Abstract
Interindividual variation in the ability of aspirin to inhibit platelet cyclooxygenase-1 (COX-1) could account for some on-treatment cardiovascular events. Here, we sought to determine whether there are clinical phenotypes that are associated with a suboptimal pharmacological effect of aspirin. In a prospective, 2-week study, we evaluated the effect of aspirin 81 mg on platelet COX-1 in 135 patients with stable CAD by measuring serum thromboxane B2 (sTxB2) as an indicator of inhibition of platelet COX-1. A nested randomized study compared enteric-coated with immediate-release formulations of aspirin. We found that sTxB2 was systematically higher among the 83 patients with metabolic syndrome than among the 52 patients without (median 4.0 ng/mL vs. 3.02 ng/mL, P=0.013). Twelve (14%) patients with metabolic syndrome, but none without metabolic syndrome, had sTxB2 levels consistent with inadequate inhibition of COX (sTxB2 ≥13 ng/mL). In linear regression models, metabolic syndrome (but none of its individual components) significantly associated with higher levels of log-transformed sTxB2 (P=0.006). Higher levels of sTxB2 associated with greater residual platelet function measured by aggregometry-based methods. Among the randomized subset, sTxB2 levels were systematically higher among patients receiving enteric-coated aspirin. Last, urinary 11-dehydrothromboxane B2 did not correlate with sTxB2, suggesting that the former should not be used to quantitate aspirin’s pharmacological effect on platelets. In conclusion, metabolic syndrome, which places patients at high risk for thrombotic cardiovascular events, strongly and uniquely associates with less effective inhibition of platelet COX-1 by aspirin.
Keywords: aspirin, thromboxane, platelets, coronary disease, metabolic syndrome
Introduction
Therapy with low-dose aspirin for secondary prevention of cardiovascular disease reduces the risk of major coronary events by 20–25%.1, 2 Despite treatment with aspirin, however, approximately 10–20% of patients suffer recurrent arterial thrombotic events.3 This led to consideration of whether interindividual variation in the effect of aspirin could contribute to those recurrent thrombotic events.
Aspirin exerts its anti-platelet effect by acetylating serine 529 of platelet cyclooxygenase-1(COX-1), which inhibits the enzyme and prevents formation of the platelet activator thromboxane (Tx) A2.4 We found that acetylation of the COXs by aspirin is inhibited by the redox cycling of the enzyme that occurs when hydroperoxides are reduced in the peroxidase active site of the COXs.5 Accordingly, the response to aspirin varies considerably among different cell types, based on the hydroperoxide concentration of the cells. This suggested that the extent to which aspirin inhibits platelet COX-1 could vary among patients. Because activation of platelets and/or lipid peroxidation could lead to formation of several peroxides, platelet activation might attenuate aspirin’s effect by this mechanism as well as by increased platelet turnover.
Although low-dose aspirin is almost uniformly effective in inhibiting platelet COX-1 among healthy, young individuals,6 there could be pathophysiologic states that impair aspirin’s effect. Here, we investigated the relationship of the pharmacologic effect of low-dose (81 mg) aspirin to a number of clinical phenotypic characteristics in a group of patients with documented coronary artery disease (CAD). Both obesity and diabetes have been associated with increased platelet activation ex vivo and in vivo7–12 and with increased oxidative stress,9, 13, 14 and the efficacy of aspirin in preventing cardiovascular events is marginal among diabetics compared with non-diabetics.15 Body weight has been inversely correlated with suppression of sTxB2. 16,17,18 Metabolic syndrome constitutes a subset of both the diabetic and obese populations that confers a substantial increase in the risk of thrombotic cardiovascular events compared with both the general population and patients with diabetes.19, 20 Accordingly, metabolic syndrome was a predetermined phenotype of interest in this study.
Drug formulation could also contribute to interindividual variation in response to aspirin. A summary of bioequivalence studies demonstrated that the enteric-coated formulations investigated were less potent in inhibiting sTxB2 than immediate-release formulations among healthy volunteers.16 Because these studies evaluated neither the McNeil formulation of enteric-coated aspirin nor the extent to which enteric coating affects platelet COX-1 inhibition among patients with CAD, we nested a randomized substudy within our cohort to study the effects of formulation in this population.
Furthermore, we evaluated the validity of measuring the urinary metabolite of TxA2, 11-dehydrothromboxane B2 (Tx-M),21 to assess aspirin’s efficacy. Higher levels of Tx-M have been associated with adverse cardiovascular events in high-risk patients, 22 but we previously found that this biomarker reflects TxA2 derived from both platelet and non-platelet (e.g. COX-2) sources in cigarette smokers.23
Methods
Study Population
This study was approved by the Vanderbilt University Institutional Review Board and registered on ClinicalTrials.gov (NCT00753935). Participants provided written informed consent. Recruitment occurred between June 2006 and May 2009. Patients with known CAD were approached if they appeared to satisfy inclusion and exclusion criteria based on review of their medical record. Inclusion criteria included ≥ 40 year-old males or post-menopausal females who were receiving aspirin 81–325 mg as part of their outpatient regimen. Exclusion criteria included concurrent use of other antiplatelet drugs, NSAIDs or COX-2 inhibitors, coronary artery bypass grafting or percutaneous coronary intervention within 6 months of enrollment, uncontrolled hypertension (systolic blood pressure > 180 mmHg), decompensated congestive heart failure, acute coronary syndrome within 6 months, significant GI bleeding, creatinine ≥ 176.8 μmol/L (2 mg/dL), hematocrit < 30%, or platelet count < 135,000/μL. Approximately 25% of patients approached declined participation, the majority citing an unacceptable travel distance to complete the study.
