Abstract
Constriction and dilation of large arteries of brain regulates cerebral vascular resistance and cerebral microvascular pressure, which play key roles in regulation of cerebral circulation. We investigated the effect of ischemic stroke on vascular reactivity of middle cerebral artery (MCA) using a rat transient focal cerebral ischemia model. Focal cerebral ischemia was induced by 1 hour MCA occlusion followed by reperfusion. MCAs were dissected from ischemic or contralateral hemisphere at 2 days or 2 weeks post reperfusion and mounted on 2 glass micropipettes for assessment of vascular reactivity. MCAs from brains of sham surgeries were used as control. At 2 days post reperfusion, a significant alteration of myogenic reactivity was found in MCAs dissected from both ischemic and non-ischemic hemispheres, which could still be identified at 2 weeks after reperfusion. Phenylephrine (PE) induced remarkable vasoconstriction in MCAs from animals that underwent sham surgery. No significant alteration of vasoconstrictive response to PE was found in MCAs isolated from either ischemic or contralateral hemisphere at 2 days or 2 weeks after ischemic stroke, as compared with MCAs from sham animals. Acetylcholine (ACh) induced mild dilation in normal MCAs, which was reversed in MCAs from both ischemic and non-ischemic hemispheres at 2 weeks after ischemic stroke. Sodium nitroprusside (SNP) induced vasodilation in MCAs from animals with sham operation, which was diminished in MCAs from both ischemic and non-ischemic hemisphere at 2 days and 2 weeks after ischemic stroke. These results demonstrated that focal cerebral ischemia could induce long-term global cerebral vasculature dysfunction.
Keywords: Cerebral ischemia, stroke, vasculature, myogenic tone, vasoreactivity
INTRODUCTION
Ischemic stroke, comprising over 80% of total stroke cases, is caused by an embolus or in situ thrombus in a cerebral artery which induces ischemic cascades that ultimately leads to irreversible brain tissue damage [1, 2]. The effort to develop effective therapies for ischemic stroke has achieved several important successes related thrombolytic therapy [3]. In addition, great effort has been invested to decipher the cellular mechanisms induced by ischemic stroke and to further the quest for neuroprotective therapy [2]. Surprisingly, relatively few studies have addressed mechanisms of vascular protection in terms of ischemic stroke [4, 5].
As a cerebral vascular event, ischemic stroke induces not only a complex array of pathogenic cellular cascades in brain parenchyma, but also impairment of autoregulation in brain vasculature. Previous studies have demonstrated that transient focal cerebral ischemia affects myogenic response of cerebrovasculature at acute stage after ischemic stroke [6–8]. The myogenic response, of healthy cerebral arteries, is characterized by the constriction of these arteries to pressure and contributes significantly to autoregulation in cerebral circulation. Thus, impairment of myogenic response of cerebral vasculature could have a significant effect on cerebral blood flow (CBF) and brain function. In the present study, we investigated the long-term effect of ischemic stroke on myogenic tone of middle cerebral artery (MCA) and its reactivity in response to vasoactive agents using a transient MCA occlusion model in rats. Our study demonstrated that focal cerebral ischemia could induce long-term cerebral vasculature dysfunction in both ischemic and non-ischemic hemispheres.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (body weight: 225 to 250g) were housed in a facility under 12:12 hour light/dark conditions and maintained on a standard diet and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee and carried out under the guidelines of the Guide for the Care and Use of Laboratory Animals.
Experimental focal cerebral ischemia and isolated artery preparation
Focal cerebral ischemia was induced by transient middle cerebral artery occlusion (MCAO) as described previously [9]. Animals were anesthetized by intraperitoneal (i.p.) injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Rectal temperature was maintained at 37.0 ± 0.5 °C during stroke procedure. For MCAO, the left MCA was occluded by a 3–0 monofilament suture introduced via the internal carotid artery. After 1 hour, the suture was withdrawn for reperfusion. For sham control, sham surgeries were performed without insertion of the suture.
