are required for the production of surface-assembled fimbriae, we constructed deletions in each of the genes encoding these proteins and determined if the bacteria possess type 1 fimbriae on their surfaces. In addition, we constructed a FimI mutant since it has been proposed that the E. coli FimI gene product may play a role in fimbrial assembly in these bacteria (43).
The absence of any fim gene encoding a major or minor fimbrial subunit in S. Typhimurium resulted in the inability of bacteria to assemble fimbriae on their surfaces. Reintroduction of a single functional homologous gene into mutants resulted in restoration of fimbrial assembly. The nonfimbriate phenotype is predicted for the FimA mutant since the absence of the major fimbrial subunit would be expected to result in the inability to form an appendage that is comprised primarily of this component. This phenotype has also been reported for FimA mutants of E. coli (20, 26). However, in S. Typhimurium the absence of adaptor (FimF) and adhesin (FimH) gene products also results in the lack of detectable surface-assembled fimbriae. This is in contrast to observations in E. coli in which single deletion mutants produced fimbriae as detected by techniques similar to those used in the studies described here (30, 37). Although the numbers and morphology of the fimbriae were altered in the E. coli mutants, it was still possible to detect type 1 fimbriae on their surfaces, and this was not the case for the Salmonella mutants. Our results would indicate that initiation of fimbrial assembly in S. Typhimurium requires all three components (FimA, FimF, and FimH) and that this process does not occur in the absence of any one of these subunits. Similar results for non-type 1 fimbriae have been reported in other species of enterobacteria (23). The E. coli fim gene cluster possesses a single additional adaptor protein (FimG) that is not found in the S. Typhimurium gene cluster. Therefore, it is possible that the presence of FimG in the absence of either FimF or FimH is sufficient to assemble type 1 fimbrial filaments. However, a report of the detection of surface-assembled fimbriae in an E. coli FimFG double mutant would indicate that these bacteria need only FimA and FimH to assemble the fimbriae (37). This is not the case for S. Typhimurium. We have previously shown that fimbrial components from E. coli and S. Typhimurium cannot complement each other to facilitate appendage formation (8, 14). Consequently, it appears that the molecular interactions between the E. coli and S. Typhimurium subunits with their respective chaperones and assembly scaffolding proteins, processes involving donor strand complementation and donor strand exchange, respectively, have evolved differently in the two species (2, 22, 28, 35).
Of particular interest from our studies was the observation that a FimA mutant could be complemented to produce functional fimbriae only with either the entire cloned fim gene cluster or the presence of a single copy of fimA integrated onto the Salmonella chromosome. Multiple copies of fimA alone carried on a recombinant plasmid did not facilitate fimbrial assembly even though the gene is being expressed. These results suggest that in S. Typhimurium the correct ratio of fim genes and, therefore, most likely, the stoichiometric ratio of Fim proteins are required for detectable surface production of type 1 fimbriae. It is possible that an excess of FimA relative to other components results in saturation of the periplasmic chaperone such that little FimF/FimC or FimH/FimC complex is formed. Considering that our data indicate that both FimF and FimH are necessary for detectable fimbrial production, this is consistent with the observations reported here.
We also examined whether FimA subunits accumulated in the Fim mutants even though no fimbriae are produced. Using either specific antisera raised against purified FimA subunits or serum raised against purified native fimbriae and whole-cell lysates, it was not possible to detect significant amounts of FimA in the mutants. Consequently, even when the fimA gene is transcribed in FimH or FimF mutants, as detected by RT-PCR, the gene products are not present. One possibility is that the fimbrial protein is rapidly turned over intracellularly, or alternatively, posttranscriptional regulation of the fimA may occur. Further analyses will be required to differentiate between these two possibilities.
In E. coli, mutations in the fimI gene have been reported to result in loss of fimbrial production (43). Mutations in the S. Typhimurium fimI gene had no effect on fimbria formation except to increase the reactivity of surface-assembled fimbrial antigen with antiserum. This would suggest that either FimI mutants produce greater numbers of surface-assembled fimbriae than does the parental strain or the fimbriae are longer. Electron microscopic observation of the fimbriae produced by the FimI mutants did not indicate any significant increase in fimbrial length compared to SL1344, a result that would suggest that FimI plays little role in the termination of fimbrial assembly to regulate the length of the appendage. Increased surface-exposed fimbrial antigen in the FimI mutant may be due to more efficient production or initiation of fimbrial biosynthesis, and this may be due to FimI modulating this stage of the assembly process.