Study design
A prospective observational study was conducted to evaluate the phenotypic characteristics of patients with stable CAD in whom inhibition of platelet COX-1 by aspirin was suboptimal. Patients received a blister pack containing a 2-week supply of a daily dose of aspirin 81 mg (McNeil Pharmaceuticals) administered in the evening. The importance of strict adherence to therapy was emphasized and participants were contacted by the research coordinator mid-study to assess and encourage continued compliance. A pill count was performed at the conclusion of the study. After 2 weeks, patients returned for phlebotomy and provided a first-morning urine specimen.
We enrolled 181 patients with CAD in the observational study. Of these, 135 fulfilled the criteria for inclusion in the cohort for analysis (see http://hyper.ahajournals.com).
From the above 181 patients, 106 consecutive subjects were enrolled in a nested randomized controlled investigation of enteric-coated aspirin. Of the 54 patients randomized to enteric-coated aspirin, nine were withdrawn: three for unsuccessful phlebotomy, two for use of other antiplatelet agents mid-study, two for self-reported use of systemic anti-inflammatory medication, one for percutaneous coronary intervention with stent placement during the study, and one for an error in enrollment (CABG within 6 months). Of the 52 patients randomized to immediate-release aspirin, five were withdrawn: two for laboratory abnormalities discovered on the day of recruitment but after enrollment, one for self-reported NSAID use, one for loss to follow-up, and one for withdrawal for personal reasons. Therefore, the final analytic cohort of 135 patients in the observational study included 45 randomized to enteric-coated aspirin and 47 randomized to immediate-release aspirin. We assigned the metabolic syndrome phenotype in accord with the AHA/NHLBI criteria.24 Additional prospectively selected phenotypic characteristics of interest were BMI, diabetes, smoking status, and age.
Laboratory Measurements
Serum TxB2
Serum TxB2 was measured as an indicator of inhibition of platelet COX activity. Non-anticoagulated blood was incubated at 37°C for 45 minutes immediately after phlebotomy.25 Serum was separated by centrifugation and stored at −80°C until analysis. Serum TxB2 was assayed by stable isotope dilution gas chromatography/mass spectrometry (GC/MS) with selective ion monitoring.26 Suboptimal inhibition of platelet COX, the primary endpoint of the study, was defined prospectively as failure to reduce sTxB2 to less than 5% of the mean level obtained in normal individuals taking no anti-platelet drugs; using the analytical techniques described herein, this equated to ≤ 13 ng/mL. The rationale and supporting evidence for this criterion for a suboptimal effect of aspirin is presented in the online supplement (please see http://hyper.ahajournals.org).
Urinary 11-dehydrothromboxane B2 (Tx-M)
Urine was stored at −80°C until analysis. Urinary Tx-M was assayed by stable isotope dilution GC/MS.27
Platelet Aggregation Studies
Citrated platelet-rich plasma (PRP), adjusted to 2.5×108 cells/mL with autologous platelet-poor plasma, was used to assess turbidimetric platelet aggregation induced by 2 μg/mL collagen (Chrono-log Corp., Havertown, PA) in a dual-channel aggregometer (Model 460VS, Chrono-log Corp.) as previously described.28 As a functional measure of residual platelet COX-1 activity in these patients treated with aspirin, we compared the extent of collagen-induced aggregation in PRP with or without pre-incubation with the highly selective thromboxane receptor antagonist SQ 29,548 (10 μM final concentration; Cayman Chemical, Ann Arbor, MI). These measurements were added to the protocol mid-study but then were performed on all subsequent patients except when difficulties with phlebotomy precluded aggregation studies.
Statistical Analysis
Data are expressed as median (interquartile range [IQR]) or frequency (percentage). For bivariate group comparisons, Wilcoxon rank-sum tests were used for continuous data and chi-square or Fisher’s exact tests were used for categorical data. Spearman rank correlations were used to study relationships between pairs of continuous variables. Linear models were used to study the relationships between log-sTxB2 and aspirin formulation, age, sex, smoking status, platelet count, BMI, and metabolic syndrome (or each of its component criteria). In addition, a linear model was used to study the association between sTxB2 and residual platelet function as measured by the difference in collagen-induced aggregation with or without the addition of SQ 29,548. Analyses were conducted by using R 2.10.1 (r-project.org). Two-sided p-values less than 0.05 were considered statistically significant.
Results
Study Population
Clinical characteristics for the patients included in the analysis are shown in Table 1. 28% had type 2 diabetes mellitus, and all but one patient carried a diagnosis of hypertension. Metabolic syndrome was present in 83 (61%) of the 135 patients. These patients were younger, had higher blood pressure at baseline, were more likely to have diabetes, and had larger median BMI and waist circumference. In addition, lower HDL, higher triglycerides, higher fasting glucose, and higher platelet count accompanied the metabolic syndrome (Table 1). Patients with metabolic syndrome were more likely to be taking antidiabetic drugs, but otherwise the use of medications was similar between those with and without metabolic syndrome (Table S1, http://hyper.ahajournals.com). There were no significant differences in clinical characteristics between patients in the randomized and nonrandomized cohorts.
Table 1.