At 2 days or 2 weeks after stroke, rats were anesthetized with ketamine/xylazine (90 mg/kg and 10 mg/kg, i.p.). They were then bled out by closed chest cardiac puncture, decapitated, and their brain removed and placed in Krebs buffer at 4 °C. The MCAs from both ischemic and contralateral hemispheres were removed with the aid of a dissecting microscope. The proximal end of MCA closest to the bifurcation was manipulated onto a glass micropipette and securely tied with 11–0 surgical nylon suture. The working physiology saline solution-cerebral artery (WPSS-CA) was allowed to flow into the vessel in the original direction of flow. The vessel was pressurized to 60 mmHg, lengthened to a length approximate to its in situ length, and checked for leaks. All side vessels were secured with suture and complete closure was confirmed by the stable maintenance of pressure. The vessel baths holding the cannulated MCAs were placed on inverted microscopes equipped with imaging system and micro caliper measuring device. A branch-free section of each vessel was chosen for the measurement site. Isolated arteries were allowed to warm-up to 37 °C and equilibrate at 60 mmHg intraluminal pressure for 45–60 minutes, with the bathing media changed every 15 minutes. Following warm-up period, vessels were subjected to WPSS-80K for 1–2 minutes and their contractility noted. Vessels that did not contract were considered non-viable and were not used. WPSS-80K was washed out and replaced with WPSS-CA and the vessels were allowed to equilibrate before beginning the vessel reactivity analysis in response to vasoconstrictor and vasodilators. Luminal diameter, wall thickness, and pressure were monitored continuously throughout the experiment [10, 11].
MCA Reactivity Analysis
Vessels were exposed to vasoconstrictor phenylepherine (PE) (10−9 to 10−4 M), endothelium-dependent dilator acetylcholine (ACh) (10−9 to 10−4 M), as well as endothelium-independent dilator sodium nitroprusside (SNP) (10−9 to 10−4 M). Each drug was administered in whole log doses to determine the dose-response relationship for each vessel to the various vasoactive chemicals. Between dose-response curves vessels were washed 3 times at 15 minute intervals with WPSS-CA. After the last wash, vessels were allowed to equilibrate for at least 5 minutes before exposure to the next drug. Before exposure to ACh and SNP vessels that did not spontaneously attain 70% tone were pre-constricted using PE. After completion of the dose-response curves the bathing media was removed and replaced with calcium-free media containing caffeine (1.94 mg/ml) and thapsigargin (TG, 1 μM). Vessels were allowed to bathe in this media for 1 hour in order to remove all stored calcium and properly assess their maximum calcium-free diameter. Calcium-free diameter reflects the maximum possible diameter for a given pressure and is limited only by vessel structure.
Drugs and Solutions
The Krebs buffer contained the following (in mM): NaCl 131.5, KCl 5.0, NaH2PO4 1.2, MgCl2*6H2O 1.2, CaCl2*2H2O 2.5, pH = 7.4. WPSS-CA buffer contained the following: NaCl 145.0, KCl 4.7, CaCl2 2.0, MgSO4 1.17, MOPS 3.0, KH2PO 41.25, Glucose 8.0, Pyruvate 3.0, NaHCO3 25, pH= 7.4 at 37 °C. The 80 mM KCl contraction solution (WPSS-80K) was composed of following (in mM): NaCl 65, KCl 84.7, CaCl2*2H2O 2.0, MgSO4*7H2O, MOPS 3.0, NaH2PO4 1.2, Glucose 5.0, pyruvate 2.0, EDTA 0.02, pH= 7.4 at 37 °C. Calcium free solution contained the following (in mM): NaCl 147.0, KCl 4.7, MgSO4 41.17, MOPS 3.0, NaH2PO4 1.2, Glucose 5.0, Pyruvate 2.0, EDTA (di salt) 2.0, pH= 7.4 at 37 °C. Phenylephrine, acetylcholine and sodium nitroprusside were all diluted from their stock solutions in WPSS-CA. Caffeine was diluted in calcium-free WPSS-CA. Thapsigargin was diluted in ethanol such that in the bath the final concentration of ethanol was not more than 1% of the total bath volume. All chemicals and drugs were purchased from Sigma (St. Louis, MO, USA).
