Abstract
Proteomic studies of post-translational modifications by metal affinity or antibody-based methods often employ data-dependent analysis, providing rich data sets that consist of randomly sampled identified peptides because of the dynamic response of the mass spectrometer. This can complicate the primary goal of programs for drug development, mutational analysis, and kinase profiling studies, which is to monitor how multiple nodes of known, critical signaling pathways are affected by a variety of treatment conditions. Cell Signaling Technology has developed an immunoaffinity-based LC-MS/MS method called PTMScan Direct for multiplexed analysis of these important signaling proteins. PTMScan Direct enables the identification and quantification of hundreds of peptides derived from specific proteins in signaling pathways or specific protein types. Cell lines, tissues, or xenografts can be used as starting material. PTMScan Direct is compatible with both SILAC and label-free quantification. Current PTMScan Direct reagents target key nodes of many signaling pathways (PTMScan Direct: Multipathway), serine/threonine kinases, tyrosine kinases, and the Akt/PI3K pathway. Validation of each reagent includes score filtering of MS/MS assignments, filtering by identification of peptides derived from expected targets, identification of peptides homologous to expected targets, minimum signal intensity of peptide ions, and dependence upon the presence of the reagent itself compared with a negative control. The Multipathway reagent was used to study sensitivity of human cancer cell lines to receptor tyrosine kinase inhibitors and showed consistent results with previously published studies. The Ser/Thr kinase reagent was used to compare relative levels of kinase-derived phosphopeptides in mouse liver, brain, and embryo, showing tissue-specific activity of many kinases including Akt and PKC family members. PTMScan Direct will be a powerful quantitative method for elucidation of changes in signaling in a wide array of experimental systems, combining the specificity of traditional biochemical methods with the high number of data points and dynamic range of proteomic methods.
The development of efficacious compounds to fight diseases including cancer, developmental defects, neurodegenerative disease, infectious disease, and metabolic disorders is an area of intense focus in both academic and industrial laboratories. An understanding of the cellular signaling pathways underlying these various disease states is critical to effective drug development programs, both in predicting response to compounds and in anticipating off target effects. Post-translational modification of signaling proteins involved in these pathways is a critical factor in determination of activity, localization, and protein-protein interactions in disease as well as other experimental systems such as protein overexpression, knockdown, or studies of the effects of tissue microenvironment.
Decades of work have provided insight into some of the mechanisms underlying various disease states, such as the dependence on tyrosine kinase activity for growth and survival of some cancer types (1–6). The fact that some cancers initially controlled by a single tyrosine kinase can develop resistance to inhibition of that kinase (2, 6–10) lends credence to the idea that it is the synthesis of inputs from many different pathways that controls disease progression (11–13). Methods that quantitatively monitor changes in these pathways and their respective signaling molecules will be ideal for the study of disease progression and drug development.
Genetic methods have long been available to profile many genes or whole genomes simultaneously, such as comparative genomic hybridization arrays, single-nucleotide polymorphism analysis, or whole genome sequencing (14–19). These methods have the disadvantage that many changes observed at the genetic level do not necessarily affect progression of the disease (so-called passenger mutations). Quantitative proteomic methods represent a more direct measure of changes that affect various disease states and can therefore be complementary or preferable to genetic methods.
In the past, the study of protein activity in complex diseases and cellular signaling pathways has either focused on a few proteins known to be critical to the system being studied or has employed proteomic methods that provide rich data sets that randomly sample the proteome. The detailed study of one or a few specific proteins has the advantage of focusing on known pathway components but suffers from an inability to sample many data points from complex systems. Previous proteomic analyses using liquid chromatography-tandem mass spectrometry (LC-MS/MS)1 have allowed simultaneous profiling of many thousands of proteins and post-translational modifications but can suffer from a lack of specificity (20–33), for example the difficulty of effectively profiling tyrosine phosphorylation using whole phosphoproteome methods such as immobilized metal affinity chromatography (28, 29, 34–38). These methods tend to sample the more abundant proteins present in a sample, whereas critical signaling may occur through proteins expressed at exquisitely low levels.
The use of antibodies to immunoprecipitate post-translationally modified peptides allows for more complete analysis of a group of related peptides, such as those sharing a consensus phosphorylation motif (39–41) or against a particular post-translational modification such as ubiquitin or acetylated lysine (21, 23, 25–27). Employing these antibody-based strategies has yielded many insights into signaling pathways and key regulators of disease (4, 21, 42–44). Current methods allow for simultaneous identification and quantification of thousands of post-translationally modified peptides across serine, threonine, and tyrosine phosphorylation, as well as ubiquitination, neddylation, ISGylation (ISG15 modification), and acetylation (www.cellsignal.com/services). A limitation of these strategies is that the list of peptides to be quantified is variable (data-dependent) and based upon factors such as the cell line or tissue type profiled, treatment conditions, motif antibody employed, and duty cycle limitations of the mass spectrometer. Development of methods that focus on a defined set of peptides from important signaling proteins would bypass this limitation and provide complimentary data to traditional data-dependent proteomic analysis.
To address this need, a novel antibody-based method, called PTMScan Direct, has been developed for identification and quantitation of post-translationally modified peptides. This method, rather than targeting specific peptide sequence motifs, targets peptides derived from proteins that are critical signaling nodes of various pathways or derived from a single protein type, such as kinases. Four PTMScan Direct reagents have been generated to date, including PTMScan Direct: Multipathway, Ser/Thr Kinase, Akt/PI3K Pathway, and Tyr Kinase reagents. The Multipathway reagent detects core proteins of many different cell signaling pathways, such as Akt signaling, MAP kinase signaling, cell cycle regulation, apoptosis, and transforming growth factor-β signaling, among others. The Ser/Thr Kinase reagent profiles over 300 phosphorylation sites on 130 serine and threonine kinases. The Tyr Kinase reagent detects over 600 tyrosine phosphorylation sites on 120 tyrosine kinases and PI3Ks. The Akt/PI3K Pathway reagent detects 296 phosphorylation sites on 105 proteins involved in Akt and PI3K-dependent signaling (more detailed information for each reagent is available at http://www.cellsignal.com/services/direct_overview.html).
The proteins and corresponding sites detected by each reagent have been determined by a validation strategy that includes profiling human cancer cell lines and mouse tissues. The utility of PTMScan Direct is demonstrated with an investigation of signaling changes in human cancer cell lines in response to receptor tyrosine kinase inhibition using the Multipathway reagent, as well as profiling of kinase phosphorylation in mouse tissues using the Ser/Thr Kinase reagent. Together, this work demonstrates that PTMScan Direct is a powerful method that combines the high number of data points assayed and sensitivity of LC-MS/MS analysis with the specificity of antibody-based methods, allowing quantitative profiling of hundreds of data points that is focused on the signaling pathway or pathways of interest.
EXPERIMENTAL PROCEDURES
Overview
PTMScan Direct is adapted from the PhosphoScan method developed at Cell Signaling Technology (39). The method and reagent validation strategy are outlined in Fig. 1.
Fig. 1.
PTMScan Direct method and validation strategy. The method is an adaptation of the original PhosphoScan method (39). Cell lines or tissues are harvested under denaturing conditions, digested with trypsin, desalted over C18, and dried under vacuum. Peptides are resuspended and immunoprecipitated with the appropriate PTMScan Direct reagent or with empty protein G beads. Immunoprecipitated peptides are desalted over C18 and run on an LTQ-Orbitrap Velos hybrid mass spectrometer. MS/MS spectra are assigned with SEQUEST 3G and SORCERER 2 v.4.0 (46), and relative quantitation is from the chromatographic peak apex intensity or integrated peak area in the MS1 channel. Validated target lists are assembled by score filtering, search for intended targets, search for peptides homologous to intended targets, a minimum MS1 peak intensity filter, and PTMScan Direct reagent dependence.
Cell Lines and Tissues
HCT-116, HeLa, NCI-H1703, NCI-H1299, Jurkat, and A549 cells were from the American Type Culture Collection (Manassas, VA). MKN-45 cells were from DSMZ (German Collection of Microorganisms and Cell Cultures). NCI-H3255 cells were from Dr. Lewis Cantley (Harvard Medical School, Boston, MA). The cells were cultured in the appropriate media supplemented with 10% fetal bovine serum and penicillin/streptomycin at 37 °C with 5% CO2. Mouse liver and brain were obtained from mature BALB/c mice. Mouse embryos were harvested at day 16 from BALB/c mice. Sodium pervanadate treatment was for 20 min with 50 ng/ml. All imatinib (Novartis), gefitinib (AstraZeneca), and SU11274 (Sigma, catalog number S9820) treatments were for 2.5 h with 1 μm of indicated compound. The same volume of DMSO was used as a control. 10 mg of protein from each cell line or tissue was used for a single immunoprecipitation. For the pervanadate-treated cell line mixture, 10 mg of protein from the mix of all cell lines was used for a single immunoprecipitation. 10 mg corresponds to ∼1 × 108 cells for cell lines or roughly 200 mg of wet weight for tissue samples, although absolute amounts are cell line- and tissue-specific.
