Abstract
Purpose
To investigate the effects of increased syntactic complexity and utterance length demands on speech production and comprehension in individuals with Parkinson’s disease (PD) using behavioral and physiological measures.
Method
Speech response latency, interarticulatory coordinative consistency, accuracy of speech production, and response latency and accuracy on a receptive language task were analyzed in 16 individuals with PD and 16 matched control participants.
Results
Individuals with PD had higher oral motor coordination variability, took a longer time to initiate speech, and made more errors on the speaking task compared with the control group. They also received lower scores on the 2 complex conditions of the receptive language task. Increased length and syntactic complexity negatively affected performance in both groups of speakers.
Conclusions
These findings provide a novel window into the speech deficits associated with PD by examining performance on longer, sentence-level utterances in contrast to earlier investigations of single-word or nonword productions. Speech motor control processes and language comprehension were adversely affected in the majority of our participants with mild to moderate PD compared to the control group. Finally, increased syntactic complexity and sentence length affected both the healthy aging and PD groups’ speech production performance at the behavioral and kinematic levels.
Keywords: Parkinson’s disease, speech production, linguistic complexity, language comprehension, speech motor control
Linguistic Complexity, Speech Production, and Comprehension in Parkinson’s Disease: Behavioral and Physiological Indices
Parkinson’s disease (PD) affects approximately 1% of the general population over the age of 65 years and 2% of those over the age of 85 years (Burn, 2000). The central pathology of PD is the progressive degeneration of dopaminergic cells of the substantia nigra of the basal ganglia. This results in dopamine depletion in striatal structures as well as in other areas of the brain. The basal ganglia are widely recognized as a network of nuclei supporting the planning and execution of movement. Our understanding of basal ganglia function has evolved, in part, through research from the last 2 decades of individuals with neurological diseases such as PD. Multiple distributed cortico-striatal-cortical circuits are thought to mediate aspects of cognition, speech, and language (e.g., Alexander, DeLong, & Strick, 1986; Crosson et al., 2003; Cummings, 1993; Friederici, Kotz, Werheid, Hein, & von Cramon, 2003; Grossman et al., 2003; Lashley, 1951; Lieberman, 2001; Mesulam, 1990; Murdoch, 2001; Zgaljardic, Borod, Foldi, & Mattis, 2003). However, the precise role that the basal ganglia play in these higher order processes is not well understood. As our view of the basal ganglia has progressed, we have seen an increasing number of studies focusing on cognitive deficits in PD and, to a far lesser extent, on speech and language impairments.
Speech Production in PD
Dysarthria, which is a disturbance of speech caused in part by neurological disease, is a prominent characteristic of PD; nearly 90% of these individuals develop speech and voice disorders (Ho, Iansek, Marigliani, Bradshaw, & Gates, 1998; Logemann, Fisher, Boshes, & Blonsky, 1978). Clinical characteristics of hypokinetic dysarthria in PD include hypophonia, monotonicity, breathiness/hoarseness, imprecise articulation, and rate problems (for a review, see Ramig, Fox, & Sapir, in press). Most studies of hypokinetic dysarthria in PD have relied upon perceptual measures and, to a lesser extent, on acoustic and physiological ones. Of particular relevance to this research is the fact that there have been few studies into the nature of the complex interarticulatory movements underlying the articulation deficits in PD. Connor, Abbs, Cole, and Gracco (1989) analyzed the sequencing of upper and lower lip and jaw peak velocities during the production of the nonword sapapple and reported decreased coordination across these articulators in nine participants with mild-to-moderate PD. Kleinow, Smith, and Ramig (2001) examined lower lip and jaw movement during production of a syntactically simple phrase using a composite measure of spatial and temporal patterning. They found that eight adults with mild-to-moderate PD performed similarly to age-matched controls on this measure. However, when speakers were asked to repeat the same test phrase at different rates and loudness levels, the patterns of movement associated with a particular speaking condition were the least distinctive in the participants with PD compared to the young adult and age-matched controls.
Language and Cognitive Influences on Speech Motor Control
Most studies of human communication have concentrated on speech production or language processing separately. A. Smith and Goffman (2004) posited that language and motor speech functions interact to a much greater extent than earlier speech/language production models suggest (e.g., Levelt, 1989; Levelt, Roelofs, & Meyer, 1999). To understand motor functions during speech, they suggest that the linguistic structure and goals of the utterance spoken be taken into account. In support of their hypothesis, earlier work from our laboratories has confirmed that linguistic factors indeed affect speech motor coordination. Specifically, increasing the length and syntactic complexity of experimental speaking tasks results in increased variability in lip and jaw kinematics in children and adults with no neurological impairments (Kleinow & Smith, 2006; Maner, Smith, & Grayson, 2000).
In populations with neurological speech disorders, there is evidence that increased linguistic demands produce more disruptions to the speech system. Several studies have reported higher disfluency rates under conditions of increased length and syntactic complexity in typically fluent children and in children who stutter (e.g., Logan & Conture, 1997; Silverman & Bernstein Ratner, 1997). Kleinow and Smith (2000) found that adults who stutter showed increased variability in articulatory movement patterns as syntactic complexity and length increased. Taken together, these studies support the hypothesis that higher order linguistic processes influence speech motor control even in adults and children with no neurological impairments. Those adults with a communication disorder may be more vulnerable to increased linguistic demands.
There have been no previous studies of the direct influence of linguistic complexity on speech production in PD. Research in individuals with this disease provides a vital opportunity to learn how deficient subcortical networks impact speech and language processes. Lieberman et al. (1992) found a significant correlation between an increase in syntactic errors on a language assessment task and voice onset timing errors on a single-word production task in participants with PD. The effect was pronounced in the moderate compared to the mild group of participants with PD. Lieberman et al. hypothesized that these deficits could be attributed to a common pathology—namely, damage to the neural circuitry connecting prefrontal and basal ganglia areas.
Language Production and Comprehension in PD
Studies focusing on semantic, syntactic, and prosodic aspects of language, collectively, suggest that 50%–60% of individuals with PD without dementia have a mild-to-moderate impairment in their language comprehension, even in the earlier stages of the disease (for reviews, see Grossman, 1999; Lieberman, 2000; Murray, 2008). With respect to spoken language, Illes, Metter, Hanson, and Iritani (1988) and Cummings, Darkins, Mendez, Hill, and Benson (1988) elicited speech samples from individuals with mild-to-moderate PD and found reduced utterance length and syntactic complexity compared with controls. In contrast, Murray (2000) and Murray and Lenz (2001) found that the utterance length and syntactic structure of spoken language samples were similar in their mild to moderate-to-severe PD and in healthy control groups; however, these researchers documented that the individuals with PD produced a smaller proportion of grammatically intact sentences.
Relevant to the present experiment, comprehension of complex syntactic structures appears to be disrupted in individuals with PD (Angwin, Chenery, Copland, Murdoch, & Silburn, 2006; Colman, Koerts, van Beilen, Leenders, & Bastiaanse, 2006; Grossman et al., 1991, 2002; Lee, Grossman, Morris, Stern, & Hurtig, 2003; Hochstadt, Nakano, Lieberman, & Friedman, 2006; Lieberman et al., 1992; Natsopoulos et al., 1991). For example, individuals with PD were significantly impaired compared with controls on a sentence-to-picture matching task, suggesting deficient syntactic processing (Colman et al., 2006; Hochstadt et al., 2006; Lieberman et al., 1992; Natsopoulous et al., 1991). Grossman and colleagues (Grossman et al., 1991, 2002; Lee et al., 2003) found that individuals with PD had decreased accuracy answering probes paired with complex sentences (i.e., with central or terminal clauses). Participants in these studies had mild PD and were not taking dopaminergic medications at the time of testing (Grossman et al., 1991) or, alternatively, were on their Parkinson’s medications (Grossman et al., 2002; Lee et al., 2003). Angwin et al. (2006) extended this line of research to show that participants with PD had poorer accuracy comprehending sentences with object-relative embedded clauses compared with subject-relative clauses and longer reading times for object-relative sentences on a self-paced reading task.
Summary and Hypotheses
There have been few studies of the complex interarticulatory movements underlying articulation deficits in PD (Connor et al., 1989; Kleinow et al., 2001). These studies included a small number of participants and assessed nonword or short-phrase production. This may be problematic, given that individuals with PD are often able to produce perceptually accurate speech at the single-word level; however, breakdowns in articulatory precision are more likely to occur at the phrase or discourse level (Robertson & Thomson, 1987; Weismer, Jeng, Laures, Kent, & Kent, 2001; Yorkston & Beukelman, 1980). We investigated interarticulatory coordination during the production of sentences that varied in length and syntactic complexity to assess how higher order linguistic processes affected speech motor performance. We chose to manipulate syntactic complexity given that previous research in the areas of language production and comprehension has shown that syntax may be especially problematic for individuals with PD. Specifically, they are less accurate at comprehending sentences that are syntactically more complex (e.g., Angwin et al., 2006; Colman et al., 2006; Grossman et al., 1991, 2002; Lee et al., 2003; Hochstadt et al., 2006; Lieberman et al., 1992; Natsopoulos et al., 1991), and they use simplified syntactic structure (Cummings, Darkins, Mendez, Hill, & Benson, 1988; Illes et al., 1988) and/or a smaller proportion of grammatically intact sentences (Murray, 2000; Murray & Lenz, 2001) during conversational speech.
