Abstract
It has long been recognized that lesions of the basal ganglia frequently result in dysarthria, in part because many individuals with Parkinson’s disease (PD) have impaired speech. Earlier studies of speech production in PD using perceptual, acoustic, and/ or kinematic analyses have yielded mixed findings about the characteristics of articulatory movements underlying hypokinetic dysarthria associated with PD: in some cases reporting reduced articulatory output, and in other instances revealing orofacial movement parameters within the normal range. The central aim of this experiment was to address these inconsistencies by providing an integrative description of basic kinematic and acoustic parameters of speech production in individuals with PD. Recordings of lip and jaw movements and acoustic data were collected in 16 individuals with PD and 16 age- and sex-matched neurologically healthy older adults. Our results revealed a downscaling of articulatory dynamics in the individuals with PD, evidenced by decreased amplitude and velocity of lower lip and jaw movements, decreased vocal intensity (dB sound pressure level [SPL]), and reduced second formant (F2) slopes. However, speech rate did not differ between groups. Our finding of an overall downscaling of speech movement and acoustic parameters in some participants with PD provides support for speech therapies directed at increasing speech effort in individuals with PD.
Keywords: Parkinson’s disease, speech acoustics, speech kinematics, speech production
Acoustic and perceptual studies of speech production in individuals with Parkinson’s disease (PD) have shown that hypokinetic dysarthria, a disturbance of speech and voice characterized by decreased intensity, articulatory imprecision, diminished prosody, and a breathy/hoarse voice, occurs in nearly 90% of this population.1,2 Yet the neurological and physiological underpinnings of dysarthria in PD are not well understood. It has been suggested that speech motor deficits in speakers with PD (eg, rigidity, hypokinesia, and/or bradykinesia) could contribute to dysarthria.3,4 However, there has been little research on articulatory movements in individuals with PD. Moreover, earlier physiological accounts of speech production in PD have yielded equivocal findings. For example, several studies have reported hypokinesia and/or bradykinesia of upper lip,5 lower lip (LL) and jaw,6–11 velar,12 and thyroarytenoid muscle13 during speech production. Conversely, unimpaired amplitude and velocity of LL and jaw articulatory movement,6,8,14,15 as well as increased lip muscle activity16 have also been noted in the literature. Interpretation of findings from earlier studies is difficult because of differences in experimental protocols. For example, in Caligiuri’s studies,9,10 the participants wore bite blocks to constrain jaw motion and to artificially exaggerate lip movement. Moreover, many of these studies required participants to produce syllable trains, nonsense words, or words in isolation.6,9–12,14 Individuals with PD are often able to produce perceptually accurate speech at the single-word level; however, based on perceptual evaluations, decreases in speech intelligibility are more likely to occur for longer words and phrases, or at the discourse level.17–19 Therefore, it is critical to examine articulatory kinematics during longer productions. Finally, most of the studies reviewed reported data from few (between 5 and 9) subjects with PD. Because variability is typically higher in clinical populations, it is essential to include more participants in order to obtain a better appreciation of performance range relative to age-matched controls.
The central aim of this study is to provide a better assessment of the articulatory movement characteristics of individuals with PD. This is an important goal, because current speech treatment protocols for dys-arthria in PD are founded on the basic assumption that these individuals need to “scale up” phonatory/ articulatory effort (eg, Freed,20 Ramig21,22). To this end, we combined acoustic and kinematic analyses to contribute to a cohesive picture of how fundamental aspects of speech are affected by the disease processes of PD. We examined amplitude and velocity of articulatory movements, and from the acoustic speech signal we analyzed speech rate, intensity, and second formant (F2) transition measures (ie, extent, duration, and rate/slope). F2 transitions were included as an index of articulatory movement because they are important dynamic speech acoustic cues signaling both consonant and vowel identities and are highly related to speech intelligibility.18,23–25
Patients and Methods
Participants
Sixteen participants diagnosed by a neurologist with idiopathic PD (11 males and 5 females) (mean [M] = 73 years; range 62–82 years) and 16 neurologically normal adults (M = 73 years; range 63–80 years) matched for age (±3 years), sex, and level of education (±3 years) to the individuals with PD participated in the study. Data from these subjects were collected as a part of a larger study of the effects of length and linguistic complexity on speech production in PD.26 Although the severity of their motor symptoms was not formally assessed, all participants with PD were ambulatory and living independently at the time of testing suggesting the participants fell into the mild to moderate range of disease severity. Summary characteristics for these participants are provided in Table 1. All participants were native speakers of American English, passed the Mini-Mental State Examination,27 had normal hearing,28 and reported normal or corrected-to-normal vision. Participants with PD were tested within 1 to 2 hours of taking their medication to control for possible on-off effects. It should be noted, however, that most studies of drug cycle effects have found no significant changes to speech when subjects were undergoing dopaminergic therapies.29–33 Participants were excluded from the experiment if they reported neurological conditions other than PD (eg, stroke), wore dentures that might interfere with articulatory movement tracking, took medication likely to affect motor performance (eg, muscle relaxants, narcotics), or had problems with hearing, speech, language, or reading unrelated to PD. The Purdue University Committee on the use of Human Subjects (Institutional Review Board [IRB]) approved all recruitment and experimental procedures.
