Abstract
Placental infection with Plasmodium falciparum is associated with increased levels of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ), and previous studies have associated increased levels of these cytokines with low birth weight (LBW), especially for malaria-infected primigravidae. To define the contribution of TNF-α and IFN-γ networks to placental-malaria-associated LBW, we measured chemokines induced by TNF-α and IFN-γ and related them to birth weight in a birth cohort of 782 mother-infant pairs residing in an area of P. falciparum holoendemicity in Tanzania. Among primigravidae, levels of CCL2, CXC ligand 9 (CXCL9), and CXCL13 were significantly higher during malaria infection in both the placenta and peripheral blood. Placental CXCL9 and CXCL13 levels were also higher in placental blood from secundigravidae and multigravidae. In multivariate analyses adjusted for known predictors of birth weight, malaria-infected primigravidae with placental CXCL9 levels in the lowest tertile gave birth to babies who weighed 610 g more than babies born to mothers with high CXCL9 levels. CXCL9 expression is induced by IFN-γ, and the strong association between birth weight and placental CXCL9 is consistent with previous observations relating IFN-γ to poor pregnancy outcomes.
INTRODUCTION
Pregnancy malaria causes severe anemia in the mother and low birth weight in the child, and these sequelae alone may lead to 10,000 maternal deaths and as many as 200,000 infant deaths annually in Africa (5, 24). In areas where malaria is endemic, pregnant women are more susceptible to malaria infection than their nonpregnant counterparts (14). Susceptibility diminishes over successive pregnancies, when women develop specific antibodies that inhibit the adhesion of the parasite to chondroitin sulfate A, explaining why multigravidae are resistant while primigravidae are susceptible (7, 22). As a consequence, malaria infection is more frequent and more intense in primigravidae than in multigravidae.
Chronic placental malaria, which is characterized by an inflammatory infiltrate, is more common in primigravidae (3, 27), presumably due to their lack of immunity (8). In nonimmune women, the initial wave of parasites sequestered in the placenta is followed by the accumulation of macrophages in the intervillous spaces; high parasite and immune cell densities are rare in immune women (8). The accumulation of macrophages in the placenta has been associated with maternal anemia, low-birth-weight (LBW) deliveries, and intrauterine growth retardation (9, 21, 27). B cells also appear in the placental infiltrate, where their products may play a role in the inflammatory engine and are associated with poor pregnancy outcomes (18).
At the molecular level, placental malaria is associated with increased levels of cytokines and chemokines such as tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-10 (IL-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and CXC ligand 8 (CXCL8)(1, 4, 6, 10, 17, 18, 23, 25, 26). In addition to soluble factors released by maternal immune cells, fetal tissue may also release mediators that contribute to placental inflammation, for example, IFN-γ secreted by placental villi during maternal malaria (26).
Several studies have related increased placental TNF-α levels to LBW, particularly in primigravid women (6, 10, 23), and intrauterine growth retardation has been associated with the upregulation of IL-8 and TNF-α transcription in the placenta (17). The role of IFN-γ has been controversial (6, 10, 15, 23). In our genomewide expression profiles of malaria-infected placentas from primigravidae, transcription of chemokines, including CXCL13, CXCL9, and CCL18, was significantly upregulated and was negatively correlated with birth weight (18).
The TNF-α network involves activated macrophages and B cells, resulting in strong upregulation in the gene expression of several chemokines, in particular CXCL13 (19). The IFN-γ network results in the upregulation of CXCL9 in various cells, including macrophages (13). We reported previously that placental IFN-γ increases the risk of LBW (6, 10), and CXCL9 transcript levels during inflammatory placental malaria (PM) are negatively associated with birth weight (18).