Cell Lysate Preparation
Adherent cells were washed once with cold PBS. PBS was removed, and the cells were scraped in urea lysis buffer (9 m sequanol grade urea, 20 mm HEPES, pH 8.0, 1 mm β-glycerophosphate, 1 mm sodium vanadate, 2.5 mm sodium pyrophosphate). Suspension cells were centrifuged at 1,100 rpm in a Rotanta 460R clinical centrifuge (Hettich) for 5 min, rinsed with PBS, and resuspended in urea lysis buffer. The cells were sonicated three times for 20 s each at 15-W output power with a 1-min cooling on ice between each burst. Sonicated lysates were centrifuged 15 min at 4 °C at 13,000 rpm (∼20,000 × g in a Beckman JA25.50 rotor). An aliquot of each supernatant was reserved for Western blotting and stored at −80 °C. Supernatants were collected and reduced with 4.5 mm DTT for 30 min at 55 °C. Reduced lysates were alkylated with iodoacetimide (0.095 g/5 ml of H2O) for 15 min at room temperature in the dark. The samples were diluted 1:4 with 20 mm HEPES, pH 8.0, and digested overnight with 10 μg/ml trypsin-tosylphenylalanyl chloromethyl ketone (Worthington) in 1 mm HCl. Digested peptide lysates were acidified with 1% TFA, and peptides were desalted over 360-mg SEP PAK Classic C18 columns (Waters, catalog number WAT051910). Peptides were eluted with 40% acetonitrile in 0.1% TFA, dried under vacuum, and stored at −80 °C.
Immunoprecipitation
PTMScan Direct reagents were produced by a combination of individual antibodies produced and tested at Cell Signaling Technology. Each reagent contains a unique mixture of antibodies that can immunoprecipitate the peptides of interest for the pathway or protein type targeted. Saturating amounts of the indicated PTMScan Direct reagents were bound to 50 μl of packed protein G-agarose beads (Roche Applied Science) overnight at 4 °C. Lyophilized peptides were resuspended in MOPS immunoaffinity purification buffer (50 mm MOPS, pH 7.2, 10 mm KH2PO4, 50 mm NaCl) and centrifuged for 5 min at 12,000 rpm in a MiniSpin microcentrifuge (Eppendorf). Supernatants were mixed with PTMScan Direct reagent bead slurries 2.5 h at 4 °C. The beads were pelleted by centrifugation for 30 s at 5,400 rpm in a MiniSpin microcentrifuge at 4 °C. The beads were washed twice with 1 ml of MOPS immunoaffinity purification buffer and four times with 1 ml of water (Burdick and Jackson). Peptides were eluted from beads with 0.15% TFA (sequential elutions of 65 μl followed by 55 μl, 10 min each at room temperature). Eluted peptides were desalted over tips packed with Empore C18 (Sigma) and eluted with 60% acetonitrile in 0.1% TFA. Eluted peptides were dried under vacuum.
LC-MS/MS Analysis
Immunoprecipitated peptides were resuspended in 0.125% formic acid and separated on a reversed phase C18 column (75-μm inner diameter × 10 cm) packed into a PicoTip emitter (∼8 μm inner diameter) with Magic C18 AQ (100 Å × 5 μm). Peptides were eluted using a 45-, 72-, or 90-min linear gradient of acetonitrile in 0.125% formic acid delivered at 280 nl/min. Tandem mass spectra were collected in a data-dependent manner with an LTQ-Orbitrap Velos mass spectrometer running XCalibur 2.0.7 SP1 using a top twenty MS/MS method, a dynamic repeat count of one, and a repeat duration of 30 s. Real time recalibration of mass error was performed using lock mass (45) with a singly charged polysiloxane ion m/z = 371.101237. The data associated with this manuscript may be downloaded from ProteomeCommons Tranche network using the following hash code: d8z1DpepUqH1NsYdXJJ3yJrtrMsuDC3FZdkx1gClzt2pd4imrhifdGVRSfUzJUtKmiqTWss8D/y/wOG88nW7PLDM+Y8AAAAAAAAB8g==.
MS/MS spectra were evaluated using SEQUEST 3G and the SORCERER 2 platform from Sage-N Research (v4.0, Milpitas CA) (46). All of the labeled MS/MS spectra can be viewed by clicking on the MS2 spectrum numbers in the qualitative tabs of supplemental Tables S1–S6. Human cell lines were searched against the NCBI Homo sapiens FASTA database updated on September 6, 2010 (release 43) containing 39,112 sequences. Mouse samples were searched against the NCBI Mus musculus FASTA database updated on September 6, 2010 (release 43) containing 36,483 sequences. A mass accuracy of ±50 ppm was used for precursor ions and 1 Da for product ions. Enzyme specificity was limited to trypsin, with at least one tryptic (Lys- or Arg-containing) terminus required per peptide and up to four miscleavages allowed. Cysteine carboxamidomethylation was specified as a static modification, oxidation of methionine residues was allowed, and phosphorylation was allowed on serine, threonine, and tyrosine residues. Reverse decoy databases were included for all searches to estimate false positive rates and filtered using a 5% false discovery rate in the Peptide Prophet module of SORCERER 2. The results were further narrowed by mass accuracy based on clustering of forward and reverse assignments in Xcorr versus mass error plots. Typically forward database assignments cluster within ±5 ppm of calculated m/z, so results were limited to peptides that fall within that range. A larger mass error range (±50 ppm) was used for the searches to allow for identification even if the lock mass signal was not adequate for accurate mass calibration. Peptides were also manually filtered using reagent-specific criteria. For the multipathway and Akt/PI3K reagents, the results were limited to peptides known to be targeted by the reagent and peptides homologous to those targets. For the Ser/Thr Kinase and Tyr Kinase reagents, the results were limited to the kinase class being targeted. For peptides not originally targeted by the reagent, the results were further limited to peptides that were at least 3-fold higher intensity in samples immunoprecipitated with protein G beads plus PTMScan Direct reagent than immunoprecipitation of the same sample with empty protein G beads.
All of the quantitative results were generated using proprietary software and were produced from either the apex peak height or peak area of the corresponding peptide assignments according to previously published protocols (42, 44). Quantification was based on a single peptide ion for each protein/site. The software incorporates a chromatographic alignment (or time warping) algorithm that performs multiple binary comparisons to generate an overall clustering strategy for the complete data set of all identified peptides on the basis of a mass precision (within ± 5 ppm) and a retention time deviation threshold (within ± 5 min). The clustering algorithm calculates retention time deviations for identical peptides across all replicates and samples and determines the expected retention time shift for a given set of peptides at any given moment in time throughout the chromatogram. Extracted ion chromatograms for peptide ions that changed in abundance between samples were manually reviewed to ensure accurate quantitation. Where necessary, fold changes were normalized using a technique in which the median log2 ratio is set to 0 and all fold changes are adjusted relative to the median.
Data Analysis
Quantitative data was evaluated and clustered in Spotfire DecisionSite (TIBCO Software AB) version 9.1.2. Hierarchical clustering was performed using complete linkage and Euclidean distance. Protein-protein interactions from the STRING database (Version 9.0, string.embl.de) (47) were limited to the experimental and database interaction categories with a minimum score of 0.700 (referred to as “high scoring” interactions in STRING). Kinase-substrate relationships were identified from the substrate search page of PhosphoSitePlus (www.phosphosite.org).
Western Blotting
Protein concentration for lysate supernatants was determined by Bradford assay using Coomassie Plus Protein Assay Reagent (Pierce), and protein amounts were normalized between samples. Samples were mixed with SDS-PAGE sample buffer (buffer 7723;Cell Signaling Technology) and run on 4–20% gradient Tris-glycine gels (Invitrogen). Proteins were transferred to nitrocellulose (Millipore) and blocked for 1 h in 5% nonfat dry milk (Sigma) in TBS. Primary antibodies were incubated in 5% BSA in TBS plus 0.1% Tween 20 (TBS-T) overnight at 4 °C. The membranes were washed three times with TBS-T, incubated with anti-rabbit or anti-mouse secondary antibody (antibodies 5366 and 5470; Cell Signaling Technology) for 1 h at room temperature in 5% milk TBS-T, washed three times with TBS-T, dried, and developed on the Odyssey nearly infrared imaging system (LI-COR). All of the antibodies used were from Cell Signaling Technology. For gel stains, gels were washed three times with deionized H2O, stained with GelCode blue stain reagent (Thermo Scientific; reagent 24592), and destained with deionized H2O.
RESULTS
PTMScan Direct Reagent Validation
PTMScan Direct reagents were designed to probe cellular signaling pathways and/or protein classes. The PTMScan Direct: Multipathway reagent targets critical signaling nodes from more than 20 cellular signaling pathways. The second reagent, PTMScan Direct: Ser/Thr Kinase, targets sites of modification on serine/threonine kinases that affect kinase activity, localization, or protein-protein interaction. More recently, PTMScan Direct reagents have been created that target the Akt-PI3K pathway (PTMScan Direct: Akt/PI3K Pathway) and tyrosine kinases (PTMScan Direct: Tyr Kinase). New reagents are under development to target other pathways such as DNA damage, cell cycle, apoptosis, and autophagy. More information for each reagent and sample data can be found at http://www.cellsignal.com/services/direct_overview.html.