TABLE 1.
Descriptive characteristics of the participants with PD
| Participant | Age | Years since diagnosis | Sex | PD medications | Reduced loudness ratinga | Reduced articulatory precision ratinga |
|---|---|---|---|---|---|---|
| PD1 | 70 | 10 | F | Selegiline, Pramipexole | 1.3 | 1.8 |
| PD2 | 71 | 5 | M | C-dopa/L-dopa, Pramipexole, Entacapone | 1.7 | 1.8 |
| PD3 | 73 | 5 | F | C-dopa/L-dopa, Pramipexole | 4.0 | 1.5 |
| PD4 | 75 | 15 | M | C-dopa/L-dopa | 4.7 | 3.0 |
| PD5 | 80 | 6 | F | C-dopa/L-dopa, Entacapone, Ropinirole | 1.7 | 1.0 |
| PD6 | 75 | 5 | F | C-dopa/L-dopa, Bromocriptine | 1 | 1.0 |
| PD7 | 77 | 4 | M | C-dopa/L-dopa | 5.8 | 3.3 |
| PD8 | 79 | 5 | M | C-dopa/L-dopa, Entacapone, Ropinirole | 4.7 | 3.0 |
| PD9 | 70 | 6 | M | C-dopa/L-dopa | 1.3 | 2.3 |
| PD10 | 70 | 5 | F | C-dopa/L-dopa, Bromocriptine | 4.0 | 2.0 |
| PD11 | 79 | 5 | M | C-dopa/L-dopa, Mirapex | 3.0 | 1.5 |
| PD12 | 82 | 4 | M | C-dopa/L-dopa, Amantadine | 1.0 | 1.7 |
| PD13 | 74 | 5 | M | C-dopa/L-dopa, Selegiline, Pramipexole | 1.7 | 1.8 |
| PD14 | 62 | 5 | M | C-dopa/L-dopa, Amantadine | 1.7 | 1.5 |
| PD15 | 67 | 12 | M | C-dopa/L-dopa | 2.7 | 1.3 |
| PD16 | 71 | 6 | M | C-dopa/L-dopa | 2.7 | 1.0 |
Seven-point perceptual rating scale: 1 =normal; 2–3 =mild severity; 4–5 =moderate; and 6–7 =severe.
PD, Parkinson’s disease; F, female; M, male; C-dopa, carbidopa; L-dopa, levodopa.
In general, the individuals with PD were judged to be intelligible but had mild to moderate dysarthria; for example, reduced articulatory precision and loudness based on a 7-point perceptual rating scale by 3 speech-language pathologists with experience assessing individuals with dysarthria (Table 1). A detailed description regarding the perceptual ratings is provided in Walsh and Smith.26
Speech Production Task
Participants produced 2 sentences containing predominantly bilabial consonants, so that lip and jaw movements were targeted. Sentence 1: “The boys and the pipers baked moist pumpkin pies,” contained 11 syllables; and sentence 2: “The messy boys whom the merry pipers saw baked many moist pumpkin pies,” contained 17 syllables.
Equipment
Kinematic data were collected with a Northern Digital Optotrak 3020 system (Waterloo, Ontario, Canada). The camera system recorded 3-dimensional positions of small infrared markers affixed to the vermilion border of the lower lip at midline and affixed to a splint attached under the chin (for jaw movement) with adhesive collars (accuracy = 0.1 mm). Movement data collection parameters described by Smith et al.34 were followed. Lip and jaw articulatory movements were corrected for head motion and digitized at 250 samples/sec. The speech signal for acoustic measurements was recorded with a condenser microphone placed at a 45-degree angle 6 inches away from the participant’s mouth; this distance was reassessed throughout the experimental session to ensure that the mouth-to-microphone distance remained constant. The acoustic signal was digitized using Praat, a software program developed for speech acoustic analysis (available for download at http://www.praat.org).35
Experimental Protocol
Participants practiced producing each sentence 1 time as it appeared on computer monitor in large font. During data collection, the 2 sentences analyzed for this experiment were quasirandomized within a block of 6 sentences, so that each sentence appeared in a particular position within a block (out of 15) an approximately equal number of times. Participants were instructed to read each sentence as soon as it appeared on the monitor using their typical speaking voice.
Kinematic Data Analysis
In the analysis, we included 10 accurate and fluent productions of each sentence for each participant (both the practice and first productions were discarded and the next 10 out of a possible 15 total acceptable productions were utilized). Overall accuracy scores are provided in Table 2. Two separate measures of displacement and velocity were computed. First, measures of peak amplitude and velocity of LL (plus jaw) opening and closing (O-C) movements during production of the syllable /paIp/ in the word “pipers” were calculated for the 10 repetitions of each sentence then averaged together for each participant. The duration of the O-C LL movement sequence for the syllable /paIp/ was also determined for all 10 repetitions of each sentence then averaged. These measures were chosen as representative spatial and temporal parameters for internal components of the sentence. Second, we employed a dynamic range measure to characterize the amplitude and velocity of LL (plus jaw), and jaw movement across the entire spoken phrase.36 The dynamic ranges included 80% of the points in the displacement or velocity trajectory for the whole utterance. The upper and lower 10% of these points were excluded, thus revealing the primary operating range of motion independent of the peak values of displacement and velocity.36 For each participant, the 10 displacement and 10 velocity dynamic ranges obtained for each sentence were averaged for each articulator.