Demographics of Study Population
| Patient Characteristic | All Subjects (N=135) | Randomized Cohort Only (N=92) | |||
|---|---|---|---|---|---|
| MetSyn (N=83) | No MetSyn (N=52) | P* | Immediate-release (N=47) | Enteric-coated (N=45) | |
| Age (y) | 64[58, 71] | 68[61, 76] | 0.007 | 68[59, 75] | 62[56, 69] |
| Male sex | 58 (70%) | 36 (69%) | 0.94 | 33 (70%) | 33 (73%) |
| Race – White | 69 (83%) | 49 (94%) | 0.082 | 42 (89%) | 36 (80%) |
| Black | 13 (16%) | 2 (4%) | 4 (9%) | 8 (18%) | |
| Other | 1 (1%) | 1 (2%) | 1 (2%) | 1 (2%) | |
| SBP (mmHg) | 126[119,136] | 117[110, 131] | 0.008 | 126[112, 133] | 124[116, 136] |
| DBP (mmHg) | 74[70, 80] | 70[62, 78] | 0.003 | 72[64, 78] | 74[70, 80] |
| Diabetes | 33 (40%) | 5 (10%) | <0.001 | 9 (19%) | 12 (27%) |
| Hypertension | 83 (100%) | 51 (98%) | 0.21 | 47 (100%) | 45 (100%) |
| Current or Former Smoker | 52 (63%) | 26 (50%) | 0.15 | 33 (70%) | 24 (53%) |
| BMI (kg/m2) | 31.2[27.7, 34.6] | 26.2[23.7, 29.2] | < 0.001 | 28.8[25.8, 32.9] | 30.3[27.3, 33.3] |
| Waist circumference (cm) | 109.2[100.3, 118.1] | 99.1[90.8, 105.7] | < 0.001 | 100.3[94.0, 111.8] | 108.0[99.1, 114.3] |
| Total cholesterol (mmol/L) | 3.67[3.23, 4.19] | 3.74 [3.43, 4.24] | 0.28 | 3.67 [3.34, 4.09] | 3.67 [3.41, 3.98] |
| LDL (mmol/L) | 1.99[1.63, 2.28] | 1.89 [1.68, 2.33] | 0.82 | 1.84 [1.66, 2.28] | 1.99 [1.55, 2.30] |
| HDL (mmol/L) | 0.98[0.83, 1.22] | 1.29 [1.11, 1.62] | < 0.001 | 1.11 [0.93, 1.40] | 1.03 [0.91, 1.32] |
| Triglycerides (mmol/L) | 1.22[1.00, 2.05] | 1.03 [0.76, 1.26] | < 0.001 | 1.14 [0.86, 1.66] | 1.14 [0.91, 1.60] |
| Platelet count (x103/mL) | 223[190, 256] | 184[168, 219] | 0.0002 | 191[176, 244] | 222[184, 243] |
| Fasting glucose (mmol/L) | 5.94[5.44, 6.94] | 5.16 [4.88, 5.44] | < 0.0001 | 5.50 [5.17, 6.22] | 5.55 [5.11, 6.16] |
| Metabolic Syndrome | - | - | - | 27 (57%) | 30 (67%) |
| Enteric-coated formulation | 56 (67%) | 32 (62%) | 0.48 | - | - |
Values are median[IQR] or counts (column %). MetSyn = metabolic syndrome; SBP = systolic blood pressure; DBP = diastolic blood pressure; BMI = body mass index; LDL = low-density lipoprotein; HDL = high-density lipoprotein.
P values compare patients with and without metabolic syndrome.
Metabolic Syndrome Associates with Inadequate Inhibition of Platelet Cyclooxygenase
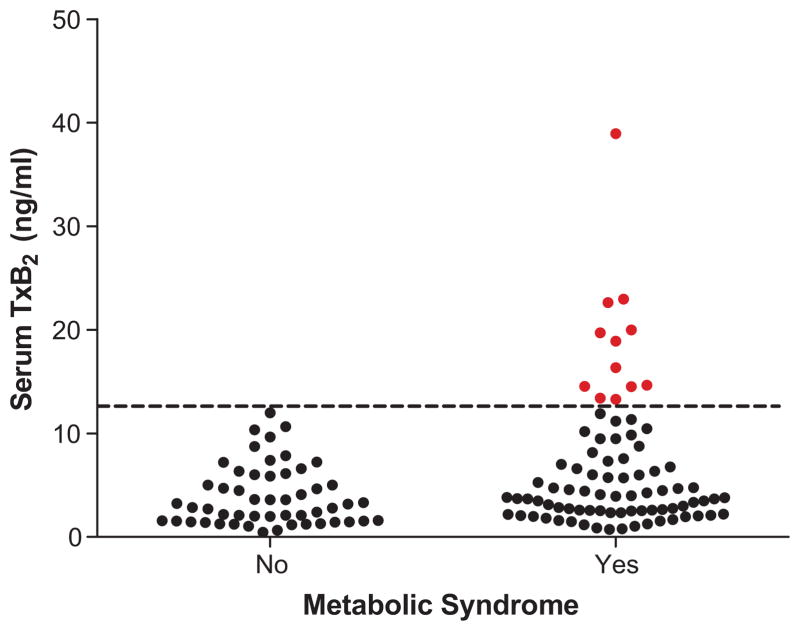
To determine whether metabolic syndrome associates with apparent biochemical resistance of cyclooxygenase to aspirin, sTxB2 was compared across metabolic syndrome status. Serum TxB2 was systematically higher among the 83 patients with metabolic syndrome than among the 53 patients without (median 3.98 ng/mL [IQR 2.52–8.46 ng/mL] vs. 3.02 ng/mL [1.55–5.92 ng/mL], P=0.013) (Fig. 1).
Figure 1.
Comparison of serum TxB2 levels between patients with and without metabolic syndrome (P=0.013), all of whom had known coronary artery disease. The dashed line (13 ng/mL) represents an estimate of 95% inhibition of cyclooxygenase.
It is generally accepted that at least 95% inhibition of platelet cyclooxygenase is required for aspirin to confer a significant antiplatelet effect.6, 29, 30 Because we could not ethically discontinue aspirin in these patients with known CAD for this study, we estimated that a serum TxB2 level of 13 ng/ml represented 95% inhibition of platelet COX based on the mean sTxB2 in 83 healthy volunteers. Biochemical resistance to aspirin (sTxB2 ≥13 ng/ml) was significantly more common among patients with metabolic syndrome than without. In the absence of the metabolic syndrome, no patient had a sTxB2 level ≥ 13 ng/mL after 2 weeks of monitored therapy whereas 12 (14%) patients with metabolic syndrome had sTxB2 levels above this threshold (P=0.003).