In the present study, we examined the independent roles of IFN-γ and TNF-α networks in placental malaria by measuring chemokines induced by TNF-α (CCL20, CCL18, CXCL1, and CXCL13), IFN-γ (CXCL9), or both (CCL2). In univariate analyses, CXCL9 and CXCL13 were associated with LBW. In multivariate analysis, only CXCL9 remained significantly associated with reductions in birth weight. These results are consistent with the model in which IFN-γ and its downstream mediator CXCL9 play key roles in poor pregnancy outcomes associated with placental malaria.
MATERIALS AND METHODS
Human subjects and clinical procedures.
Women participating in this study were recruited between September 2002 and October 2005 into a longitudinal cohort conducted by the Mother-Offspring Malaria Studies (MOMS) Project in the Muheza district, northeastern Tanzania. Pregnant women 18 years old or older without clinical evidence of chronic or debilitating illness were asked to participate in the study and gave signed informed consent after receiving a study explanation form and an oral explanation from a nurse in their native language as described previously (10, 20).
The protocol and study procedures were approved by the International Clinical Studies Review Committee of the Division of Microbiology and Infectious Diseases at the U.S. National Institutes of Health. Ethical clearance was obtained from the institutional review boards of the Seattle Biomedical Research Institute and the National Institute for Medical Research in Tanzania.
Peripheral blood was obtained by venipuncture from women at delivery and was anticoagulated with citrate phosphate dextrose. Placental blood samples were obtained by manual compression of the placental tissue in a grinder using EDTA as an anticoagulant. Plasma from placental and peripheral blood was obtained by centrifugation at 2,800 rpm for 5 min, and samples were stored at −80°C until use.
Placental malaria was defined as the identification of any parasite in a placental blood smear. A placental blood smear was defined as negative after examination of 100 high-power fields in the thick smear. Low birth weight (LBW) was defined as less than 2.5 kg.
Multiplex chemokine assays.
Plasma samples were analyzed using a multiplex bead-based platform (Bioplex; Bio-Rad, Irvine, CA). The multiplex assay was developed in-house using Duoset ELISA development reagents (R&D Systems, Minneapolis, MN). All pipetting and sample identification were performed with a bar code-enabled, high-speed pipetting robot (Megaflex; Tecan, Research Triangle Park, NC). The detection limits for the different soluble factors were as follows: for CCL18, CCL2, CXCL1, and CXCL13, 2.44 pg/ml; for CXCL9, 4.88 pg/ml; and for CCL20, 1.22 pg/ml.
Statistical analysis.
Analyses were performed using Statview, version 5.0.1 (SAS Institute, Cary, NC). Differences in chemokine levels between groups were analyzed by a Mann-Whitney test. Univariate linear regression and multivariate linear regression were used to analyze the relationship between soluble mediators coded as either a continuous variable or as tertiles of their distribution and birth weight. In continuous analyses, levels of chemokines were log transformed to obtain normal distributions. P values of <0.05 were considered significant or were adjusted for multiple comparisons in the multivariate analysis.
RESULTS
Study population.
The study population included 882 (254 primigravid, 201 secundigravid, and 427 multigravid) mother-infant pairs, after the exclusion of twins, triplets, and women infected with HIV. The placental malaria rate was higher in primigravid and secundigravid than in multigravid women (18.9%, 18.4%, and 7.2%, respectively), as described in a previous report on this cohort (10). PM was associated with a reduction in birth weight in all parity groups (P < 0.001). A total of 782 placenta blood samples and 738 peripheral blood samples were available for the analyses described here (Table 1).
Table 1.