Data Calculations and Statistical Analysis
Spontaneous myogenic tone was calculated as percent increase of wall thickness or change of lumen diameter from the fully relaxed vessels at baseline by the equation: [(wall thickness at baseline– wall thickness in WPSS) / wall thickness at baseline] × 100 or [(lumen diameter in baseline-lumen diameter in WPSS)/lumen diameter at baseline] × 100. Vessel dilation was calculated as percent possible dilation by the equation: [(lumen diameter after response - preconstricted lumen diameter)/(lumen diameter at calcium free - preconstricted lumen diameter)] × 100, where the preconstricted lumen diameter was the lumen diameter before the application of the drug for that curve. Values are presented as mean ± S.E.M. Differences between groups were determined by ANOVA and considered significant at p<0.05.
RESULTS
Transient focal cerebral ischemia induces long-term changes in myogenic reactivity of MCAs in both ischemic and non-ischemic hemispheres
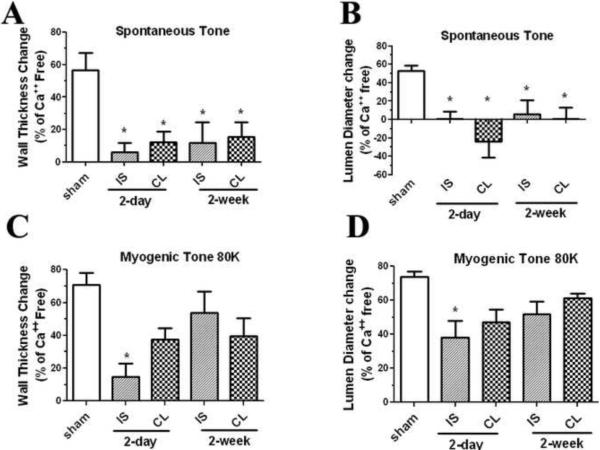
In the first experiments, we investigated the effect of transient focal cerebral ischemia on myogenic tone of MCAs. Prior to the addition of WPSS-80K, lumen diameter and outside diameter of each vessel were measured. The lumen diameters of MCAs from sham controls (n=5) were significantly smaller than that of ischemic and contralateral MCAs at 2 days (n=6) (Figure 1) as well as 2 weeks post-reperfusion (n=7). Conversely, the wall thicknesses of MCAs from sham animals were much larger than that of MCAs from ischemic and contralateral hemisphere at either 2 days or 2 weeks post reperfusion. Myogenic tone was calculated in terms of both wall thickness change, as well as lumen diameter change. A significant lack of spontaneous myogenic tone was observed in MCAs from both ischemic and non-ischemic hemispheres at 2 days and 2 weeks following ischemic stroke (Figure 2A & B). On the other hand, only a mild impairment of myogenic tone of MCAs from ischemic hemisphere was found at 2 days after ischemic stroke when 80 mM KCl contraction solution was applied (Figure 2C & D).
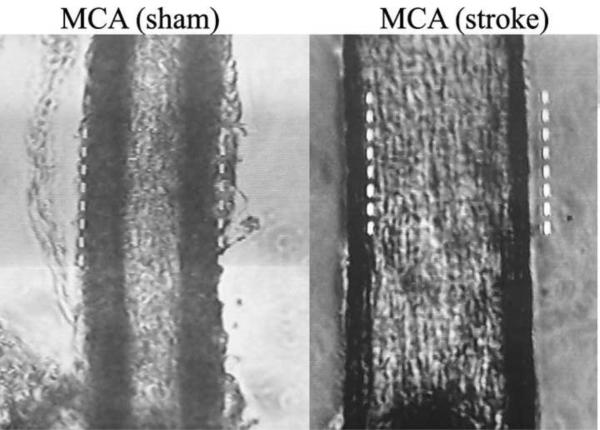
Figure 1.
Microscopic images of MCAs from rats at 2 days after sham surgery or transient MCAO.
Figure 2.
Effect of transient focal cerebral ischemia on myogenic properties of MCAs from sham-operated and ischemic stroke rats at 2 days or 2 weeks after transient MCAO. Myogenic tone was calculated in terms of wall thickness and lumen diameter change as: (Calcium free – spontaneous) / calcium free × 100. Significant impairment of spontaneous myogenic tone of MCAs from both ischemic (IS) and non-ischemic (CL) hemispheres was observed at 2 days and 2 weeks after ischemic stroke (A, B). Only a mild impairment of myogenic tone of MCAs from the ischemic hemisphere was found at 2 days after ischemic when 80 mM KCl solution (80K) was applied (C, D). Sham: n=5; 2 days ischemic MCA: n=6; 2 days non-ischemic MCA: n=6; 2 weeks ischemic MCA: n=7; 2 weeks non-ischemic MCA: n=7. * p<0.05 vs sham.