TABLE 2.
Means and standard deviations in parentheses for the group and condition factors
| Measure | Control | PD | ||
|---|---|---|---|---|
| SPL (dB) | 79.1 (3.7) | 74.5 (2.2) | ||
| Syllable duration (s) | 0.25 (0.03) | 0.24 (0.03) | ||
| Speech rate (syllable/s) | 4.2 (0.24) | 4.1 (0.08) | ||
| Accuracy | 0.86 (0.13) | 0.95 (0.03) | ||
| Direction
|
Direction
|
|||
| Open | Close | Open | Close | |
|
| ||||
| LL displacement (mm) | 11.3 (2.8) | 11.3 (2.0) | 9.5 (2.7) | 9.1 (2.4) |
| LL velocity (mm/s) | 139.1 (47.4) | 162.0 (29.6) | 107.8 (33.7) | 141.1 (34.4) |
| Articulator
|
Articulator
|
|||
| Lower lip | Jaw | Lower lip | Jaw | |
|
| ||||
| Displacement dynamic range (mm) | 8.7 (2.1) | 7.8 (2.3) | 7.0 (1.5) | 6.0 (1.6) |
| Velocity dynamic range (mm/s) | 172.0 (46.8) | 158.7 (50.4) | 132.6 (38.3) | 112.0 (39.1) |
| Word
|
Word
|
|||
| Boy | Moist | Boy | Moist | |
|
| ||||
| F2 slopea (Hz/ms) | 10.8 (3.3) | 14.4 (2.7) | 8.4 (1.7) | 11.2 (2.8) |
| F2 extent (Hz) | 890.7 (228.5) | 775.2 (197.2) | 735.7 (219.1) | 676.1 (222.5) |
| F2 duration (ms) | 96.0 (27.0) | 58.7 (16.3) | 99.5 (30.0) | 65.0 (26.8) |
Values are means and standard deviations for each variable for both participant groups.
Before log-transformation to correct for heterogeneity of variances.
PD, Parkinson’s disease; SPL, sound pressure level; LL, lower lip; F2, second formant.
Acoustic Data Analysis
Acoustic analyses were completed on the same 10 fluent and accurate productions of each sentence as described above for the kinematic analyses. Individual sentences were extracted by using the combined waveform and wideband spectrographic displays of Praat. For each speaker, the mean speech intensity in dB sound pressure level (SPL) was computed across each sentence repetition for both sentences then averaged together. The mean speech rate (syllables/s) was calculated for each repetition then averaged for a given sentence condition. F2 transition measures for the diphthong / I/ in the words “boys” and “moist” for the 10 repetitions from each sentence were computed according to conventional criteria (eg, Kent et al.23). In order to define F2, the first and last glottal pulse of the vocalic nucleus for each diphthong was identified in Praat. For each vowel nucleus, formant traces for F2 were computed in 5-msec intervals using linear predictive coding. The formant trajectories were analyzed with a custom written program in MATLAB (Math-Works, Natick, MA). The onset of an F2 slope was defined as the portion of the vowel nucleus from which a 20-msec increment was accompanied by at least a 20-Hz frequency increase, while the offset was the point from which a 20-msec increment did not have a 20-Hz or greater change.23 Other second formant trajectory measures (transition extent [TE], the amount of frequency change along the transition, and transition duration [TD], the duration of the transitional segment) were also derived using the MATLAB program. The mean F2 slope value was computed across 20 productions of the words “boy” and “moist” (10 for each sentence) for each participant.
Statistical Analysis
Values for the variables dB SPL, speech rate, and syllable duration were entered into separate analyses of variance (ANOVAs) to detect potential group differences. Separate repeated measures ANOVAs were computed to examine potential between-group differences for the following variables: O-C displacement and O-C peak velocity, LL and jaw displacement, and LL and jaw velocity dynamic ranges. The within-subject factors in these ANOVAs was direction (2: open and close) and articulator (2: LL and jaw). An ANOVA with repeated measures on word (2: boy and moist) was also computed to analyze between group differences in F2 characteristics. In cases in which the parametric assumption of homogeneous variances was violated and not amenable through transformation (eg, log, arc-sine, or square-root); between-group differences were assessed with Mann-Whitney U analyses.
Approximately 10% of the acoustic data was reanalyzed through random selection of 2 participants with PD and 1 control. Reliability was assessed by computing Pearson’s r correlations between the first and second measurement for each variable. The correlation coefficients (r) and mean differences (MDs) between the original and reanalyzed data were as follows: rate: r = 0.99, MD = 0.01 seconds; dB SPL: r = 0.98, MD = 0.03 dB; and F2 slope: r = 0.97, MD = 113.26 Hz/s.