Because setting an absolute sTxB2 threshold to define biochemical resistance to aspirin could be considered arbitrary, log-transformed sTxB2 was analyzed as a continuous variable in a linear regression model. Metabolic syndrome significantly associated with higher sTxB2 even after adjusting for age, sex, smoking history, platelet count, and aspirin formulation (P=0.006). Replacing the metabolic syndrome variable with each of its component criteria in four otherwise identical models (hypertension could not be analyzed given its 99% prevalence in this cohort) demonstrated that none of the individual components significantly associated with sTxB2 when considered alone (Table 2). The diabetic patients had higher sTxB2 levels than non-diabetic patients (median 4.47 ng/mL [IQR 2.79–10.11 ng/mL] vs. 3.55 ng/mL [2.11–6.35 ng/mL]; P=0.061); although this difference was not statistically significant, this study was not powered for this comparison. With the adjustment of age, sex, smoking history, platelet count, and aspirin formulation in the linear regression model, the association of metabolic syndrome was significant (P=0.011) and the association of diabetes was not significant (P=0.82) (Fig. S1, http://hyper.ahajournals.com).
Table 2.
Comparison of metabolic syndrome with its components in separate multivariable linear models of log-transformed sTxB2
| Model* | Estimate | P |
|---|---|---|
| Model 1: Metabolic Syndrome† | 0.441 | 0.006 |
| Model 2: Low HDL‡ | 0.172 | 0.26 |
| Model 3: Elevated triglycerides§ | 0.118 | 0.52 |
| Model 4: Elevated fasting glucose|| | 0.244 | 0.09 |
| Model 5: Elevated waist circumference¶ | 0.282 | 0.09 |
In addition to the variable listed, all models adjust for age, sex, smoking status (current/former vs. never), platelet count, and aspirin formulation (enteric-coated vs. immediate-release). The P value corresponds to the listed variable in the model.
AHA/NHLBI criteria.24
HDL < 1.03 mmol/L (40mg/dL) for men, < 1.29 mmol/L (50 mg/dL) for women.
Triglycerides ≥ 1.69 mmol/L (150 mg/dL)
Fasting glucose ≥ 5.56 mmol/L (100 mg/dL) or diagnosis of diabetes
Waist circumference ≥ 102 cm (40 inches) for men, ≥ 88 cm (35 inches) for women
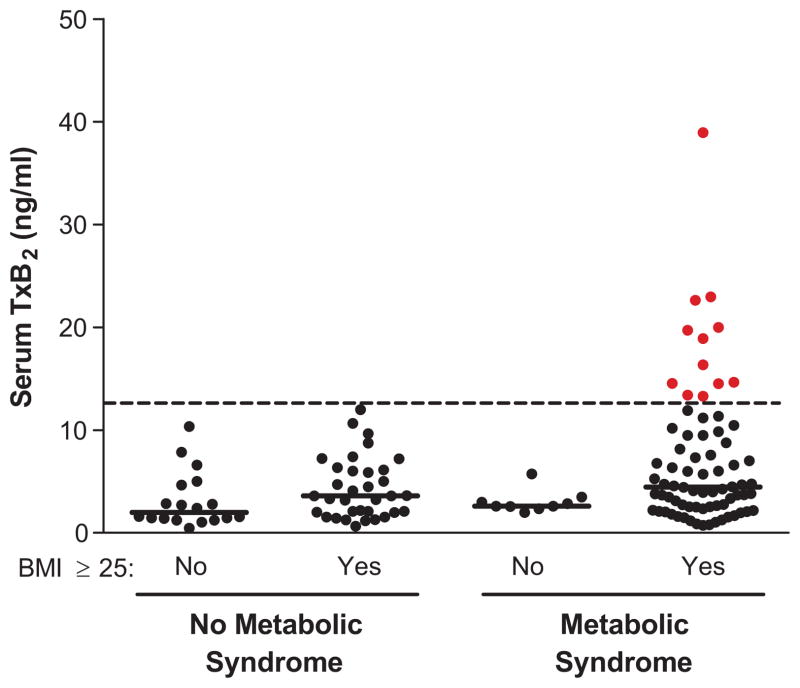
To further investigate the contributions of BMI and metabolic syndrome to inadequate suppression of cyclooxygenase, sTxB2 levels were compared across metabolic syndrome stratified by BMI category. Serum TxB2 levels ≥ 13 ng/mL were only observed among patients who were both overweight/obese (BMI ≥ 25 kg/m2) and had metabolic syndrome (16% of this population). Conversely, the 9 patients with metabolic syndrome and normal BMI (< 25 kg/m2) had well-suppressed sTxB2 levels (Fig. 2). Taken together, these data suggest that the metabolic syndrome associates with higher sTxB2 levels among patients with CAD receiving aspirin 81mg daily, with 14% (95% CI: 7% – 22%) elevated to levels generally considered to reflect biochemical resistance to aspirin. This resistance is even more common among those who have metabolic syndrome and are also overweight/obese.
Figure 2.
Serum TxB2 levels by body mass index (BMI) across metabolic syndrome (MetSyn) status. Solid lines indicate medians. No significant differences were detected between BMI categories among patients with or without metabolic syndrome (P=0.08 and P=0.11, respectively).
Because higher gastric pH could reduce the bioavailability of aspirin, we examined whether concomitant use of proton pump inhibitors (PPIs), H2 antagonists, or antacids associated with higher levels of sTxB2. Of the 37 patients taking PPIs, 3 (8%) had sTxB2 > 13 ng/mL compared with 9 (9%) of the 98 patients not taking PPIs. Similarly, the use of neither H2 antagonists nor antacids associated with sTxB2 > 13 ng/mL. Additional data regarding concomitant medications are summarized in Table S2 (see http://hyper.ahajournals.com).