The validation strategy is outlined in Fig. 1. The various reagents were used to immunoprecipitate peptides from a mixture of human cell line lysates (HCT-116, NCI-H1299, HeLa, A549, Jurkat, and MKN-45) treated with pervanadate to artificially increase levels of phosphorylation through inhibition of tyrosine phosphatase activity (48, 49). The cell line mix was also immunoprecipitated with empty protein G beads (no antibody). In addition to passing score filtering criteria in SORCERER 2 (SAGE-N), peptides had to be at least 3-fold more abundant in the PTMScan Direct eluate than in the beads alone eluate (thus reagent-dependent) to be considered validated. Typical fold changes were much higher than 3-fold, as seen in the quantitative tab of supplemental Table S1. The median log2 fold change between PTMScan Direct: multipathway reagent immunoprecipitation and beads alone immunoprecipitation was 6.87, corresponding to a 116.6-fold change. The analytical reproducibility of the assay is also shown, with a median % coefficient of variation of 5.1 between analytical replicates of the PTMScan Direct: multipathway sample. A minimum signal intensity of 20,000 was required in a validation run for inclusion of the peptide in the final, validated list.
Reagents were designed to immunoprecipitate specific peptides, and search results were compared with the list of targeted proteins/sites and further filtered to include only peptides from proteins/sites initially targeted or peptides with sequence homology to initial targets. Multiple replicate analyses of the cell line mix allowed assembly of a list of validated human peptide targets for each reagent. This same strategy was used with the three mouse tissues (liver, brain, and embryo) to create lists of validated mouse peptide targets for each reagent. A representative table for the human 6-cell mixture with associated MS/MS scoring metrics is provided for each reagent (supplemental Tables S1–S4). For each table, the number of total MS/MS spectra, number of spectra passing score filtering (5% peptide prophet cutoff), number of phosphopeptides, and number of validated peptides are given. In each case, the number of validated peptides represents a small percentage of the total number of spectra (less than 5%), reflecting the high standard necessary for inclusion in the validated list. Although initially filtered at a 5% false discovery rate, the false discovery rate in the final, validated lists was 0 for all reagents.
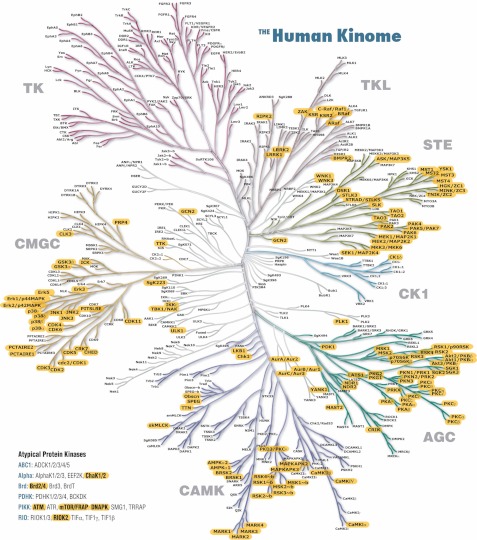
Fig. 2 illustrates PTMScan Direct: Multipathway coverage of the MAP kinase and AKT/PI3K signaling pathways. A partial list of signaling pathways targeted by the reagent is shown at the top of Fig. 2, and the purple-shaded ellipses in the MAP kinase and AKT/PI3K pathways indicate proteins from which peptides are identified using the PTMScan Direct: Multipathway reagent. A full list of proteins and sites detected by the Multipathway reagent is provided in supplemental Table S1. Other highlighted pathways are provided in supplemental File S1. Fig. 3 highlights serine/threonine kinases identified by the PTMScan Direct: Ser/Thr Kinase reagent mapped onto the human kinome tree (50). Proteins highlighted in yellow with bold text are identified by the reagent in human cell lines and/or mouse tissues. Tyrosine kinases, in the top left quadrant of the kinome tree, are detected by the PTMScan Direct: Tyr Kinase reagent. A complete list of validated proteins and sites detected by the Ser/Thr Kinase reagent is available in supplemental Table S2.
Fig. 2.
PTMScan Direct: Multipathway targets. Examples of signaling pathways targeted by the PTMScan Direct: Multipathway reagent are shown at the top. Two pathways, MAP kinase signaling and AKT/PI3K pathway, are shown in detail as examples of pathway coverage by the reagent. The reagent targets proteins highlighted in purple. A list of phosphorylation sites from the targeted proteins is provided in supplemental Table S1.
Fig. 3.
PTMScan Direct: Ser/Thr Kinase reagent coverage of the human kinome. Kinases marked with bold text and yellow highlighting are targeted by the PTMScan Direct: Ser/Thr Kinase reagent. Atypical protein kinases are shown at bottom left. The kinome tree was originally published by Manning et al. (50) and is available for download at www.cellsignal.com.
PTMScan Direct: Multipathway Receptor Tyrosine Kinase Inhibitor Sensitivity Study
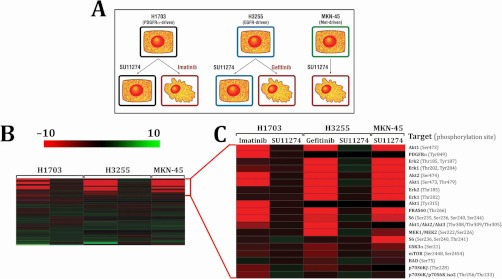
Profiling signaling changes downstream of tyrosine kinase inhibitors used in treatment of cancers and other diseases is a major goal of drug development and discovery programs (6, 7, 51–55). The PTMScan Direct: Multipathway reagent was used to quantify changes in peptide abundance in human cancer cell lines dependent on receptor tyrosine kinase activity for growth (NCI-H1703 dependent on PDGFRα, NCI-H3255 dependent on EGFR, and MKN-45 dependent on Met receptor) (3, 4, 56–59). The cell lines were treated with DMSO as a control or with compounds to which they are known to be sensitive and insensitive, as indicated in Fig. 4A. Label-free quantification was performed using chromatographic peak heights, and fold changes for each drug treatment compared with DMSO control were determined. Relative abundance in this study and all other studies was verified by manual review of each peptide peak in the LCMS chromatogram files. Fold changes across all cell lines and treatments were input into Spotfire DecisionSite (TIBCO Software AP) and sorted using Euclidean distance and complete linkage hierarchical clustering. Fig. 4B shows the complete clustering result, in which each row represents a different protein/site identified by the multipathway reagent and each column a different cell line/treatment. Fig. 4C is a zoomed in view of the top rows of Fig. 4B focused on the peptides that decreased the most with a given inhibitor treatment. The more intense the red color of the cell, the larger the decrease in abundance with compound treatment compared with DMSO control. Some protein/sites decreased in intensity in all cell lines with appropriate treatment, such as ribosomal protein S6 Ser-235/Ser-236/Ser-240/Ser-244 and Akt1 Ser-473. Other changes were cell type-specific, such as the decrease in PDGFRα Tyr-849 peptide intensity in NCI-H1703 cells treated with imatinib. This peptide did not change with SU11274 treatment in NCI-H1703 cells and was not observed in the other two cell lines, because PDGFRα is not expressed/phosphorylated in NCI-H3255 or MKN-45 cells. Qualitative and quantitative data tables for each comparison are provided in supplemental Table S5.
Fig. 4.
PTMScan Direct: Multipathway reagent receptor tyrosine kinase inhibitor sensitivity study. A, experimental design. All of the cell lines were treated with DMSO or 1 μm of the indicated compound for 2.5 h. Irregular cell shapes denote previously reported sensitivity to the indicated compound. B, hierarchical clustering of data collected for all treatments with the three cell lines. Each row represents a different peptide ion in a list made nonredundant by protein/site. Each column represents a different cell line and treatment as indicated in C. Red indicates a decrease in abundance relative to DMSO control, and green indicates an increase. Clustering was performed in Spotfire DecisionSite version 9.1.2 (TIBCO Software AB) using Euclidean distance and complete linkage. C, detailed view of the top rows from B. Cell lines and treatments are indicated at the tops and bottoms of the columns, respectively. The protein name and PhosphoSitePlus phosphorylation site numbers for each peptide are shown at right.
PTMScan Direct: Ser/Thr Kinases Mouse Tissue Profiling
The PTMScan Direct: Ser/Thr Kinase reagent was used to profile mouse liver, brain, and embryo tissue. 5 mg of each tissue was processed with the Ser/Thr Kinase reagent, and peptide ion intensity in each sample was determined using label-free quantification. The highest intensity measurement across the three tissues was set to 1, and the other two tissue intensities were normalized relative to that value. The normalized intensity values were plotted in Spotfire DecisionSite (TIBCO Software AB), with each row representing a different serine/threonine kinase peptide ion and each column representing a different tissue. The more intense the green color of a particular cell, the higher the intensity in that tissue. Hierarchical clustering of intensity data was performed using Euclidean distance and complete linkage and is shown in Fig. 5A. This clustering grouped peptides that were higher in one specific tissue, such as brain (indicated with a blue bracket in Fig. 5A), embryo (indicated with a red bracket in Fig. 5A), or liver (indicated with a green bracket in Fig. 5A). Peptides in other rows were of higher intensity in two tissues than the third or equal among all three tissues. The qualitative and quantitative results from Fig. 5 are provided in supplemental Table S6.