Chemokine levels in placental blood and maternal peripheral blood stratified by parity and placental malaria status
| Parity and location | Chemokine | PM− |
PM+ |
Pa | ||
|---|---|---|---|---|---|---|
| No. of samples | Median (interquartile range) | No. of samples | Median (interquartile range) | |||
| Primigravid | ||||||
| Placenta | CCL2 | 180 | 295.5 (200.6–414.6) | 44 | 386.5 (253.6–640.8) | 0.007 |
| CCL18 | 180 | 25,049.9 (9,039.3–106) | 44 | 1,000,000 (11,949.4–106) | 0.07 | |
| CCL20 | 180 | 170.8 (99.9–319.9) | 44 | 183.65 (134.6–359.8) | NS | |
| CXCL1 | 180 | 1,302.6 (833.7–1,872.7) | 44 | 1,291.4 (832.3–2,603.9) | NS | |
| CXCL9 | 180 | 2,139.9 (697.3–3,939.1) | 44 | 4,665 (2,084.5–12,100.2) | <0.0001 | |
| CXCL13 | 180 | 461.9 (313.7–670.7) | 44 | 1,454.8 (535.3–2,356.3) | <0.0001 | |
| Peripheral | CCL2 | 166 | 126.8 (96.9–174.6) | 45 | 162.6 (117.5–206.4) | 0.003 |
| CCL18 | 166 | 23,782.7 (9,663.3–106) | 45 | 1,000,000 (16,434.9–106) | 0.02 | |
| CCL20 | 166 | 48.35 (32.4–77.5) | 45 | 57.69 (38.3–80.9) | NS | |
| CXCL1 | 166 | 183.6 (0–377.3) | 45 | 332.8 (101.4–494.5) | 0.03 | |
| CXCL9 | 166 | 2,203.8 (883.9–3,290.4) | 45 | 2,462.1 (2,140.9–3,794.9) | 0.08 | |
| CXCL13 | 166 | 557.8 (385.5–825.7) | 45 | 759.1 (518.4–1,185.4) | 0.003 | |
| Secundigravid | ||||||
| Placenta | CCL2 | 148 | 314.7 (195.0–449.9) | 32 | 347.7 (224.9–609.6) | NS |
| CCL18 | 148 | 17,362 (8,884.3–106) | 32 | 15,551 (7,328.8–758,714) | NS | |
| CCL20 | 148 | 167.2 (95.5–290.0) | 32 | 159.3 (102.4–312.1) | NS | |
| CXCL1 | 148 | 1,064.3 (679.8–1,768.0) | 32 | 1,413.3 (721.1–2,243.1) | NS | |
| CXCL9 | 148 | 1,701.6 (330.3–3,151.8) | 32 | 2,652.2 (1,433.1–5,957.7) | 0.002 | |
| CXCL13 | 148 | 363.7 (258.1–526.9) | 32 | 515.3 (363.2–701.1) | 0.009 | |
| Peripheral | CCL2 | 136 | 127.0 (92.7–165.3) | 32 | 125.0 (92.7–155.4) | NS |
| CCL18 | 136 | 18,191.6 (10,257.8–106) | 32 | 24,680.5 (8,852.3–106) | NS | |
| CCL20 | 136 | 47.8 (31.0–79.7) | 32 | 36.12 (23.8–55.7) | 0.02 | |
| CXCL1 | 136 | 161.0 (0–342.9) | 32 | 183.9 (130.5–351.2) | NS | |
| CXCL9 | 136 | 1,968.4 (919.7–2,651.8) | 32 | 2,086.1 (177.1–2,509.1) | NS | |
| CXCL13 | 136 | 459.1 (323.6–459.0) | 32 | 449.2 (298.6–582.4) | NS | |
| Multigravid | ||||||
| Placenta | CCL2 | 357 | 382.9 (239.0–602.1) | 21 | 380.0 (208.1–715.6) | NS |
| CCL18 | 357 | 21,248.4 (8,568.7–106) | 21 | 15,448.4 (10,540.4–106) | NS | |
| CCL20 | 357 | 214.6 (115.9–442.9) | 21 | 128.9 (69.9–357.7) | NS | |
| CXCL1 | 357 | 1,366.8 (865.4–2,039.4) | 21 | 1,821.8 (995.2–2,285.0) | NS | |
| CXCL9 | 357 | 1,701.7 (274.3–2,484.7) | 21 | 3,329.0 (2,199.1–6,633.8) | <0.0001 | |
| CXCL13 | 357 | 319.2 (205.4–495.5) | 21 | 473.8 (213.6–901.4) | 0.06 | |
| Peripheral | CCL2 | 343 | 127.0 (93.2–162.6) | 16 | 143.8 (118.9–194.8) | NS |
| CCL18 | 343 | 17,032.4 (9,365.6–106) | 16 | 20,022.8 (10,077.9–759,256) | NS | |
| CCL20 | 343 | 42.0 (28.1–69.1) | 16 | 39.2 (28.1–80.8) | NS | |
| CXCL1 | 343 | 376.1 (0–376.1) | 16 | 183.9 (26.9–317.6) | NS | |
| CXCL9 | 343 | 2,140.9 (833.9–3,173.6) | 16 | 2,509.1 (1,391.6–4,596.4) | 0.09 | |
| CXCL13 | 343 | 385.9 (264.4–570.0) | 16 | 521.9 (313.7–623.9) | NS | |
NS, not significant.