In summary, the purpose of the present investigation was to perform multilevel assessments of speech production and comprehension abilities in individuals with PD. We included both behavioral and physiological assessments of speech production as well as behavioral measures of language comprehension. Because of the obvious motor deficiencies caused by PD, we hypothesized that the individuals with PD would show higher coordination variability in a speech production task. Previous research has also revealed that these individuals are less accurate at comprehending syntactically complex sentences and may use simplified syntactic structure in their spontaneous speech. Therefore, we also hypothesized that increases in utterance length and complexity would have greater deleterious effects on oral motor coordination in the group with PD. In order to assess these two hypotheses, we used a measure of speech motor coordination that captures the consistency of temporal and spatial relations among the upper lip, lower lip, and jaw (Kleinow & Smith, 2006; A. Smith & Zelaznik, 2004; Walsh, Smith, & Weber-Fox, 2006). We also examined the effect of syntactic complexity and utterance length on behavioral measures of speech production—namely, speech response latency and the number of production errors. Finally, we employed a sentence comprehension task designed to parallel the speech production task, as the syntactic complexity of the stimuli was manipulated similarly for both tasks. We adopted this strategy to replicate earlier findings (but employing a visual rather than an auditory paradigm to remove the reliance on memory to generate the sentences) and to explore whether the integrity of language comprehension and speech production was similarly compromised in individuals with PD.
Method
Participants
Participants were 11 men and five women (M = 73 years, range = 62–82 years) with idiopathic PD diagnosed by a neurologist and 16 healthy adults (M = 73 years, range = 63–80 years) matched for age (±3 years), gender, and level of education (±3 years) to the individuals with PD. Education ranged from a total of 12 years to 20 years (M = 16.0 years) for the participants with PD, and from a total of 12–20 years (M = 15.9 years) for the control participants. All participants were native speakers of American English. The individuals with PD were tested within 1–2 hr of taking their medication to control for possible on–off effects. Although the severity of their motor symptoms was not formally assessed, all participants with PD were ambulatory and were living independently at the time of testing. Summary characteristics for the participants with PD are provided in Table 1.
Table 1.
General characteristics of the participants with Parkinson’s disease (PD).
| Participant | Gender | Age | Time since diagnosis (in years) |
Medication for PD | SMMSE score (out of 30) |
Antidepressant |
|---|---|---|---|---|---|---|
| PD1 | F | 70 | 10 | Selegiline, Pramipexole | 30 | Y |
| PD2 | M | 71 | 5 | C-dopa/L-dopa, Pramipexole, Entacapone | 28 | N |
| PD3 | F | 73 | 5 | C-dopa/L-dopa, Pramipexole | 30 | Y |
| PD4 | M | 75 | 15 | C-dopa/L-dopa | 26 | Y |
| PD5 | F | 80 | 6 | C-dopa/L-dopa, Entacapone, Ropinirole | 29 | Y |
| PD6 | F | 75 | 5 | C-dopa/L-dopa, Bromocriptine | 30 | N |
| PD7 | M | 77 | 4 | C-dopa/L-dopa | 27 | N |
| PD8 | M | 79 | 5 | C-dopa/L-dopa, Entacapone, Ropinirole | 26 | N |
| PD9 | M | 70 | 6 | C-dopa/L-dopa | 30 | N |
| PD10 | F | 70 | 5 | C-dopa/L-dopa, Bromocriptine | 28 | Y |
| PD11 | M | 79 | 5 | C-dopa/L-dopa, Mirapex | 30 | N |
| PD12 | M | 82 | 4 | C-dopa/L-dopa, Amantadine | 30 | N |
| PD13 | M | 74 | 5 | C-dopa/L-dopa, Selegiline, Pramipexole | 29 | N |
| PD14 | M | 62 | 5 | C-dopa/L-dopa, Amantadine | 28 | Y |
| PD15 | M | 67 | 12 | C-dopa/L-dopa | 30 | Y |
| PD16 | M | 71 | 6 | C-dopa/L-dopa | 27 | N |
Note. SMMSE = Standardized Mini Mental State Examination; F = female; M = male; Y = yes; N = no.
Screening Measures and Dysarthria Severity Assessment
Participants had a negative history of dementia per self-report and passed screenings on the Standardized Mini Mental State Examination (SMMSE; Molloy, 1999), a global measure of cognitive ability. A score below 24 on this measure indicates cognitive impairment (see Table 1). All participants passed a pure-tone hearing screening at 40 dB for 500, 1000, and 1500 Hz, bilaterally (Ventry & Weinstein, 1983), and reported normal or corrected-to-normal vision. The participants with PD completed a history form providing information about the time since diagnosis and medication regimens. Prospective participants were excluded from the experiment if they reported other neurological conditions or had problems with speech, language, or reading unrelated to their PD. The control participants also completed a medical history form providing information about illnesses, medication, and/or speech, language, and learning problems. Control participants were excluded if they reported neurological disorders, took medication likely to affect performance (e.g., muscle relaxants, antidepressants), or reported problems with speech, language, reading, or hearing.
In general, the individuals with PD had mild-to-moderate speech and voice impairment based on the perceptual ratings of three speech-language pathologists with experience assessing individuals with dysarthria. In order to assess whether the participants spoke with reduced loudness, the expert listeners heard recordings of participants reading aloud a portion of “The Papa Passage” (Sapienza & Stathopoulos, 1995). Selected portions of this speaking passage were also used to assess speech rate and articulatory precision, equated for peak intensity at 70 dB to ensure that intensity differences did not influence listener judgments of rate and precision. To assess each dysarthria characteristic, the first recording that the listeners heard was from a control participant whose speech was determined to be normal by the experimenter. They were instructed to compare each subsequent recording to this exemplar by circling the number corresponding to severity (1 = normal, 2–3 = mild, 4–5 = moderate, and 6–7 = severe). The exemplar was replayed after every five participant recordings; however, the listeners were encouraged to replay it as often as needed. Specific dysarthria characteristics for each participant with PD are provided in Table 2.
Table 2.
Relevant speech severity ratings for participants with PD.
| Participant | Degree of reduced loudness |
Reduced articulatory precision |
Impairment of speech rate |
|---|---|---|---|
| PD1 | Normal–mild | Mild | Normal |
| PD2 | Mild | Mild | Mild |
| PD3 | Moderate | Mild | Moderate |
| PD4 | Moderate | Mild | Mild |
| PD5 | Mild | Normal | Normal–Mild |
| PD6 | Normal | Normal | Mild |
| PD7 | Severe | Mild–moderate | Mild–Moderate |
| PD8 | Moderate | Mild | Mild–Moderate |
| PD9 | Normal–mild | Mild | Moderate |
| PD10 | Moderate | Mild | Mild |
| PD11 | Mild | Mild | Mild |
| PD12 | Normal | Mild | Mild |
| PD13 | Mild | Mild | Mild–Moderate |
| PD14 | Mild | Mild | Normal–Mild |
| PD15 | Mild | Normal–mild | Mild |
| PD16 | Mild | Normal | Normal–Mild |
Experimental Procedure Overview
All data were collected during one experimental session, which was 1½–2 hr in length. During the first portion of the session, the speech production protocol was completed, and kinematic data were collected. Next, the participants were given the pure-tone hearing screening and produced speaking samples, which we recorded in order to assess dysarthria severity (as described above). During the final portion of the session, the SMMSE was administered, and participants then completed the sentence comprehension protocol. All experimental and screening sessions were video recorded.
Speech Production Task
Speech production: Stimuli
Stimuli consisted of six sentences that varied in both length and syntactic complexity; the sentences (with their acronym codes, for brevity) are listed in Table 3. They were designed such that they contained predominantly bilabial consonants, so that lip and jaw movements were targeted and constrained. These stimuli were developed to include different levels of syntactic complexity and contained either 11 or 12 (short conditions) or 16 or 17 (long conditions) syllables, creating a length contrast.
Table 3.
Sentence stimuli for speech production tasks.
| Description | Sentence | Sentence code |
|---|---|---|
| Simple, short | The boys and the pipers baked moist pumpkin pies. | SS |
| Simple, long | The messy boys and the merry pipers baked many moist pumpkin pies. | SL |
| Complex, subject-relative, short | The boys who saw pipers baked moist pumpkin pies. | CSS |
| Complex, subject-relative, long | The messy boys who saw merry pipers baked many moist pumpkin pies. | CSL |
| Complex, object-relative, short | The boys whom the pipers saw baked moist pumpkin pies. | COS |
| Complex, object-relative, long | The messy boys whom the merry pipers saw baked many moist pumpkin pies. | COL |
We manipulated sentence complexity so that there were declarative sentences (Simple 1); sentences containing a center-embedded, subject-relative clause (Complex 2); and sentences containing a center-embedded, object-relative clause (Complex 3). This strategy was used on the basis of the results from earlier studies employing behavioral, functional magnetic resonance imaging (fMRI), and/or event-related potential (ERP) analyses of syntactic complexity and comprehension, which showed distinctions between these sentence types (Just & Carpenter, 1993; Just, Carpenter, Keller, Eddy, & Thulborn, 1996; King & Just, 1991; King & Kutas, 1995; Lee et al., 2003; Miyake, Just, & Carpenter, 1994).
Speech production: Recording equipment
Speech response latencies (i.e., the time between the visual presentation of the sentences on a computer monitor and the onset of the participant’s speech acoustic signal) were recorded with E-Prime software (Psychology Software Tools) and a Serial Response Box (Model 200a) from Psychology Software Tools. The speech acoustic signal was detected with a condenser microphone positioned 6 in. away from the participant’s mouth and at a 45° angle to the lips.
Kinematic data were collected with a Northern Digital Optotrak 3020 system. The camera system recorded three-dimensional movements of small infrared markers affixed to the upper lip, lower lip, and jaw with adhesive collars. The lip markers were attached to the vermilion border of the lips at midline. Jaw movement was recorded with a marker affixed to a splint attached under the chin; however, this signal was not analyzed for this experiment. Optotrak data collection parameters described by A. Smith, Johnson, McGillem, and Goffman (2000) were followed. Additional markers were used to compute a head coordinate system so that extraneous movements—due to dyskinesias, for example—did not interfere with the collection of movement from the articulators. Marker motions were digitized by the Optotrak system at 250 Hz. The participants’ acoustic signal was digitized at an 18-kHz sampling rate by an analog-to-digital unit within the Optotrak system so that it was synchronized with the movement signals. We used the acoustic signal to ensure that (a) the target sentences were spoken correctly and, thus, to tabulate production accuracy scores, and (b) the kinematic data segmented for analysis corresponded to the appropriate speech sample (i.e., was not inadvertently cut off).