Fig. 5.
PTMScan Direct: Ser/Thr kinase mouse tissue study. Equal amounts of mouse brain, liver, and embryo were probed with the PTMScan Direct: Ser/Thr Kinase reagent. For each peptide ion, the highest peak intensity across the three tissues was set to 1, and the other two intensities were normalized relative to the maximum intensity. The more intense the green shading for a particular cell, the closer the intensity is to the maximum value of 1. Each row represents a different peptide ion made nonredundant by protein/site, and each column is a different tissue. Clustering was performed in Spotfire DecisionSite v.9.1.2 (TIBCO Software AB) using Euclidean distance and complete linkage. Blue bracket, kinase peptides elevated in brain. Red bracket, kinase peptides elevated in embryo. Green bracket, kinase peptides elevated in liver. B, Akt kinase family peptides. Heat map is organized as in A. C, PKC isoforms. D, Western blots of tissues used in PTMScan Direct in A–C. 20 μg of total protein for each tissue was run per lane and blotted with the indicated antibodies. β-Actin was run as a loading control. E, selected Akt isoform phosphopeptides. Peptides from different isoforms can be distinguished by different sequences, MH+ values, charge states, and chromatographic retention times.
The data in Fig. 5A were then organized by kinase family to illustrate relative peptide abundance between tissues for specific serine/threonine kinases. Fig. 5B includes the Akt kinases Akt1, Akt2, and Akt3. Phosphorylated peptides from all three Akt kinases were relatively higher in embryonic tissue than in brain or liver. Akt3 peptides, especially those phosphorylated at Ser-472/Ser-474, were as abundant in brain tissue as in embryo, whereas Akt1 and Akt2 peptides were lower abundance. In liver tissue, Akt2 peptides phosphorylated at Ser-474/Ser-478 were close to the same intensity as in embryo, whereas other Akt-derived phosphopeptides were of much lower intensity in liver.
Phosphopeptides derived from PKC isoforms (Fig. 5C) also showed differential abundance between the mouse tissues. Unlike the Akt phosphopeptides, for which each peptide was of the highest abundance in embryo, some PKC peptides were lower abundance in embryo than other tissues, such as peptides from PKCβ, PKCγ, and PKCε, which were more abundant in brain. Other family members, such as PKCδ and PKCι, were higher abundance in embryonic tissue. Still other family members such as PKCζ appeared to be equally abundant across all three tissues.
Western blotting of the mouse tissue samples used in PTMScan Direct was performed using available Akt and PKC isoform-specific antibodies (Fig. 5D). Equal amounts of total protein were loaded for each tissue type, as indicated by β-actin blot, SDS-PAGE gel stain (Fig. 5D), and Coomassie protein assay (data not shown). Akt isoform expression differed by tissue, with Akt1 highest in embryo, Akt2 more evenly expressed across all three tissues, and Akt3 highest in brain. A pan-Akt antibody, which detects the combined signal from all three isoforms, showed nearly equal signal in brain and embryo and lower signal in liver. Phosphorylation of all three Akt isoforms together at Thr-308 and Ser-473 was also probed. For both sites, the signals in brain and embryo were similar, whereas the intensity in liver was lower. Akt Thr-308 and Ser-473-derived phosphopeptides identified by PTMScan Direct are shown in Fig. 5E. For each site, peptides derived from the various isoforms can be distinguished by their distinct sequences, MH+ values, charge states, and chromatographic retention times. More complete information for each peptide is given in supplemental Table S6.
All of the PKC isoforms showed highest signal in brain tissue by western blot in Fig. 5D. PKCα showed higher relative signal than other isoforms in liver and embryo, and PKCι showed equal expression in brain and embryonic tissue. A phospho-PKC-pan antibody (recognizing all PKC isofoms) against the activation loop site (Thr-411 in PKCζ) was also used and showed highest signal in brain, followed by liver and embryo. Multiple bands were detected by western blot because of different molecular weights of various PKC isoforms.
PTMScan Direct: Akt/PI3K Pathway and Tyr Kinases
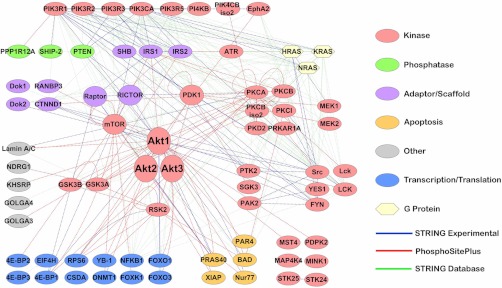
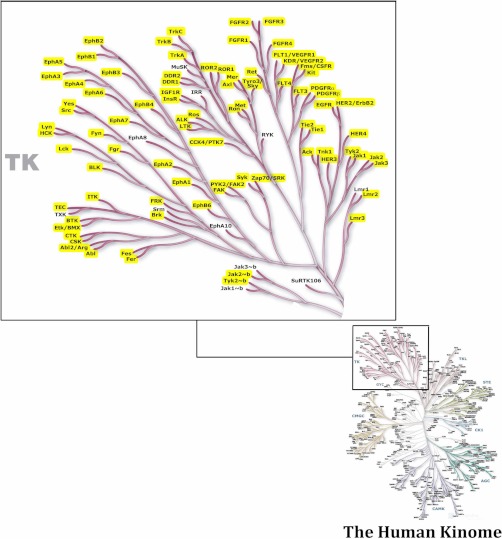
In addition to the two PTMScan Direct reagents previously discussed, two new reagents have been prepared for use in elucidating signaling pathways. As with the Multipathway and Ser/Thr Kinase reagents, these new reagents were validated with both human cancer cell lines and mouse tissues. Fig. 6 and supplemental Table S3 show the proteins detected by the Akt/PI3K Pathway reagent. The interaction list used to produce Fig. 6 was created by combining known interactions found in the STRING database (string.embl.de) and those in the PhosphoSitePlus database (www.phosphosite.org). The interaction map was constructed in Cytoscape version 2.8.1 (60). The protein class information in Fig. 6 was derived from PhosphoSite Plus. The PTMScan Direct: Tyr Kinase reagent can identify and quantify over 600 phosphorylation sites on 120 proteins including receptor tyrosine kinases, cytoplasmic tyrosine kinases, and phosphatidylinositol 3-kinases (Fig. 7 and supplemental Table S4). Fig. 7 focuses on the tyrosine kinase branch of the human kinome tree (50), and kinases for which tyrosine-phosphorylated peptides have been identified and are highlighted in yellow.
Fig. 6.
Interaction Map for PTMScan Direct: Akt/PI3K pathway reagent. Protein-protein interactions were derived from both the STRING database (string.embl.de) (47) using experimental and database lines of evidence and “high scoring” interactions (score > 0.700) and the substrate search function in the PhosphoSitePlus database (www.phosphosite.org). The interaction map was generated using Cytoscape 2.8.1 (www.cytoscape.org) (60). Protein class information is from PhosphoSitePlus.
Fig. 7.
PTMScan Direct: Tyr Kinase reagent kinome coverage. The tyrosine kinase branch of the human kinome tree (50) is shown in detail. Kinases highlighted in yellow are targeted by the Tyr Kinase reagent.
DISCUSSION
PTMScan Direct Reagent Validation
Validation of the peptides and corresponding proteins/sites that can be identified and quantified by each reagent produced by combination of individual antibodies was a critical step in ensuring accuracy and robustness of the reagents. The final, validated lists (shown in Figs. 2, 3, 6, and 7 and supplemental Tables S1–S4) were synthesized from many LC-MS/MS runs and incorporate not only quality of the MS/MS assignments but other factors such as intensity of the chromatographic peptide peak, reagent dependence (lower abundance or absence of the peak in the protein G beads only control sample), whether the protein/site was an initial target, and whether the peptide sequence was homologous to another protein that was an initial target. In some cases, particularly for the PTMScan Direct: Ser/Thr Kinase reagent, the list of validated targets may be an under-representation of what can actually be identified, because not all serine/threonine kinases are expressed in the cell lines/tissues used. At the time of publication, use of the Multipathway reagent in the PTMScan Direct method detects 103 sites on 69 proteins. The Ser/Thr Kinase reagent can detect over 300 sites of phosphorylation on 130 different kinases when used in the PTMScan Direct method. Use of the Akt/PI3K Pathway reagent in the method detects 296 sites on 105 proteins, whereas performing PTMScan Direct with the Tyr Kinase reagent can detect over 600 sites on 120 tyrosine and PI3Ks.