PM modifies chemokine levels.
We related chemokine levels in both placental and peripheral blood to the presence or absence of PM (Table 1). In all parity groups (primigravid, secundigravid, or multigravid women), levels of CXCL9 and CXCL13 were higher in the placental blood of PM+ than of PM− mothers. These differences were significant, except for placental CXCL13 levels in multigravidae, where the difference approached significance. CCL2 levels were significantly higher in the placental blood of PM+ primigravidae compared to that of PM− primigravidae but not in the placental blood of other women (Table 1). Placental CCL18, CCL20, and CXCL1 levels were similar for PM+ and PM− women.
Among PM+ primigravidae, levels of both CXCL13 and CXCL9 in the placenta correlated with the macrophage count in placental blood (r, 0.373 [P = 0.02] for CXCL13; r, 0.457 [P = 0.004] for CXCL9). Other chemokines were not significantly related to the macrophage count.
Placental malaria was also associated with increased chemokine levels in the peripheral blood. CCL2, CCL18, CXCL1, and CXCL13 levels were significantly higher in PM+ than in PM− primigravidae. In both primigravidae and multigravidae, CXCL9 levels in peripheral blood were higher during PM, but the differences did not achieve significance. Among secundigravidae, PM was associated with a reduction in CCL20 levels in the peripheral blood.
CXCL9 is associated with reduction in birth weight.
We examined the association between placental chemokine levels that were significantly elevated in PM+ women and low birth weight (LBW). LBW was associated with 5.5-fold-higher median levels of placental CXCL9 and 4.6-fold-higher median levels of CXCL13 among PM+ primigravidae (Fig. 1). Only two cases of LBW deliveries occurred among PM+ multigravidae, and none among PM+ secundigravidae, preventing meaningful analysis of the relationships between chemokines and LBW in these groups. Among PM+ primigravidae, CCL2 levels were not associated with LBW.
Fig 1.
Placental CXCL9 (A) and CXCL13 (B) levels in normal-birth-weight (open boxes) and low-birth-weight (shaded boxes) deliveries stratified by gravidity and malaria infection status. The number of samples tested (normal birth weight, low birth weight) and the significance of differences between normal birth weight and low birth weight, respectively, for CXCL9 (P values analyzed by Mann-Whitney U test) was as follows: for primigravid PM− women, 169 and 11 (P = 0.8); for primigravid PM+ women, 35 and 8 (P = 0.015); for multigravid PM− women, 345 and 11 (P = 0.15); for multigravid PM+ women, 19 and 2. The corresponding values for CXCL13 were as follows: for primigravid PM− women, 169 and 11 (P = 0.7); for primigravid PM+ women, 35 and 8 (P = 0.008); for multigravid PM− women, 345 and 11 (P = 0.03); for multigravid PM+ women, 19 and 2.