Speech production: Experimental protocol
In order to familiarize the participants with the six-sentence stimuli, they practiced producing each sentence one time as it appeared on a 20-in. monitor positioned 7 ft. in front of the participants. The sentences were presented on three lines of text for the short-sentence conditions and on five lines of text for the long-sentence conditions in 48-point Arial boldfaced font using E-Prime software. During data collection, 15 blocks of six sentences were presented. The six sentences were pseudorandomized within each block so that each sentence appeared in a particular position within the block an approximately equal number of times. Ninety trials (15 productions of each of the six sentences) were produced by each participant. There were pauses of approximately 2 s between presentation of each sentence. Participants were instructed to read each sentence as soon as it appeared on the monitor using their typical speaking voice and habitual rate.
Speech production: Kinematic data analysis
To systematically select 10 productions of each sentence for the kinematic analysis, we adhered to the following rules: The first production of each sentence was discarded for all participants. The next 10 error-free productions of each sentence were then selected. A production of a sentence was judged to be error free when it did not contain substitutions, omissions, additions, distortions, dis-fluencies, aberrant prosody, or inappropriate pauses. These judgments were made by the first author and one other experimenter online and were confirmed by the first author later during offline data analysis.
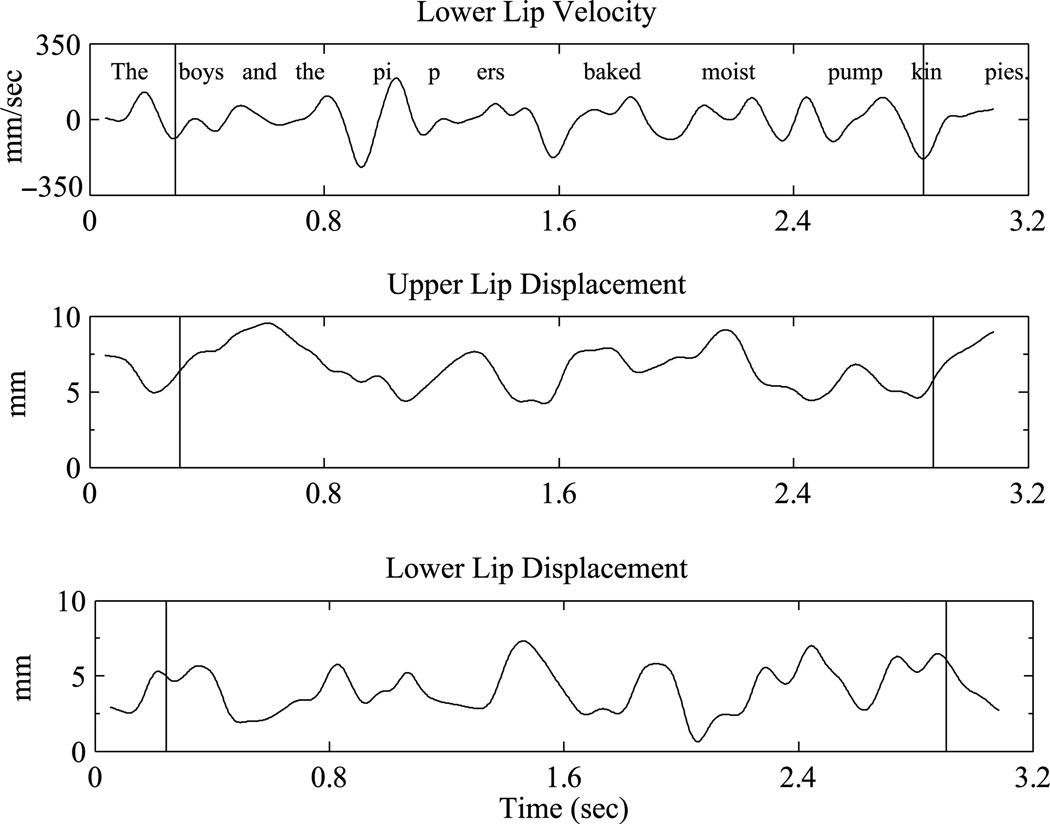
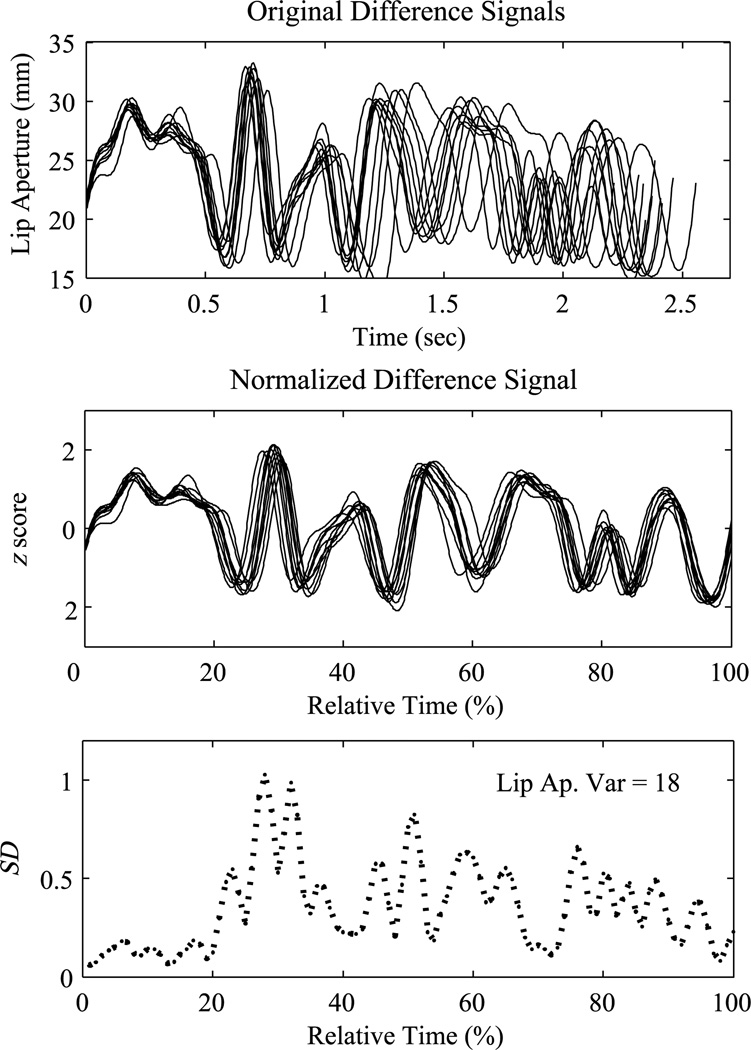
Only superior–inferior articulatory movements were analyzed because this is the primary dimension of movement for bilabial stop consonants. The kinematic records for the 10 exemplars of each sentence were imported into MATLAB (The Math Works) for analysis. As illustrated in Figure 1, the lower lip (plus jaw) velocity signal (superior–inferior dimension) was used to define the beginning and end points (peak velocity of the initial and final opening movements) of each sentence. This interval excluded “the” and started at the peak velocity of the bilabial opening movement (opening from /b/ to /ɔI/ in the word boys or /m/ to /e/ in the word messy) to the peak velocity of the last opening movement for the utterance (release of the /p/ to /aI/ in pies). These indices were used to extract the upper lip and lower lip (plus jaw) displacement interval for analysis (see Figure 1). The lower lip (plus jaw) displacement signal was subtracted from the upper lip signal to produce the lip aperture (LA) signal. Thus, the LA signal reflects the coordination of the upper lip, lower lip, and jaw to control oral opening as a function of time for the entire utterance (A. Smith & Zelaznik, 2004). We acknowledge that our signal is not an exact representation of actual LA, which is a complex three-dimensional measure (see Westbury & Hashi, 1997). However, it is a reasonable and frequently utilized estimate (e.g., Kleinow & Smith, 2006; A. Smith & Zelaznik, 2004; Walsh et al., 2006). As illustrated in Figure 2, the LA signals for each sentence were then linearly time and amplitude normalized (e.g., A. Smith et al., 2000). Finally, following A. Smith and Zelaznik (2004), an LA variability index was computed for each participant and each sentence by calculating theSDacross the sets of 10 time-and amplitude-normalized displacement waveforms. These SDs were then summed to produce the LA variability index (see inset, Figure 2). This value reflects the dispersion of the set of normalized LA trajectories for the entire utterance.
Figure 1.
Sample raw data: Kinematic data from a control participant during one production of the following sentence: The boys and the pipers baked moist pumpkin pies. Displacement trajectories from the upper lip and lower lip are plotted under the lower lip velocity signal. In this figure, the vertical lines pass through the velocity peaks of the initial bilabial opening movement for the /b/ in boys and last opening movement for the /p/ in pies to demonstrate segmentation. mm = millimeters; sec = seconds.
Figure 2.
Lip aperture (LA) variability index calculation. The top panel shows 10 LA displacement trajectories of the sentence, The boys and the pipers baked moist pumpkin pies, spoken by a control participant. Zero on the y-axis of this panel represents minimal interlip distance or lip closure. Because the markers were placed on the vermilion border of the participant’s upper and lower lip, intermarker distance should not be 0 at closure. In the middle panel, the trajectories have been time- and amplitude-normalized and, as a result, closely align. The bottom panel shows the SDs of the 10 normalized LA trajectories computed at successive intervals as a function of relative time. The LA variability index is reported in the inset. Lip Ap. Var = lip aperture variability.
