Abstract
Background:
Esophageal pressure monitoring during polysomnography in children offers a gold-standard, “preferred” assessment for work of breathing, but is not commonly used in part because prospective data on incremental clinical utility are scarce. We compared a standard pediatric apnea/hypopnea index to quantitative esophageal pressures as predictors of apnea-related neurobehavioral morbidity and treatment response.
Methods:
Eighty-one children aged 7.8 ± 2.8 (SD) years, including 44 boys, had traditional laboratory-based pediatric polysomnography, esophageal pressure monitoring, multiple sleep latency tests, psychiatric evaluations, parental behavior rating scales, and cognitive testing, all just before clinically indicated adenotonsillectomy, and again 7.2 ± 0.8 months later. Esophageal pressures were used, along with nasal pressure monitoring and oronasal thermocouples, not only to identify respiratory events but also more quantitatively to determine the most negative esophageal pressure recorded and the percentage of sleep time spent with pressures lower than −10 cm H2O.
Results:
Both sleep-disordered breathing and neurobehavioral measures improved after surgery. At baseline, one or both quantitative esophageal pressure measures predicted a disruptive behavior disorder (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-defined attention-deficit/hyperactivity disorder, conduct disorder, or oppositional defiant disorder) and more sleepiness and their future improvement after adenotonsillectomy (each P < .05). The pediatric apnea/hypopnea index did not predict these morbidities or treatment outcomes (each P > .10). The addition of respiratory effort-related arousals to the apnea/hypopnea index did not improve its predictive value. Neither the preoperative apnea/hypopnea index nor esophageal pressures predicted baseline hyperactive behavior, cognitive performance, or their improvement after surgery.
Conclusions:
Quantitative esophageal pressure monitoring may add predictive value for some, if not all, neurobehavioral outcomes of sleep-disordered breathing.
Trial registry:
ClinicalTrials.gov; No.: NCT00233194; URL: www.clinicaltrials.gov
Esophageal pressure monitoring during sleep was recognized by 1982 to identify abnormalities in breathing that are more subtle than frank apneas and hypopneas, yet are still responsible for serious neurobehavioral and mental health consequences in children.1 Esophageal pressure monitoring also offers a gold-standard assessment of upper airway resistance and work of breathing during polysomnography.2 However, exactly how esophageal pressure monitoring should be used has not been well defined. Early literature on the upper airway resistance syndrome illustrated use of both quantitative measurements, such as the most negative esophageal pressure recorded during a polysomnogram, and respiratory effort-related arousals (RERAs) identified by more qualitative crescendo increases in esophageal pressure swings prior to arousals.1,3‐7
In practice, nasal pressure monitoring is simpler than esophageal pressure monitoring and has been adopted more commonly to identify rates of hypopneas and RERAs,8 which in combination with rates of obstructive apneas offer an overall respiratory disturbance index with increased sensitivity to the subtlest forms of pediatric sleep-disordered breathing (SDB). Although one report has suggested that nasal pressure in comparison with esophageal pressure monitoring identifies RERAs equally well,9 with data derived from 15 adults rather than children, studies have rarely examined the clinical value of quantitative esophageal pressures, and the two monitoring approaches have not been compared for prediction of morbidity or treatment outcomes. More broadly, the degree to which any data beyond frank obstructive apneas effectively predict pediatric SDB-related morbidity or treatment response remains inadequately studied. Current guidelines for polysomnographic scoring of events more subtle than apneas have been established largely by consensus for the pediatric age group.8 Esophageal pressure monitoring is listed as “preferred” for identification of RERAs, but its unique potential as a more quantitative measure is not mentioned.
We prospectively used both esophageal and nasal pressure monitoring during polysomnography, and paired results with intensive evaluations of behavior, cognition, mental health, and sleepiness before and after clinically indicated adenotonsillectomies. Approximately two-thirds of the children in this Washtenaw County Adenotonsillectomy Cohort II tolerated esophageal pressure monitoring for ≥ 2 h of sleep before and after surgery. The primary hypothesis was that quantitative esophageal pressures would help to predict baseline neurobehavioral morbidity and treatment outcomes. Secondary hypotheses were that RERAs and hypopneas would augment the predictive value of obstructive apneas.