We evaluated the relationships between chemokine levels and birth weight as a continuous variable in univariate linear regression models. Placental CXCL9 and CXCL13 were each negatively associated with birth weight in PM+ primigravidae and secundigravidae (Table 2). Changes in other chemokine levels were not significantly associated with birth weight.
Table 2.
Univariate associations between chemokines upregulated during PM and birth weight
| Factor | Primigravid |
Secundigravid |
Multigravid |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM− |
PM+ |
PM− |
PM+ |
PM− |
PM+ |
|||||||
| β | P | β | P | β | P | β | P | β | P | β | P | |
| CXCL9 | 0.077 | NSa | −0.515 | 0.0004 | 0.023 | NS | −0.452 | 0.01 | 0.036 | NS | −0.229 | NS |
| CXCL13 | −0.102 | NS | −0.548 | 0.0001 | −0.145 | NS | −0.471 | 0.007 | −0.092 | NS | −0.428 | 0.05 |
| CCL2 | 0.114 | NS | −0.15 | NS | −0.056 | NS | −0.211 | NS | 0.044 | NS | −0.033 | NS |
NS, not significant.
In an earlier report, levels of several soluble factors (cord leptin, cord ferritin, placental TNF-α, and placental IFN-γ) were associated with birth weight in this cohort (10). We performed multivariate linear regression analysis of the factors previously associated with reduction in birth weight together with the chemokines measured here in order to examine their independent contributions to birth weight. In PM+ primigravidae, CXCL9 was strongly related to decreased birth weight after adjustment for multiple comparisons (β = −0.499; P = 0.002) (Table 3).
Table 3.
Multivariate linear regression analysis of soluble mediators associated with birth weight
| Parity | PM status | No. of samples | Factor | β | P |
|---|---|---|---|---|---|
| Primigravid | PM− | 145 | Cord leptin | 0.464 | <.0001 |
| Cord ferritin | 0.103 | NS | |||
| Placental TNF-α | −0.061 | NS | |||
| Placental IL-10 | 0.159 | NS | |||
| Placental IFN-γ | −0.111 | NS | |||
| Placental CXCL9 | 0.010 | NS | |||
| Placental CXCL13 | −0.128 | NS | |||
| PM+ | 35 | Cord leptin | 0.232 | NS | |
| Cord ferritin | 0.154 | NS | |||
| Placental TNF-α | −0.160 | NS | |||
| Placental IL-10 | 0.434 | 0.005 | |||
| Placental IFN-γ | −0.117 | NS | |||
| Placental CXCL9 | −0.499 | 0.002 | |||
| Placental CXCL13 | −0.317 | NS | |||
| Secundigravid | PM− | 126 | Cord leptin | 0.273 | 0.002 |
| Cord ferritin | 0.142 | NS | |||
| Placental TNF-α | −0.438 | 0.002 | |||
| Placental IL-10 | 0.191 | NS | |||
| Placental IFN-γ | 0.202 | NS | |||
| Placental CXCL9 | 0.054 | NS | |||
| Placental CXCL13 | −0.162 | NS | |||
| PM+ | 27 | Cord leptin | 0.320 | NS | |
| Cord ferritin | −0.503 | NS | |||
| Placental TNF-α | 0.340 | NS | |||
| Placental IL-10 | 0.068 | NS | |||
| Placental IFN-γ | −0.010 | NS | |||
| Placental CXCL9 | 0.053 | NS | |||
| Placental CXCL13 | −0.466 | 0.05 | |||
| Multigravid | PM− | 297 | Cord leptin | 0.281 | <0.0001 |
| Cord ferritin | −0.078 | NS | |||
| Placental TNF-α | 0.092 | NS | |||
| Placental IL-10 | −0.007 | NS | |||
| Placental IFN-γ | 0.019 | NS | |||
| Placental CXCL9 | 0.060 | NS | |||
| Placental CXCL13 | −0.113 | NS | |||
| PM+ | 18 | Cord leptin | 0.374 | NS | |
| Cord ferritin | −0.328 | NS | |||
| Placental TNF-α | −0.045 | NS | |||
| Placental IL-10 | −0.164 | NS | |||
| Placental IFN-γ | −0.136 | NS | |||
| Placental CXCL9 | −0.186 | NS | |||
| Placental CXCL13 | −0.136 | NS |
Because IFN-γ levels were detectable in only half of the women, the groups with and without detectable IFN-γ were analyzed separately (10). CXCL9 was related to decreased birth weight in PM+ primigravidae with (β = −0.655; P = 0.01) or without (β = −0.924; P = 0.04) detectable IFN-γ (see Table S1 in the supplemental material). CXCL9 is regulated by IFN-γ and is a marker of functional IFN-γ (13). CXCL9 may be a more sensitive measure of inflammatory pathways associated with poor outcomes than IFN-γ. CXCL9 was not associated with birth weight in PM+ secundigravidae and multigravidae. Among PM+ primigravidae, placental IL-10 was significantly associated with increased birth weight (Table 3).