Functional Consequence of Incomplete sTxB2 Inhibition
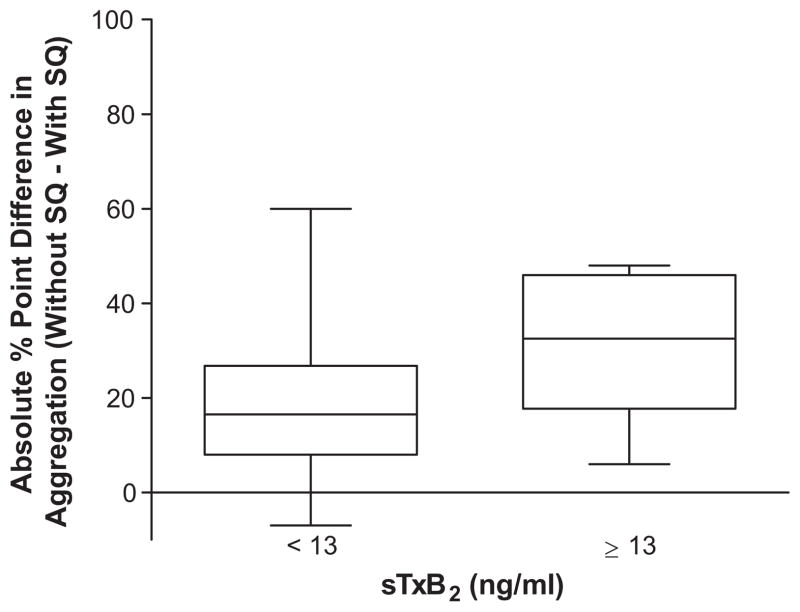
The functional consequence of incomplete inhibition of sTxB2 was evaluated with light-transmission aggregometry. The extent of collagen-induced aggregation was compared with and without pre-incubation of the platelet-rich plasma with the highly selective thromboxane receptor antagonist SQ 29,548 (“SQ”). In the setting of complete inhibition of platelet COX-1 by aspirin, pre-incubation with SQ does not attenuate collagen-induced aggregation further, producing a “without SQ – with SQ” difference of 0. In the setting of incomplete inhibition of platelet COX-1 by aspirin, however, pre-incubation with SQ attenuates collagen-induced aggregation, producing a “without SQ – with SQ” difference greater than 0. By this functional measure, patients with sTxB2 ≥ 13 ng/mL had significantly greater residual platelet function (P=0.02) (Fig. 3). Furthermore, simple linear regression suggested that a 1 ng/mL increase in sTxB2 is associated with a 1.14 absolute percentage point increase in difference between collagen-induced aggregation without and with SQ pre-incubation, on average (P<0.0001).
Figure 3.
Positive values of “without SQ – with SQ” suggest greater residual thromboxane receptor-mediated platelet aggregability. The bounds of the boxes indicate the 1st and 3rd quartiles; the line within the box indicates the median.
Enteric-coated Aspirin Affects Suppression of sTxB2 by Aspirin
The effect of enteric-coating on the inhibition of sTxB2 by aspirin was studied in a randomized subgroup of the patients. Serum TxB2 levels were systematically higher and exhibited greater variability among patients randomized to enteric-coated aspirin compared with those randomized to immediate-release aspirin (median 5.02 ng/mL [IQR 3.36–7.86 ng/mL] vs. 2.78 ng/mL [1.60–4.76]; P=0.005) (Fig. S2, http://hyper.ahajournals.com). Despite randomization, the median age was slightly lower and the median waist circumference was higher in the group receiving enteric-coated aspirin. Adjusting for these potential confounders in a linear model of log-transformed sTxB2, enteric-coated aspirin remained associated with higher sTxB2 levels (P=0.030).
Urinary 11-dehydrothromboxane B2 does not correlate with sTxB2
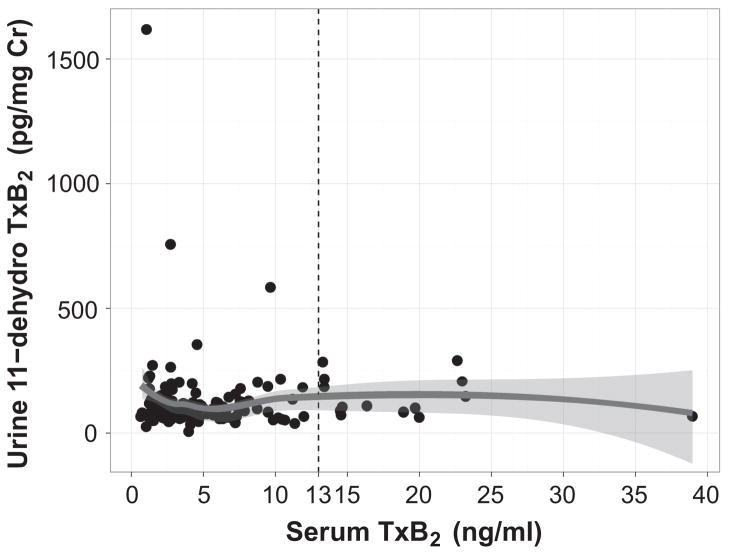
Because sTxB2 is the most direct measure of the pharmacological effect of aspirin, we compared each patient’s sTxB2 level with their urinary Tx-M to determine the extent to which Tx-M predicted inhibition of platelet COX-1. Urinary Tx-M did not correlate with sTxB2 in this population (Spearman’s ρ=0.04, P=0.63) (Fig. 4).
Figure 4.