Receptive Language Task
Sentence comprehension: Stimuli
The participants completed a sentence comprehension task consisting of 54 sentences paired with a probe question. Mirroring the speech production stimuli, the syntactic complexity of the task was manipulated by the grammatical phrase structure of the target sentence. One third of the sentences were simple in structure (e.g., The playful puppy nipped the frightened kitten.). One third of the sentences included a center-embedded, subject-relative clause (e.g., The submarine that bumped the yacht was reckless.). The remaining third of the target sentences included a center-embedded, object-relative clause (e.g., The dean that the professor criticized was sheepish.). Sentences from the three conditions contained approximately the same number of words to control for length confounds. Half the probes for each sentence condition were in the active voice (e.g., “What did the nipping?”), and half were in the passive voice (e.g., “Whowas criticized?”). These stimuli were adapted and were used with permission from Grossman and colleagues (Grossman et al., 1991).
Sentence comprehension: Experimental protocol
The participants were seated in front of a 17-in. monitor for the sentence comprehension task. The sentence stimuli were randomized and presented in 20-point Arial boldfaced font using E-Prime software, which was used to detect the participants’ accuracy and response times (RTs). The participants were informed that their RT and accuracy were being measured and were encouraged to answer each item as quickly as possible without compromising accuracy. Each sentence item appeared on the computer screen for 5 s before the accompanying probe, and two answer choices appeared beneath the sentence. The participants were asked to answer each probe by pressing either the number 1 or number 2 key on the number pad of the computer keyboard. The participants rested their preferred hand on a pad in order to be as close as possible to the keyboard. In addition, brightly colored stickers with the numbers printed on them in large font were placed on the number 1 and number 2 answer keys to facilitate responding. Once an answer key was pressed, the next sentence automatically appeared on the screen. The sentences remained on the screen throughout the duration of each trial so that the participants could review the sentence as needed to answer the item. This step ensured that the task assessed the comprehension of syntactic structure rather than working memory processes. If performance fell below 60% on a 10-item practice test, instructions were re-administered to ensure that the participant understood the task.
Statistical Analysis
The reliability of the expert listeners’ dysarthria assessments was determined by repeating recordings from one pseudorandomly chosen female and male speaker for each of the six dysarthria characteristics. Correlation coefficients were then computed between the first and second rating. The correlation coefficient and mean difference between the original and reanalyzed data were r = .90, p < .01, indicating good reliability.
Speech production response latencies less than 500 ms or greater than 4,000 ms were considered outliers and were removed from the data set. For the participants with PD, 3.55% of the trials were removed from the data set as outliers. For the control participants, 1.25% of the trials were removed from the data set as outliers. Before conducting statistical analyses on data from the sentence comprehension task, those responses counted as outliers (i.e., extremely short [< 700 ms] or prolonged [> 15 s]) were eliminated. For two participants with PD, this resulted in a 46% and 67% loss of their data, respectively. Because these individuals were extreme outliers, their RT and accuracy data on the sentence comprehension task were removed from the data set. For the other participants with PD, error responses accounted for approximately 1% of the total number of trials and were removed from the data set. For the control participants, an average of 0.7% of the trials was removed from the data set. Finally, three participants with PD did not produce 10 accurate and fluent repetitions of each sentence. In these cases, LA variability index calculations were derived from the eight or nine available tokens. However, in one instance, a participant produced only three tokens of the complex, subject-relative long sentence. In this case, the average sentence LA variability index for the group with PD for this particular sentence condition was substituted for the missing value in the statistical analyses.
For the speech production task, group differences (PD vs. control) in the LA variability index, production accuracy, and speech production response latencies were assessed by performing separate analyses of variance (ANOVAs) with repeated measures on sentence complexity (3 levels: simple, subject-relative, and object-relative) and sentence length (2 levels: short and long). For the receptive sentence comprehension task, group differences were examined for sentence comprehension accuracy and sentence comprehension RT with separate ANOVAs, with repeated measures on sentence complexity (3 levels: simple, subject-relative, and objective relative). Log-transformed speech response latencies for fluent productions and log-transformed sentence comprehension RTs for correctly answered items were used in the repeated-measures ANOVA (Ratcliff, 1993).
For all ANOVAs, degrees of freedom and F values are reported utilizing the Greenhouse–Geisser probability adjustment for cases in which the assumption of sphericity was violated. The alpha level was set at p < .05. In cases in which the parametric assumption of homogeneous variances was violated and not amenable through transformation, between-group differences were assessed with Mann–Whitney U analyses (p < .05).
We wished to perform correlational analyses between variables related to PD severity (i.e., time postonset of PD [TPO], general cognitive status [SMMSE score], age, level of dysarthria [degree of reduced loudness, articulatory imprecision, level of speech rate impairment] and performance on comprehension and speech motor tasks in the PD group). To reduce the number of correlations involved, as there were six sentences on the speaking task, the average LA index and average production accuracy score were calculated for each individual with PD for the analyses. Justification for doing so stemmed from the fact that pairs of LA index values and production accuracy scores were correlated (all rs between .51–.91; all ps < .05). In addition, scatterplots of these correlations were inspected to ensure homoscedasticity and a linear relationship of the data before Pearson’s r correlations (p < .05) were computed to summarize the strength of the relationship between these variables of interest. Finally, it is possible that antidepressant use could influence performance in the participants with PD, especially on response-timed tasks. Therefore, we divided the individuals with PD into two groups (those taking antidepressant medication and those not taking an antidepressant) and compared performance on speech RT, comprehension accuracy, and comprehension RT using separate repeated-measures ANOVAs.
Results
Speech Production Task
Speech production latencies
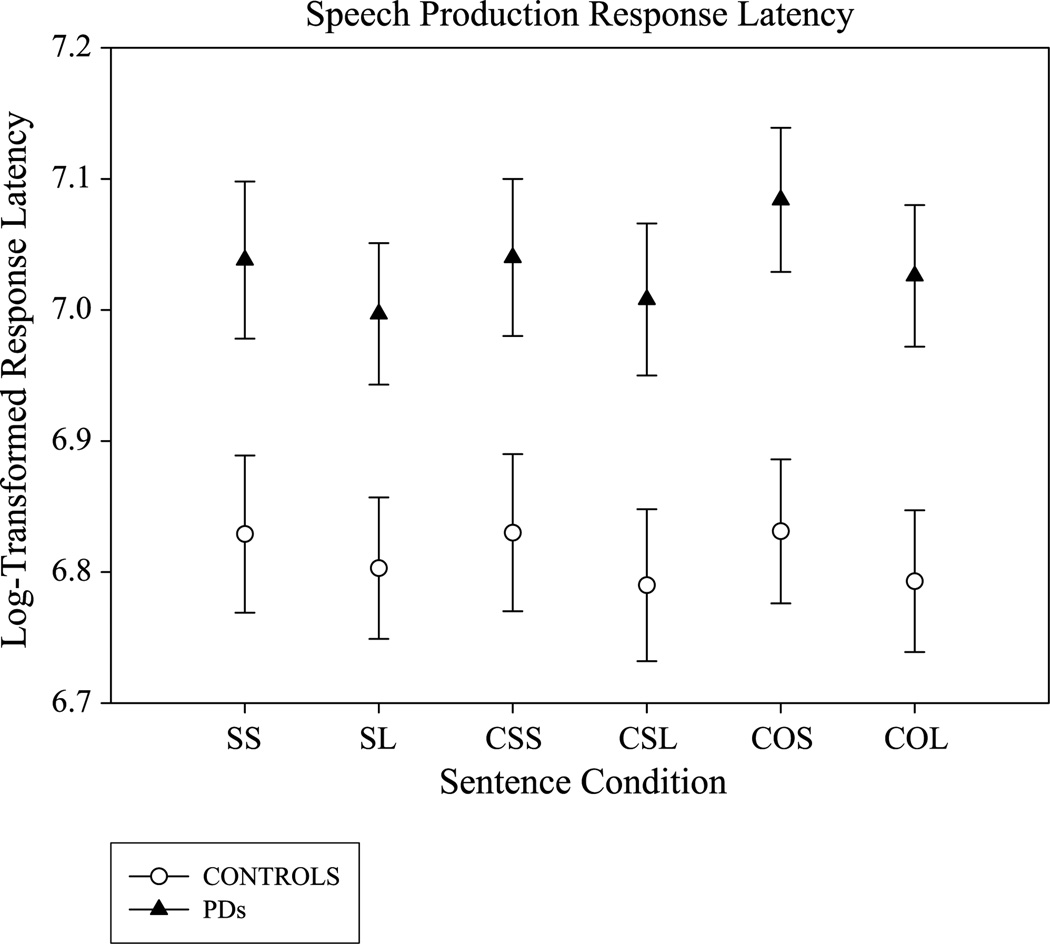
In Figure 3, mean response latencies (and standard error of the mean [SEM]) are plotted for the PD and control groups. The group with PD was, on average, significantly slower to produce the sentences than the control group, F(1, 30) = 7.8, p = .009, , by approximately 246 ms. There was also a sentence length effect, F(1, 30) = 16.7, p ≤ .001, , across both groups. The shorter sentences resulted in longer speech response latencies by approximately 44 ms. There was no significant effect of sentence complexity on this measure and no significant interactions.
Figure 3.
Mean and standard error bars for log-transformed speech production response latencies are plotted for the individuals with Parkinson’s disease (PD; filled triangles) and control participants (open circles) as a function of the six sentence conditions (simple, short [SS]; simple, long [SL]; complex, subject-relative, short [CSS]; complex, subject-relative, long [CSL]; complex, object-relative, short [COS]; and complex, object-relative, long [COL]).