Transient focal cerebral ischemia induces long-term changes in reactivity to vasoactive agents in MCAs of both ischemic and non-ischemic hemispheres
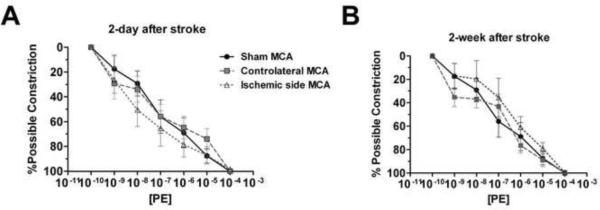
When the vessels were exposed to increasing doses of vasoconstrictor PE, a dose-dependent vasoconstriction was observed in MCAs derived from sham stroke and stroke animals at 2 days or 2 weeks after stroke. No significant difference was found between MCAs from sham controls and MCAs from either ischemic or the contralateral hemisphere of stroke animals at 2 days or 2 weeks post-reperfusion (Figure 3A and B).
Figure 3.
Dose-response curve of MCAs from sham or stroke animals to phenylephrine (PE) at 2 days (A, sham: n=5; ischemic MCA: n=6; non-ischemic MCA: n=6) and 2 weeks (B, sham: n=5; ischemic MCA: n=7; non-ischemic MCA: n=7) after transient MCAO. PE induced a similar dose-dependent vasoconstriction in MCAs from sham animals (Sham MCA), ischemic (Ischemic side MCA), or contralateral hemisphere (Contralateral MCA).
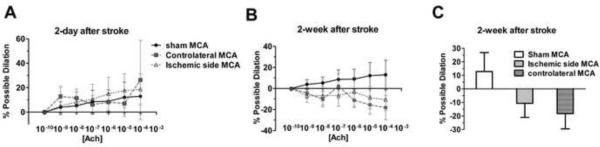
A mild dilation was induced by ACh in MCAs from sham stroke and stroke animals at 2 days after stroke (Figure 4A). On the other hand, the dilatory response to ACh was replaced by a mild dose-dependent constriction in MCAs from both ischemic and contralateral hemispheres of stroke rats at 2 weeks after reperfusion (Figure 4B). At 2 weeks after ischemia/reperfusion injury, the possible dilation induced by the highest dose of ACh (10−4 M) was 12.89 ± 13.96% in MCAs from sham, −10.69 ± 10.34% and −18.26 ± 11.29% in MCAs from ischemic and non-ischemic hemisphere, respectively (Figure 4C).
Figure 4.
Dose-response curve of MCAs from sham animals, ischemic side, and contralateral side to acetylcholine (ACh) at 2 days (A, sham: n=5; ischemic MCA: n=6; non-ischemic MCA: n=6) and 2 weeks (B & C, sham: n=5; ischemic MCA: n=7; non-ischemic MCA: n=7) after transient MCAO. Instead of dilation, ACh induced a mild dose-dependent constriction in the MCAs from both the ischemic and contralateral hemispheres at 2 weeks after stroke (B). At 2 weeks after ischemic stroke, the highest dose of ACh (10−14 M) induced 12.89 ± 13.96% dilation in MCAs from sham controls (sham MCA), −10.69 ± 10.34% and −18.26 ± 11.29% constriction in the MCAs from the ischemic (ischemic side MCA) and non-ischemic hemispheres (contralateral MCA), respectively.
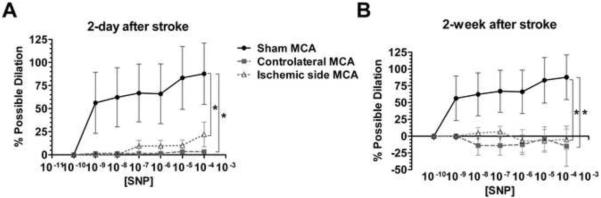
We further determined the effect of ischemic stroke on SNP-induced cerebrovascular dilation. SNP induced a robust vasodilation in MCAs from sham controls, which was markedly diminished in MCAs from both ischemic and non-ischemic hemispheres of stroke animals at both 2 days and 2 weeks post-reperfusion (Figure 5A and B).