PTMScan Direct: Multipathway Receptor Tyrosine Kinase Inhibitor Sensitivity Study
A main goal of drug discovery and development programs is to profile the response of known, critical cellular signaling pathways to the compound of interest. Often, the response of sensitive and resistant cell lines to a compound or group of compounds is measured to determine mechanism of action as well as potential off target effects. Here, we used the PTMScan Direct: Multipathway reagent to probe receptor tyrosine kinase (RTK) inhibitor-induced changes in signaling in three cell lines whose growth is controlled by RTK activity. NCI-H1703 cell growth is controlled by the RTK PDGFRα (4), NCI-H3255 is dependent on EGFR (58, 59), and MKN-45 is controlled by Met receptor (57, 61). These cell lines were treated with RTK inhibitors to which they should be sensitive and resistant, as outlined in Fig. 4A. NCI-H1703 cells, for example, were treated with imatinib (sensitive) and SU11274 (insensitive).
The heat map in Fig. 4B demonstrates the specificity of the RTK inhibitor treatment as well as the ability of the PTMScan Direct: Multipathway reagent to identify changes in signaling in responsive cell lines. NCI-H1703 cells were sensitive to the PDGFRα inhibitor imatinib but not the Met receptor inhibitor SU11274. Likewise, EGFR-driven NCI-H3255 cells were sensitive to EGFR inhibitor gefitinib but not SU11724. Finally, the Met receptor-driven cell line MKN-45 was sensitive to the Met inhibitor SU11274. Sensitivity here is illustrated by the decrease in peptide abundance with a given treatment (red cells in Fig. 4B).
Further analysis of the data in Fig. 4C demonstrates the specificity of the PTMScan Direct: Multipathway reagent. PDGFRα is only expressed and phosphorylated in NCI_H1703 cells (4), and indeed this is the only cell line in which phosphorylated PDGFRα peptides were identified. Other changes in phosphorylated peptide levels were consistent across all three cell lines, such as the decrease in phosphorylated Akt1 at Ser-473 and Ser-473/Ser-478 and phosphorylated Akt2 at Ser-474 (see first, fifth, and sixth rows of Fig. 4C). Akt1 Ser-473 and Akt2 Ser-474 are critical sites of Akt kinase activation (62–64). The decrease in phosphorylation at these sites is consistent with the observation that Akt signaling is active downstream of receptor tyrosine kinases in these cell lines (4, 43). Phosphorylation of direct substrates of Akt was also affected, as observed for Thr-266 of PRAS40 (65, 66), which decreased in all three cell lines with the appropriate inhibitor treatment. Because the Akt pathway is known to be critical to cellular growth and survival signaling (62, 67–69), these changes may partially explain the previously observed decreases in cell growth caused by the inhibitors (3, 4, 43).
The decrease in extracellular signal-regulated kinase (Erk) phosphorylation observed for both singly phosphorylated and dually phosphorylated Erk1 and Erk2 (see third, fourth, seventh, and eighth rows of Fig. 4C) was more pronounced in NCI-H3255 and MKN-45 cells than in NCI-H1703 cells. This is because Erk 1/2 expression levels are lower in NCI-H1703 cells than the other two cell lines (43). Together, these data demonstrate that changes in critical signaling pathways in RTK-dependent cancer cell lines correlates with sensitivity of the cell lines to particular inhibitors. Previous work has established the effect of these inhibitors on growth of the cell lines (3, 4, 43) and is supported by the changes observed in Fig. 4.
PTMScan Direct: Ser/Thr Kinases Mouse Tissue Profiling
The PTMScan Direct: Ser/Thr Kinase reagent was used to profile relative levels of serine/threonine kinase phosphopeptide abundance in mouse liver, brain, and embryo. Because phosphorylation of many of these sites correlates with kinase activity, the levels of the phosphopeptides can sometimes be used to estimate relative kinase activity. The wide variance of phosphopeptide abundance between tissues in Fig. 5A shows the diversity of kinase expression and activity in these three tissues. The highest level of phosphopeptide abundance for many of the peptides (red bracket in Fig. 5A) was found in embryonic tissue, which is expected given the high level of growth and developmental programs active in this tissue relative to adult tissue (70). There are, however, some kinase peptides that were more abundant in brain tissue (see peptides within the blue bracket in Fig. 5A) and yet others that were higher in liver (green bracket in Fig. 5A).
Specific kinase families are shown in Fig. 5 (B and C) to demonstrate the power of PTMScan Direct in elucidating relative expression/activation of kinase family members that may be difficult or impossible to distinguish by other methods such as western blotting. The Akt family consists of three members, Akt1, Akt2, and Akt3 (www.phosphosite.org). In this study all three members were expressed and phosphorylated at both critical sites (Ser-473 and Thr-308) (62–64, 68, 69, 71) in embryonic tissue. Akt3 phosphopeptides were relatively higher in brain tissue than Akt1 or Akt2. Specifically, peptides containing the critical Ser-472 site of Akt3 were more abundant in brain. This correlates well with known biology of Akt3 in brain, because Akt3 is necessary for normal brain development and is expressed in adult brain tissues (72–75). Akt2 Ser-474 and Ser-474/Ser-478 peptides were higher in liver than other Akt peptides. Akt2 is expressed in the liver (75–77), although the differential abundance of Ser-474-containing peptides and Thr-308-containing peptides perhaps indicates a difference in activation state of Akt2 in liver as well.
The PKC family contains at least 11 members (www.phosphosite.org). A number of PKC isoforms were detected by the Ser/Thr Kinase reagent in mouse tissue, including PKCα, PKCβ, PKCβ iso2, PKCγ, PKCδ, PKCδ iso2, PKCε, PKCη, PKCι, and PKCζ. Phosphopeptides from these PKC isoforms encompass both the activation loop of the kinases (phosphopeptides including residues 408–531 in Fig. 5C), critical for kinase activity, as well as a C-terminal domain known to be important for kinase localization, activity, and protein-protein interaction (residues 655–729 in Fig. 5C) (78, 79). Some PKC isoform phosphopeptides identified by PTMScan Direct are higher in a specific tissue, such as PKCβ, γ, and ε in the brain, and PKCδ, η, and ι in the embryo. PKCζ-derived phosphopeptides, by contrast, are more equally abundant across all three tissues. Again, these observations correlate well with known biology, as PKCβ, γ, and ε are known to be highly expressed in brain tissue, whereas PKCζ is widely expressed across many tissues (80).
Western blotting of the same mouse tissue samples, shown in Fig. 5D, confirmed the differences in expression and phosphorylation of Akt and PKC isoforms observed using PTMScan Direct. Using the Ser/Thr kinase reagent, Akt1 phosphopeptide abundance was highest in embryo, and indeed Akt1 expression is highest in embryo. Akt2 Ser-474 phosphopeptides were closer in abundance to embryonic levels than other Akt peptides in liver, and Akt2 signal in Fig. 5D was closer in liver and embryo than it was for Akt 1 or Akt3. Akt3 peptides were higher in brain relative to embryo than Akt1 or Akt2 peptides, and the western blots showed that Akt3 was expressed at the highest level in brain. These results highlight an advantage of PTMScan Direct over traditional western blotting, in that the total and phospho-Akt Thr-308 and Ser-473 signals in Fig. 5D represent the coalescence of signal for Akt1, Akt2, and Akt3, whereas the sequence specificity of LC-MS/MS analysis in PTMScan Direct allows the behavior of the individual isoforms to be tracked independently, in this case highlighting the relative differences in Akt2 phosphorylation in liver and Akt3 phosphorylation in brain. Fig. 5E shows Akt phosphopeptides identified in the LCMS run. Akt1, Akt2, and Akt3 phosphopeptides could be distinguished by their different amino acid sequences, MH+ values, charge states, and chromatographic retention times.
As with the Akt isoforms, the behavior of individual PKC isoforms was obscured in a western blot using an antibody that detects the activation loop site of all PKC isoforms (phospho-PKC-pan Thr-410 in Fig. 5D). Here the combined signal of all isoforms showed higher phosphorylation in brain than in liver or embryo. When blots were performed with the various PKC isoform-specific antibodies, expression was indeed higher in brain than the other two tissues. However, two isoforms with phosphopeptides more abundant in embryo than brain, PKCδ and PKCι, showed higher relative expression in embryo than the other isoforms. In fact, expression of PKCι was roughly equivalent in brain and embryo. This again illustrates the utility of PTMScan Direct in that it can differentiate between highly related proteins such as protein kinase isoforms based on minor sequence differences. This is an improvement over traditional methods that either cannot make those distinctions, such as western blotting, or require more involved experimentation, such as immunoprecipitation-western or two-dimensional gel analysis.
The new PTMScan Direct reagents shown in Fig. 6 and supplemental Table S3 (Akt/PI3K Pathway) and Fig. 7 and supplemental Table S4 (Tyr Kinase) will provide even more targeted information for critical signaling proteins. Whereas the Multipathway reagent focuses on a few central nodes of the Akt/PI3K pathway such as Akt, mTOR, and ribosomal protein S6, the Akt/PI3K Pathway reagent provides a much more complete picture of Akt/PI3K-dependent signaling, including activation of upstream components such as PTEN and PI3K; activity of core pathway components such as Akt, mTOR, Raptor, and Rictor; and activation of downstream substrates such as GSK3, PRAS40, XIAP, Foxo1, Foxo3, Bad, and others.