Serum TxB2 did not correlate with urinary 11-dehydrothromboxane B2 among patients with coronary artery disease taking aspirin 81mg daily (Spearman’s ρ=0.04, P=0.63).
Discussion
The effect of aspirin on platelet COX-1 is substantially and significantly diminished in patients with the metabolic syndrome. Twelve (14%) patients with metabolic syndrome, but none without metabolic syndrome, had sTxB2 levels consistent with inadequate inhibition of COX. These 12 poorest responders also were overweight/obese, constituting 16% of that BMI subgroup.
Several abnormalities in ex vivo platelet function have been demonstrated in patients with metabolic syndrome who are not on aspirin. Closure time in the flow-based clotting system, PFA-100®, is prolonged,31 platelets exhibit increased P-selectin expression in response to ADP,32 and aggregation in response to ADP, collagen and arachidonic acid is modestly but significantly increased.33 Increased platelet surface expression of P-selectin and GP IIb/IIIa has been demonstrated,31, 34 as well as elevated levels of circulating soluble P-selectin, soluble CD40 ligand,35, 36 and conjugates of leukocytes with platelets and/or platelet microparticles.34 Increased numbers of reticulated platelets suggest accelerated platelet turnover.32 Furthermore, platelet count is higher in metabolic syndrome as demonstrated by both Jesri et al.37 and the present study.
Increased in vivo platelet activation in metabolic syndrome can be hypothesized based on several observations. Platelet activation could be a basis for impaired acetylation of platelet COX-1 by aspirin. The biosynthesis of peroxides (e.g., 12-HPETE, PGG2, and peroxynitrite) during platelet activation would lead to redox cycling of COX-1, which we have shown to inhibit acetylation of COX-1 by aspirin.5 Moreover, increased platelet turnover resulting from abnormal platelet activation in vivo would generate more young platelets with active COX-1 between the aspirin dosing interval, thus impairing accumulation of the effect of low-dose aspirin. If it can be shown that increased platelet activation and suboptimal effect of aspirin are associated in the same population, this would mean that aspirin’s therapeutic effect is impaired in the patients who need it most. In this regard, it is of note that aspirin produces only a slight and non-significant reduction in thrombotic cardiovascular events in patients with diabetes, many of whom have metabolic syndrome.1, 38, 39
Although a pharmacokinetic effect of obesity is a conceivable basis for increased sTxB2 levels in aspirin-treated patients with metabolic syndrome, direct evidence is elusive given the substantial acetylation of platelet COX-1 in the portal circulation and the extent of first-pass metabolism. Moreover, our data indicate that waist circumference, the parameter that measures central obesity, does not alone explain the relation of metabolic syndrome to elevated sTxB2 (Table 2).
This represents the first evidence that the effect of aspirin on its pharmacologic target, platelet COX-1, is reduced in metabolic syndrome. Previously, investigations have found that inhibition of the function of platelets by aspirin is diminished in metabolic syndrome.32, 33, 40, 41 However, platelet function assays (e.g., ADP- and collagen-induced aggregation, PFA-100®, and VerifyNow®) indirectly measure the net effects of both thromboxane-dependent and thromboxane-independent pathways. Although these tests may provide information about platelet reactivity, they cannot determine the success or failure of aspirin to inhibit platelet COX-1 in an individual patient.6 Indeed, a major predictor of hyper-function of platelets during aspirin treatment is elevated function in the absence of aspirin,42 which is clearly the case in metabolic syndrome; therefore, it is not surprising that multiple studies have suggested that platelet reactivity in patients receiving aspirin is a harbinger of increased cardiovascular risk.22, 43–45 It cannot be inferred that failure of aspirin to acetylate platelet COX-1 is the principal cause of this increased risk but COX-1-dependent residual platelet activity is certainly a contributor.17
The most effective treatment strategy for patients with metabolic syndrome who have an impaired response to low-dose aspirin remains to be determined. Certainly, an increase in the dose of aspirin is a consideration, and evidence supports an advantage of twice-daily dosing to compensate for the accelerated rate of entry of platelets with unacetylated platelet COX that results from the increased platelet turnover in diabetes39, 46 and metabolic syndrome.32 Higher doses, however, also would further inhibit prostacyclin biosynthesis,30 which may be deleterious and could increase risk for gastrointestinal bleeding. Further research must identify the optimal anti-platelet therapy for patients with metabolic syndrome in whom aspirin’s pharmacologic effect is suboptimal.
Enteric-coated formulations have several potential pharmacokinetic disadvantages: there is significant variability in the pill-coating process, possibly affecting bioavailability; delivery of aspirin to the more alkaline small bowel increases the likelihood of intra-intestinal deacetylation; and slower absorption allows for more efficient first-pass hepatic clearance. In healthy volunteers, Cox et al. demonstrated that 75-mg enteric-coated preparations had higher median sTxB2 than a 75-mg immediate-release preparation.16 The reduction in aspirin effect also varied between the different enteric-coated formulations, prompting us to study the McNeil 81-mg enteric-coated formulation in a target population for the drug, patients with CAD. We found that this formulation also has a diminished and more variable effect on platelet COX-1 compared with an immediate-release formulation. The cumulative evidence indicating a reduced pharmacological effect of enteric-coated formulations is cogent in light of the fact that the basis for the use of low-dose aspirin for prevention of thrombotic cardiovascular disease largely derives from a meta-analysis that comprised studies employing immediate-release formulations with the exception of two studies that used a 100-mg enteric-coated preparation.1 Thus, no evidence exists to support the use of 81-mg enteric-coated aspirin to prevent cardiovascular events.
Interest in the possibility that urinary Tx-M could mark suboptimal suppression of platelet TxA2 biosynthesis by aspirin followed the demonstration that higher levels of urinary Tx-M associate with an increased risk for cardiovascular events in a high-risk population.22 Multiple subsequent studies used urinary Tx-M as an indicator of “aspirin resistance,”45 even though urinary Tx-M could indicate resistance to aspirin’s pharmacological effect, noncompliance, or non-platelet sources of thromboxane. For example, we found that 22% of Tx-M derives from COX-2 among smokers.23 In healthy volunteers, Tx-M has not been found to correlate with sTxB2.42 In the present study of patients with CAD, we did not detect a correlation between these two measures, indicating that Tx-M is not a reliable biomarker of suboptimal inhibition of platelet COX-1 by aspirin. The concerted evidence, therefore, indicates that urinary Tx-M should not be used to interpret whether a patient is resistant to the pharmacological effects of aspirin.
In conclusion, metabolic syndrome strongly and uniquely associates with suboptimal inhibition of platelet COX-1 by aspirin. The increasing prevalence of the metabolic syndrome and its association with greater risk for cardiovascular events highlight the importance of optimizing anti-platelet therapy to reduce cardiovascular risk for these patients.
Perspective
In patients with metabolic syndrome, platelet hyperactivity likely contributes to the risk for acute coronary syndrome. This emphasizes the need for effective anti-platelet therapy in the very patients in whom we found the effect of low-dose aspirin to be suboptimal. Inhibition of platelet cyclooxygenase by aspirin may be substantially inadequate in approximately 14% of patients with metabolic syndrome and coronary artery disease. Because a growing proportion of the 18 million patients in the United States with coronary artery disease have metabolic syndrome, this suggests that hundreds of thousands of patients may be receiving inadequate treatment for their hyperactive platelets.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the excellent technical assistance of Joseph Chambers, Bharati Kakkad, Richard Gray, and Audra Judd.
Funding Sources: This project was supported by a SCCOR in Hemostatic and Thrombotic Diseases (P50HL81009), by the Vanderbilt CTSA (UL1RR024975), a grant from McNeil Pharmaceuticals, and T32DK007569 (JPS), 5T32GM07569 (JPS and EVH). JAO is the Thomas F. Frist, Sr. Professor of Medicine.
Footnotes
Disclosures: JAO was an ad hoc consultant to McNeil Pharmaceuticals prior to 2007.
References
- 1.Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ (Clinical research ed) 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs: The relationships among dose, effectiveness, and side effects: The seventh accp conference on antithrombotic and thrombolytic therapy. Chest. 2004;126:234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 4.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: A new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bala M, Chin CN, Logan AT, Amin T, Marnett LJ, Boutaud O, Oates JA. Acetylation of prostaglandin h2 synthases by aspirin is inhibited by redox cycling of the peroxidase. Biochem Pharmacol. 2008;75:1472–1481. doi: 10.1016/j.bcp.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, Pettinella C, Recchiuti A, Ferrante E, Ciabattoni G, Davi G, Patrono C. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: Implications for aspirin “Resistance”. J Am Coll Cardiol. 2009;53:667–677. doi: 10.1016/j.jacc.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Bridges JM, Dalby AM, Millar JH, Weaver JA. An effect of d-glucose on platelet stickiness. Lancet. 1965;1:75–77. doi: 10.1016/s0140-6736(65)91656-9. [DOI] [PubMed] [Google Scholar]
- 8.Davi G, Catalano I, Averna M, Notarbartolo A, Strano A, Ciabattoni G, Patrono C. Thromboxane biosynthesis and platelet function in type ii diabetes mellitus. N Engl J Med. 1990;322:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 9.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 10.Kubisz P, Arabi A, Seghier F, Cronberg S. Investigations on the effect of thrombocytin on platelets. Thromb Res. 1984;33:225–227. doi: 10.1016/0049-3848(84)90183-x. [DOI] [PubMed] [Google Scholar]
- 11.McDonagh PF, Hokama JY, Gale SC, Logan JJ, Davis-Gorman G, Goldman S, Copeland JG. Chronic expression of platelet adhesion proteins is associated with severe ischemic heart disease in type 2 diabetic patients: Chronic platelet activation in diabetic heart patients. J Diabetes Complications. 2003;17:269–278. doi: 10.1016/s1056-8727(02)00246-5. [DOI] [PubMed] [Google Scholar]
- 12.Trovati M, Anfossi G. Mechanisms involved in platelet hyperactivation and platelet-endothelium interrelationships in diabetes mellitus. Curr Diab Rep. 2002;2:316–322. doi: 10.1007/s11892-002-0020-7. [DOI] [PubMed] [Google Scholar]
- 13.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 14.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the framingham study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Sun A, Zhang P, Wu C, Zhang S, Fu M, Wang K, Zou Y, Ge J. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2010;87:211–218. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Cox D, Maree AO, Dooley M, Conroy R, Byrne MF, Fitzgerald DJ. Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke. 2006;37:2153–2158. doi: 10.1161/01.STR.0000231683.43347.ec. [DOI] [PubMed] [Google Scholar]
- 17.Frelinger AL, 3rd, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, Furman MI, Michelson AD. Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–2596. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]
- 18.Maree AO, Curtin RJ, Dooley M, Conroy RM, Crean P, Cox D, Fitzgerald DJ. Platelet response to low-dose enteric-coated aspirin in patients with stable cardiovascular disease. J Am Coll Cardiol. 2005;46:1258–1263. doi: 10.1016/j.jacc.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES. Prevalence of the metabolic syndrome in us populations. Endocrinol Metab Clin North Am. 2004;33:333–350. doi: 10.1016/j.ecl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Roberts LJ, 3rd, Sweetman BJ, Oates JA. Metabolism of thromboxane b2 in man. Identification of twenty urinary metabolites. J Biol Chem. 1981;256:8384–8393. [PubMed] [Google Scholar]
- 22.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 23.McAdam BF, Byrne D, Morrow JD, Oates JA. Contribution of cyclooxygenase-2 to elevated biosynthesis of thromboxane a2 and prostacyclin in cigarette smokers. Circulation. 2005;112:1024–1029. doi: 10.1161/CIRCULATIONAHA.105.542696. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An american heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 25.Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, Satta MA, Peskar BA. Low dose aspirin and inhibition of thromboxane b2 production in healthy subjects. Thromb Res. 1980;17:317–327. doi: 10.1016/0049-3848(80)90066-3. [DOI] [PubMed] [Google Scholar]
- 26.Parsons WG, 3rd, Roberts LJ., 2nd Transformation of prostaglandin d2 to isomeric prostaglandin f2 compounds by human eosinophils. A potential mast cell-eosinophil interaction. J Immunol. 1988;141:2413–2419. [PubMed] [Google Scholar]
- 27.Morrow JD, Minton TA. Improved assay for the quantification of 11-dehydrothromboxane b2 by gas chromatography-mass spectrometry. J Chromatogr. 1993;612:179–185. doi: 10.1016/0378-4347(93)80161-v. [DOI] [PubMed] [Google Scholar]
- 28.Adler DH, Cogan JD, Phillips JA, 3rd, Schnetz-Boutaud N, Milne GL, Iverson T, Stein JA, Brenner DA, Morrow JD, Boutaud O, Oates JA. Inherited human cpla(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J Clin Invest. 2008;118:2121–2131. doi: 10.1172/JCI30473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: Implications for therapy with platelet inhibitory drugs. Blood. 1987;69:180–186. [PubMed] [Google Scholar]
- 30.Serebruany VL, Malinin A, Ong S, Atar D. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. J Thromb Thrombolysis. 2008;25:207–213. doi: 10.1007/s11239-007-0047-3. [DOI] [PubMed] [Google Scholar]
- 31.Vaduganathan M, Alviar CL, Arikan ME, Tellez A, Guthikonda S, DeLao T, Granada JF, Kleiman NS, Ballantyne CM, Lev EI. Platelet reactivity and response to aspirin in subjects with the metabolic syndrome. Am Heart J. 2008;156:1002 e1001–1002 e1007. doi: 10.1016/j.ahj.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya D, Yanek LR, Faraday N, Moy TF, Becker LC, Becker DM. Native platelet aggregation and response to aspirin in persons with the metabolic syndrome and its components. Metab Syndr Relat Disord. 2009;7:289–296. doi: 10.1089/met.2008.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–74. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 34.Gokulakrishnan K, Deepa R, Mohan V, Gross MD. Soluble p-selectin and cd40l levels in subjects with prediabetes, diabetes mellitus, and metabolic syndrome--the chennai urban rural epidemiology study. Metabolism. 2006;55:237–242. doi: 10.1016/j.metabol.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Natal C, Restituto P, Inigo C, Colina I, Diez J, Varo N. The proinflammatory mediator cd40 ligand is increased in the metabolic syndrome and modulated by adiponectin. J Clin Endocrinol Metab. 2008;93:2319–2327. doi: 10.1210/jc.2007-2491. [DOI] [PubMed] [Google Scholar]
- 36.Jesri A, Okonofua EC, Egan BM. Platelet and white blood cell counts are elevated in patients with the metabolic syndrome. J Clin Hypertens (Greenwich) 2005;7:705–711. doi: 10.1111/j.1524-6175.2005.04809.x. quiz 712–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: A randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 38.Spectre G, Arnetz L, Ostenson CG, Brismar K, Li N, Hjemdahl P. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascular complications. Thromb Haemost. 2011:106. doi: 10.1160/TH11-04-0216. [DOI] [PubMed] [Google Scholar]
- 39.Cagirci G, Ozdemir O, Geyik B, Cay S, Ozturk S, Aras D, Topaloglu S. The prevalence of aspirin resistance in patients with metabolic syndrome. Turk Kardiyol Dern Ars. 2009;37:461–466. [PubMed] [Google Scholar]
- 40.Kahraman G, Sahin T, Kilic T, Baytugan NZ, Agacdiken A, Ural E, Ural D, Komsuoglu B. The frequency of aspirin resistance and its risk factors in patients with metabolic syndrome. International journal of cardiology. 2007;115:391–396. doi: 10.1016/j.ijcard.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 41.Frelinger AL, Li Y, Linden MD, Tarnow I, Barnard MR, Fox ML, Michelson AD. Aspirin ‘resistance’: Role of pre-existent platelet reactivity and correlation between tests. J Thromb Haemost. 2008;6:2035–2044. doi: 10.1111/j.1538-7836.2008.03184.x. [DOI] [PubMed] [Google Scholar]
- 42.Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 43.Grotemeyer KH, Scharafinski HW, Husstedt IW. Two-year follow-up of aspirin responder and aspirin non responder. A pilot-study including 180 post-stroke patients. Thromb Res. 1993;71:397–403. doi: 10.1016/0049-3848(93)90164-j. [DOI] [PubMed] [Google Scholar]
- 44.Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin “Resistance” And risk of cardiovascular morbidity: Systematic review and meta-analysis. BMJ (Clinical research ed. 2008;336:195–198. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, Tomasello SD, Capranzano P, Seecheran N, Darlington A, Tello-Montoliu A, Desai B, Bass TA, Angiolillo DJ. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv. 2011;4:180–187. doi: 10.1161/CIRCINTERVENTIONS.110.960187. [DOI] [PubMed] [Google Scholar]
- 46.FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, 2nd, Lawson JA, Brash AR. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catella F, FitzGerald GA. Paired analysis of urinary thromboxane b2 metabolites in humans. Thromb Res. 1987;47:647–656. doi: 10.1016/0049-3848(87)90103-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.