In summary, our studies demonstrate that unlike the E. coli system, the presence of the major and minor fimbrial subunits of S. Typhimurium is required for production of detectable amounts of appendage on the bacterial surface. A second difference between the two systems appears to be the role of FimI, since mutants lacking the ability to produce this gene product exhibit opposite phenotypes in E. coli and S. Typhimurium. However, as for the other fimbrial proteins, there is little relatedness between the two FimI gene products, and designation of the S. Typhimurium gene was originally based upon its location between the genes encoding the major fimbrial subunit and the chaperone. Finally, our studies indicate that the ratio of the fim genes encoding the structural and assembly components of the type 1 fimbriae influences the fimbrial phenotype, indicating that excessive production of FimA in comparison to the other proteins results in inhibition of efficient fimbria production.
ACKNOWLEDGMENTS
This work was supported by NIH grants RO1GM084318 and RO1AI074693 to S.C.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Adegbola RA, Old DC. 1987. Antigenic relationships among type-1 fimbriae of Enterobacteriaceae revealed by immuno-electronmicroscopy. J. Med. Microbiol. 24:21–28 [DOI] [PubMed] [Google Scholar]
- 2. Allen WJ, Phan G, Waksman G. 6 March 2012. Pilus biogenesis at the outer membrane of Gram-negative bacterial pathogens. Curr. Opin. Struct. Biol. [Epub ahead of print.] doi:10.1016/j.sbi.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 3. Althouse C, Patterson S, Fedorka-Cray P, Isaacson RE. 2003. Type 1 fimbriae of Salmonella enterica serovar Typhimurium bind to enterocytes and contribute to colonization of swine in vivo. Infect. Immun. 71:6446–6452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blanco M, et al. 1997. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: relationship with expression of adhesins and production of toxins. Res. Microbiol. 148:745–755 [DOI] [PubMed] [Google Scholar]
- 5. Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S. 2002. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol. Microbiol. 45:1255–1265 [DOI] [PubMed] [Google Scholar]
- 6. Chouikha I, Bree A, Moulin-Schouleur M, Gilot P, Germon P. 2008. Differential expression of iutA and ibeA in the early stages of infection by extra-intestinal pathogenic E. coli. Microbes Infect. 10:432–438 [DOI] [PubMed] [Google Scholar]
- 7. Clegg S, Hughes KT. 2002. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clegg S, Hull S, Hull R, Pruckler J. 1985. Construction and comparison of recombinant plasmids encoding type 1 fimbriae of members of the family Enterobacteriaceae. Infect. Immun. 48:275–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duguid JP, Campbell I. 1969. Antigens of the type-1 fimbriae of salmonellae and other enterobacteria. J. Med. Microbiol. 2:535–553 [DOI] [PubMed] [Google Scholar]
- 11. Duguid JP, Clegg S, Wilson MI. 1979. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J. Med. Microbiol. 12:213–227 [DOI] [PubMed] [Google Scholar]
- 12. Ewen SW, et al. 1997. Salmonella enterica var Typhimurium and Salmonella enterica var Enteritidis express type 1 fimbriae in the rat in vivo. FEMS Immunol. Med. Microbiol. 18:185–192 [DOI] [PubMed] [Google Scholar]
- 13. Frohman MA, Dush MK, Martin GR. 1988. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. U. S. A. 85:8998–9002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerlach GF, et al. 1989. Expression of type 1 fimbriae and mannose-sensitive hemagglutinin by recombinant plasmids. Infect. Immun. 57:764–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh S, Mittal A, Vohra H, Ganguly NK. 1996. Interaction of a rat intestinal brush border membrane glycoprotein with type-1 fimbriae of Salmonella typhimurium. Mol. Cell. Biochem. 158:125–131 [DOI] [PubMed] [Google Scholar]
- 16. Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183:6384–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoiseth SK, Stocker BA. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238–239 [DOI] [PubMed] [Google Scholar]
- 18. Johnson JG, Clegg S. 2010. Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J. Bacteriol. 192:3944–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kisiela DI, et al. 2011. Allosteric catch bond properties of the FimH adhesin from Salmonella enterica serovar Typhimurium. J. Biol. Chem. 286:38136–38147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klemm P, Jorgensen BJ, van Die I, de Ree H, Bergmans H. 1985. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol. Gen. Genet. 199:410–414 [DOI] [PubMed] [Google Scholar]