LA variability index
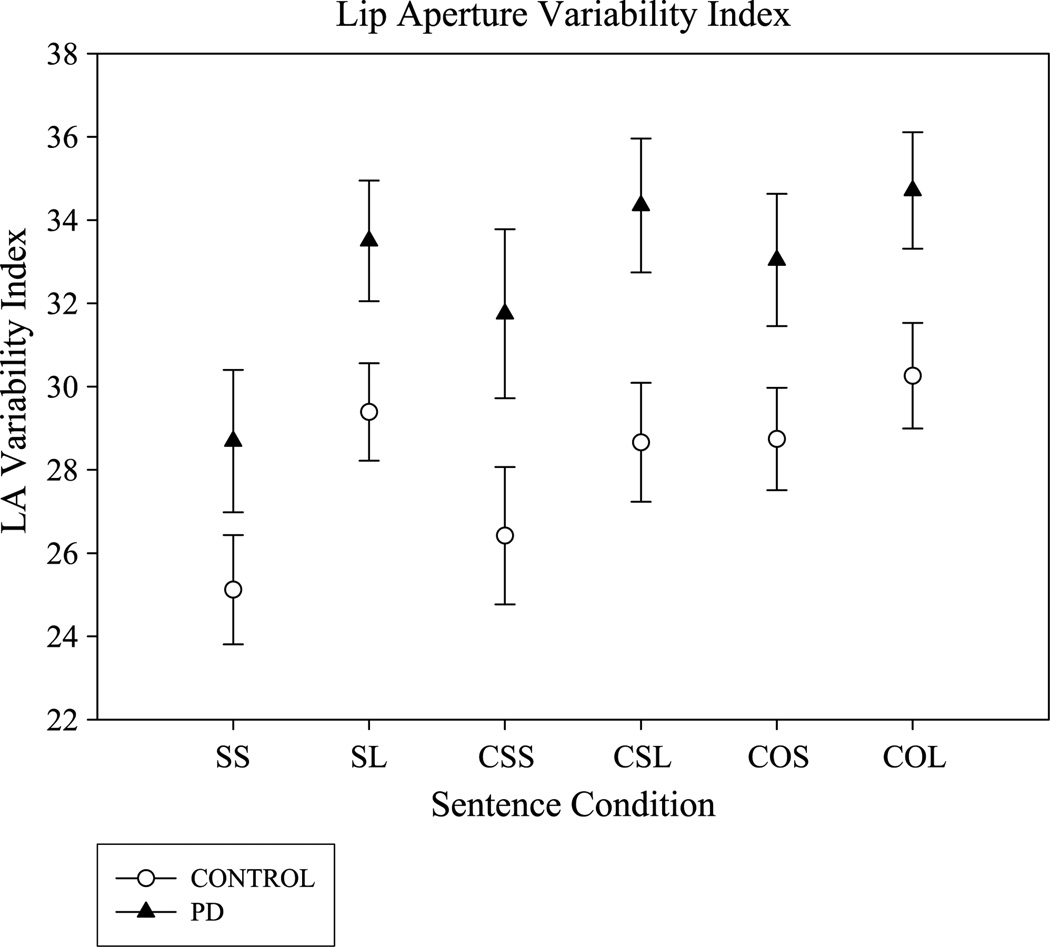
Figure 4 shows the mean LA variability indices as a function of sentence condition (SEM) for each group of participants. As this figure shows, there was a significant group effect, F(1, 30) = 6.3, p = .02, . For all sentences combined, individuals with PD (M = 32.7, SD = 6.5) had higher LA variability indices, denoting less consistent oral motor coordination than the control participants (M = 28.1, SD = 5.4). The main effects of length and complexity were significant, and the interaction between complexity and length was also significant, F(2, 60) = 5.1, p = .009, . Post hoc tests revealed that longer and/or more complex sentence conditions resulted in higher LA variability indices in both groups of participants. Specifically, the LA index for the SL, CSL, COS, and COL sentence conditions was significantly higher than the LA index for the SS and CSS conditions (Tukey’s honestly significant difference [HSD], p < .05). There was no interaction between group and sentence length or complexity.
Figure 4.
Mean and standard error bars for the LA variability index are plotted for the individuals with PD (filled triangles) and controls (open circles) as a function of the six sentence conditions (SS, SL, CSS, CSL, COS, and COL).
Production accuracy
Table 4 lists the percentage correct (out of a possible 15) and theSDs for the six sentences for each participant group. These data confirm that the control group performed near ceiling across all sentence conditions. Examples of errors included productions that had omissions, additions, distortions, disfluencies, false starts, aberrant prosody, or inappropriate pauses. Because a Levene’s test of homogeneity of variance was significant, a nonparametric Mann–Whitney U analysis was used to assess group differences. The individuals with PD had significantly lower production accuracy scores than did the control participants across all sentence conditions (all Us between 39.5 and 75.0; all ps < .05).
Table 4.
Means (and SDs) for the percentage of accurate productions for each sentence condition of the speech production task.
| Sentence condition |
Control participants (n = 16) |
Participants with PD n ( = 16) |
|---|---|---|
| SS | 97 (0.01) | 92 (0.03) |
| SL | 98 (0.01) | 89 (0.03) |
| CSS | 97 (0.01) | 80 (0.04) |
| CSL | 96 (0.01) | 80 (0.05) |
| COS | 99 (0.01) | 91 (0.03) |
| COL | 91 (0.02) | 80 (0.04) |
Receptive Language Task
Sentence comprehension: Response time
Table 5 shows that on average, the participants with PD were slower to respond to the question probes on the sentence comprehension task by approximately 700 ms compared with the control participants; however, this difference was not statistically significant F(1, 28) = 1.4, p = .2. RT means and SDs (in ms) for correctly answered items on the sentence comprehension task are reported for the two subject groups for each sentence condition in the first two columns of Table 5. There was a significant effect of sentence complexity on RT, F(1.5, 42.8) = 16.6, G-G p < .001, . For both groups combined, comprehension RT was significantly slower for the two complex sentence conditions, subject-relative (M = 4,441 ms, SD = 2,066 ms) and object-relative (M = 4,786 ms, SD = 2,101 ms), compared to the simple sentence condition (M = 3,725 ms, SD = 1,603 ms) (Tukey’ sHSD, p < .001). The interaction between group and sentence complexity was not significant.
Table 5.
Means (and SDs) for accuracy percentage and response time (in ms) for the sentence comprehension task.
| RT | % accuracy | |||
|---|---|---|---|---|
| Sentence condition | Control participants (n = 16) |
Participants with PD (n = 14) |
Control participants n ( = 16) |
Participants with PD n ( = 14) |
| Simple | 3,561.5 (1,624.0) | 3,911.8 (1,617.2) | 95.0 (6.0) | 86.4 (18.1) |
| Subject-relative | 4,065.9 (1,981.9) | 4,869.6 (2,150.1) | 96.8 (4.5) | 83.6 (14.6) |
| Object-relative | 4,335.7 (2,123.3) | 5,301.5 (2,123.3) | 93.3 (10.8) | 79.8 (18.3) |
Note. RT = response time.
Sentence comprehension: Accuracy
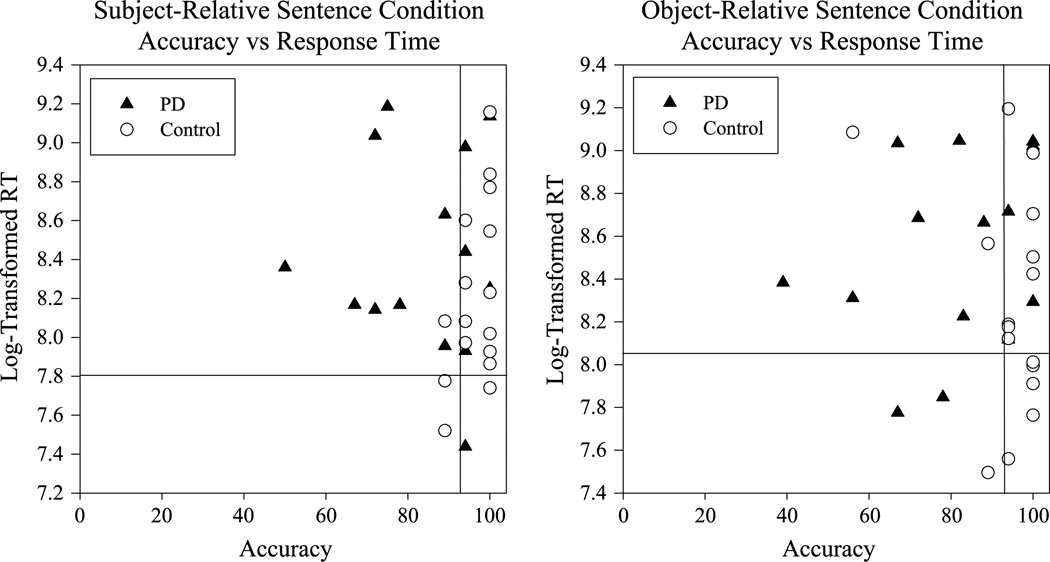
The last two columns of Table 5 list the sentence comprehension accuracy means and SDs for the individuals with PD and controls for each sentence condition. A Levene’s test revealed a violation of the homogeneity of variance assumption; thus, a Mann–Whitney U test confirmed that the individuals with PD had lower comprehension accuracy scores on the subject-relative condition (U = 43; p < .01) and the object-relative condition (U = 59.5; p = .02). However, the groups received statistically similar scores on the simple sentence condition (U = 79.5; p = .16). Figure 5 shows the mean sentence comprehension accuracy score for the subject- and object-relative conditions for each participant plotted against his/her log-transformed mean sentence comprehension RT. The inset lines show the median value for each variable across all participants. The lower right quadrant of each graph, therefore, contains data from participants with the highest accuracy scores and fastest RTs, whereas the upper left quadrant of this graph represents data from participants with lower accuracy scores and slower RTs. From these graphs, it is apparent that the majority of participants with PD are clustered in the upper left quadrants, whereas a number of control participants are clustered in the lower right quadrants.
Figure 5.
Data points for sentence comprehension accuracy (x-axis) are plotted for 14 of the individuals with PD (filled triangles) and 16 control participants (open circles) against his or her log-transformed average sentence comprehension response time (y-axis). The left graph in this figure shows data from the subject-relative sentence condition, and the right graph shows data from the object-relative sentence condition. In these graphs, the vertical and horizontal solid lines indicate the median value across all participants for each variable. RT = response time.