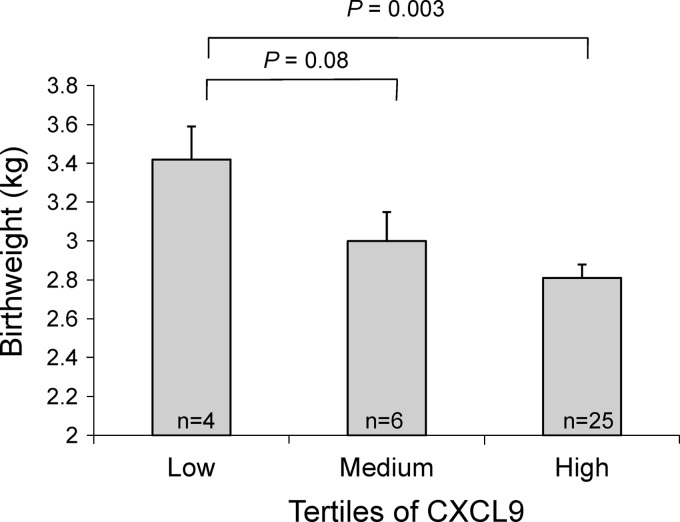
To better quantify these effects, we evaluated the difference in birth weight (analyzed as a continuous variable) between infants born to mothers with low, medium, and high levels of placental CXCL9. In multivariate analysis adjusted for cord ferritin, cord leptin, placental TNF-α, placental IL-10, and placental IFN-γ, infants born to PM+ primigravidae with high CXCL9 levels weighed 610 g less than infants born to PM+ primigravidae with low CXCL9 levels (P = 0.003) (Fig. 2). Similarly, infants born to PM+ primigravidae with medium CXCL9 levels weighed 420 g less than infants born to PM+ primigravidae with low CXCL9 levels (P = 0.08). The association between high CXCL9 levels and reduced birth weight was not observed among infants born to PM+ secundigravidae or multigravidae.
Fig 2.
Placental CXCL9 among PM+ primigravidae is associated with reduced birth weight. In multivariate analysis adjusted for soluble factors (cord ferritin and leptin, placental TNF-α, IL-10, and IFN-γ), women with high CXCL9 levels gave birth to infants weighing 610 g less than infants born to PM+ primigravidae with low CXCL9 levels. Error bars represent standard errors of the means.
DISCUSSION
In areas where malaria is endemic, pregnancy malaria is associated with an increased incidence of LBW deliveries, placing the newborn at risk for morbidity and mortality during infancy (5, 24). Both the inflammatory infiltrate and inflammatory cytokines, such as TNF and IFN, in the placenta have been associated with LBW (6, 10, 17, 23). In an earlier multivariate analysis of this cohort, only IFN-γ was significantly associated with reduced birth weight among infected primigravidae (10). Here we show that placental CXCL9 and CXCL13 levels are negatively associated with birth weight in PM+ primigravidae and secundigravidae in univariate analyses. In multivariate analyses that included factors previously associated with birth weight, only CXCL9 was independently associated with reduced birth weight among the offspring of infected primigravidae, and this relationship was observed even when IFN-γ levels were below the level of detection.