Figure 5.
Dose-response curve of MCAs from sham animals, ischemic side, and contralateral side to sodium nitroprusside (SNP) at 2 days (sham: n=5; ischemic MCA: n=6; non-ischemic MCA: n=6) and 2 weeks (sham: n=5; ischemic MCA: n=7; non-ischemic MCA: n=7) after transient MCAO. SNP induced a robust vasodilation in MCAs from the sham controls (Sham MCA), which was almost non-existent in MCAs from ischemic (Ischemic side MCA) and non-ischemic (Contralateral side MCA) hemisphere at 2-days (A) and 2-weeks (B) after ischemic stroke. * p<0.05 vs Sham MCA.
DISCUSSION
Our current study has identified two important findings. First, ischemic stroke induces long-term changes in myogenic tone of MCAs at both ischemic and non-ischemic hemispheres. Second, ischemic stroke induces long-term changes in reactivity to vasoactive agents in MCAs at both ischemic and non-ischemic hemispheres. These findings may provide insight for the development of novel therapies stroke treatment.
Increasing evidence has demonstrated that transient focal cerebral ischemia affects myogenic tone not only in local cerebrovasculature but also contralateral vasculature at acute stage after ischemic stroke [6–8]. In the present study, we determined myogenic response of MCAs at 2 days or 2 weeks after experimental ischemic stroke as magnetic resonance imaging study has indicated that postischemic hyperperfusion peaked at 2 days and resolved 1 week after ischemic stroke using a similar model [12]. Our result concurs with the previous publications, demonstrating a bilateral vasculature dysfunction after transient focal cerebral ischemia [6–8, 13, 14]. In addition, our study identified a long-term impairment of myogenic response in MCAs after focal cerebral ischemia.
Focal cerebral ischemia induced not only impairment of myogenic response but also a change in reactivity to vasoactive agents in MCAs. Constriction and dilation of large arteries in the brain regulates cerebral vascular resistance and cerebral microvascular pressure [15]. Diminished cerebral arterial responsiveness to vasoconstrictor 5-HT, abolition of endothelium-dependent relaxation, and diminished distensibility of cerebral vasculatures have been observed after transient focal cerebral ischemia in experimental stroke models [6, 14]. In the present study, MCAs from both ischemic and contralateral hemispheres of stroke animals had similar dilation responses to ACh as MCAs from sham controls at 2 days post-reperfusion. However, at 2 weeks post-reperfusion, both ischemic and contralateral MCAs from stroke animals showed a lesser dilatory response than MCAs from sham controls. Interestingly, the dilative action of SNP, an endothelium-independent vasodilator induces a robust dilation in normal MCAs, was significantly diminished in MCAs after transient focal cerebral ischemia, suggesting that an endothelium-independent mechanism could also contribute to the ischemic stroke-induced vasculature dysfunction. Consistently, studies have shown that loss of myogenic activity after ischemic stroke was associated with a decrease in filamentous actin in vascular smooth muscle [14, 16]. In ischemic stroke patients, SNP treatment has been found to improve local CBF given at a dose which reduced mean arterial blood pressure by 10 mmHg [17]. In the present ex vivo analysis, we have identified a markedly diminishment of vasodilative action of SNP in MCAs after ischemic stroke. Further in vivo studies are needed to validate our finding and its significance in term of ischemic stroke.