Results
Speech Kinematic Measures
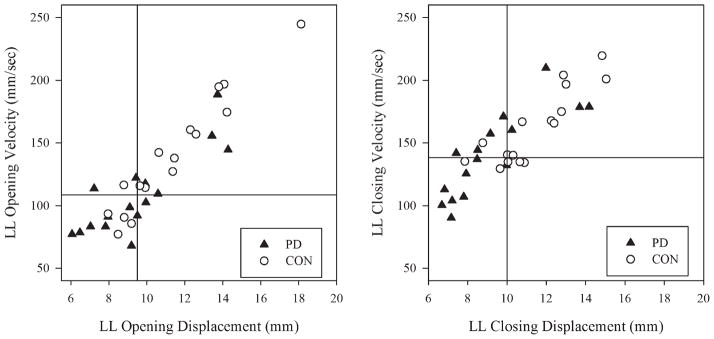
In Table 2, means and standard deviations for each variable for both participant groups are listed. Table 3 provides a statistical summary of all the results from the ANOVAs. The 2 graphs in Figure 1 show individual data points for O-C LL displacement and velocity. The lower left quadrant of each graph, therefore, contains data from participants with the smallest movement amplitudes and lowest velocities, while the upper right quadrants represent data from participants with larger movement amplitudes and higher velocities. Although there is overlap in the data from the 2 groups, on average, the individuals with PD had significantly smaller LL O-C displacements and lower velocities than the control participants. There was a within-subjects effect of direction. As expected, on the basis of earlier reports (eg, Max et al.37), closing velocities were significantly higher than opening velocities. Finally, the group effect for duration of the LL open-close movement sequence for the syllable /paIp/ was not significant.
TABLE 3.
Statistical summary for group, factor, and group by factor effects from ANOVA
| Group
|
||||||
|---|---|---|---|---|---|---|
| Measures | F | P | ||||
| Sound pressure level Syllable duration | 18.2 | <.001* | ||||
| 0.6 | 0.4 | |||||
| Group
|
Direction
|
Group × Direction
|
||||
| F | P | F | P | F | P | |
|
| ||||||
| LL open/close displacement | 5.5 | .03* | <1 | <1 | ||
| LL open/close velocity | 4.5 | .04* | 38.4 | <.001* | 1.3 | .26 |
| Group
|
Articulator
|
Group × Articulator
|
||||
| F | P | F | P | F | P | |
|
| ||||||
| Displacement dynamic range Velocity dynamic range | 7.4 | .01* | 21.5 | <.001* | <1 | |
| 8.5 | .01* | 12.4 | <.001* | <1 | ||
| Group
|
Word
|
Group × Word
|
||||
| F | P | F | P | F | P | |
|
| ||||||
| F2 slope | 9.58 | .004* | 97.3 | <.001* | <1 | |
Significant at P <.05.
ANOVA, analysis of variance; LL, lower lip; F2, second formant.
FIG. 1.
Individual data points for lower lip opening (left graph) and closing (right graph) displacement and velocity are shown. Because opening velocities are negative values, the absolute value of velocity is plotted in this figure. The inset lines show the median value for each variable for all participants.
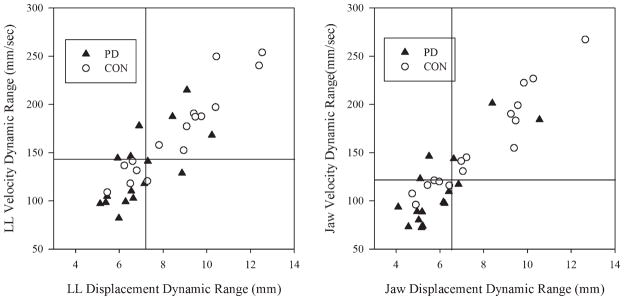
Figure 2 shows individual data points for LL and jaw velocity and displacement dynamic ranges for both groups. Similar to findings for single-movement components, the PD group had significantly reduced LL and jaw displacement dynamic ranges across whole-phrase productions compared to the control participants. The PD group also had significantly reduced velocity dynamic ranges for the LL and jaw than the control group. There was a significant effect of articulator. For both groups, jaw motion was typically associated with smaller displacement and reduced velocity dynamic ranges than LL. The interaction between articulator and group was not significant.
FIG. 2.
Individual data point for lower lip (left graph) and jaw (right graph) displacement and velocity dynamic ranges are shown. The inset lines in these graphs show the median value for each variable for all participants.