IFN-γ induces macrophages and other cells to express CXCL9 (13). During pregnancy, the placenta can be a source of CXCL9 (12). Changes in the levels of CXCL9, CXCL10, CXCL11, and CXCL13 have been previously associated with villitis of unknown etiology (VUE) (12). In immunofluorescence studies, expression of these chemokines in placental tissue is higher during VUE and localizes to Hofbauer cells, stromal cells, and endothelial cells (12).
Secretion of CXCL9 by macrophages and other cells reflects biologically active IFN-γ, and CXCL9 has been described as a sensitive measure of IFN-γ activity (2). In the present study, IFN-γ was no longer significant in the multivariate model that included CXCL9, most likely due to colinearity of these factors (R = 0.43; P = 0.01). The results are consistent with a causal pathway leading from IFN-γ to CXCL9 and resulting in reduced birth weight.
Our multivariate analysis suggests that the CXCL9 induced by IFN-γ plays a major role in the pathogenesis of placental-malaria-associated LBW. In addition to its role as a chemoattractant, CXCL9 also induces the proliferation of IFN-γ-producing T cells (28), which, in turn, could amplify the production of TNF-α by macrophages. We acknowledge that additional, unmeasured factors may contribute to the negative consequences of IFN-γ on birth outcomes.
Placental IL-10 was positively associated with birth weight in PM+ primigravidae. IL-10 levels increase during PM in the peripheral and placental blood of women of all parities; however, among PM+ primigravidae, IL-10 levels were higher in the presence of inflammation than in the absence of inflammation (11). Among PM+ primigravidae, IL-10 correlated positively with TNF-α, IFN-γ, CXCL9, and CXCL13. During the inflammatory process, IL-10 may function to attenuate the production or effects of proinflammatory cytokines (16), resulting in improved birth weights.
In summary, in this study we find that CXCL9 is significantly associated with lower birth weight among malaria-infected primigravidae. CXCL9 expression is induced by IFN-γ and may represent a marker for functional IFN-γ (2); hence, our results are consistent with the earlier finding that IFN-γ increases the risk of LBW during pregnancy malaria (10). In this model, the inflammatory engine during placental malaria involves IFN-γ, produced by placental and/or maternal T cells, that stimulates macrophages, resulting in higher levels of CXCL13 and CXCL9.
Supplementary Material
ACKNOWLEDGMENTS
We thank the clinical team and the laboratory staff of the MOMS Project for their efforts in collecting clinical data and sample processing. Connor McCoy and Wong-Jong Moon managed the clinical data.
This work was supported by grants from the U.S. National Institutes of Health (grants AI52059 to P.E.D. and HD058005 to M.F.) and the Bill & Melinda Gates Foundation (grant 47029 to P.E.D.). This research was supported in part by the Intramural Research Program of NIAID-NIH.