Combined with the Ser/Thr Kinase reagent, the PTMScan Direct: Tyr Kinase reagent will allow simultaneous detection and quantitation of peptides containing critical modification sites of 250 serine, threonine, tyrosine, and phosphatidylinositol kinases. These reagents target phosphorylation sites both within the activation loop of the kinases, which are critical for kinase activity, and also other modification sites on the kinase(s) of interest outside of the activation loop. Changes in phosphorylation of these other sites may affect protein stability, localization, or protein-protein interaction, which can be equally important in determining kinase activity.
Summary
PTMScan Direct has been used in preliminary validation studies, investigation of RTK inhibitor sensitivity in human cancer cells, and mouse tissue profiling. In all of these studies, PTMScan Direct provided quantitative data for hundreds of post-translationally modified peptides and yet is focused on peptides from proteins critical to cellular signaling pathways. PTMScan Direct therefore combines the specificity of traditional biochemical methods such as western blotting with the richness of data sets, sensitivity, and dynamic range of LC-MS/MS-based proteomic methods. PTMScan Direct will be broadly applicable to drug discovery and development programs, mutational analysis, kinase profiling, and tissue profiling.
PTMScan Direct can also be used in conjunction with technologies such as multiple reaction monitoring (81, 82) or high mass accuracy methods such as LC-MSE or Sequential Windowed Acquistion on TOFM Analyzer with High Resolution (83–85). This could allow high throughput screening of response biomarkers or other peptides of interest in hundreds to thousands of samples. PTMScan Direct will also provide data focused on post-translational modifications that will be complimentary to whole proteome analysis such as stable isotope standard capture with anti-peptide antibodies (SISCAPA) or single-run analysis (86–90). The ability to quantitatively profile proteins from many signaling pathways simultaneously represents an important step toward complete, quantitative disease biology.
Footnotes
* The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- EGFR
- epidermal growth factor receptor
- PDGFR
- platelet-derived growth factor receptor
- PI3K
- phosphatidylinositol 3-kinase
- PKC
- protein kinase C
- RTK
- receptor tyrosine kinase
- MAP
- mitogen-activated protein
- mTOR
- mammalian target of rapamycin.
REFERENCES
- 1. Dowell J. E., Minna J. D. (2005) Chasing mutations in the epidermal growth factor in lung cancer. N. Engl. J. Med. 352, 830–832 [DOI] [PubMed] [Google Scholar]
- 2. Gazdar A. F. (2009) Activating and resistance mutations of EGFR in non-small-cell lung cancer: Role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 28, (Suppl. 1) S24–S31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo A., Villén J., Kornhauser J., Lee K. A., Stokes M. P., Rikova K., Possemato A., Nardone J., Innocenti G., Wetzel R., Wang Y., MacNeill J., Mitchell J., Gygi S. P., Rush J., Polakiewicz R. D., Comb M. J. (2008) Signaling networks assembled by oncogenic EGFR and c-Met. Proc. Natl. Acad. Sci. U.S.A. 105, 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rikova K., Guo A., Zeng Q., Possemato A., Yu J., Haack H., Nardone J., Lee K., Reeves C., Li Y., Hu Y., Tan Z., Stokes M., Sullivan L., Mitchell J., Wetzel R., Macneill J., Ren J. M., Yuan J., Bakalarski C. E., Villen J., Kornhauser J. M., Smith B., Li D., Zhou X., Gygi S. P., Gu T. L., Polakiewicz R. D., Rush J., Comb M. J. (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 [DOI] [PubMed] [Google Scholar]
- 5. Weinstein I. B. (2002) Cancer. Addiction to oncogenes: The Achilles heal of cancer. Science 297, 63–64 [DOI] [PubMed] [Google Scholar]
- 6. Antonescu C. R. (2011) The GIST paradigm: Lessons for other kinase-driven cancers. J. Pathol. 223, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodgkinson V. C., Eagle G. L., Drew P. J., Lind M. J., Cawkwell L. (2010) Biomarkers of chemotherapy resistance in breast cancer identified by proteomics: Current status. Cancer Lett. 294, 13–24 [DOI] [PubMed] [Google Scholar]
- 8. Sasaki T., Koivunen J., Ogino A., Yanagita M., Nikiforow S., Zheng W., Lathan C., Marcoux J. P., Du J., Okuda K., Capelletti M., Shimamura T., Ercan D., Stumpfova M., Xiao Y., Weremowicz S., Butaney M., Heon S., Wilner K., Christensen J. G., Eck M. J., Wong K. K., Lindeman N., Gray N. S., Rodig S. J., Jänne P. A. (2011) A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 71, 6051–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi H., Kong X., Ribas A., Lo R. S. (2011) Combinatorial treatments that overcome PDGFRβ-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res. 71, 5067–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisberg E., Barrett R., Liu Q., Stone R., Gray N., Griffin J. D. (2009) FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist. Updat. 12, 81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones S., Zhang X., Parsons D. W., Lin J. C., Leary R. J., Angenendt P., Mankoo P., Carter H., Kamiyama H., Jimeno A., Hong S. M., Fu B., Lin M. T., Calhoun E. S., Kamiyama M., Walter K., Nikolskaya T., Nikolsky Y., Hartigan J., Smith D. R., Hidalgo M., Leach S. D., Klein A. P., Jaffee E. M., Goggins M., Maitra A., Iacobuzio-Donahue C., Eshleman J. R., Kern S. E., Hruban R. H., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V. E., Kinzler K. W. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo J., Solimini N. L., Elledge S. J. (2009) Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell 136, 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vogelstein B., Kinzler K. W. (2004) Cancer genes and the pathways they control. Nat. Med. 10, 789–799 [DOI] [PubMed] [Google Scholar]
- 14. LaFramboise T. (2009) Single nucleotide polymorphism arrays: A decade of biological, computational and technological advances. Nucleic Acids Res. 37, 4181–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maciejewski J. P., Tiu R. V., O'Keefe C. (2009) Application of array-based whole genome scanning technologies as a cytogenetic tool in haematological malignancies. Br. J. Haematol. 146, 479–488 [DOI] [PubMed] [Google Scholar]
- 16. Greenman C., Stephens P., Smith R., Dalgliesh G. L., Hunter C., Bignell G., Davies H., Teague J., Butler A., Stevens C., Edkins S., O'Meara S., Vastrik I., Schmidt E. E., Avis T., Barthorpe S., Bhamra G., Buck G., Choudhury B., Clements J., Cole J., Dicks E., Forbes S., Gray K., Halliday K., Harrison R., Hills K., Hinton J., Jenkinson A., Jones D., Menzies A., Mironenko T., Perry J., Raine K., Richardson D., Shepherd R., Small A., Tofts C., Varian J., Webb T., West S., Widaa S., Yates A., Cahill D. P., Louis D. N., Goldstraw P., Nicholson A. G., Brasseur F., Looijenga L., Weber B. L., Chiew Y. E., DeFazio A., Greaves M. F., Green A. R., Campbell P., Birney E., Easton D. F., Chenevix-Trench G., Tan M. H., Khoo S. K., Teh B. T., Yuen S. T., Leung S. Y., Wooster R., Futreal P. A., Stratton M. R. (2007) Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kan Z., Jaiswal B. S., Stinson J., Janakiraman V., Bhatt D., Stern H. M., Yue P., Haverty P. M., Bourgon R., Zheng J., Moorhead M., Chaudhuri S., Tomsho L. P., Peters B. A., Pujara K., Cordes S., Davis D. P., Carlton V. E., Yuan W., Li L., Wang W., Eigenbrot C., Kaminker J. S., Eberhard D. A., Waring P., Schuster S. C., Modrusan Z., Zhang Z., Stokoe D., de Sauvage F. J., Faham M., Seshagiri S. (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873 [DOI] [PubMed] [Google Scholar]
- 18. Puente X. S., Pinyol M., Quesada V., Conde L., Ordóñez G. R., Villamor N., Escaramis G., Jares P., Beà S., González-Díaz M., Bassaganyas L., Baumann T., Juan M., López-Guerra M., Colomer D., Tubío J. M., López C., Navarro A., Tornador C., Aymerich M., Rozman M., Hernández J. M., Puente D. A., Freije J. M., Velasco G., Gutiérrez-Fernández A., Costa D., Carrió A., Guijarro S., Enjuanes A., Hernández L., Yagüe J., Nicolás P., Romeo-Casabona C. M., Himmelbauer H., Castillo E., Dohm J. C., de Sanjosé S., Piris M. A., de Alava E., San Miguel J., Royo R., Gelpí J. L., Torrents D., Orozco M., Pisano D. G., Valencia A., Guigó R., Bayés M., Heath S., Gut M., Klatt P., Marshall J., Raine K., Stebbings L. A., Futreal P. A., Stratton M. R., Campbell P. J., Gut I., López-Guillermo A., Estivill X., Montserrat E., López-Otín C., Campo E. (2011) Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475, 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin K., DeVries S., Fridlyand J., Spellman P. T., Roydasgupta R., Kuo W. L., Lapuk A., Neve R. M., Qian Z., Ryder T., Chen F., Feiler H., Tokuyasu T., Kingsley C., Dairkee S., Meng Z., Chew K., Pinkel D., Jain A., Ljung B. M., Esserman L., Albertson D. G., Waldman F. M., Gray J. W. (2006) Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10, 529–541 [DOI] [PubMed] [Google Scholar]