Speech Acoustic Measures
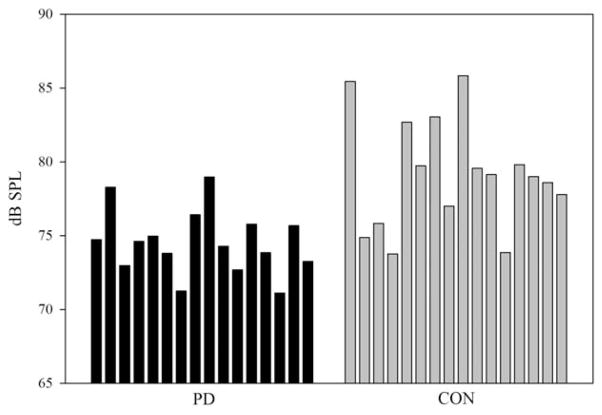
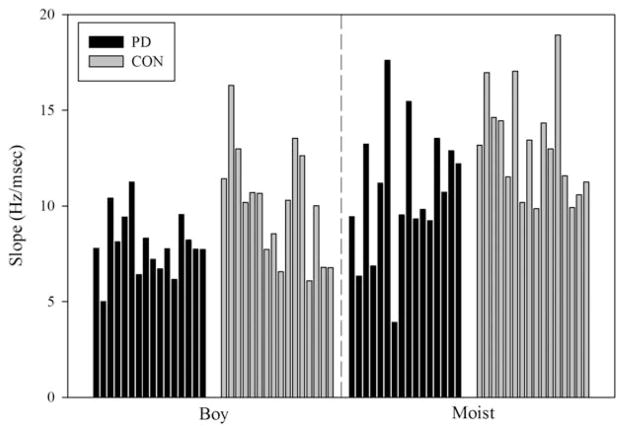
A Mann-Whitney U test confirmed that there were no differences between the groups with respect to their speaking rates (U = 102.0; P =.33). There was a significant group effect for speech intensity (dB SPL). Figure 3 shows individual dB SPL values for each group. The group with PD had, on average, SPL levels that were 4.6 dB lower than the control group. Descriptive statistics for transition extent (Hz), transition duration (msec), and F2 slope (Hz/ms) are reported in Table 2 for the words “boy and “moist.” Only slope measures, however, were considered in the statistical analysis to avoid redundancy.38 In Figure 4, the individual slopes for both groups are plotted as a function of the word produced. As Figure 4 suggests, there was a significant group effect; the slopes for both words combined were significantly reduced in the participants with PD. The PD group had shallower F2 slopes by approximately 2.79 Hz/msec compared to the control group. There was a word effect. Figure 4 shows that the diphthong in the word “moist” was consistently associated with increased slopes compared to the diphthong in the word “boy” in both groups of speakers. The group by word interaction was not significant.
FIG. 3.
Individual data bars for speech intensity in dB SPL are shown. The acoustic signal was recorded with a microphone positioned 6 inches away from the participant’s mouth.
FIG. 4.
Individual data bars for F2 slope in Hz/ms are shown for production of the words “boy” and “moist.”
Discussion
Taken together, our kinematic and acoustic analyses provide a more complete picture of how speech production is affected by PD. We found that on average, individuals with PD spoke with reduced lower lip and jaw movement amplitudes and velocities, decreased vocal intensity, and had shallower formant slopes compared to controls. However, speech rates and syllable durations were similar between the 2 groups.
As reported in several earlier studies,7,9–11 on average, individuals with PD had lower amplitudes and velocities of opening and closing lower lip movements within single articulatory movements as well as for multiple movement sequences for phrase-length productions. The participants with PD also had reduced dynamic ranges for jaw displacement and velocity during phrase-level productions. This finding is in agreement with some earlier studies.6,7 On the other hand, Connor and Abbs14 found no differences between PD and control participants with respect to jaw movement. However, their findings were based on data from only 5 individuals with PD. Furthermore, although they measured jaw velocity and amplitude separately, they only reported that the ratio between these variables was unimpaired.
On average, the participants with PD had significantly lower speech intensities and shallower F2 transition slopes for diphthong production (eg, the “oi” in moist) compared to the controls. These findings are corroborated by earlier studies.23,34 The values in Table 2 imply that shallower slopes in the PD group were due to reduced transition extents rather than reduced transition durations (which were nearly identical between the 2 groups). Reduced F2 transitions in the group with PD reflects a reduction in the rate of change of the oral cavity (eg, reduced tongue and/or jaw movement) to produce the diphthong,34 and mirrors the finding of reduced LL and jaw displacement in the kinematic analyses.
Similar to findings from several other studies,6,18,38–40 we found no significant difference in the average speaking rate between the 2 subject groups. There are several accounts of faster speech rates in participants with PD.41,42 In our task, the participants repeated sentences in response to a visual prompt. We found that not only was the overall rate comparable between the 2 groups, but that the duration of internal sentence components (ie, syllables and F2 transitions) was similar in both groups; however, the PD group had a larger range of speaking rates. The kinematic data showed that the group with PD had lower velocities on average; however, they were moving smaller distances, thus maintaining similar durations as the control group. Precise timing of rapid sequential movements of multiple structures is critical for intelligible speech production. As Connor et al.6 pointed out, varying the duration of components in the speech acoustic signal affects the auditory perception of consonants.43 Thus, it is possible that greater importance is placed on the preservation of timing mechanisms which are essential for intelligible speech production.