Maintenance of constant CBF as a result of autoregulation caused by a combination of myogenic, neuronal, and metabolic mechanisms is critical for brain function [15, 18]. Thus, it might not be surprising that pathological conditions such as ischemic stroke could disrupt neurovascular coupling and consequently induce cerebral vasculature dysfunction in the ischemic hemisphere. Increasing evidence has indicated occlusion of a cerebral artery could induce both hemodynamic and cellular change beyond the ischemic territory [19]. During focal cerebral artery occlusion, the circulatory system can compensate for the reduction of focal CBF using collateral circulation and autoregulatory mechanisms, which causes hemodynamic perturbations and a redistribution of CBF [20] [21]. Flow territory maps in patients of internal carotid artery occlusion showed significant differences in flow territories of contralateral internal carotid artery and vertebrobasilar arteries compared with those in control subjects [22]. In experimental stroke models, transient unilateral hemodynamic stress has found to induce a transient increase in CBF velocities in the opposite hemisphere and delayed cleavage of neuronal caspase 3 in both ischemic and contralateral side cortices, although their cause-effect relationship at the non-ischemic side remained unknown. [23]. In addition, endothelial dysfunction has been found in the peripheral mesenteric resistance artery after experimental ischemic stroke in rats [24]. It is till unclear how focal cerebral ischemic damage induces a global vasculature dysfunction. A recent study has indicated that ischemic stroke could trigger an inflammatory process and an increase of cytokine expression in plasma, which might contribute to the impairment of vasculature function [8].
The acute damage of myogenic reactivity and autoregulatory capacity of cerebral vasculature after ischemic stroke might contribute to reperfusion injury and edema formation at the ischemic territory [5, 25]. On the other hand, the long-term impairment of autoregulation of global cerebral vasculature might not only make the patients prone to recurrence of ischemic stroke but also contribute to the progression of vascular dementia after ischemic stroke. Stroke is the first leading cause of long-term disability in the United States. Epidemiological studies have shown that the prevalence of dementia in ischemic stroke patients is 4 to 12 times higher than controls [26]. In addition, a progressive course of cognitive function decline has been suggested [27–30]. Brain function is tightly coupled with CBF autoregulation and thus cognitive function is by default coupled to the functional capacity of brain's autoregulatory capabilities. Cognitive and sensorimotor stimulation induces increases in CBF that are associated with increased metabolic demand [31]. Ischemic stroke-induced long-term vascular dysfunction might compromise the coupling between the metabolic requirements of brain and CBF, thereby affecting cognitive function. Indeed, evidence shows that drugs used for the treatment of systemic vascular disorders such as hypertension have effects on cerebral vasculature function and modify the progression of dementia [32].
In summary, the present study demonstrates that transient focal cerebral ischemia could induce long-term impairment of spontaneous myogenic tone in MCAs at both ischemic and non-ischemic areas. The loss of dilatory responses of MCAs to ACh and SNP suggest that both endothelial-dependent and -independent mechanisms could contribute to the global vascular dysfunction induced by ischemic stroke. Our study places further emphasis on studies of vascular protection for the treatment of ischemic stroke. Chronic vascular protection through enhancement of endothelial function and suppression of thrombosis has been a traditional approach for primary and secondary prevention of stroke [5]. Understanding the molecular mechanisms underlying the long-term effect of ischemic stroke on cerebral vasculature could eventually lead to development of more effective therapeutics for both acute treatment of ischemic stroke and prevention of vascular dementia after stroke.
Acknowledgements
Supported partly by NIH grants R01NS054687 (SY), R01NS054651 (SY), R01DK079968 (RM), JCT was a postdoctoral trainee supported by T32 AG020494.