Materials and Methods
Overview
Subjects were recruited from local otolaryngology practices. Staff helped to identify potential subjects, aged 3.0 to 12.9 years, who were scheduled for adenotonsillectomy for any clinical indication, but as usual,10 were not thought to need polysomnography prior to surgery. Sleep and neurobehavioral assessments were completed up to 3 days before adenotonsillectomy, and again about 6 months thereafter. A child psychiatrist, child psychologist, or behavioral developmental pediatrician interviewed each family prior to a full, nocturnal, laboratory-based polysomnogram. The next day, a multiple sleep latency test was administered. Between naps, children underwent neuropsychologic testing, and a parent completed behavioral rating scales and a standard socioeconomic survey.11
As detailed online (e-Appendix 1 (424.2KB, pdf) ), pediatric polysomnography followed standard recommendations,8 published after the start of this research protocol, except that piezoelectric strain gauges rather than inductance plethysmography were used to monitor thoracic and abdominal excursion. Both oronasal thermocouples and nasal pressure monitoring were used to assess airflow. Esophageal pressure was recorded through a water-filled, 6F pediatric feeding tube.12,13 Multiple sleep latency tests followed standard procedures,14 but nap opportunities were lengthened from the adult standard of 20 min to 30 min.15,16
Scoring
All sleep studies were scored, or in a minority of cases, thoroughly rescored, by a single pediatric-experienced sleep and electroencephalography-registered chief technologist. Sleep staging followed standard criteria.8 Esophageal pressure swings were scored as the difference between peak inspiratory and peak expiratory readings. The technologist recorded the percentage of sleep epochs spent with most esophageal pressure swings more negative than −10 cm H2O (low Pes time), and also the most negative esophageal pressure swing (Pes nadir) for each study. Obstructive apneas (2 breaths or longer), hypopneas, RERAs scored by nasal pressure or esophageal pressure, and central apneas were scored according to pediatric criteria recommended by the American Academy of Sleep Medicine (AASM) in 2007.8 The AASM-2007 apnea/hypopnea index was calculated as the number of apneas and hypopneas per hour of sleep, and the AASM-2007 respiratory disturbance index was calculated similarly, with the addition of RERAs. In multiple sleep latency tests, the mean sleep latency across all nap opportunities provided an objective measure of daytime sleepiness.17
Neurobehavioral Outcomes
Standardized, well-validated assessments were used to evaluate Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnoses, behavioral problems, and cognitive deficits long thought to represent the most important morbidity in childhood SDB.1,18‐23 Psychiatric diagnoses were guided in large part by the Computerized Diagnostic Interview Schedule for Children–Parent,24‐26 and the Children’s Psychiatric Rating Scale.27‐29 The final categorical diagnostic outcome variable was presence or absence of a DSM-IV-defined disruptive behavior disorder—attention-deficit/hyperactivity disorder, conduct disorder, or oppositional-defiant disorder—as concluded by the interviewing clinician. Behavioral outcome variables were generated by two validated parental rating scales for inattention and hyperactivity: the Conners’ Parent Rating Scales30 and the Child Symptom Inventory-431 (or the Early Childhood Inventory-432 for children between 3 and 5 years). A behavioral hyperactivity index33 (mean 50; SD 10; with higher scores indicating more significant symptoms) was constructed from the average of the inattention and hyperactivity T scores produced by the two instruments. Finally, cognitive testing for a total of about 2 h included the NEPSY,34 a developmental neuropsychologic test battery created for children ages 3 to 12 years of age: a memory and learning score and an attention/executive functions score were averaged to create a cognitive index (mean 100; SD 15; with higher scores indicating better performance).
Analyses
Baseline and follow-up measures were compared initially using a nonparametric signed rank test for paired continuous data or the McNemar test for dichotomous data. Associations between baseline sleep and neurobehavioral morbidity measures were assessed with the nonparametric Spearman correlation coefficient ρ, or by Wilcoxon two-sample tests. Each association that showed statistical significance in these bivariate comparisons was then examined in a multiple regression of the neurobehavioral measure at baseline, or its change after adenotonsillectomy, on the baseline sleep measure. General linear models or logistic regression models were used as appropriate to take several potential confounders into account. The level of significance was set at P < .05.
Results
Subjects
Subject recruitment (Fig 1) generated the sample of 81 children whose demographics and findings are summarized in Table 1. Clinically suspected SDB was an indication for adenotonsillectomy for 70 (86%), and only 18 (22%) had or also had chronic tonsillitis or throat infections. The baseline AASM-2007 apnea/hypopnea index ranged from 0.20 (normal < 1) to 81.20 (severe). The AASM-2007 apnea/hypopnea index on average was seven times higher than the obstructive apnea index. The AASM-2007 respiratory disturbance index, which added RERAs to the apnea/hypopnea index, exceeded the latter on average by only 8.5%. The obstructive apnea index, apnea/hypopnea index, and respiratory disturbance index were strongly intercorrelated (ρ = 0.70 for the first and second variable, ρ = 0.68 for the first and third, and ρ = 0.99 for the second and third, all P < .0001).
Figure 1.
Recruitment of 81 subjects who provided data for the current report. Among 147 children whose families agreed to participate in the study, comparisons between the 81 children whose full sleep and neurobehavioral outcome data (at baseline and follow-up) were available for analysis in this study, and the 66 children excluded because full data were not available, revealed that the following baseline variables showed no statistically significant difference (all P > .05, Wilcoxon signed rank test or χ2, as appropriate): sex, socioeconomic status, BMI z score, total baseline polysomnogram recording time, sleep latency, sleep efficiency, arousal index, percent of time spent in each sleep stage, obstructive apnea index, apnea/hypopnea index, respiratory disturbance index, minimum oxygen saturation, percentage of sleep time spent with oxygen saturation < 90%, most negative recorded esophageal pressure (available for some time in n = 15 excluded children), percentage of sleep time spent with esophageal pressure more negative than −10 cm H2O, behavioral hyperactivity index, cognitive index, and mean sleep latency on the multiple sleep latency test. In contrast, the following variables did differ (P < .05) at baseline between the two groups of 81 and 66 children: age (7.8 ± 2.8 y vs 6.4 ± 1.8 y), race (84% vs 67% white), total sleep time (513 ± 62 min vs 534 ± 46 min), and frequency of a disruptive behavior disorder diagnosis (35% vs 58%).
Table 1.