- 21. Lane MC, Mobley HL. 2007. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int. 72:19–25 [DOI] [PubMed] [Google Scholar]
- 22. Le Trong I, et al. 2010. Donor strand exchange and conformational changes during E. coli fimbrial formation. J. Struct. Biol. 172:380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, et al. 1997. Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly. Infect. Immun. 65:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindberg F, Lund B, Johansson L, Normark S. 1987. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature 328:84–87 [DOI] [PubMed] [Google Scholar]
- 25. Lindberg FP, Lund B, Normark S. 1984. Genes of pyelonephritogenic E. coli required for digalactoside-specific agglutination of human cells. EMBO J. 3:1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maurer L, Orndorff PE. 1987. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J. Bacteriol. 169:640–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mobley HL, et al. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1–4) beta Gal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143–155 [DOI] [PubMed] [Google Scholar]
- 28. Morrissey B, et al. 27 February 2012. The role of chaperone-subunit usher domain interactions in the mechanism of bacterial pilus biogenesis revealed by ESI-MS. Mol. Cell. Proteomics [Epub ahead of print.] doi:10.1074/mcp.M111.015289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naughton PJ, et al. 2001. Expression of type 1 fimbriae (SEF 21) of Salmonella enterica serotype enteritidis in the early colonisation of the rat intestine. J. Med. Microbiol. 50:191–197 [DOI] [PubMed] [Google Scholar]
- 30. Norgren M, et al. 1984. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 3:1159–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nuccio SP, Baumler AJ. 2007. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 71:551–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Old DC, Duguid JP. 1970. Selective outgrowth of fimbriate bacteria in static liquid medium. J. Bacteriol. 103:447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Old DC, Duguid JP. 1971. Selection of fimbriate transductants of Salmonella typhimurium dependent on motility. J. Bacteriol. 107:655–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Old DC, Payne SB. 1971. Antigens of the type-2 fimbriae of salmonellae: “cross-reacting material” (CRM) of type-1 fimbriae. J. Med. Microbiol. 4:215–225 [DOI] [PubMed] [Google Scholar]
- 35. Poole ST, et al. 2007. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol. Microbiol. 63:1372–1384 [DOI] [PubMed] [Google Scholar]
- 36. Rosen DA, et al. 2008. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect. Immun. 76:3337–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Russell PW, Orndorff PE. 1992. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J. Bacteriol. 174:5923–5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saini S, Pearl JA, Rao CV. 2009. Role of FimW, FimY, and FimZ in regulating the expression of type i fimbriae in Salmonella enterica serovar Typhimurium. J. Bacteriol. 191:3003–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swenson DL, Kim KJ, Six EW, Clegg S. 1994. The gene fimU affects expression of Salmonella typhimurium type 1 fimbriae and is related to the Escherichia coli tRNA gene argU. Mol. Gen. Genet. 244:216–218 [DOI] [PubMed] [Google Scholar]
- 40. Thanassi DG, et al. 1998. The PapC usher forms an oligomeric channel: implications for pilus biogenesis across the outer membrane. Proc. Natl. Acad. Sci. U. S. A. 95:3146–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tinker JK, Clegg S. 2001. Control of FimY translation and type 1 fimbrial production by the arginine tRNA encoded by fimU in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 40:757–768 [DOI] [PubMed] [Google Scholar]
- 42. Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valenski ML, Harris SL, Spears PA, Horton JR, Orndorff PE. 2003. The product of the fimI gene is necessary for Escherichia coli type 1 pilus biosynthesis. J. Bacteriol. 185:5007–5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yeh KS, Hancox LS, Clegg S. 1995. Construction and characterization of a fimZ mutant of Salmonella typhimurium. J. Bacteriol. 177:6861–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeh KS, Tinker JK, Clegg S. 2002. FimZ binds the Salmonella typhimurium fimA promoter region and may regulate its own expression with FimY. Microbiol. Immunol. 46:1–10 [DOI] [PubMed] [Google Scholar]