Conclusions
The results of our study support an overall “down-scaling” of speech production in some individuals with PD. These participants with PD spoke with decreased amplitude and velocity of LL and jaw movements, presumably had decreased tongue/jaw excursions as indicated by the F2 measures, and spoke at lower intensities than controls. Our findings provide support for pursuing treatment programs for individuals with PD such as LSVT® (LSVT Global, Tucson, AZ), an intensive dysarthria treatment program in which the goal is an overall “scaling up” of vocal and articulatory effort.21,22 It is important, however, to note that some participants with PD produced speech parameters within the range obtained for control participants. If dysarthria therapy is warranted, these individuals may benefit from alternative treatments that target, for example, other parameters such as rate, articulatory precision, and intelligibility.
Decreased amplitude and/or slowed movements in PD are well documented in studies of gait, handwriting, and hand pointing/reaching,44–49 and in voluntary facial expression.50 Hypokinesia and bradykinesia in PD in limb motor control is hypothesized to result from inadequate excitation of motor neuron pools due to deficient discharge from the basal ganglia to cortical areas (eg, Albin et al.,51 Berardelli et al.,52 Desmurget et al.53) and/or impaired sensory integration/ proprioception, which in turn affects motor program execution (eg, Abbruzzese and Berardelli,54 Demirci et al.,55 Fellows et al.,56 Klockgether et al.57). There is a paucity of data on facial muscle function during speech production in PD, as the underlying sensory motor system deficits that produce this downscaling of movement in speech production are unknown. It is interesting, however, to note that several studies indicate there is no muscular insufficiency preventing individuals with PD from speaking with larger lip and jaw movements and/or at a louder volume when externally cued to do so.58,59 Further research is needed to clarify how sensory processing and/or reduced cortical discharge to motor neuron pools affect speech motor control, contributing to the downscaling of articulatory movements in individuals with PD.
Acknowledgments
We expressly thank all participants whose contributions made this research possible.
Funding agencies: National Institute on Deafness and Other Communication Disorders, National Institutes of Health (F31DC007267-01 and R01DC00559); Indiana Lions Club (67313533762); Parkinson’s Awareness Association of Central Indiana (201116).
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behav Neurol. 1998;11:131–137. [PubMed] [Google Scholar]
- 2.Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and co-occurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- 3.Ramig L, Fox C, Sapir S. Speech and voice disorders in Parkinson’s disease. In: Olanow CW, Stocchi F, Lang AE, editors. Parkinson’s DISEASE: non-motor and non-dopaminergic features. Oxford: Wiley-Blackwell Inc; 2011. pp. 346–360. [Google Scholar]
- 4.Sapir S, Ramig L, Fox C. Speech therapy for Parkinson’s disease. In: Pfieffer R, Wszolek ZK, Edabi M, editors. Parkinson’s disease. 2. Boca Raton: Taylor and Francis Group; (in press) [Google Scholar]
- 5.Netsell R, Daniel B, Celesia GG. Acceleration and weakness in parkinsonian dysarthria. J Speech Hear Disord. 1975;40:170–178. doi: 10.1044/jshd.4002.170. [DOI] [PubMed] [Google Scholar]
- 6.Connor NP, Abbs JH, Cole KJ, Gracco VL. Parkinsonian deficits in serial multiarticulate movements for speech. Brain. 1989;112:997–1009. doi: 10.1093/brain/112.4.997. [DOI] [PubMed] [Google Scholar]
- 7.Forrest K, Weismer G, Turner GS. Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric speakers. J Acoust Soc Am. 1989;85:2608–2622. doi: 10.1121/1.397755. [DOI] [PubMed] [Google Scholar]
- 8.Svensson P, Henningson C, Karlsson S. Speech motor control in Parkinson’s disease: a comparison between a clinical assessment protocol and a quantitative analysis of mandibular movements. Folia Phoniatr (Basel) 1993;45:157–164. doi: 10.1159/000266243. [DOI] [PubMed] [Google Scholar]
- 9.Caligiuri MP. Labial kinematics during speech in patients with Parkinsonian rigidity. Brain. 1987;110:1033–1044. doi: 10.1093/brain/110.4.1033. [DOI] [PubMed] [Google Scholar]
- 10.Caligiuri MP. The influence of speaking rate on articulatory hypokinesia in Parkinsonian dysarthria. Brain Lang. 1989;36:493–502. doi: 10.1016/0093-934x(89)90080-1. [DOI] [PubMed] [Google Scholar]
- 11.Forrest K, Weismer G. Dynamic aspects of lower lip movement in Parkinsonian and neurologically normal geriatric speakers’ production of stress. J Speech Hear Res. 1995;38:260–272. doi: 10.1044/jshr.3802.260. [DOI] [PubMed] [Google Scholar]
- 12.Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson’s disease. Ann Neurol. 1986;19:283–284. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- 13.Baker K, Ramig L, Luschei E, Smith M. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51:1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]
- 14.Connor NP, Abbs JH. Task-dependent variations in Parkinsonian motor impairments. Brain. 1991;114:321–332. [PubMed] [Google Scholar]
- 15.Ackermann H, Hertrich I, Daum I, Scharf G, Spieker S. Kinematic analysis of articulatory movements in central motor disorders. Mov Disord. 1997;12:1019–1027. doi: 10.1002/mds.870120628. [DOI] [PubMed] [Google Scholar]
- 16.Leanderson R, Meyerson BA, Persson A. Lip muscle function in parkinsonian dysarthria. Acta Otolaryngol. 1972;74:350–357. doi: 10.3109/00016487209128462. [DOI] [PubMed] [Google Scholar]
- 17.Robertson SJ, Thomson F. Working with dysarthric clients: a practical guide to therapy for dysarthria. Tucson, AZ: Communication Skill Builders, Inc; 1987. [Google Scholar]
- 18.Weismer G, Jeng JY, Laures J, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatr Logop. 2001;52:201–219. doi: 10.1159/000052649. [DOI] [PubMed] [Google Scholar]
- 19.Yorkston KM, Beukelman DR. An analysis of connected speech samples of aphasic and normal speakers. J Speech Hear Disord. 1980;45:27–36. doi: 10.1044/jshd.4501.27. [DOI] [PubMed] [Google Scholar]
- 20.Freed D. Motor speech disorders diagnosis and treatment. San Diego: Singular Publishing Group; 2000. [Google Scholar]
- 21.Ramig L, Sapir S, Fox C, Countryman S. Changes in vocal intensity following intensive voice treatment (LSVT®) in individuals with Parkinson’s disease: a two-year follow-up. J Neurol Neurosurg Psychiatry. 2001;71:493–498. doi: 10.1136/jnnp.71.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramig L, Sapir S, Fox C. Changes in vocal intensity following intensive voice treatment (LSVT®) in individuals with Parkinson’s disease: a comparison with untreated patients and normal age-matched controls. Mov Disord. 2011;16:79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Kent RD, Kent JF, Weismer G, et al. Relationships between speech intelligibility and the slope of second-formant transitions in dysarthric subjects. Clin Linguist Phon. 1989;3:347–358. [Google Scholar]
- 24.Kim Y, Weismer G, Kent RD, Duffy JR. Statistical models of F2 slope in relation to severity of dysarthria. Folia Phoniatr Logop. 2009;61:329–335. doi: 10.1159/000252849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulligan M, Carpenter J, Riddel J, et al. Intelligibility and the acoustic characteristics of speech in amyotrophic lateral sclerosis. J Speech Hear Res. 1994;37:496–503. doi: 10.1044/jshr.3703.496. [DOI] [PubMed] [Google Scholar]
- 26.Walsh B, Smith A. Linguistic complexity, speech production, and comprehension in Parkinson’s disease: behavioral and physiological indices. J Speech Hear Res. 2011;54:787–802. doi: 10.1044/1092-4388(2010/09-0085). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37–42. [PubMed] [Google Scholar]
- 29.Baker KK, Ramig LO, Johnson AB, Freed CR. Preliminary voice and speech analysis following fetal dopamine transplants in 5 individuals with Parkinson disease. J Speech Lang Hear Res. 1997;40:615–626. doi: 10.1044/jslhr.4003.615. [DOI] [PubMed] [Google Scholar]
- 30.Daniels N, Oates J, Phyland DJ, Feiglin A, Hughes A. Vocal characteristics and response to levodopa in Parkinson’s disease. Mov Disord. 1996;11:117. [Google Scholar]
- 31.Gamboa J, Jimenez-Jimenez FJ, Nieto A. Acoustic voice analysis in patients with Parkinson’s disease treated with dopaminergic drugs. J Voice. 1997;11:314–320. doi: 10.1016/s0892-1997(97)80010-0. [DOI] [PubMed] [Google Scholar]
- 32.Plowman-Prine EK, Okun MS, Sapienza CM, et al. Perceptual characteristics of Parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. Neurorehabilitation. 2009;24:131–144. doi: 10.3233/NRE-2009-0462. [DOI] [PubMed] [Google Scholar]
- 33.Skodda S, Visser W, Schlegel U. Short- and long-term dopaminergic effects on dysarthria in early Parkinson’s disease. J Neural Transm. 2010;117:197–205. doi: 10.1007/s00702-009-0351-5. [DOI] [PubMed] [Google Scholar]
- 34.Smith A, Johnson M, McGillem C, Goffman L. On the assessment of stability and patterning of speech movements. J Speech Lang Hear Res. 2000;43:277–286. doi: 10.1044/jslhr.4301.277. [DOI] [PubMed] [Google Scholar]
- 35.Boersma P, Weenink D. Praat. Amsterdam: Institute of Phonetic Sciences; 2006. (version, 4.5 ed) [Google Scholar]