—Demographic, Sleep, and Neurobehavioral Measures at Baseline and Follow-up After Adenotonsillectomy for 81 Children
| Variable | Baseline Mean (SD) | Follow-up Mean (SD) | Effect Size | P Valuea |
| Demographics | ||||
| Age, y | 7.8 (2.8) | 8.4 (2.8) | ... | ... |
| Sex, male, % | 54 | ... | ... | ... |
| Race, white, % | 84 | ... | ... | ... |
| Socioeconomic statusb | 2.4 (0.9) | ... | ... | ... |
| BMI z score | 0.52 (1.36) | 0.81 (1.04) | 0.24 | < .0001 |
| Sleep | ||||
| Total recording time, min | 597 (38) | 598 (44) | 0.02 | .9489 |
| Total sleep time, min | 513 (62) | 528 (57) | 0.24 | .0746 |
| Sleep latency, min | 26 (23) | 23 (31) | 0.13 | .0193 |
| Sleep efficiency,b % | 86 (8) | 88 (7) | 0.30 | .0118 |
| Arousal indexb | 12 (6) | 9 (3) | 0.60 | < .0001 |
| Stage 1 sleep, % | 12 (5) | 9 (3) | 0.62 | < .0001 |
| Stage 2 sleep, % | 44 (7) | 47 (7) | 0.44 | < .0001 |
| Stage 3 sleep, % | 25 (5) | 23 (6) | 0.25 | .0105 |
| REM sleep, % | 19 (5) | 20 (4) | 0.20 | .1198 |
| Sleep apnea measures | ||||
| Obstructive apnea indexb | 0.97 (3.15) | 0.17 (0.27) | 0.47 | < .0001 |
| Apnea/hypopnea indexb | 6.9 (10.9) | 1.9 (1.7) | 0.79 | < .0001 |
| Respiratory disturbance indexb | 7.5 (11.1) | 2.1 (1.8) | 0.84 | < .0001 |
| Minimum oxygen saturation, % | 90.1 (6.1) | 92.2 (4.0) | 0.42 | < .0001 |
| Sleep time with oxygen saturation < 90%, % | 0.55 (2.92) | 0.02 (0.16) | 0.34 | .0684 |
| Minimum esophageal pressure, cm H2O | −37 (17) | −22 (9) | 1.11 | < .0001 |
| Sleep time with esophageal pressure more negative than −10 cm H2O, % | 50 (32) | 27 (31) | 0.74 | < .0001 |
| Neurobehavioral measures | ||||
| Disruptive behavior disorder,b % with positive diagnosis | 35 | 17 | ... | .0017 |
| Behavioral hyperactivity index | 57 (11) | 53 (9) | 0.39 | < .0001 |
| Cognitive index | 103 (13) | 112 (12) | 0.73 | < .0001 |
| Mean sleep latency on multiple sleep latency test, min | 24 (6) | 25 (5) | 0.23 | .0516 |
REM = rapid eye movement.
Nonparametric signed rank test for difference between baseline and follow-up (for continuous data), or the McNemar test (for disruptive behavior disorder categorical diagnosis at baseline vs follow-up).
Index = events per hour of sleep, scored following American Academy of Sleep Medicine 2007 recommendations; disruptive behavior disorder = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition-consistent, psychiatrist- and scheduled interview-determined diagnosis of attention-deficit/hyperactivity disorder, oppositional defiant disorder, or conduct disorder; sleep efficiency = (total sleep time/total recording time); socioeconomic status = Hollingshead (1965) rank (1 = highest, 5 = lowest).