Relationship Among PD Severity Measures, Speech Production, and Receptive Language Performance
We wanted to determine whether performance on the speech production and receptive language tasks correlated with cognitive status, dysarthria severity, TPO, and age of the participants with PD. Half of the participants with PD scored at ceiling on the SMMSE screener. These seven individuals also represented the full range of data values on the other variables of interest, so that significant correlations would not be expected. Given the moderate sample size, these preliminary findings should be interpreted cautiously.
As expected, ratings for the three dysarthria characteristics—reduced loudness, articulatory imprecision, and speech rate impairment—correlated with one another (see Table 6). The dysarthria characteristic of reduced loudness significantly correlated with average LA variability index (see Table 6). This signifies that greater interarticulatory variability is associated with greater degree of reduced loudness. The dysarthria characteristic reduced articulatory precision that was negatively correlated with production accuracy (see Table 6), such that poorer accuracy on the speech production task was associated with a greater degree of articulatory imprecision. Reduced articulatory precision and reduced loudness also correlated with subject-relative and object-relative comprehension task conditions, respectively. As anticipated, subject-relative and object-relative scores were correlated. No correlation between degree of speech rate impairment and any performance variable was significant. Unpredictably, we found that age did not correlate with any dysarthria characteristic or performance variable. Speech production accuracy correlated with the two complex conditions of the receptive language task. Production accuracy also correlated with LA index and TPO. Finally, no significant performance differences were found between (a) those seven participants taking antidepressant medication and (b) those nine participants not taking antidepressant medication (see Table 1) with respect to their speech RT, F(1, 14) = 0.3, p = .59; their comprehension RT, F(1, 12) = 2.1, p = .17; or their comprehension accuracy, F(1, 12) = 0.19, p = .67.
Table 6.
Correlations for individuals with PD.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Agea | 1 | −.24 | .18 | .18 | .19 | .35 | .04 | −.14 | .01 | −.13 |
| 2. TPOa | −.24 | 1 | .13 | .15 | −.19 | .06 | −.51* | −.30 | .06 | .23 |
| 3. Reduced loudnessa | .18 | .13 | 1 | .68** | .46* | .54* | −.39 | −.24 | −.37 | −.48* |
| 4. Reduced articulatory precisiona | .18 | .15 | .68** | 1 | .52* | .35 | −.53* | −.38 | −.53* | −.38 |
| 5. Speech ratea | .19 | −.19 | .46* | .52* | 1 | .14 | −.02 | .20 | .13 | −.04 |
| 6. LA indexa | .35 | .06 | .54* | .35 | .14 | 1 | −.45* | .25 | −.15 | −.16 |
| 7. Production accuracya | .04 | −.51* | −.39 | −.53* | −.02 | −.45* | 1 | .43 | .70** | .72** |
| 8. Simple comprehension accuracyb | −.14 | −.30 | −.24 | −.38 | .20 | .25 | .43 | 1 | .72** | .59* |
| 9. Subject-relative comprehensionb | .01 | .06 | −.37 | −.53* | .13 | −.15 | .70** | .72** | 1 | .80** |
| 10. Object-relative comprehensionb | −.13 | .23 | −.48* | −.38 | −.04 | −.16 | .72** | .59* | .80** | 1 |
Note. TPO = time postonset of PD; LA = lip aperture.
n = 16.
n = 14.
p < .05 (one-tailed).
p < .01 (one-tailed).
Discussion
We used both behavioral and physiological measures to analyze the planning and execution of longer, sentence-level productions and thus to provide a novel window onto the speech production deficits associated with PD. This study, which contained the largest number of participants in a kinematic study of speech production in PD to date, showed that individuals with PD had higher oral motor coordination variability for all sentence conditions, required a longer period of time to initiate speech, and made more errors on the speaking task compared with the control group. These findings indicate the presence of a significant level of speech motor impairment characteristic of individuals with PD. We also hypothesized that the participants with PD would be disproportionately vulnerable to effects of increased sentence length and syntactic complexity. We were surprised to find that both the PD and control groups showed equally robust effects of syntactic complexity and sentence length on their speech motor coordination, as evidenced by higher LA variability indices and lower production accuracy scores for the production of more complex, longer sentences. In addition, increasing the syntactic complexity of sentences in the comprehension task yielded longer RTs in both groups of participants. Thus, the present findings clearly demonstrate that speech production and comprehension processes are challenged by increased utterance length and complexity in older, healthy adults. One result, however, revealed differential impairment in individuals with PD; they scored significantly lower than the controls on the two complex conditions of the receptive language task.
Speech Production: Speech Response Latency, LA Variability Index, and Production Accuracy Scores
It is well-documented in the limb motor control literature that individuals with PD not only have difficulty with movement execution but also manifest movement planning deficits resulting in delayed movement onset to achieve specific task goals (e.g., Chan, 1986; Harrington & Haaland, 1991; Rafal, Winhoff, Friedman, & Bernstein, 1987; Stelmach, Worringham, & Strand, 1986). We also found that individuals with PD had longer speech response latencies compared with control participants on the speech production task. Spencer and Rogers (2005) measured speech onset time as a window onto speech motor programming in individuals with hypokinetic dysarthria (including those with PD). Using an RT paradigm, they reported that individuals with PD had slower speech onset times when cued to produce the target—a monosyllabic word—compared with control participants, although the trend of delayed speech onset did not reach statistical significance for syllabic strings. Ho, Iansek, and Bradshaw (2002) also found that individuals with PD had longer speech latencies compared with control participants in an oral counting task.
Contrary to our predictions, the individuals with PD did not demonstrate longer speech response latencies as a result of increasing syntactic complexity. For both groups of participants, the effect of sentence complexity on speech response latency was not significant; however, there was a robust effect of sentence length. Paradoxically, the presentation of the shorter sentences resulted in longer speech response latencies in both groups of participants. In contrast to our findings, Ferreira (1991) showed that increasing the length and syntactic complexity of sentences resulted in longer speech initiation time in healthy young adults. A critical difference, however, is that the participants in Ferreira’s study were required to reproduce the sentence stimuli from memory. In the present study, the sentence was available on the computer screen throughout the trial. Because we used a reading paradigm, it is possible that our participants adopted a “chunking” strategy for speech motor programming—that is, they programmed the longer sentences in “chunks” online as they read them, allowing them to begin speaking the long sentences more rapidly and thus accounting for shorter speech response latencies. Conversely, programming the short sentences as a whole would result in longer speech response latencies due to the greater demand placed on preparatory processes. We acknowledge that this argument is speculative, but our paradigm was substantially different from Ferreira’s (1991) RT study reporting longer RTs for longer sentences in healthy young adults.
The LA variability index captures the consistency of upper lip, lower lip, and jaw coordination for repeated productions of the same utterance. Thus, a higher index reflects higher interarticulatory coordination variability across repeated productions. The individuals with PD had consistently higher LA variability indices compared with the age-matched control participants. One might speculate that increased oral motor coordination variability in the individuals with PD is simply an epiphenomenon of a slower speaking rate (e.g., Crystal & House, 1988; B. L. Smith, Sugarman, & Long, 1983). However, acoustic analyses of speech rate collected from these two groups of participants undertaken for a separate publication showed that the participants with PD (M = 3.71 syllables/s) demonstrated similar speech rates as the control participants (M = 3.60 syllables/s; Walsh & Smith, 2011). Thus, we conclude that the higher LA variability indices are clear evidence of more variable speech motor execution processes in speakers with PD and did not arise from speaking rate differences.
The finding of delayed speech response latencies and higher interarticulatory coordination variability on the speech production task in the PD group could be interpreted as evidence of longer and more variable speech motor planning processes as well. Using a pattern recognition analysis, Kleinow et al. (2001) reported that patterns of lip movement associated with speaking conditions (loud vs. habitual) were less distinctive in individuals with PD compared with a young and an aged control group. However, the individuals with PD and aged control participants had similar values on a variability index for the lower lip motion. This result for a single articulator is in contrast to the present findings regarding interarticulatory coordination in individuals with PD. Kleinow et al. (2001) included only eight participants in their study, five of whom were in the earliest stages of the disease. Furthermore, these researchers analyzed lower lip movement during the production of a simple short phrase, which would be less likely to tax the speech production system (Maner et al., 2000). Because we used an index to capture the consistency in the coordination of multiple articulators to produce more complex stimuli, we believe that the probability of observing differences in performance of the participants with PD was higher in the present study. Our finding of higher variability in articulatory movement coordination is consistent with that of an earlier study by Connor et al. (1989), in which individuals with PD had more variable sequencing of lip and jaw motions to produce oral closure compared with control participants. Thus, examining the more sensitive measure of interarticulatory coordination—especially in more complex production tasks—is more likely to reveal differences between speakers with and without a speech disorder.
For studies of the effects of increasing syntactic complexity, length is always a difficult confound, as more complex sentences tend to be longer. The stimuli in the present study were designed to disambiguate the potential effects of length and complexity. When both groups of speakers produced the longest and/or most syntactically complex sentences, they manifested the greatest degree of articulatory variability and made a greater number of production errors. Kleinow and Smith (2006) reported that as the length and syntactic complexity of sentences increased, oral motor coordination decreased in typically developing children and healthy young adults (also assessed with the LA variability index). We hypothesized that the individuals with PD would be especially vulnerable to increased linguistic demands resulting in disproportionately higher LA indices for the more complex and longer sentences; however, there was no interaction between group and sentence length or sentence complexity. One alternative explanation to consider is that our reading paradigm greatly reduced formulation demands for the complex sentences. It is possible that we would have seen a Complexity × Group interaction if we elicited speech production using an auditory paradigm. However, an auditory task introduces a substantial working memory load, which would confound results, as individuals with PD have deficient working memory capabilities (Gabrieli, Singh, Stebbins, & Goetz, 1996; Kensinger, Shearer, Locascio, Growdon, & Corkin, 2003). Thus, we opted to use a reading task so that our participants would be able to complete the experiment. Clearly, a preferable approach would be to examine speech motor processes in speakers with PD compared with healthy aging individuals using a generative language production task in which the speaker is formulating, planning, and producing speech. The present kinematic analysis cannot be applied to spontaneous speech, but we are currently exploring methods that may allow us to do this in the future.