Reference
- 1.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 2.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M, Schaebitz W. An overview of acute stroke therapy: past, present, and future. Arch Intern Med. 2000;160:3196–3206. doi: 10.1001/archinte.160.21.3196. [DOI] [PubMed] [Google Scholar]
- 4.Faraci FM. Vascular protection. Stroke. 2003;34:327–329. doi: 10.1161/01.str.0000054052.52510.2c. [DOI] [PubMed] [Google Scholar]
- 5.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 6.Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–180. doi: 10.1161/01.str.28.1.176. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–2099. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez-Altayo F, Martin A, Rojas S, Justicia C, Briones AM, Giraldo J, Planas AM, Vila E. Transient middle cerebral artery occlusion causes different structural, mechanical, and myogenic alterations in normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H628–635. doi: 10.1152/ajpheart.00165.2007. [DOI] [PubMed] [Google Scholar]
- 9.Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH. 17beta-Estradiol attenuates blood-brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol. 2008;586:1649–1667. doi: 10.1113/jphysiol.2007.149567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor JC, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic and neuropeptide Y Y1 receptor control of collateral circuit conductance: influence of exercise training. J Physiol. 2008;586:5983–5998. doi: 10.1113/jphysiol.2008.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Q, Du F, Huang S, Duong TQ. Spatiotemporal characteristics of postischemic hyperperfusion with respect to changes in T1, T2, diffusion, angiography, and blood-brain barrier permeability. J Cereb Blood Flow Metab. 2011;31:2076–2085. doi: 10.1038/jcbfm.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr. Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 14.Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- 15.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 16.Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke. 2006;37:894–899. doi: 10.1161/01.STR.0000204043.18592.0d. [DOI] [PubMed] [Google Scholar]
- 17.Butterworth RJ, Cluckie A, Jackson SH, Buxton-Thomas M, Bath PM. Pathophysiological assessment of nitric oxide (given as sodium nitroprusside) in acute ischaemic stroke. Cerebrovasc Dis. 1998;8:158–165. doi: 10.1159/000015842. [DOI] [PubMed] [Google Scholar]
- 18.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 19.Liu RYH, Yuan F, Yang SH. Neuroprotection trageting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurological Research. 2012 doi: 10.1179/1743132812Y.0000000020. in press. [DOI] [PubMed] [Google Scholar]
- 20.Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. doi: 10.1161/01.STR.0000086465.41263.06. [DOI] [PubMed] [Google Scholar]
- 21.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Laar PJ, Hendrikse J, Klijn CJ, Kappelle LJ, van Osch MJ, van der Grond J. Symptomatic carotid artery occlusion: flow territories of major brain-feeding arteries. Radiology. 2007;242:526–534. doi: 10.1148/radiol.2422060179. [DOI] [PubMed] [Google Scholar]
- 23.Villapol S, Bonnin P, Fau S, Baud O, Renolleau S, Charriaut-Marlangue C. Unilateral blood flow decrease induces bilateral and symmetric responses in the immature brain. Am J Pathol. 2009;175:2111–2120. doi: 10.2353/ajpath.2009.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Revelles S, Jimenez-Altayo F, Caracuel L, Perez-Asensio FJ, Planas AM, Vila E. Endothelial dysfunction in rat mesenteric resistance artery after transient middle cerebral artery occlusion. J Pharmacol Exp Ther. 2008;325:363–369. doi: 10.1124/jpet.107.134619. [DOI] [PubMed] [Google Scholar]
- 25.Kuroiwa T, Nagaoka T, Ueki M, Yamada I, Miyasaka N, Akimoto H. Different apparent diffusion coefficient: water content correlations of gray and white matter during early ischemia. Stroke. 1998;29:859–865. doi: 10.1161/01.str.29.4.859. [DOI] [PubMed] [Google Scholar]
- 26.Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, Remien RH, Williams JB, Mohr JP, Hauser WA, et al. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology. 1992;42:1185–1193. doi: 10.1212/wnl.42.6.1185. [DOI] [PubMed] [Google Scholar]
- 27.Henon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–1222. doi: 10.1212/wnl.57.7.1216. [DOI] [PubMed] [Google Scholar]
- 28.Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 2002;33:2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- 29.Altieri M, Di Piero V, Pasquini M, Gasparini M, Vanacore N, Vicenzini E, Lenzi GL. Delayed poststroke dementia: a 4-year follow-up study. Neurology. 2004;62:2193–2197. doi: 10.1212/01.wnl.0000130501.79012.1a. [DOI] [PubMed] [Google Scholar]
- 30.Yang SH, Shetty RA, Liu R, Sumien N, Heinrich KR, Rutledge M, Thangthaeng N, Brun-Zinkernagel AM, Forster MJ. Endovascular middle cerebral artery occlusion in rats as a model for studying vascular dementia. Age (Dordr) 2006;28:297–307. doi: 10.1007/s11357-006-9026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moody M, Panerai RB, Eames PJ, Potter JF. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1581–1588. doi: 10.1152/ajpregu.00837.2004. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson J. Cerebrovascular structure and dementia: new drug targets. Trends Pharmacol Sci. 2001;22:630–635. doi: 10.1016/s0165-6147(00)01866-6. [DOI] [PubMed] [Google Scholar]