At baseline, at least one disruptive behavior disorder was diagnosed in 28 of the 81 children (35%): 25 (31%) had attention-deficit/hyperactivity disorder, 12 (15%) had oppositional defiant disorder, and none had conduct disorder. The 81 subjects had somewhat elevated behavioral hyperactivity indices, 0.67 SD above age- and sex-specific norms. Cognitive indices reflected performance just better than average. The average mean sleep latency of 24 ± 6 min on the 30-min multiple sleep latency test was similar to that published previously for a series of children with obstructive sleep apnea, primary snoring, or no SDB.15
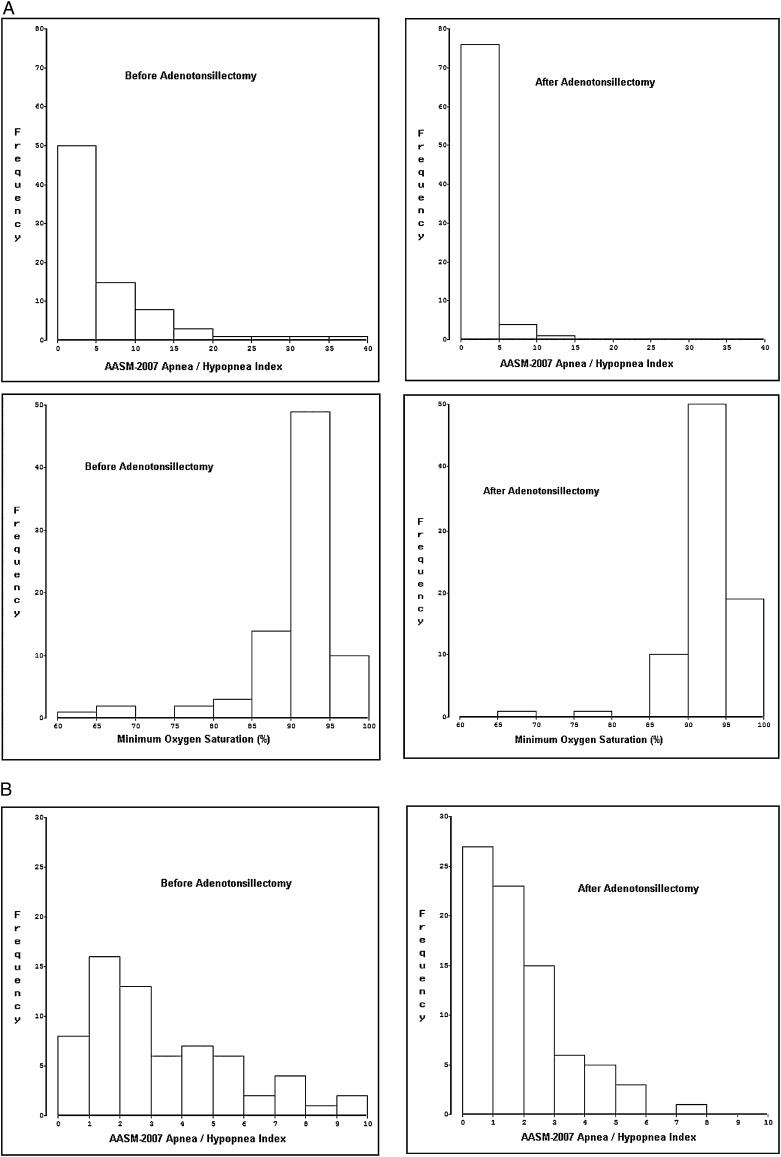
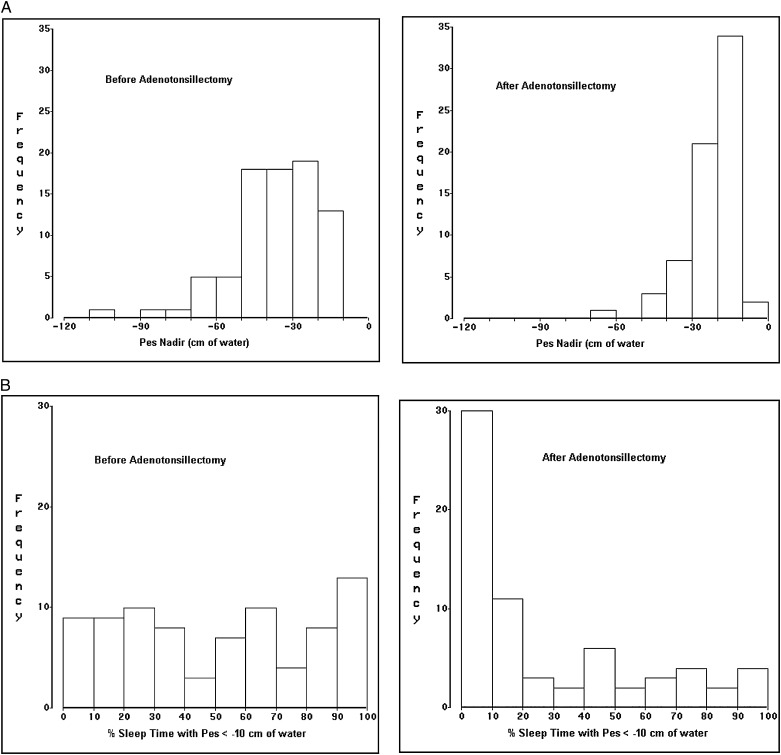
Follow-up studies were performed 0.60 ± 0.07 years (7.2 months) after baseline studies (range 0.46 to 0.80 years). Postoperative apnea severity measures improved (Table 1), but on average still met diagnostic criteria for pediatric sleep apnea.35 Respiratory event indices (Fig 2) improved more dramatically than did the minimum oxygen saturation or the two quantitative esophageal pressure measures (Fig 3). Neurobehavioral measures also improved, to an extent that ranged from robust (eg, 51% reduction in frequency of disruptive behavior disorders) to marginally nonsignificant (1 minute on the multiple sleep latency test).
Figure 2.
Distributions of two common measures of apnea severity during baseline and postadenotonsillectomy polysomnograms in 81 children. A, The AASM-2007 apnea/hypopnea index (first row) and minimum oxygen saturation (second row). One subject’s apnea/hypopnea index (81.2) is not shown. B, Distributions in finer detail for AASM-2007 apnea/hypopnea indices of 10 or less. AASM = American Academy of Sleep Medicine.
Figure 3.
Distributions of two quantitative Pes measures during baseline and postadenotonsillectomy polysomnograms in 81 children. A, Pes nadir values before and after adenotonsillectomy. B, Percentage of sleep time spent with Pes more negative than −10 cm H2O before and after adenotonsillectomy. Pes = esophageal pressure; Pes nadir = most negative esophageal pressure swing recorded during a polysomnogram.
Sleep Apnea Measures and Neurobehavioral Morbidity at Baseline
The 28 children with baseline disruptive behavior disorders, in comparison with remaining children, on average had a low Pes time of 61% rather than 44% (P = .010, Table 2). These 28 also trended toward higher obstructive apnea indices (P = .090), but the difference between group means was small (1.00 ± 2.66 vs 0.96 ± 3.40). Trends emerged for mean sleep latency (sleepiness) to be predicted by the previous-night obstructive apnea index, percent of sleep time with oxygen saturation < 90%, Pes nadir, and low Pes time. The behavioral hyperactivity index and cognitive index were not predicted by any previous-night SDB measures.