- 20. Cantin G. T., Yi W., Lu B., Park S. K., Xu T., Lee J. D., Yates J. R., 3rd (2008) Combining protein-based IMAC, peptide-based IMAC, and MudPIT for efficient phosphoproteomic analysis. J. Proteome Res. 7, 1346–1351 [DOI] [PubMed] [Google Scholar]
- 21. Lee K. A., Hammerle L. P., Andrews P. S., Stokes M. P., Mustelin T., Silva J. C., Black R. A., Doedens J. R. (2011) Ubiquitin ligase substrate identification through quantitative proteomics at both the protein and peptide levels. J. Biol. Chem. 286, 41530–41538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olsen J. V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 23. Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 [DOI] [PubMed] [Google Scholar]
- 24. Choudhary C., Mann M. (2010) Decoding signalling networks by mass spectrometry-based proteomics. Nat. Rev. Mol. Cell Biol. 11, 427–439 [DOI] [PubMed] [Google Scholar]
- 25. Denis N. J., Vasilescu J., Lambert J. P., Smith J. C., Figeys D. (2007) Tryptic digestion of ubiquitin standards reveals an improved strategy for identifying ubiquitinated proteins by mass spectrometry. Proteomics 7, 868–874 [DOI] [PubMed] [Google Scholar]
- 26. Xu G., Paige J. S., Jaffrey S. R. (2010) Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat. Biotechnol. 28, 868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng J., Schwartz D., Elias J. E., Thoreen C. C., Cheng D., Marsischky G., Roelofs J., Finley D., Gygi S. P. (2003) A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 21, 921–926 [DOI] [PubMed] [Google Scholar]
- 28. Villén J., Beausoleil S. A., Gerber S. A., Gygi S. P. (2007) Large-scale phosphorylation analysis of mouse liver. Proc. Natl. Acad. Sci. U.S.A. 104, 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng S., Ye M., Zhou H., Jiang X., Zou H., Gong B. (2007) Immobilized zirconium ion affinity chromatography for specific enrichment of phosphopeptides in phosphoproteome analysis. Mol. Cell. Proteomics 6, 1656–1665 [DOI] [PubMed] [Google Scholar]
- 30. Eyrich B., Sickmann A., Zahedi R. P. (2011) Catch me if you can: Mass spectrometry-based phosphoproteomics and quantification strategies. Proteomics 11, 554–570 [DOI] [PubMed] [Google Scholar]
- 31. Monetti M., Nagaraj N., Sharma K., Mann M. (2011) Large-scale phosphosite quantification in tissues by a spike-in SILAC method. Nat. Methods 8, 655–658 [DOI] [PubMed] [Google Scholar]
- 32. Rigbolt K. T., Prokhorova T. A., Akimov V., Henningsen J., Johansen P. T., Kratchmarova I., Kassem M., Mann M., Olsen J. V., Blagoev B. (2011) System-wide temporal characterization of the proteome and phosphoproteome of human embryonic stem cell differentiation. Sci. Signal. 4, rs3. [DOI] [PubMed] [Google Scholar]
- 33. Mann M., Ong S. E., Grønborg M., Steen H., Jensen O. N., Pandey A. (2002) Analysis of protein phosphorylation using mass spectrometry: Deciphering the phosphoproteome. Trends Biotechnol. 20, 261–268 [DOI] [PubMed] [Google Scholar]
- 34. Tan F., Zhang Y., Mi W., Wang J., Wei J., Cai Y., Qian X. (2008) Enrichment of phosphopeptides by Fe3+-immobilized magnetic nanoparticles for phosphoproteome analysis of the plasma membrane of mouse liver. J. Proteome Res. 7, 1078–1087 [DOI] [PubMed] [Google Scholar]
- 35. Zhou H., Tian R., Ye M., Xu S., Feng S., Pan C., Jiang X., Li X., Zou H. (2007) Highly specific enrichment of phosphopeptides by zirconium dioxide nanoparticles for phosphoproteome analysis. Electrophoresis 28, 2201–2215 [DOI] [PubMed] [Google Scholar]
- 36. Larsen M. R., Thingholm T. E., Jensen O. N., Roepstorff P., Jørgensen T. J. (2005) Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol. Cell. Proteomics 4, 873–886 [DOI] [PubMed] [Google Scholar]
- 37. Cao P., Stults J. T. (2000) Mapping the phosphorylation sites of proteins using on-line immobilized metal affinity chromatography/capillary electrophoresis/electrospray ionization multiple stage tandem mass spectrometry. Rapid Commun. Mass Spectrom. 14, 1600–1606 [DOI] [PubMed] [Google Scholar]
- 38. Stensballe A., Andersen S., Jensen O. N. (2001) Characterization of phosphoproteins from electrophoretic gels by nanoscale FeIII affinity chromatography with off-line mass spectrometry analysis. Proteomics 1, 207–222 [DOI] [PubMed] [Google Scholar]
- 39. Rush J., Moritz A., Lee K. A., Guo A., Goss V. L., Spek E. J., Zhang H., Zha X. M., Polakiewicz R. D., Comb M. J. (2005) Immunoaffinity profiling of tyrosine phosphorylation in cancer cells. Nat. Biotechnol. 23, 94–101 [DOI] [PubMed] [Google Scholar]
- 40. Stokes M. P., Rush J., Macneill J., Ren J. M., Sprott K., Nardone J., Yang V., Beausoleil S. A., Gygi S. P., Livingstone M., Zhang H., Polakiewicz R. D., Comb M. J. (2007) Profiling of UV-induced ATM/ATR signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 104, 19855–19860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang H., Zha X., Tan Y., Hornbeck P. V., Mastrangelo A. J., Alessi D. R., Polakiewicz R. D., Comb M. J. (2002) Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J. Biol. Chem. 277, 39379–39387 [DOI] [PubMed] [Google Scholar]
- 42. Bonnette P. C., Robinson B. S., Silva J. C., Stokes M. P., Brosius A. D., Baumann A., Buckbinder L. (2010) Phosphoproteomic characterization of PYK2 signaling pathways involved in osteogenesis. J. Proteomics 73, 1306–1320 [DOI] [PubMed] [Google Scholar]
- 43. Moritz A., Li Y., Guo A., Villén J., Wang Y., MacNeill J., Kornhauser J., Sprott K., Zhou J., Possemato A., Ren J. M., Hornbeck P., Cantley L. C., Gygi S. P., Rush J., Comb M. J. (2010) Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci. Signal. 3, ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brave S. R., Ratcliffe K., Wilson Z., James N. H., Ashton S., Wainwright A., Kendrew J., Dudley P., Broadbent N., Sproat G., Taylor S., Barnes C., Silva J. C., Farnsworth C. L., Hennequin L., Ogilvie D. J., Jürgensmeier J. M., Shibuya M., Wedge S. R., Barry S. T. (2011) Assessing the activity of cediranib, a VEGFR-2/3 tyrosine kinase inhibitor, against VEGFR-1 and members of the structurally related PDGFR family. Mol. Cancer Ther. 10, 861–873 [DOI] [PubMed] [Google Scholar]
- 45. Olsen J. V., de Godoy L. M., Li G., Macek B., Mortensen P., Pesch R., Makarov A., Lange O., Horning S., Mann M. (2005) Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteomics 4, 2010–2021 [DOI] [PubMed] [Google Scholar]
- 46. Lundgren D. H., Martinez H., Wright M. E., Han D. K. (2009) “Protein identification using Sorcerer 2 and SEQUEST,” Current Protocols in Bioinformatics, Chapter 13, Unit 13.13 Wiley Blackwell; USA: [DOI] [PubMed] [Google Scholar]
- 47. von Mering C., Huynen M., Jaeggi D., Schmidt S., Bork P., Snel B. (2003) STRING: A database of predicted functional associations between proteins. Nucleic Acids Res. 31, 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kadota S., Fantus I. G., Deragon G., Guyda H. J., Hersh B., Posner B. I. (1987) Peroxide(s) of vanadium: A novel and potent insulin-mimetic agent which activates the insulin receptor kinase. Biochem. Biophys. Res. Commun. 147, 259–266 [DOI] [PubMed] [Google Scholar]
- 49. Fantus I. G., Kadota S., Deragon G., Foster B., Posner B. I. (1989) Pervanadate [peroxide 49(s) of vanadate] mimics insulin action in rat adipocytes via activation of the insulin receptor tyrosine kinase. Biochemistry 28, 8864–8871 [DOI] [PubMed] [Google Scholar]
- 50. Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. (2002) The protein kinase complement of the human genome. Science 298, 1912–1934 [DOI] [PubMed] [Google Scholar]
- 51. Ackermann B. L., Hale J. E., Duffin K. L. (2006) The role of mass spectrometry in biomarker discovery and measurement. Curr. Drug Metab. 7, 525–539 [DOI] [PubMed] [Google Scholar]
- 52. Calligaris D., Villard C., Lafitte D. (2011) Advances in top-down proteomics for disease biomarker discovery. J. Proteomics 74, 920–934 [DOI] [PubMed] [Google Scholar]