It is widely accepted that lesions to the basal ganglia resulting from PD disrupt speech production. The pattern of results provides additional evidence of basal ganglia involvement in the programming and coordination of speech movement sequences. On average, the speakers with PD had longer speech response latencies, had less consistently coordinated articulatory movements, and had lower accuracy scores across all sentences on the speech production task. If the basal ganglia played a significant role in the integration of linguistic and motor processes, we should have observed a greater decrement in the performance of the PD group on the syntactically more complex sentences. Although complexity clearly affected coordination for both groups, the relative increases in coordination variability were similar. Thus, the basal ganglia may be more involved at the motor programming and production levels than at the language/speech motor interface, whereas the integration of speech motor and language processes is more likely the function of cortical networks, presumably involving supplementary, premotor, and motor areas. This suggestion is supported by the fact that adults who stutter, compared with their matched controls, do show greater decrements in speech motor performance with increased linguistic demands (Kleinow & Smith, 2000), and neuroimaging studies suggest that the neural bases of stuttering lie in the cortical networks involved in supplementary, premotor, and primary motor speech areas (Salmelin, Schnitzler, Schmitz, & Freund, 2000; Sommer, Koch, Paulus, Weiller, & Buchel, 2002).
One issue to consider when interpreting our findings is that our participants with PD were on the mild-to-moderate end of the disease continuum because they were ambulatory and were living independently. It is possible that more severely affected individuals with PD may show disproportionately larger effects of increased sentence length and complexity. Also, our participants with PD were optimally medicated during the experiment; thus, it is also possible that their dopaminergic medications moderated potential effects on speech production as well.
Sentence Comprehension: Accuracy and RT
The participants with PD had significantly lower accuracy scores than the control group on the subject- and object-relative conditions of the receptive language task (but not on the simple condition). Complex sentence processing deficits in individuals with PD have been substantiated by other studies; however, the basis for the impairment is unclear. Several investigators attribute the finding to deficient language processing—specifically, a grammatical processing deficit (e.g., Cohen, Bouchard, Scherzer, & Whitaker, 1994; Lieberman et al., 1992; Natsopoulos et al., 1991) or slowed lexical retrieval (Angwin et al., 2006). Alternatively, other researchers attribute poorer sentence comprehension to deficient executive functioning—for example, decreased working memory span (e.g., Grossman et al., 2002; Kemmerer, 1999), reduced attention and processing speed (Lee et al., 2003), and impaired set-switching and inhibitory processes (Colman et al., 2006; Hochstadt et al., 2006). We did not include sentence comprehension in our study to disambiguate these hypotheses but, rather, to parallel our speech production measure in order to learn whether increased syntactic complexity similarly affects the integrity of language comprehension and speech production in PD. It appears that slowed processing and/or working memory processes did not play a significant role in our findings. A fundamental difference between these earlier studies and ours is that our sentence comprehension task effectively minimized working memory demands by allowing the sentence to remain on the monitor throughout each trial, so that the participant could reread it as needed to answer the probe. It is evident that the participants took advantage of this, based on the fact that the two complex sentence conditions resulted in significantly longer RTs for both groups. However, whereas the controls adopted a speed/accuracy tradeoff response strategy as they maintained a consistent degree of accuracy across all sentence conditions, the participants with PD were less accurate than the controls on the complex conditions, implicating possible deficiency in basal ganglia/cortical circuitry.
Relationship Between PD Severity Measures and Speech Production and Receptive Language Performance
We did not have reliable access to our participants’ PD severity rating. However, an obvious question of interest is the relationship between the factors indicative of disease severity (i.e., TPO, SMMSE score, age, degree of reduced loudness, articulatory imprecision, and level of speech rate impairment) and performance on comprehension and speech motor tasks. For example, is TPO related to poorer comprehension accuracy? Is interarticulatory coordination variability associated with greater articulatory imprecision? Although we had 16 participants with PD, as an exploratory probe into these questions, we examined the correlations between severity factors and performance on the speech and receptive language tasks. The results from these preliminary analyses, reported in Table 6, confirmed that the dysarthria characteristic of reduced loudness correlated with the LA variability index such that those individuals with PD who were judged to speak more softly were likely to have greater interarticulatory variability. The dysarthria characteristic of articulatory imprecision correlated with production accuracy. Perhaps not surprisingly, the individuals with PD who were judged to have less precise articulation also had greater difficulty producing fluent, error-free productions on the speaking task. TPO also correlated with production accuracy but, unexpectedly, not with any other variable. Age and the dysarthria characteristic of rate impairment were not associated with any of the performance variables. Interestingly, several speech production variables correlated with comprehension variables. Greater accuracy on the two complex conditions of the comprehension task was associated with greater accuracy on the speaking task, whereas the participants who were judged to have a greater degree of reduced articulatory precision and reduced loudness also performed more poorly on the complex conditions of the sentence comprehension task. These preliminary results point to potentially important relationships between production and comprehension processes in individuals with PD.
Finally, it is estimated that approximately 40% of individuals with PD suffer symptoms of depression (for a review, see Reijnders, Ehrt, Weber, Aarsland, & Leentjens, 2008), which could conceivably confound performance, especially on timed tasks. Although depression was not formally evaluated, we documented that seven of 16 of our participants with PD reported taking antidepressants (see Table 1). We found that there was no difference between the group taking antidepressants and the group not taking antidepressants with respect to their response time on the speaking task and their response time or accuracy on the sentence comprehension task.
The disease processes of PD clearly affect motor control, including the control and execution of articulatory movements for speech production. We found that both speech production and language comprehension were adversely affected in the majority of our participants who had mild-to-moderate PD. We observed a constant level of deficit on speech motor planning and production regardless of syntactic complexity, suggestive of a general disease factor impacting speech production. Increasing the syntactic complexity of the receptive language task resulted in decreased accuracy in the PD group, which may be indicative of cognitive deficiency. It is important to note, however, that not all of our participants with PD were impaired on all measures of comprehension and speech motor performance. There was a range of individual differences within the group of participants with PD. The heterogeneity in the course of the degenerative processes of PD is the likely explanation of differences in task performance and suggests that studies of speech and language parameters in this group of participants with neurological disorders must include enough participants so that the full range of their behavior is observed.
Acknowledgments
This article is based on a dissertation submitted by the first author in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Neuroscience and Speech, Language, and Hearing Sciences from Purdue University. Support for this research was made possible through National Institute on Deafness and Other Communication Disorders Grant F31DC007267-01, Indiana Lions Club Grant 67313533762, and Parkinson’s Awareness Association of Central Indiana Grant 201116. We wish to express our sincere appreciation to each of our participants; without their efforts, this research would not have been possible. We would like to acknowledge the guidance and input of Jessica Huber, Bob Meisel, and Chris Weber-Fox. Finally, we are grateful to Janna Berlin, Lisa Beehler, and Jessie Grskovic for their help with data collection and analysis.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Angwin AJ, Chenery HJ, Copland DA, Murdoch BE, Silburn PA. Self-paced reading and sentence comprehension in Parkinson’s disease. Journal of Neurolinguistics. 2006;19:239–252. [Google Scholar]
- Burn D. Parkinson’s disease: An overview. The Pharmaceutical Journal. 2000;264:333–337. Retrieved from www.pharmj.com/Editorial/20000226/education/parkinsons1.html. [Google Scholar]
- Chan CW. Could Parkinsonian akinesia be attributable to a disturbance in the motor preparatory process? Brain Research. 1986;386:183–196. doi: 10.1016/0006-8993(86)90155-1. [DOI] [PubMed] [Google Scholar]
- Cohen H, Bouchard S, Scherzer P, Whitaker H. Language and verbal reasoning in Parkinson’s disease. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1994;7:166–175. [Google Scholar]
- Colman K, Koerts J, van Beilen M, Leenders KL, Bastiaanse R. The role of cognitive mechanisms in sentence comprehension in Dutch speaking Parkinson’s disease patients: Preliminary data. Brain and Language. 2006;99:120–121. [Google Scholar]
- Connor NP, Abbs JH, Cole KJ, Gracco VL. Parkinsonian deficits in serial multiarticulate movements for speech. Brain. 1989;112:997–1009. doi: 10.1093/brain/112.4.997. [DOI] [PubMed] [Google Scholar]
- Crosson B, Benefield H, Cato MA, Sadek JR, Moore BA, Wierenga CE, Briggs RW. Left and right basal ganglia and frontal activity during language generation: Contributions to lexical, semantic, and phonological processes. Journal of the International Neuropsychological Society. 2003;9:1061–1077. doi: 10.1017/S135561770397010X. [DOI] [PubMed] [Google Scholar]
- Crystal TH, House AS. A note on the variability of timing control. Journal of Speech and Hearing Research. 1988;31:497–502. doi: 10.1044/jshr.3103.497. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Darkins A, Mendez M, Hill MA, Benson DF. Alzheimer’s disease and Parkinson’s disease: Comparison of speech and language alterations. Neurology. 1988;38:680–684. doi: 10.1212/wnl.38.5.680. [DOI] [PubMed] [Google Scholar]