Table 2.
—Unadjusted Associations Among 81 Children Between Sleep Apnea Measures at Baseline and Concurrent Neurobehavioral Morbidity
| Disruptive Behavior Disorder, Present vs Absent | Behavioral Hyperactivity Index | Cognitive Index | Mean Sleep Latency on Multiple Sleep Latency Test | |||||
| Apnea Measure | Wilcoxon Z | One-sided P Value | Spearman ρ | P Value | Spearman ρ | P Value | Spearman ρ | P Value |
| Obstructive apnea indexa | −1.35b | .090b | −0.16 | .16 | −0.066 | .56 | −0.19b | .083b |
| Apnea/hypopnea indexa | 0.0099 | .50 | −0.022 | .85 | −0.082 | .47 | −0.078 | .49 |
| Respiratory disturbance indexa | 0.0000 | .50 | −0.007 | .95 | −0.119 | .29 | −0.061 | .59 |
| Minimum oxygen saturation | −0.69 | .24 | −0.102 | .37 | 0.011 | .92 | 0.16 | .15 |
| Sleep time with oxygen saturation < 90%, % | 0.66 | .25 | 0.047 | .68 | −0.094 | .40 | −0.19b | .083b |
| Esophageal pressure nadir | −0.90 | .18 | −0.030 | .79 | −0.028 | .80 | 0.19b | .092b |
| Sleep time with esophageal pressure below −10 cm H2O, % | 2.36b | .010b | 0.097 | .39 | −0.176 | .12 | −0.19b | .087b |
Index = events per hour of sleep, scored following American Academy of Sleep Medicine 2007 recommendations.
Results that show statistical significance or trends.
Logistic regression of disruptive behavior disorder (present vs absent) on both low Pes time and the AASM-2007 apnea/hypopnea index showed that the former, but not the latter, retained statistical significance (OR = 1.023, 95% CI [1.005, 1.042], vs 0.969 [0.913, 1.028]). Thus, on average after accounting for the AASM-2007 apnea/hypopnea index, each 10 percentage point increase in low Pes time resulted in a 23% increase in the odds of having a disruptive behavior disorder. Similar results emerged from logistic regression of disruptive behavior disorder on low Pes time and the obstructive apnea index (1.020 [1.003, 1.037] vs 0.940 [0.793, 1.113]). Logistic regression of disruptive behavior disorder on several potential confounders simultaneously—age, sex, white non-Hispanic vs other race/ethnicity, socioeconomic status, and BMI z score—in addition to low Pes time rendered the latter variable only marginally nonsignificant (1.015 [0.998, 1.032]). No other explanatory variable showed a significant association in this model.
Prediction of Neurobehavioral Improvement After Adenotonsillectomy
The 17 children whose disruptive behavior disorder was destined to resolve by follow-up, in comparison with the remaining 64 children, had increased baseline low Pes time (64% vs 47%, P = .021) and a trend toward lower Pes nadirs (−42 vs −35 cm H2O, P = .087, Table 3). No other baseline SDB measures predicted which children would experience resolution. Improvement in mean sleep latency was predicted by the baseline obstructive apnea index and Pes nadir; low Pes time showed a trend. Improvements in behavior and cognition were not predicted significantly by any sleep apnea measure.
Table 3.
—Unadjusted Associations Among 81 Children Between Sleep Apnea Measures at Baseline and Changes in Neurobehavioral Outcomes (Follow-up Minus Baseline)
| Baseline Apnea Measure | Resolution of Disruptive Behavior Disordera | Change in Behavioral Hyperactivity Index | Change in Cognitive Index | Change in Mean Sleep Latency on Multiple Sleep Latency Test | ||||
| Wilcoxon Z | One-sided P Value | Spearman ρ | P Value | Spearman ρ | P Value | Spearman ρ | P Value | |
| Obstructive apnea indexb | −1.12 | .13 | 0.125 | .27 | 0.076 | .50 | 0.22c | .045c |
| Apnea/hypopnea indexb | 0.15 | .44 | 0.049 | .66 | 0.077 | .49 | 0.15 | .19 |
| Respiratory disturbance indexb | 0.28 | .39 | 0.029 | .80 | 0.11 | .33 | 0.14 | .22 |
| Minimum oxygen saturation | −1.02 | .16 | 0.095 | .40 | 0.013 | .91 | −0.14 | .22 |
| Sleep time with oxygen saturation < 90%, % | 0.11 | .46 | 0.054 | .63 | 0.070 | .54 | 0.17 | .12 |
| Esophageal pressure nadir | −1.37c | .087c | 0.076 | .50 | 0.090 | .42 | −0.30c | .0059c |
| Sleep time with esophageal pressure below −10 cm H2O, % | 2.08c | .021c | −0.104 | .36 | 0.095 | .40 | 0.21c | .055c |
Children whose disruptive behavior disorder at baseline resolved by follow-up (coded as 1, n = 17) were compared with all other children (coded as 0, n = 64).
Index = events per hour of sleep, scored following American Academy of Sleep Medicine 2007 recommendations.
Results that show statistical significance or trends.