- 53. Sawyers C. L. (2002) Rational therapeutic intervention in cancer: Kinases as drug targets. Curr. Opin. Genet. Dev. 12, 111–115 [DOI] [PubMed] [Google Scholar]
- 54. Veenstra T. D. (2007) Global and targeted quantitative proteomics for biomarker discovery. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 847, 3–11 [DOI] [PubMed] [Google Scholar]
- 55. Wu Z., Doondeea J. B., Moghaddas Gholami A., Janning M. C., Lemeer S., Kramer K., Eccles S. A., Gollin S. M., Grenman R., Walch A., Feller S. M., Kuster B. (2011) Quantitative chemical proteomics reveals new potential drug targets in head and neck cancer. Mol. Cell. Proteomics 12, 10.1074/mcp.M111.011635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lynch T. J., Bell D. W., Sordella R., Gurubhagavatula S., Okimoto R. A., Brannigan B. W., Harris P. L., Haserlat S. M., Supko J. G., Haluska F. G., Louis D. N., Christiani D. C., Settleman J., Haber D. A. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 [DOI] [PubMed] [Google Scholar]
- 57. Smolen G. A., Sordella R., Muir B., Mohapatra G., Barmettler A., Archibald H., Kim W. J., Okimoto R. A., Bell D. W., Sgroi D. C., Christensen J. G., Settleman J., Haber D. A. (2006) Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc. Natl. Acad. Sci. U.S.A. 103, 2316–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tracy S., Mukohara T., Hansen M., Meyerson M., Johnson B. E., Jänne P. A. (2004) Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 64, 7241–7244 [DOI] [PubMed] [Google Scholar]
- 59. Paez J. G., Jänne P. A., Lee J. C., Tracy S., Greulich H., Gabriel S., Herman P., Kaye F. J., Lindeman N., Boggon T. J., Naoki K., Sasaki H., Fujii Y., Eck M. J., Sellers W. R., Johnson B. E., Meyerson M. (2004) EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 [DOI] [PubMed] [Google Scholar]
- 60. Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaji M., Yonemura Y., Harada S., Liu X., Terada I., Yamamoto H. (1996) Participation of c-Met in the progression of human gastric cancers: Anti-c-Met oligonucleotides inhibit proliferation or invasiveness of gastric cancer cells. Cancer Gene Ther. 3, 393–404 [PubMed] [Google Scholar]
- 62. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 63. Bellacosa A., Chan T. O., Ahmed N. N., Datta K., Malstrom S., Stokoe D., McCormick F., Feng J., Tsichlis P. (1998) Akt activation by growth factors is a multiple-step process: The role of the PH domain. Oncogene 17, 313–325 [DOI] [PubMed] [Google Scholar]
- 64. Stephens L., Anderson K., Stokoe D., Erdjument-Bromage H., Painter G. F., Holmes A. B., Gaffney P. R., Reese C. B., McCormick F., Tempst P., Coadwell J., Hawkins P. T. (1998) Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279, 710–714 [DOI] [PubMed] [Google Scholar]
- 65. Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat. Cell Biol. 9, 316–323 [DOI] [PubMed] [Google Scholar]
- 66. Kovacina K. S., Park G. Y., Bae S. S., Guzzetta A. W., Schaefer E., Birnbaum M. J., Roth R. A. (2003) Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 278, 10189–10194 [DOI] [PubMed] [Google Scholar]
- 67. Franke T. F., Kaplan D. R., Cantley L. C. (1997) PI3K: Downstream AKTion blocks apoptosis. Cell 88, 435–437 [DOI] [PubMed] [Google Scholar]
- 68. Burgering B. M., Coffer P. J. (1995) Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 [DOI] [PubMed] [Google Scholar]
- 69. Franke T. F., Yang S. I., Chan T. O., Datta K., Kazlauskas A., Morrison D. K., Kaplan D. R., Tsichlis P. N. (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 [DOI] [PubMed] [Google Scholar]
- 70. Unsicker K., Krieglstein K. (2006) Cell signaling and growth factors in development: From molecules to organogenesis, Wiley-VCH, Weinheim, Germany [Google Scholar]
- 71. Stokoe D., Stephens L. R., Copeland T., Gaffney P. R., Reese C. B., Painter G. F., Holmes A. B., McCormick F., Hawkins P. T. (1997) Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 [DOI] [PubMed] [Google Scholar]
- 72. Easton R. M., Cho H., Roovers K., Shineman D. W., Mizrahi M., Forman M. S., Lee V. M., Szabolcs M., de Jong R., Oltersdorf T., Ludwig T., Efstratiadis A., Birnbaum M. J. (2005) Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol. Cell. Biol. 25, 1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tschopp O., Yang Z. Z., Brodbeck D., Dummler B. A., Hemmings-Mieszczak M., Watanabe T., Michaelis T., Frahm J., Hemmings B. A. (2005) Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132, 2943–2954 [DOI] [PubMed] [Google Scholar]
- 74. Masure S., Haefner B., Wesselink J. J., Hoefnagel E., Mortier E., Verhasselt P., Tuytelaars A., Gordon R., Richardson A. (1999) Molecular cloning, expression and characterization of the human serine/threonine kinase Akt-3. Eur. J. Biochem. 265, 353–360 [DOI] [PubMed] [Google Scholar]
- 75. Coffer P. J., Jin J., Woodgett J. R. (1998) Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Altomare D. A., Guo K., Cheng J. Q., Sonoda G., Walsh K., Testa J. R. (1995) Cloning, chromosomal localization and expression analysis of the mouse Akt2 oncogene. Oncogene 11, 1055–1060 [PubMed] [Google Scholar]
- 77. Altomare D. A., Lyons G. E., Mitsuuchi Y., Cheng J. Q., Testa J. R. (1998) Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene 16, 2407–2411 [DOI] [PubMed] [Google Scholar]
- 78. Keranen L. M., Dutil E. M., Newton A. C. (1995) Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 5, 1394–1403 [DOI] [PubMed] [Google Scholar]
- 79. Freeley M., Kelleher D., Long A. (2011) Regulation of protein kinase C function by phosphorylation on conserved and non-conserved sites. Cell Signal. 23, 753–762 [DOI] [PubMed] [Google Scholar]
- 80. Wetsel W. C., Khan W. A., Merchenthaler I., Rivera H., Halpern A. E., Phung H. M., Negro-Vilar A., Hannun Y. A. (1992) Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J. Cell Biol. 117, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wolf-Yadlin A., Hautaniemi S., Lauffenburger D. A., White F. M. (2007) Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc. Natl. Acad. Sci. U.S.A. 104, 5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Anderson L., Hunter C. L. (2006) Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics 5, 573–588 [DOI] [PubMed] [Google Scholar]
- 83. Silva J. C., Gorenstein M. V., Li G. Z., Vissers J. P., Geromanos S. J. (2006) Absolute quantification of proteins by LCMSE: A virtue of parallel MS acquisition. Mol. Cell. Proteomics 5, 144–156 [DOI] [PubMed] [Google Scholar]
- 84. Schmidt A., Beck M., Malmström J., Lam H., Claassen M., Campbell D., Aebersold R. (2011) Absolute quantification of microbial proteomes at different states by directed mass spectrometry. Mol. Syst. Biol. 7, 510–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Reiter L., Rinner O., Picotti P., Hüttenhain R., Beck M., Brusniak M. Y., Hengartner M. O., Aebersold R. (2011) mProphet: Automated data processing and statistical validation for large-scale SRM experiments. Nat. Methods 8, 430–435 [DOI] [PubMed] [Google Scholar]
- 86. Thakur S. S., Geiger T., Chatterjee B., Bandilla P., Frohlich F., Cox J., Mann M. (2011) Deep and highly sensitive proteome coverage by LC-MS/MS without prefractionation. Mol. Cell. Proteomics 10.1074/mcp.M110.003699, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Whiteaker J. R., Zhao L., Abbatiello S. E., Burgess M., Kuhn E., Lin C., Pope M. E., Razavi M., Anderson N. L., Pearson T. W., Carr S. A., Paulovich A. G. (2011) Evaluation of large scale quantitative proteomic assay development using peptide affinity-based mass spectrometry. Mol. Cell. Proteomics 10.1074/mcp.M110.005645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Anderson N. L., Anderson N. G., Pearson T. W., Borchers C. H., Paulovich A. G., Patterson S. D., Gillette M., Aebersold R., Carr S. A. (2009) A human proteome detection and quantitation project. Mol. Cell. Proteomics 8, 883–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Michalski A., Damoc E., Hauschild J. P., Lange O., Wieghaus A., Makarov A., Nagaraj N., Cox J., Mann M., Horning S. (2011) Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol. Cell. Proteomics 10.1074/M111.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Geiger T., Cox J., Mann M. (2010) Proteomics on an Orbitrap benchtop mass spectrometer using all-ion fragmentation. Mol. Cell. Proteomics 9, 2252–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]