- 36.Riely RR, Smith A. Speech movements do not scale by orofacial structure size. J Appl Physiol. 2003;94:2119–2126. doi: 10.1152/japplphysiol.00502.2002. [DOI] [PubMed] [Google Scholar]
- 37.Max L, Caruso AJ, Gracco VL. Kinematic analyses of speech, oro-facial nonspeech, and finger movements in stuttering and nonstuttering adults. J Speech Lang Hear Res. 2003;46:215–232. doi: 10.1044/1092-4388(2003/017). [DOI] [PubMed] [Google Scholar]
- 38.Tjaden K, Wilding GE. Rate and loudness manipulations in dys-arthria: acoustic and perceptual findings. J Speech Lang Hear Res. 2004;47:766–783. doi: 10.1044/1092-4388(2004/058). [DOI] [PubMed] [Google Scholar]
- 39.Kleinow J, Smith A, Ramig L. Speech motor stability in IPD: effects of rate and loudness manipulations. J Speech Lang Hear Res. 2001;44:1041–1051. doi: 10.1044/1092-4388(2001/082). [DOI] [PubMed] [Google Scholar]
- 40.Skodda S, Schlegel U. Speech rate and rhythm in Parkinson’s disease. Mov Disord. 2008;23:985–992. doi: 10.1002/mds.21996. [DOI] [PubMed] [Google Scholar]
- 41.McRae PA, Tjaden K, Schoonings B. Acoustic and perceptual consequences of articulatory rate change in Parkinson disease. J Speech Lang Hear Res. 2002;45:35–50. doi: 10.1044/1092-4388(2002/003). [DOI] [PubMed] [Google Scholar]
- 42.Flint AJ, Black SE, Campbell-Taylor I, Gailey GF, Levinton C. Acoustic analysis in the differentiation between Parkinson’s disease and major depression. J Psycholinguist Res. 1992;21:383–399. doi: 10.1007/BF01067922. [DOI] [PubMed] [Google Scholar]
- 43.Faulkner A, Rosen S. Contributions of temporal encodings of voicing, voicelessness, fundamental frequency, and amplitude variation to audio-visual and auditory speech perception. J Acoust Soc Am. 1999;106:2063–2073. doi: 10.1121/1.427951. [DOI] [PubMed] [Google Scholar]
- 44.Alberts JL, Saling M, Adler CH, Stelmach GE. Disruptions in the reach-to-grasp actions of Parkinson’s patients. Exp Brain Res. 2000;134:353–362. doi: 10.1007/s002210000468. [DOI] [PubMed] [Google Scholar]
- 45.Margolin DI, Wing AM. Agraphia and micrographia: clinical manifestations of motor programming and performance disorders. Acta Psychol. 1983;54:263–283. doi: 10.1016/0001-6918(83)90039-2. [DOI] [PubMed] [Google Scholar]
- 46.McLennan JE, Nakano K, Tyler HR, Schwab RS. Micrographia in Parkinson’s disease. J Neurol Sci. 1972;15:141–152. doi: 10.1016/0022-510x(72)90002-0. [DOI] [PubMed] [Google Scholar]
- 47.Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 1994;117:1169–1181. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- 48.Morris ME, Huxham FE, McGinley J, Iansek R. Gait disorders and gait rehabilitation in Parkinson’s disease. Adv Neurol. 2001;87:347–361. [PubMed] [Google Scholar]
- 49.Van Gemmert AW, Adler CH, Stelmach GE. Parkinson’s disease patients undershoot target size in handwriting and similar tasks. J Neurol Neurosurg Psychiatry. 2003;74:1502–1508. doi: 10.1136/jnnp.74.11.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowers D, Miller K, Bosch W, et al. Faces of emotion in Parkinson’s disease: micro-expressivity and bradykinesia during voluntary facial expressions. J Int Neuropsych Soc. 2006;12:765–773. doi: 10.1017/S135561770606111X. [DOI] [PubMed] [Google Scholar]
- 51.Albin RL, Young AB, Penny JB. The functional neuroanatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 52.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124:2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 53.Desmurget M, Grafton ST, Vindras P, Gréa H, Turner RS. Basal ganglia network mediates the control of movement amplitude. Exp Brain Res. 2003;153:197–209. doi: 10.1007/s00221-003-1593-3. [DOI] [PubMed] [Google Scholar]
- 54.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 55.Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol. 1997;41:781–788. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- 56.Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain. 1998;121:1771–1784. doi: 10.1093/brain/121.9.1771. [DOI] [PubMed] [Google Scholar]
- 57.Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J. A defect of kinesthesia in Parkinson’s disease. Mov Disord. 1995;10:460–465. doi: 10.1002/mds.870100410. [DOI] [PubMed] [Google Scholar]
- 58.Dromey C, Ramig L. Intentional changes in sound pressure level and rate: their impact on measures of respiration, phonation, and articulation. J Speech Lang Hear Res. 1998;41:1003–1018. doi: 10.1044/jslhr.4105.1003. [DOI] [PubMed] [Google Scholar]
- 59.Sadagopan N, Huber JE. Effects of loudness cues on respiration in individuals with Parkinson’s disease. Mov Disord. 2007;22:651–659. doi: 10.1002/mds.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]