Logistic regression of disruptive behavior disorder resolution, from baseline to follow-up, on both low Pes time and the AASM-2007 apnea/hypopnea index showed that only low Pes time retained independent predictive value (OR = 1.023 [1.002, 1.045] vs 0.972 [0.908, 1.041]). Similar results emerged from regression on low Pes time and the obstructive apnea index (1.020 [1.001, 1.041] vs 0.943 [0.771, 1.153]). Regression of disorder resolution on low Pes time in addition to the potential confounders showed marginally nonsignificant predictive value for low Pes time (1.017 [0.997, 1.037]), but no other explanatory variable.
A general linear model with change in mean sleep latency as the outcome (follow-up minus baseline value) and both Pes nadir and AASM-2007 apnea/hypopnea index as explanatory variables showed that the Pes nadir retained significance (β = −0.091, SE = 0.043, t = −2.10, P = .039) whereas the AASM-2007 apnea/hypopnea index did not (β = −0.00050, SE = 0.067, t = −0.01, P = .99). Similarly, regression of mean sleep latency change on both Pes nadir and the obstructive apnea index showed that Pes nadir retained a trend toward association (β = −0.076, SE = 0.040, t = −1.91, P = .060) whereas the obstructive apnea index did not (β = 0.141, SE = 0.212, t = 0.67, P = .51). Regression of mean sleep latency change on baseline Pes nadir and the potential confounders showed predictive value for Pes nadir (β = −0.094, SE = 0.033, t = −2.88, P = .0052; model R2 = 0.18) but no other explanatory variable.
Discussion
This sample of 81 children studied intensively just before and about 6 months after adenotonsillectomy shows that quantitative esophageal pressures during baseline polysomnography can help to identify children who have disruptive behavior disorders, and children destined to benefit after surgery from resolution of those psychiatric disorders or improvement in excessive daytime sleepiness. In contrast, baseline esophageal pressure measures did not help to predict concurrent parent-rated hyperactive behavior; a composite cognitive measure of attention/executive functioning and memory/learning; or their improvement after surgery. Whenever SDB measures did predict neurobehavioral morbidity or treatment outcomes, quantitative esophageal pressures appeared to have more robust and independent utility than did standard rates of apneic events. Esophageal pressure monitoring was tolerated by most research volunteers in the larger cohort, as in the past,13 and again proved safe and easy to accomplish by sleep technologists. These observations, in perhaps the largest prospective sample of children studied to date with a gold-standard polysomnographic measure of work of breathing, provide insight into the strengths as well as limitations of laboratory-based sleep studies that are commonly recommended,36,37 if not always obtained,10,38 prior to treatment of suspected SDB in childhood.
Neurobehavioral problems are some of the main morbidities associated with childhood SDB.35,36 These problems show substantial improvement after adenotonsillectomy, but prior studies without quantitative esophageal pressure monitoring have largely failed to show associations between baseline apnea severity measures and neurobehavioral response to surgery. Current results are also notable because they suggest that considerable effort to identify subtle apneic events in children may not always improve clinical use. In our study, the pediatric obstructive apnea index showed predictive value as good or better than that of the AASM-2007 pediatric apnea/hypopnea index, which identified 7 times as many respiratory events. In contrast, among adults, hypopneas in comparison with apneas predict daytime sleepiness with equal strength, if less reliably.39 Our data further suggest that a respiratory disturbance index, including RERAs scored using both nasal and esophageal pressure monitoring,8 may identify negligible numbers of respiratory events beyond those captured by a highly sensitive pediatric apnea/hypopnea index derived from nasal pressure monitoring and 2-breath hypopnea durations. The AASM-2007 pediatric hypopnea and RERA scoring rules, which by necessity are based more on consensus than outcome-based evidence,8 may merit revision as more data become available.
On the other hand, our findings do suggest that quantitative esophageal pressures can supplement qualitative data commonly recorded from nasal and oral thermocouples, nasal pressure, and chest and abdominal excursion. Prolonged increased work of breathing, without discrete apneic events, can still be associated with sleepiness that improves with continuous positive airway pressure.1,4,40 Indeed, computer-identified respiratory cycle-related EEG changes, thought to represent nonvisible inspiratory microarousals outside regions of apnea or hypopnea, appear to worsen with increasing apnea severity,41,42 correlate with esophageal pressure swings,43 diminish with treatment of SDB,41 and explain sleepiness beyond that accounted for by the apnea/hypopnea index.44
Our study has several strengths, including the sample size given relatively intense evaluations of sleep, mental health, behavior, and cognition; the follow-up rate at 7 months (95%); and the rigorous scoring procedures. However, this study also has limitations. Most, but not all, children tolerated esophageal pressure monitoring, and subjects with and without complete data were not identical on every measure (Fig 1). Assessment of esophageal pressures by additional approaches not planned for this study, for example, focus on the ratio for any individual between esophageal pressure during sleep and quiet wakefulness, still merit exploration and could further improve predictive use. Nasal pressure was recorded with a transducer made for this purpose (e-Appendix 1 (424.2KB, pdf) ), but high frequency filtering built into the transducer to eliminate vibration from snoring could have impeded optimal identification of flow limitation. Finally, only neurobehavioral outcomes were examined in our study, though these are among the most salient36 and sensitive to subtle SDB that characterizes many children treated for the condition.33
Our study does confirm meaningful neurobehavioral improvement after adenotonsillectomy, but the nonrandomized design cannot prove surgery was the cause. The ongoing randomized Childhood Adenotonsillectomy Study may provide more conclusive evidence of cause-and-effect,45 but does not monitor esophageal pressures or score RERAs. If the Childhood Adenotonsillectomy Study also finds significant neurobehavioral improvement after surgery, the field may still be left with dual imperatives to improve understanding of mechanisms by which SDB affects brain function, and to devise measures that allow more informative and predictive diagnostic assessments. In the meantime, our results suggest that esophageal pressure monitoring should be considered in clinical sleep laboratory evaluations when a child with suspected SDB also has a disruptive behavior disorder or excessive daytime sleepiness that could motivate a decision to pursue treatment. Use of quantitative esophageal pressure monitoring is unlikely to have significant negative impact,13 but could provide the most useful polysomnographic predictors of whether such a child will experience neurobehavioral benefit from adenotonsillectomy.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Chervin takes responsibility for the integrity of the work.