- Ferreira F. Effects of length and syntactic complexity on initiation times for prepared utterances. Journal of Memory and Language. 1991;30:210–233. [Google Scholar]
- Friederici AD, Kotz SA, Werheid K, Hein G, von Cramon DY. Syntactic comprehension in Parkinson’s disease: Investigating early automatic and late integrational processes using event-related brain potentials. Neuropsychology. 2003;17:133–142. [PubMed] [Google Scholar]
- Gabrieli JDE, Singh J, Stebbins GT, Goetz CG. Reduced working memory span in Parkinson’s disease: Evidence for the role of a frontostriatal system in working and strategic memory. Neuropsychology. 1996;10:322–332. [Google Scholar]
- Grossman M. Sentence processing in Parkinson’s disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carvell S, Gollomp S, Stern MB, Vernon G, Hurtig HI. Sentence comprehension and praxis deficits in Parkinson’s disease. Neurology. 1991;41:1620–1626. doi: 10.1212/wnl.41.10.1620. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Lee C, Alsop D, Detre J, Hurtig HI. Grammatical and resource components of sentence processing in Parkinson’s disease: An fMRI study. Neurology. 2003;60:775–781. doi: 10.1212/01.wnl.0000044398.73241.13. [DOI] [PubMed] [Google Scholar]
- Grossman M, Zurif E, Lee C, Prather P, Kalmanson J, Stern MB, Hurtig HI. Information processing speed and sentence comprehension in Parkinson’s disease. Neuropsychology. 2002;16:174–181. doi: 10.1037//0894-4105.16.2.174. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY. Sequencing in Parkinson’s disease. Abnormalities in programming and controlling movement. Brain. 1991;114:99–115. [PubMed] [Google Scholar]
- Ho A, Iansek R, Bradshaw JL. The effect of a concurrent task on Parkinsonian speech. Clinical and Experimental Neuropsychology. 2002;24:36–47. doi: 10.1076/jcen.24.1.36.972. [DOI] [PubMed] [Google Scholar]
- Ho A, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of people with Parkinson’s disease. Behavioural Neurology. 1998;11:131–137. [PubMed] [Google Scholar]
- Hochstadt J, Nakano H, Lieberman P, Friedman J. The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain and Language. 2006;97:243–257. doi: 10.1016/j.bandl.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Illes J, Metter EJ, Hanson WR, Iritani S. Language production in Parkinson’s disease: Acoustic and linguistic considerations. Brain and Language. 1988;33:146–160. doi: 10.1016/0093-934x(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. The intensity of thought: Pupillometric indices of sentence processing. Canadian Journal of Experimental Psychology. 1993;47:310–339. doi: 10.1037/h0078820. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. (1996, October 4);274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kemmerer D. Impaired comprehension of raisingto- subject constructions in Parkinson’s disease. Brain and Language. 1999;66:311–328. doi: 10.1006/brln.1999.2022. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Shearer DK, Locascio JJ, Growdon JH, Corkin S. Working memory in mild Alzheimer’s disease and early Parkinson’s disease. Neuropsychology. 2003;17:230–239. doi: 10.1037/0894-4105.17.2.230. [DOI] [PubMed] [Google Scholar]
- King J, Just MA. Individual differences in syntactic processing: The role of working memory. Journal of Memory and Language. 1991;30:580–602. [Google Scholar]
- King J, Kutas M. Who did what and when? Using work- and clause-level ERPs to monitor working memory usage in reading. Journal of Cognitive Neuroscience. 1995;7:376–395. doi: 10.1162/jocn.1995.7.3.376. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. Journal of Speech, Language, and Hearing Research. 2000;43:548–559. doi: 10.1044/jslhr.4302.548. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Potential interactions among linguistic, autonomic, and motor factors in speech. Developmental Psychobiology. 2006;48:275–287. doi: 10.1002/dev.20141. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A, Ramig LO. Speech motor stability in IPD: Effects of rate and loudness manipulations. Journal of Speech, Language, and Hearing Research. 2001;44:1041–1051. doi: 10.1044/1092-4388(2001/082). [DOI] [PubMed] [Google Scholar]
- Lashley KS. The problem of serial order in behavior. In: Jefress LA, editor. Cerebral mechanisms in behavior. New York, NY: Wiley; 1951. pp. 112–146. [Google Scholar]
- Lee C, Grossman M, Morris J, Stern MB, Hurtig HI. Attentional resource and processing speed limitations during sentence processing in Parkinson’s disease. Brain and Language. 2003;85:347–356. doi: 10.1016/s0093-934x(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Levelt WJ. Speaking: From intention to articulation. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral Brain Science. 1999;22:1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Lieberman P. Human language and our reptilian brain. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Lieberman P. Human language and our reptilian brain. Perspectives in Biology and Medicine. 2001;44:32–51. doi: 10.1353/pbm.2001.0011. [DOI] [PubMed] [Google Scholar]
- Lieberman P, Kako E, Friedman J, Tajchman G, Feldman LS, Jiminez EB. Speech production, syntax comprehension, and cognitive deficits in Parkinson’s disease. Brain and Language. 1992;43:169–189. doi: 10.1016/0093-934x(92)90127-z. [DOI] [PubMed] [Google Scholar]
- Logan KJ, Conture EG. Selected temporal, grammatical, and phonological characteristics of conversational utterances produced by children who stutter. Journal of Speech, Language, and Hearing Research. 1997;40:107–120. doi: 10.1044/jslhr.4001.107. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Maner KJ, Smith A, Grayson L. Influences of utterance length and complexity on speech motor performance in children and adults. Journal of Speech, Language, and Hearing Research. 2000;43:560–573. doi: 10.1044/jslhr.4302.560. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Miyake A, Just MA, Carpenter PA. Working memory constraints on the resolution of lexical ambiguity: Maintaining multiple interpretations in neural contexts. Journal of Memory and Language. 1994;22:175–202. [Google Scholar]
- Molloy DW. The Standardized Mini-Mental Status Exam (SMMSE) Troy, Ontario, Canada: New Grange Press; 1999. [Google Scholar]
- Murdoch BE. Subcortical brain mechanisms in speech and language. Folia Phoniatrica et Logopaedica. 2001;53:233–251. doi: 10.1159/000052679. [DOI] [PubMed] [Google Scholar]
- Murray LL. Spoken language production in Huntington’s and Parkinson’s diseases. Journal of Speech, Language, and Hearing Research. 2000;43:1350–1366. doi: 10.1044/jslhr.4306.1350. [DOI] [PubMed] [Google Scholar]
- Murray LL. Language and Parkinson’s disease. Annual Review of Applied Linguistics. 2008;28:113–127. [Google Scholar]
- Murray LL, Lenz LP. Productive syntax abilities in Huntington’s and Parkinson’s diseases. Brain and Cognition. 2001;46:213–219. doi: 10.1016/s0278-2626(01)80069-5. [DOI] [PubMed] [Google Scholar]
- Natsopoulos D, Katsarou Z, Bostantzopoulou S, Grouios G, Mentenopoulos G, Logothetis J. Strategies in comprehension of relative clauses by Parkinsonian patients. Cortex. 1991;27:255–268. doi: 10.1016/s0010-9452(13)80130-x. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Winhoff A, Friedman JH, Bernstein E. Programming and execution of sequentialmovements in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50:1267–1273. doi: 10.1136/jnnp.50.10.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig LO, Fox C, Sapir S. Speech and voice disorders in Parkinson’s disease. In: Olanow W, Stocchi F, Lang A, editors. Parkinson’s disease: Non-motor and non-dopaminergic features. Chichester, United Kingdom: Wiley-Blackwell; (in press). [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Movement Disorders. 2008;23:183–189. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Thomson F. Working with dysarthric clients: A practical guide to therapy for dysarthria. Tucson, AZ: Communication Skill Builders; 1987. [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund HJ. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–1202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Sapienza CM, Stathopoulos ET. Speech task effects on acoustic and aerodynamic measures of women with vocal nodules. Journal of Voice. 1995;9:413–418. doi: 10.1016/s0892-1997(05)80203-6. [DOI] [PubMed] [Google Scholar]
- Silverman SW, Bernstein Ratner NB. Syntactic complexity, fluency, and accuracy of sentence imitation in adolescents. Journal of Speech, Language, and Hearing Research. 1997;40:95–106. doi: 10.1044/jslhr.4001.95. [DOI] [PubMed] [Google Scholar]
- Smith A, Goffman L. Interaction of language and motor factors in speech production. In: Maasen B, Kent RD, Peters HFM, Peters PHHM, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford, United Kingdom: Oxford University Press; 2004. pp. 225–252. [Google Scholar]
- Smith A, Johnson M, McGillem C, Goffman L. On the assessment of stability and patterning of speech movements. Journal of Speech, Language, and Hearing Research. 2000;43:277–286. doi: 10.1044/jslhr.4301.277. [DOI] [PubMed] [Google Scholar]
- Smith A, Zelaznik HN. The development of functional synergies for speech motor coordination in childhood and adolescence. Developmental Psychobiology. 2004;45:22–33. doi: 10.1002/dev.20009. [DOI] [PubMed] [Google Scholar]
- Smith BL, Sugarman MD, Long SH. Experimental manipulation of speaking rate for studying temporal variability. The Journal of the Acoustical Society of America. 1983;74:744–749. doi: 10.1121/1.389860. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Buchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Rogers MA. Speech motor programming in hypokinetic and ataxic dysarthria. Brain and Language. 2005;94:347–366. doi: 10.1016/j.bandl.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Worringham CJ, Strand EA. Movement preparation in Parkinson’s disease: The use of advance information. Brain. 1986;109:1179–1194. doi: 10.1093/brain/109.6.1179. [DOI] [PubMed] [Google Scholar]
- Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25(7):37–42. [PubMed] [Google Scholar]
- Walsh B, Smith A. Evidence of a down-scaling of speech in Parkinson’s disease. 2011 Manuscript in preparation. [Google Scholar]
- Walsh B, Smith A, Weber-Fox C. Short-term plasticity in children’s speech motor systems. Developmental Psychobiology. 2006;48:660–674. doi: 10.1002/dev.20185. [DOI] [PubMed] [Google Scholar]
- Weismer G, Jeng JY, Laures J, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica. 2001;52:201–219. doi: 10.1159/000052649. [DOI] [PubMed] [Google Scholar]
- Westbury JR, Hashi M. Lip-pellet positions during vowels and labial consonants. Journal of Phonetics. 1997;25:405–419. [Google Scholar]
- Yorkston KM, Beukelman DR. An analysis of connected speech samples of aphasic and normal speakers. Journal of Speech and Hearing Disorders. 1980;45:27–36. doi: 10.1044/jshd.4501.27. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ. A review of the cognitive and behavioral sequelae of Parkinson’s disease:Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]