Footnotes
Published ahead of print 11 June 2012
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1. Abrams ET, et al. 2003. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J. Immunol. 170:2759–2764 [DOI] [PubMed] [Google Scholar]
- 2. Brice GT, Graber NL, Hoffman SL, Doolan DL. 2001. Expression of the chemokine MIG is a sensitive and predictive marker for antigen-specific, genetically restricted IFN-γ production and IFN-γ-secreting cells. J. Immunol. Methods 257:55–69 [DOI] [PubMed] [Google Scholar]
- 3. Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. 1993. Placental malaria. I. Pathological classification. Histopathology 22:211–218 [DOI] [PubMed] [Google Scholar]
- 4. Chaisavaneeyakorn S, et al. 2003. Levels of macrophage inflammatory protein 1α (MIP-1α) and MIP-1β in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin. Diagn. Lab. Immunol. 10:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desai M, et al. 2007. Epidemiology and burden of malaria in pregnancy. Lancet Infect. Dis. 7:93–104 [DOI] [PubMed] [Google Scholar]
- 6. Fried M, Muga RO, Misore AO, Duffy PE. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-γ and TNF-α associated with pregnancy outcomes. J. Immunol. 160:2523–2530 [PubMed] [Google Scholar]
- 7. Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. 1998. Maternal antibodies block malaria. Nature 395:851–852 [DOI] [PubMed] [Google Scholar]
- 8. Garnham PCC. 1938. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans. R. Soc. Trop. Med. Hyg. 32:13–48 [Google Scholar]
- 9. Jilly P. 1969. Anaemia in parturient women, with special reference to malaria infection of the placenta. Ann. Trop. Med. Parasitol. 63:109–116 [DOI] [PubMed] [Google Scholar]
- 10. Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. 2008. Fetal responses during placental malaria modify the risk of low birth weight. Infect. Immun. 76:1527–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kabyemela ER, et al. 2008. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar. J. 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim MJ, et al. 2009. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J. Immunol. 182:3919–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liao F, et al. 1995. Human Mig chemokine: biochemical and functional characterization. J. Exp. Med. 182:1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGregor IA. 1984. Epidemiology, malaria and pregnancy. Am. J. Trop. Med. Hyg. 33:517–525 [DOI] [PubMed] [Google Scholar]
- 15. Moore JM, Nahlen BL, Misore A, Lal AA, Udhayakumar V. 1999. Immunity to placental malaria. I. Elevated production of interferon-gamma by placental blood mononuclear cells is associated with protection in an area with high transmission of malaria. J. Infect. Dis. 179:1218–1225 [DOI] [PubMed] [Google Scholar]
- 16. Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165–190 [DOI] [PubMed] [Google Scholar]
- 17. Moormann AM, et al. 1999. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J. Infect. Dis. 180:1987–1993 [DOI] [PubMed] [Google Scholar]
- 18. Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. 2007. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J. Immunol. 179:557–565 [DOI] [PubMed] [Google Scholar]
- 19. Muller G, Hopken UE, Lipp M. 2003. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 195:117–135 [DOI] [PubMed] [Google Scholar]
- 20. Mutabingwa TK, et al. 2005. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2:e407 doi:10.1371/journal.pmed.0020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ordi J, et al. 1998. Massive chronic intervillositis of the placenta associated with malaria infection. Am. J. Surg. Pathol. 22:1006–1011 [DOI] [PubMed] [Google Scholar]
- 22. Parekh FK, Davison BB, Gamboa D, Hernandez J, Branch OH. 2010. Placental histopathologic changes associated with subclinical malaria infection and its impact on the fetal environment. Am. J. Trop. Med. Hyg. 83:973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rogerson SJ, et al. 2003. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect. Immun. 71:267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steketee RW, Nahlen BL, Parise ME, Menendez C. 2001. The burden of malaria in pregnancy in malaria-endemic areas. Am. J. Trop. Med. Hyg. 64:28–35 [DOI] [PubMed] [Google Scholar]
- 25. Suguitan AL, Jr, et al. 2003. Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 69:574–581 [PubMed] [Google Scholar]
- 26. Suguitan AL, Jr, et al. 2003. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J. Infect. Dis. 188:1074–1082 [DOI] [PubMed] [Google Scholar]
- 27. Watkinson M, Rushton DI. 1983. Plasmodial pigmentation of placenta and outcome of pregnancy in West African mothers. Br. Med. J. (Clin. Res. Ed.) 287:251–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whiting D, et al. 2004. Chemokine monokine induced by IFN-γ/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production. J. Immunol. 172:7417–7424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.