Dr Chervin: contributed to designing and conducting the study, managing and analyzing data, and drafting or editing the manuscript.
Dr Ruzicka: contributed to designing and conducting the study, managing and analyzing data, and drafting or editing the manuscript.
Dr Hoban: contributed to designing the study, interpreting sleep studies, and drafting or editing the manuscript.
Ms Fetterolf: contributed to designing the study, scoring sleep studies, and drafting or editing the manuscript.
Dr Garetz: contributed to designing and conducting the study, and drafting or editing the manuscript.
Mr Guire: contributed to designing the study, managing and analyzing data, and drafting or editing the manuscript.
Dr Dillon: contributed to designing and conducting the study, and drafting or editing the manuscript.
Dr Felt: contributed to designing and conducting the study, and drafting or editing the manuscript.
Dr Hodges: contributed to designing and conducting the study, and drafting or editing the manuscript.
Dr Giordani: contributed to designing and conducting the study, and drafting or editing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Chervin has received gifts to support education from Philips Respironics and Fisher & Paykel Healthcare Limited, holds a professorship funded in part by Philips Respironics, and serves as a member of the board of directors for the American Academy of Sleep Medicine and the International Pediatric Sleep Association. The remaining authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: This work was performed at the University of Michigan, Ann Arbor, MI.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- AASM
American Academy of Sleep Medicine
- DSM IV
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- low Pes time
percentage of sleep epochs spent with most esophageal pressure swings more negative than −10 cm H2O
- Pes nadir
most negative esophageal pressure swing recorded during a polysomnogram
- RERA
respiratory effort-related arousal
- SDB
sleep-disordered breathing
Footnotes
Funding/Support: This work was supported by the National Institutes of Health [Grants R01 HL080941, R01 HL105999, UL1 RR024986].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Guilleminault C, Winkle R, Korobkin R, Simmons B. Children and nocturnal snoring: evaluation of the effects of sleep related respiratory resistive load and daytime functioning. Eur J Pediatr. 1982;139(3):165-171 [DOI] [PubMed] [Google Scholar]
- 2.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force.. Sleep. 1999;22(5):667-689 [PubMed] [Google Scholar]
- 3.Guilleminault C, Stoohs R, Clerk A, Simmons J, Labanowski M. From obstructive sleep apnea syndrome to upper airway resistance syndrome: consistency of daytime sleepiness. Sleep. 1992;15suppl 6S13-S16 [PubMed] [Google Scholar]
- 4.Guilleminault C, Stoohs R, Clerk A, Simmons J, Labanowski M. Excessive daytime somnolence in women with abnormal respiratory efforts during sleep. Sleep. 1993;16suppl 8S137-S138 [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Stoohs R, Duncan S. Snoring (I). Daytime sleepiness in regular heavy snorers. Chest. 1991;99(1):40-48 [DOI] [PubMed] [Google Scholar]
- 6.Shiomi T, Guilleminault C, Stoohs R, Schnittger I. Obstructed breathing in children during sleep monitored by echocardiography. Acta Paediatr. 1993;82(10):863-871 [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781-787 [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chesson A, Quan SF.for the American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications Vol 1 Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 9.Ayappa I, Norman RG, Krieger AC, Rosen A, O’malley RL, Rapoport DM. Non-invasive detection of respiratory effort-related arousals (REras) by a nasal cannula/pressure transducer system. Sleep. 2000;23(6):763-771 [DOI] [PubMed] [Google Scholar]
- 10.Weatherly RA, Mai EF, Ruzicka DL, Chervin RD. Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: a survey of practice patterns. Sleep Med. 2003;4(4):297-307 [DOI] [PubMed] [Google Scholar]
- 11.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale Press; 1965 [Google Scholar]
- 12.Kushida CA, Giacomini A, Lee MK, Guilleminault C, Dement WC. Technical protocol for the use of esophageal manometry in the diagnosis of sleep-related breathing disorders. Sleep Med. 2002;3(2):163-173 [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Ruzicka DL, Wiebelhaus JL, et al. Tolerance of esophageal pressure monitoring during polysomnography in children. Sleep. 2003;26(8):1022-1026 [DOI] [PubMed] [Google Scholar]
- 14.Littner MR, Kushida C, Wise M, et al. Standards of Practice Committee of the American Academy of Sleep Medicine Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113-121 [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Wang M, Pope DW., Jr Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics. 2001;108(3):693-697 [DOI] [PubMed] [Google Scholar]
- 16.Golan N, Suraya S, Pillar G. Daytime sleepiness in children with attention deficit hyperactive disorder. Sleep. 2003;26supplA127-A127 [Google Scholar]
- 17.Hoban TF, Chervin RD. Assessment of sleepiness in children. Semin Pediatr Neurol. 2001;8(4):216-228 [DOI] [PubMed] [Google Scholar]
- 18.Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung. 1981;159(5):275-287 [DOI] [PubMed] [Google Scholar]
- 19.Ali NJ, Pitson DJ, Stradling JR. Snoring, sleep disturbance, and behaviour in 4-5 year olds. Arch Dis Child. 1993;68(3):360-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen DE, Vandenberg B. Neuropsychological features and differential diagnosis of sleep apnea syndrome in children. J Clin Child Psychol. 1997;26(3):304-310 [DOI] [PubMed] [Google Scholar]
- 21.Ali NJ, Pitson D, Stradling JR. Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr. 1996;155(1):56-62 [DOI] [PubMed] [Google Scholar]
- 22.Weissbluth M, Davis AT, Poncher J, Reiff J. Signs of airway obstruction during sleep and behavioral, developmental, and academic problems. J Dev Behav Pediatr. 1983;4(2):119-121 [DOI] [PubMed] [Google Scholar]
- 23.Rhodes SK, Shimoda KC, Waid LR, et al. Neurocognitive deficits in morbidly obese children with obstructive sleep apnea. J Pediatr. 1995;127(5):741-744 [DOI] [PubMed] [Google Scholar]
- 24.Shaffer D, Fisher P, Dulcan MK, et al. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): description, acceptability, prevalence rates, and performance in the MECA Study. Methods for the Epidemiology of Child and Adolescent Mental Disorders Study. J Am Acad Child Adolesc Psychiatry. 1996;35(7):865-877 [DOI] [PubMed] [Google Scholar]
- 25.Halperin JM, Newcorn JH, Kopstein I, et al. Serotonin, aggression, and parental psychopathology in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(10):1391-1398 [DOI] [PubMed] [Google Scholar]
- 26.Swanson JM, Wigal S, Greenhill LL, et al. Analog classroom assessment of Adderall in children with ADHD. J Am Acad Child Adolesc Psychiatry. 1998;37(5):519-526 [PubMed] [Google Scholar]
- 27.Spencer EK, Alpert M, Pouget ER. Scales for the assessment of neuroleptic response in schizophrenic children: specific measures derived from the CPRS. Psychopharmacol Bull. 1994;30(2):199-202 [PubMed] [Google Scholar]
- 28.Overall JE, Campbell M. Behavioral assessment of psychopathology in children: infantile autism. J Clin Psychol. 1988;44(5):708-716 [DOI] [PubMed] [Google Scholar]
- 29.National Institute of Mental Health. Children’s Psychiatric Rating Scale–NIMH Psychopharmacol Bull. 1985;21770 [Google Scholar]
- 30.Conners CK. Conners’ Rating Scales–Revised. North Tonawanda, NY: Multi-Health Systems Publishing; 1997 [Google Scholar]
- 31.Gadow KD, Sprafkin J. Child Symptom Inventory-4. Stony Brook, NY: Checkmate Plus; 1994 [Google Scholar]
- 32.Sprafkin J, Gadow KD. Early Childhood Symptom Inventory Manual. Stony Brook, NY: Checkmate Plus; 1996 [Google Scholar]
- 33.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769-e778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korkman M, Kirk U, Kemp S. A Developmental Neuropsychological Assessment. San Antonio, TX: Harcourt Brace Jovanovich; 1998 [Google Scholar]
- 35.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed Westchester, IL: American Academy of Sleep Medicine; 2005 [Google Scholar]
- 36.Section on Pediatric Pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. American Academy of Pediatrics Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109(4):704-712 [DOI] [PubMed] [Google Scholar]
- 37.Aurora RN, Zak RS, Karippot A, et al. American Academy of Sleep Medicine Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: survey of current practice. Laryngoscope. 2006;116(6):956-958 [DOI] [PubMed] [Google Scholar]
- 39.Chervin RD, Aldrich MS. Characteristics of apneas and hypopneas during sleep and relation to excessive daytime sleepiness. Sleep. 1998;21(8):799-806 [PubMed] [Google Scholar]
- 40.Guilleminault C, Stoohs R, Kim YD, Chervin R, Black J, Clerk A. Upper airway sleep-disordered breathing in women. Ann Intern Med. 1995;122(7):493-501 [DOI] [PubMed] [Google Scholar]
- 41.Chervin RD, Burns JW, Subotic NS, Roussi C, Thelen B, Ruzicka DL. Correlates of respiratory cycle-related EEG changes in children with sleep-disordered breathing. Sleep. 2004;27(1):116-121 [DOI] [PubMed] [Google Scholar]
- 42.Chervin RD, Burns JW, Subotic NS, et al. Respiratory cycle-related EEG changes (RCREC) predict neurobehavioral outcomes in childhood OSA. Sleep. 2004;27supplA99 [Google Scholar]
- 43.Chervin RD, Malhotra RK, Burns JW. Respiratory cycle-related EEG changes during sleep reflect esophageal pressures. Sleep. 2008;31(12):1713-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chervin RD, Burns JW, Ruzicka DL. Electroencephalographic changes during respiratory cycles predict sleepiness in sleep apnea. Am J Respir Crit Care Med. 2005;171(6):652-658 [DOI] [PubMed] [Google Scholar]
- 45.Redline S, Amin R, Beebe DW, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34(11):1509-1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement