Abstract
Background:
The mast cell localization to airway smooth muscle (ASM) bundle in asthma is important in the development of disordered airway physiology. Thymic stromal lymphopoietin (TSLP) is expressed by airway structural cells. Whether it has a role in the crosstalk between these cells is uncertain. We sought to define TSLP expression in bronchial tissue across the spectrum of asthma severity and to investigate the TSLP and TSLP receptor (TSLPR) expression and function by primary ASM and mast cells alone and in coculture.
Methods:
TSLP expression was assessed in bronchial tissue from 18 subjects with mild to moderate asthma, 12 with severe disease, and nine healthy control subjects. TSLP and TSLPR expression in primary mast cells and ASM was assessed by immunofluorescence, flow cytometry, and enzyme-linked immunosorbent assay, and its function was assessed by calcium imaging. The role of TSLP in mast cell and ASM proliferation, survival, differentiation, synthetic function, and contraction was examined.
Results:
TSLP expression was increased in the ASM bundle in mild-moderate disease. TSLP and TSLPR were expressed by mast cells and ASM and were functional. Mast cell activation by TSLP increased the production of a broad range of chemokines and cytokines, but did not affect mast cell or ASM proliferation, survival, or contraction.
Conclusions:
TSLP expression by the bronchial epithelium and ASM was upregulated in asthma. TSLP promoted mast cell synthetic function, but did not contribute to other functional consequences of mast cell-ASM crosstalk.
Asthma affects 5% to 10% of adults, and 10% of those with asthma have severe disease.1 These patients consume over 50% of the health-care resources.2 Asthma is characterized by variable airflow obstruction; airway hyperresponsiveness and mast cell-airway smooth muscle (ASM) interactions are important in the development of disordered airway physiology.3,4
Thymic stromal lymphopoietin (TSLP) is implicated in both the innate and adaptive immune response.5 T-helper cell type 2 (Th2) polarization of the inflammatory response is an important component of the asthma paradigm.5,6 A major effector axis resulting in this polarization is the recognition of allergen presented by dendritic cells in local lymph nodes to the CD4+ T cell. The differentiation of naive T cells or reactivation of memory T cells is dependent upon costimulatory molecules, such as OX40, and their cognate ligand OX40L,5‐10 which is increased in the bronchial submucosa in asthma9 and upregulated by TSLP.5,8 TSLP messenger RNA (mRNA) is upregulated in the bronchial epithelium and submucosa in asthma in response to allergen, viruses, and environmental stimuli.11 TSLP is expressed by mast cells12,13 and ASM14,15 supporting the view that TSLP may have a role beyond Th2 polarization and may be important in mast cell-ASM interactions.
We hypothesized that expression of TSLP and TSLP receptor (TSLPR) is increased in asthma and that this axis plays a role in mast cell-ASM crosstalk. To test our hypothesis, we investigated TSLP expression in bronchial tissue across the spectrum of asthma severity compared with healthy control subjects, and defined TSLP and TSLPR expression and function by ASM and mast cells.
Materials and Methods
Subjects
Subjects were recruited from Leicester, England. Asthmatic subjects had a consistent history and objective evidence of asthma.16 Asthma severity was defined by Global Initiative for Asthma (GINA) treatment steps (mild-moderate GINA 1-3, severe GINA 4-5).17 Subjects underwent clinical characterization including sputum induction18 and video-assisted fiber-optic bronchoscopic examination.19 The study was approved by the Leicestershire Ethics Committee, approval number 4977, and all patients gave their written informed consent.
Cell Isolation and Culture
Pure ASM bundles were isolated from bronchoscopic samples (n = 14 asthma, n = 8 nonasthma) and from lung resection (n = 1). ASM was cultured and characterized as previously described.16,20 The human lung mast cells (HLMCs) were isolated and cultured16,20 from nonasthmatic lung (n = 10). The human mastocytoma cell line (HMC-1) was a generous gift from J. Butterfield, MD, (Mayo Clinic).
TSLP/TSLPR Expression
Sequential 2-μm sections were cut from glycomethacrylate-embedded bronchial biopsies and stained using a sheep polyclonal antihuman TSLP antibody (R&D Systems), monoclonal antibody mast cell tryptase (clone no. AA1; Dako), and appropriate isotype control sheep IgG (R&D Systems) and mouse IgG1 (Dako), respectively. The number of positively stained nucleated cells was enumerated per mm2 of the lamina propria by a blinded observer. TSLP expression by the ASM or epithelium was also assessed using (1) a semiquantitative intensity score of no staining = 0, low = 1, moderate = 2, and high = 3 and (2) thresholding of red hue using Image J software (ImageJ 1.40g/java 1.6.0_05; NIH Image). The red/green/blue image was converted to hue/saturation/brightness stack. Hue image was thresholded to include pixels with non-red hue 30-210 (scale of 0-255) for all images, and the percentage area covered by red hue pixels was calculated by deducting non-red pixels from the total.21 Tryptase was colocalized with TSLP within the ASM using sequentially cut sections and a minimum area of 0.1 mm2 was considered assessable as described previously.19
TSLP and TSLPR expression was assessed in ASM, HLMC, and HMC-1 cells by flow cytometry and immunofluorescence. Isotype control subjects were used where appropriate (Dako). TSLP protein release was measured in ASM, HLMC, HMC-1, and sputum by enzyme-linked immunosorbent assay (R&D Systems Inc). Recombinant TSLP recovery was unaffected by the mucolytic dithiothreitol. TSLP and TSLPR mRNA levels were examined in ASM cells using the Human Genome U133A probe array (GeneChip; Affymetrix).22
Functional Assays
Changes in intracellular calcium [Ca21]i concentrations in ASM cells in response to recombinant human TSLP (rh-TSLP) were measured by fluo-3/Fura Red acetoxymethyl ester ratios (Invitrogen) using flow cytometry. ASM cells were primed with rh-TSLP and collagen gel contraction assessed. Gel surface area was measured using ImageJ by a blinded observer. The concentration of a panel of cytokines and chemokines were measured in ASM and HMC-1 cells stimulated with TSLP by electrochemiluminescence detection and pattern arrays (Mesoscale Discovery).
Proliferation and Apoptosis
ASM proliferation was assessed by cell counts and the CellTiter 96 Aqueous One Solution with the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H (MTS) (Promega) according to the manufacturer’s instructions. Morphologic features of apoptosis (nuclear condensation and fragmentation) were assessed by DAPI (4′,6-diamidino-2-phenylindole) staining.
Coculture ASM and HLMC
HLMC proliferation was assessed by carboxyfluorescein succinimidyl ester (CFSE) (CellTrace Proliferation Kit; Invitrogen) in HLMC cocultured with ASM cells. Changes in ASM phenotype were studied in the presence of HLMC lysate and stained with α-smooth muscle actin (Sigma-Aldrich).
Statistical Analysis
Statistical analysis was performed using PRISM, version 4 (GraphPad Software). Parametric data were presented as mean (SEM) and nonparametric data as median (interquartile range [IQR]). Parametric data were analyzed with paired and unpaired t tests or one-way analysis of variance and the Tukey posthoc test for intergroup comparison as appropriate. Nonparametric data were analyzed using Mann-Whitney or Kruskal-Wallis tests and the Dunn test for posthoc comparison as appropriate. Correlations between parametric data were assessed by Pearson correlation and nonparametric data by Spearman rank correlation. A P value < .05 was considered significant.
Results
TSLP Expression in the ASM Bundle in Asthma
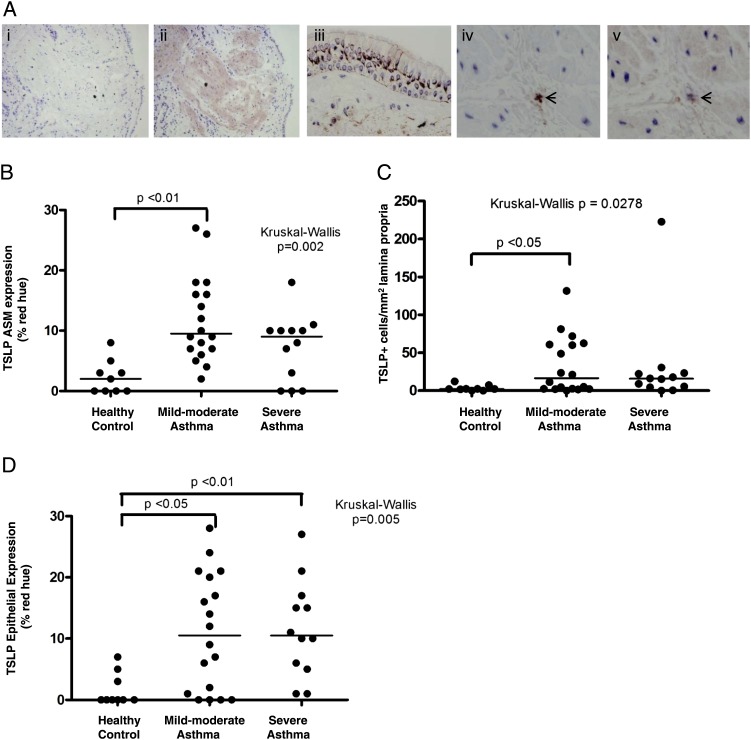
The clinical characteristics of the subjects assessed by immunohistochemistry are shown in Table 1. TSLP staining was apparent in the epithelium, ASM, and cells within the lamina propria (Fig 1A).
Table 1.
—Clinical Characteristics of Asthmatic and Healthy Control Subjects
| Normal | Mild-Moderate Asthma GINA 1-3 (1 = 11, 2 = 1, 3 = 6) | Severe Asthma GINA 4-5 (4 = 7, 5 = 5) | |
| No. | 9 | 18 | 12 |
| Age, ya | 45 (27) | 53 (29) | 51 (14) |
| Male (female) sex | 5 (4) | 7 (11) | 4 (8) |
| Never/current/ex-smokers | 8/1/0 | 14/4/0 | 10/0/2 |
| Atopy, % | 44 | 67 | 75 |
| Inhaled corticosteroids,a μg/d beclomethasone equivalent | 0 | 0 (0-500) | 1,800 (1,600-2,000) |
| Oral corticosteroid,a mg/d | 0 | 0 | 0 (0-10) |
| PC20 FEV1,b mg/mL | > 16 | 0.5 (0.2-1.3)d | 0.8 (0.3-2.3)d |
| FEV1% predictedc | 94 (3) | 82 (7) | 77 (7)d |
| Pre-BD FEV1/FVC,c % | 82 (2) | 72 (2)d | 67 (5)d |
| BD,c % | 0 (0) | 11 (4)d | 9 (2)d |
| Sputum cell counts | |||
| TCCc | 0.9 (0.1) | 2.4 (0.5) | 5.9 (1.4)d |
| Eosinophil,a % | 0.4 (0.8) | 1.0 (5.2) | 4.6 (18.0)d |
| Neutrophil,c % | 56 (12) | 48 (7) | 65 (7) |
| Macrophage,c % | 37 (12) | 39 (7) | 18 (6)d |
| Lymphocyte,a % | 1 (5) | 1 (2) | 0.3 (3.1) |
| Epithelial cells,a % | 1 (12) | 4 (11) | 1 (2) |
| TSLP expression in bronchial biopsy | |||
| Epithelium,a SQS | 0 (0.3) | 0.75 (1.9)d | 0.75 (1.3)d |
| Epithelium,a % red hue | 0 (4) | 11 (19)d | 11 (12)d |
| Lamina propria,a cells/mm2 | 2.2 (4.1) | 16.3 (59.5)d | 15.8 (17.7) |
| Airway smooth muscle,a SQS | 0.25 (0.4) | 0.88 (0.7)e | 0.75 (0.9) |
| Airway smooth muscle,a % red hue | 2 (4) | 10 (10)d | 9 (9) |
BD = bronchodilator; GINA = Global Initiative for Asthma; IQR = interquartile range; PC = provocation concentration; SQS = semiquantitative score; TCC = total cell count.
Median (IQR).
Geometric mean (95% CI).
Mean (SE).
P < .05 compared with control subjects.
P < .001 compared with control subjects.
Figure 1.
Mast cells in the ASM bundle express TSLP. A, Representative photomicrographs of a bronchial biopsy specimen from asthmatic subjects (original magnification, ×100) showing negative isotype control (i), TSLP staining in ASM bundles (ii), and epithelium (magnification ×400, iii). Sequential sections of the ASM bundle highlighting the same cells across sections showing mast cell tryptase-positive staining (iv) and TSLP (v). The short arrows illustrate the cells that are both tryptase+ and TSLP+ within the ASM bundle. B, Dot plot showing ASM TSLP expression determined by % red hue. C, TSLP-positive cells per square millimeter of lamina propria in subjects with and without asthma. D, TSLP expression in the epithelium by % red hue. Horizontal bar represents median. ASM = airway smooth muscle; TSLP = thymic stromal lymphopoietin.
TSLP expression was significantly increased in the ASM and lamina propria in mild to moderate asthma and in the epithelium across all severities (Figs 1B-D, Table 1). There was a good correlation between TSLP expression assessed by the semiquantitative score and by the percentage of hue thresholding (r = 0.94, P < .001). The intensity of TSLP expression (percentage of red hue) in the ASM and epithelium were correlated (r = 0.77, P < .0001) and both were related to the number of epithelial cells in the sputum (r = 0.58, P < .01 and r = 0.64, P < .001, respectively). TSLP+ cells/mm2 in the lamina propria was also correlated with TSLP intensity (percentage of red hue) in the ASM, epithelium (r = 0.49, P = .002 and r = 0.46, P = .003, respectively) and sputum epithelial cell counts (r = 0.55, P < .01). There were no correlations with other sputum cell counts or with lung function. The median (IQR) proportion of TSLP+ cells in the lamina propria that were mast cells was 40% (77). All the inflammatory cells in the ASM that colocalized to TSLP were mast cells. Sputum TSLP was measured in 12 patients with asthma and four healthy control subjects and was below limit of detection in all except for two asthmatics.
TSLP Expression by Primary Cells
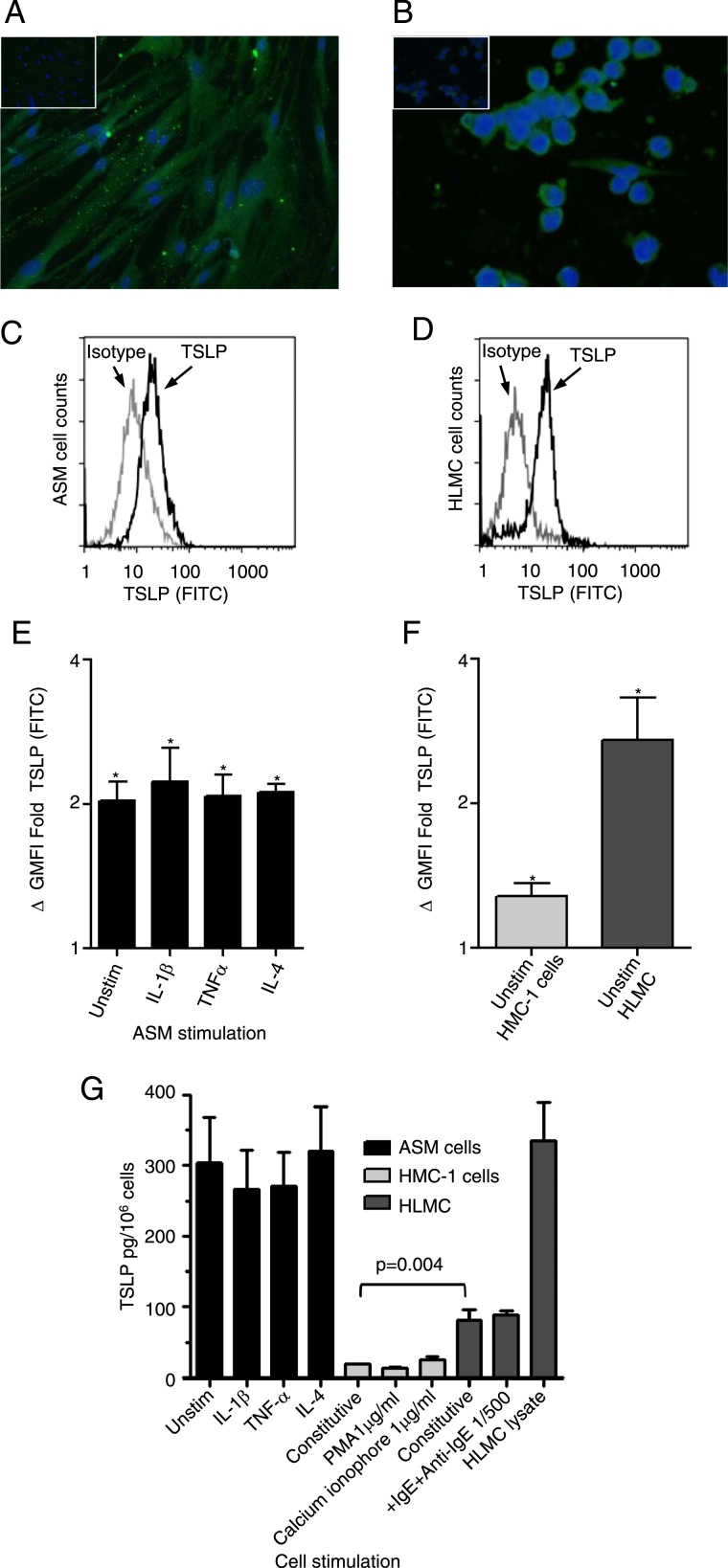
There was no difference in expression between ASM cells derived from patients with asthma and from normal control subjects in all studies (data not shown). Therefore, normal and asthmatic ASM data were combined throughout. TSLP expression was identified in ASM and human mast cells by immunofluorescence (Figs 2A, 2B) and flow cytometry (Figs 2C-F). The expression of TSLP in unstimulated ASM cells compared with isotype control was geometric mean fluorescence intensity (mean ΔGMFI) fold (95% CI) 2 (1.6-2.6); P < .001, n = 8, which was not affected by stimulation with IL-1β, tumor necrosis factor-α (TNF-α), or IL-4 (Fig 2E). TSLP expression was also increased in unstimulated HMC-1 cells ΔGMFI fold (95% CI) 1.3 (1.07-1.5), P = .01, n = 5 and HLMC 2.8 (0.6-5), P = .03, n = 3, compared with isotype control (Fig 2F). TSLP was measurable in unstimulated ASM cells (305 ± 63 pg/106 cells, n = 11), HMC-1 cell supernatants (19 ± 1 pg/106 cells, n = 4), HLMC supernatants (82 ± 14 pg/106 cells, n = 3), and HLMC cell lysates (334.8 ± 54 pg/106 cells, n = 3), but was not affected by stimulation (Fig 2G). Resting HLMC released significantly more TSLP in cell supernatant compared with the resting HMC-1 cell mean difference ([95% CI] 59 [23-153] pg/106 cells, P = .004) (Fig 2G). Quantified by gene array analysis, TSLP mRNA was present in all ASM donors (1.47% ± 0.32%) of GAPDH mRNA (n = 11).
Figure 2.
TSLP expressed by ex vivo ASM and mast cells. A, B, TSLP expression was confirmed in (A) ASM and (B) HMC-1 cells by immunofluorescence (nuclei stained blue, TSLP stained green, isotype shown as insert, magnification × 400, n = 3). C, D, The example fluorescent histograms for (C) ASM and (D) HLMC cells represent populations of TSLP (black line) plotted with the corresponding isotype control (gray line). E, The expression of intracellular TSLP was investigated by flow cytometry on unstimulated and stimulated ASM cells with 10 ng/mL proinflammatory cytokines IL-1β, TNFα, and IL-4 over 20 h (n = 8; *P < .05 compared with isotype control). F, Expression was also seen in unstimulated mast cells (n = 3-5; *P < .05 compared with isotype control). G, TSLP protein release was measured by enzyme-linked immunosorbent assay (ELISA) in ASM (n = 8-11, unstimulated and stimulated for 20 h), HMC-1 (n = 4), and HLMC (n = 3-5) supernatants (and lysate for HLMC). HMC-1 cell protein release was studied in cell supernatants following PMA stimulation with 1 μg/mL or calcium ionophore 1 μg/mL over 24 h and HLMC IgE sensitized (2.4 μg/mL) and then activated with anti-IgE (1:500) for 1 h. All data presented as mean ± SEM. Statistical differences were assessed using the t tests, and P values are as shown FITC = fluorescein isothiocyanate; GMFI = geometric mean fluorescence intensity; HLMC = human lung mast cell; HMC-1 = human mastocytoma cell line; PMA = phorbol myristate acetate; TNF = tumor necrosis factor; Unstim = unstimulated. See Figure 1 legend for expansion of other abbreviations.
TSLPR Expression by Primary Cells
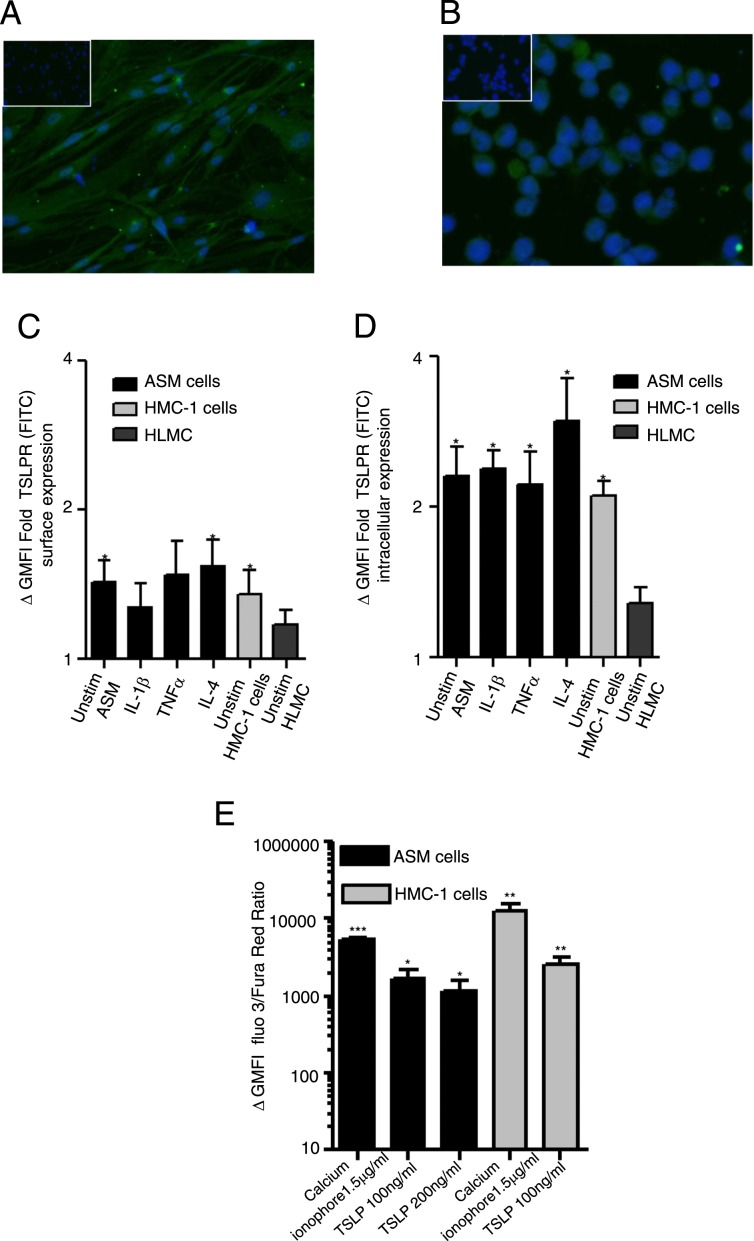
TSLPR expression was expressed in ASM and mast cells both by immunofluorescence (Figs 3A, 3B) and flow cytometry (Figs 3C, 3D). The expression of surface TSLPR in unstimulated ASM cells compared with isotype control was mean ΔGMFI fold (95% CI) 1.5 (1.1-1.8), P = .01, n = 7, which was not affected by stimulation with IL-1β, TNF-α, or IL-4 (Fig 3C), for unstimulated HMC-1 cells 1.4 (0.1-2), P = .03, n = 7, and for HLMC 1.8 (0.9-1.4); n = 4. TSLPR intracellular expression was detected in ASM and HMC-1 cells and was unaffected by stimulation (Fig 3D). Functional responses of TSLPR were studied; cells were loaded with fluo-3 and Fura Red and stimulated with rh-TSLP at 100-200 ng/mL to trigger Ca21 flux through the membrane linked receptor (TSLPR) or calcium ionophore which induces a rapid release of Ca21 from intracellular stores (Fig 3E). The response of HMC-1 cells and ASM cells stimulated with rh-TSLP showed a similar pattern with an increase in GMFI (fluo 3/Fura Red ratio, Fig 3E) for ASM cells stimulated with 100 ng/mL (P = .03, n = 5) and 200 ng/mL (P = .04, n = 8) rh-TSLP (Fig 3E) compared with baseline.
Figure 3.
TSLPR expressed by ex vivo ASM and mast cells. A, B, TSLPR expression was confirmed in (A) ASM and (B) HMC-1 cells by immunofluorescence (nuclei stained blue, TSLPR stained green, isotype shown as insert, magnification × 400, n = 3). C, D, The expression of (C) surface and (D) intracellular TSLPR was investigated by flow cytometry on unstimulated ASM, mast cells, and stimulated ASM cells with 10 ng/mL proinflammatory cytokines IL-1β, TNFα, and IL-4 over 20 h (n = 3-7, *P < .05 compared with isotype control). TSLPR activation was studied by calcium flux assays in human ASM and HMC-1 cells. Cells were loaded with fluo-3 and Fura Red and baseline calcium levels were recorded for 60 s followed by the addition of either 100-200 ng/mL recombinant human TSLP (rh-TSLP) or 1.5 μg/mL calcium ionophore, (positive control) over a further 180 s (n = 5-8). E, The ΔGMFI was determined by the difference between the total stimulated GMFI minus the matched baseline GMFI for each cell type (*P < .05, **P < .01, ***P < .001 compared with baseline GMFI). All data presented as mean ± SEM. Statistical differences were assessed using the t tests. TSLPR = thymic stromal lymphopoietin receptor. See Figure 1 and 2 legends for expansion of other abbreviations.
Neutralization of TSLP in Primary Cells
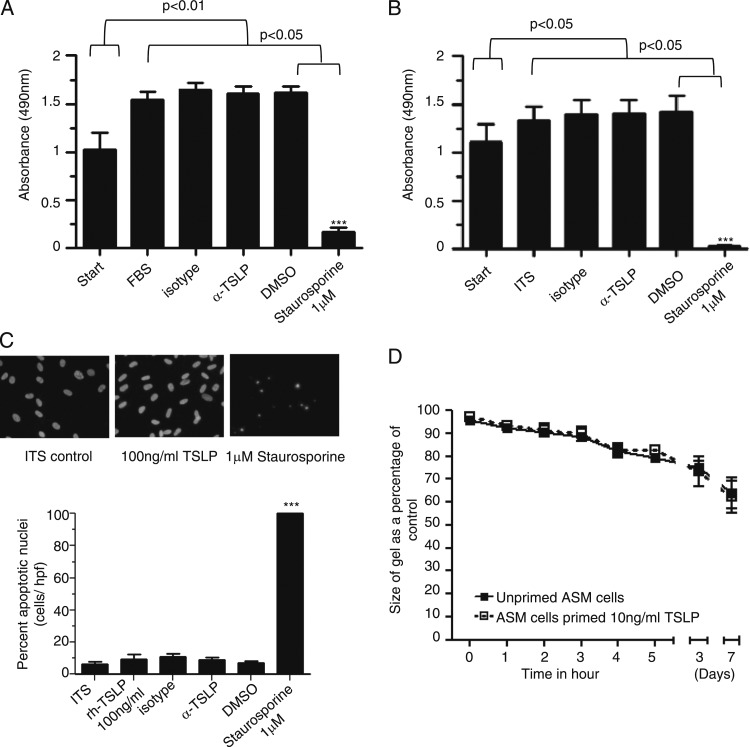
ASM assessed by the MTS assay demonstrated a significant increase in cell proliferation/metabolic activity after 96 h for cells cultured in either 10% fetal bovine serum (FBS) (P = .004) or ITS media compared with ASM at 0 h (P = .04) (Figs 4A, 4B). Recombinant TSLP (12.5-100 ng/mL) had no effect on the MTS assay in the presence of FBS and ITS media (data not shown). Neutralizing TSLP also had no effect on ASM metabolic activity both in FBS and ITS media (Figs 4A, 4B), and in contrast to the staurosporine positive control in FBS and ITS media (P < .0001, n = 6).
Figure 4.
Neutralization of TSLP in ex vivo human cells. A, B, ASM cell metabolic activity or proliferation in the presence of (A) 10% FBS media and (B) serum-free ITS media was assessed over 96 h in the presence of isotype control, α-TSLP 1 μg/mL, DMSO, and 1μM staurosporine (positive control, n = 6). C, Representative micrographs of ASM cells showing DAPI (4′,6-diamidino-2-phenylindole) staining of cells in the presence of ITS media alone,100 ng/mL rh-TSLP, and 1μM staurosporine over 96 h. The percentage of apoptotic nuclei of ASM cells identified by nuclear morphology over 96 h of ASM cells alone in ITS, presence of rh-TSLP 100 ng/mL, α-TSLP, isotype, 1μM staurosporine, and DMSO control (n = 6). Comparisons were made between DMSO control vs staurosporin treated cells, ***P < .001. ASM cells were primed with TSLP (10 ng/mL) over 48 h and impregnated in the collagen gel and left in the gel without stimulation for 7 days (n = 4) to assess collagen gel contraction (D). All data presented as mean ± SEM. Statistical differences were assessed using the t tests and P values are as shown. DMSO = dimethyl sulfoxide; FBS = fetal bovine serum; ITS = insulin transferrin sodium selenite. See Figure 1-3 legends for expansion of other abbreviations.
The percentage of ASM nuclei showing nuclear condensation and fragmentation characteristic of apoptosis, detected by DAPI staining, was unaffected by incubation with 100 ng/mL TSLP for 96 h (untreated, 6.3% ± 1.4% vs 100 ng/mL TSLP 9.6 ± 2.7, n = 6, P = .2). In the presence of staurosporine (1 μM, 96 h), a positive control ASM cells showed nuclear morphology characteristic of cells undergoing apoptosis compared with dimethyl sulfoxide (DMSO) control (P < .0001, n = 6) (Fig 4C). ASM cells primed for 48 h with rh-TSLP (10 ng/mL) embedded within collagen gels did not result in altered gel contraction compared with unprimed ASM over 7 days (n = 4) (Fig 4D).
ASM-Mast Cell Coculture
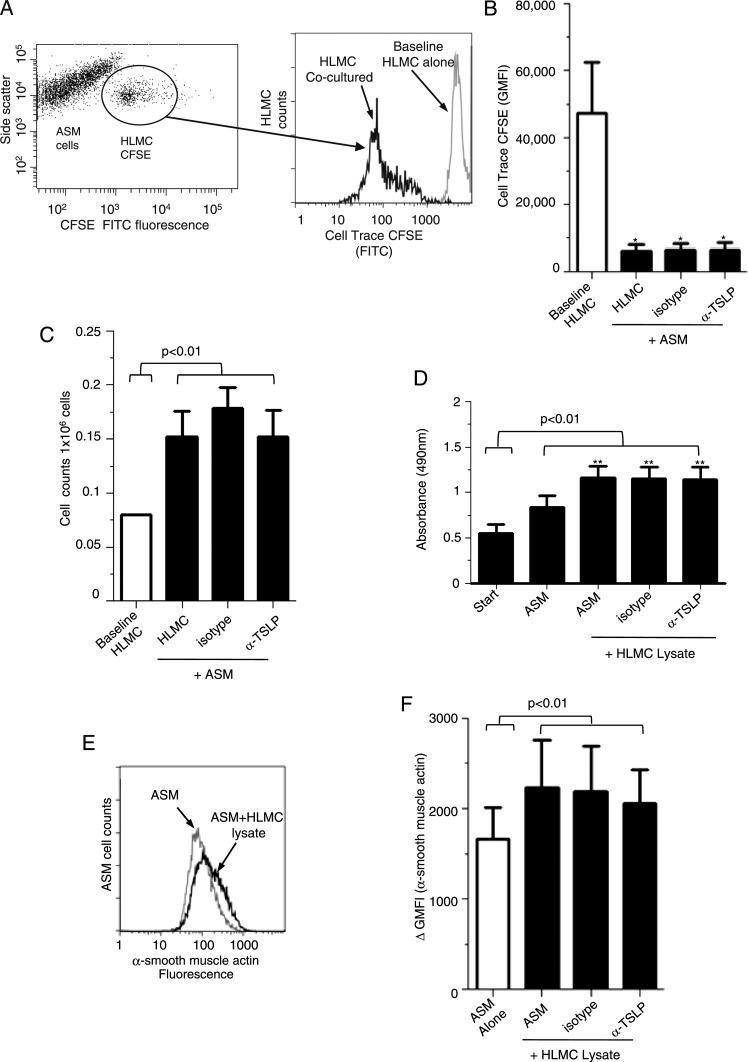
To track mast cells cocultured with ASM over 7 days, mast cells were labeled with fluorescent marker CFSE, a stable dye that is not passed between cells upon adhesion. Using flow cytometry, CFSE-labeled mast cells were gated (Fig 5A) and CFSE GMFI analyzed compared with cells cocultured with isotype and anti-TSLP (Fig 5B). Cocultured HLMC survived and proliferated with ASM cells alone and in the presence of anti-TSLP determined by CFSE (Fig 5B) and cell counts (Fig 5C).
Figure 5.
Mast cell coculture and lysate. A, Representative dot plot for ASM and HLMC (prelabeled with CFSE-FITC) cocultured for 7 days. After 7 days, coculture-labeled CFSE HLMC were gated and analyzed for CFSE GMFI intensity compared with HLMC baseline. Flow cytometric histogram illustrating CFSE fluorescence at baseline for HLMC alone and then cocultured for 7 days in the presence of ASM cells. B, HLMC CFSE proliferation was assessed over 7 days for HLMC cocultured with ASM with and without isotype control and α-TSLP (1 μg/mL) n = 6). Comparisons were made between HLMC GMFI at baseline compared with HLMC cocultured over 7 days. C, The number of HLMC present over 7 days in coculture ± isotype, α-TSLP 1 μg/mL was assessed and comparisons were made between baseline HLMC counts vs cocultured HLMC. D, ASM cell metabolic activity/proliferation was assessed over 7 days in the presence of ASM1HLMC lysate, ± isotype, α-TSLP 1 μg/mL (n = 4). E, Example fluorescent histogram of ASM cells stained with α-smooth muscle actin with ASM cells alone (gray) or ASM coculture with HLMC lysate (ratio-1 HLMC lysate:4 ASM, black line). F, The ΔGMFI of α-smooth muscle actin in ASM cells with and without neutralizing TSLP over 7 days (n = 4). All data presented as mean ± SEM. Statistical differences were assessed using the t tests and P values are as shown *P < .05, **P < .01. CFSE = carboxyfluorescein succinimidyl ester. See Figure 1-4 legends for expansion of other abbreviations.
ASM cells cocultured with HLMC lysate (1:4 ratio of mast cells:ASM) showed a significant increase in metabolic activity compared with ASM alone over 7 days compared with baseline (n = 4) (Fig 5D). TSLP neutralization had no effect on ASM metabolic activity mediated by HLMC lysates (Fig 5D). ASM cells cultured with HLMC lysate showed increased α-smooth muscle actin GMFI compared with ASM cells, but was also unaffected by anti-TSLP (Figs 5E, 5F).
Chemokine and Cytokine Release in Human Cultured Cells
HMC-1 cell release of most chemokines and cytokines was significantly upregulated following TSLP (1 ng/mL) activation for 24 h (Table 2). ASM release of cytokines and chemokines was not upregulated by TSLP activation over 24 h (1-10 ng/mL, data not shown).
Table 2.
—Mean (SEM) Chemokine and Cytokine Release by HMC-1 (pg/106 Cells) With and Without TSLP Stimulation (1 ng/mL) Over 24 h
| Chemokine/Cytokine | Unstimulated | Stimulated | P Value |
| CCL11 | 585 (110) | 2,994 (60) | < .001 |
| CCL4 | 374 (9) | 11,362 (267) | < .001 |
| CCL26 | 411 (121) | 2,618 (256) | .003 |
| CCL17 | 966 (52) | 2,146 (123) | .003 |
| CXCL10 | 798 (30) | 2,380 (112) | < .001 |
| CXCL8 | 63 (15) | 4,970 (128) | < .001 |
| CCL2 | 5,616 (173) | 16,308 (242) | < .001 |
| CCL22 | 922 (97) | 2,074 (66) | < .001 |
| CCL13 | 419 (143) | 1,553 (9) | .004 |
| CCL5 | 4.5 (6) | 219 (14) | < .001 |
| IFN-γ | 125 (52) | 105 (9) | .74 |
| IL-1β | 0 | 4.8 (2) | .121 |
| IL-2 | 102 (2) | 292 (39) | .016 |
| IL-4 | 36 (18) | 25 (14) | .662 |
| IL-5 | 0 | 132 (8) | < .001 |
| IL-10 | 80 (4) | 354 (6) | < .001 |
| IL-12p70 | 84 (22.84) | 96 (35) | .79 |
| IL-13 | 36 (36) | 325 (19) | .005 |
| TNF-α | 81 (4) | 236 (34) | .0138 |
HMC-1 = human mastocytoma cell line; IFN = interferon; TNF = tumor necrosis factor; TSLP = thymic stromal lymphopoietin.
Discussion
We report for the first time, to our knowledge, that TSLP expression by ASM is increased in mild to moderate asthma, and that mast cells within the ASM bundle express TSLP. We confirm that the bronchial epithelium is an important source of TSLP. Primary ASM and mast cells also express TSLP and TSLPR constitutively. We confirmed that mast cells cocultured with ASM cells survive and proliferate, but that this was not affected by TSLP. Additionally, ASM contraction and synthetic capacity was not modulated by TSLP. In contrast, TSLP potently activated mast cells to release an array of cytokines and chemokines, suggesting that ASM-derived TSLP may play a role in mast cell activation.
TSLP is both necessary and sufficient for the development of Th2 cytokine-associated inflammation of the airways in rodents. Mice expressing a TSLP transgene in the airway epithelium develop a spontaneous, progressive inflammatory disease with all the characteristics of human asthma,23 whereas direct intranasal delivery of TSLP (in the presence of antigen) leads to rapid onset of severe disease.24 In human disease, genetic analysis has shown an association of polymorphisms in TSLP with asthma and airway hyperresponsiveness, IgE concentrations, and eosinophilia.25‐27 In addition, asthmatics have higher concentrations of TSLP in their lungs.13 TSLP is expressed mainly by epithelial cells at barrier surfaces.28 Factors known to be involved in either the development of asthma or the exacerbation of existing disease can induce TSLP expression in airway epithelial cells such as inflammatory cytokines and respiratory viruses.28,29 We confirm here that TSLP expression was upregulated by the bronchial epithelium in asthma, independent of disease severity. Interestingly, the intensity of TSLP expression was related to the number of epithelial cells in the sputum supernatant, suggesting that TSLP expression was associated with epithelial damage. Other cells express TSLP including mast cells12,13 and ASM.14,15 TSLP expression was upregulated in the ASM bundle in chronic obstructive pulmonary disease14 and increases with exposure to cigarette smoke.30 Here, we extend these observations to demonstrate for the first time that the number of cells in the lamina propria and the intensity of TSLP staining in the ASM bundle are also upregulated in mild to moderate, but not severe, asthma. Whether the relatively attenuated TSLP expression in severe disease represents the response to high-dose corticosteroid therapy or a feature of disease severity warrants further investigation.
Mast cell-ASM crosstalk has been implicated in the development of disordered airway physiology in asthma. Indeed, the number of mast cells within the ASM bundle is related to the degree of airway hyperresponsiveness.19 Coculture of primary mast cells with ASM promotes mast cell activation,31 differentiation,32 survival and proliferation,31,32 and ASM contractility.33 We considered here whether the TSLP/TSLPR axis plays a role in these interactions. We confirmed that mast cells and ASM express both TSLPR and its ligand and that the receptor is functional using calcium imaging. Interestingly, activation of ASM TSLPR did not affect the function of these cells in terms of proliferation, survival, contraction, or synthetic response. Similarly, TSLP did not affect mast cell survival or proliferation. In contrast TSLP had a marked effect upon mast cell synthesis of chemokines and cytokines. This is consistent with previous work using cord11 or peripheral blood-derived differentiated mast cell progenitors.34 Interestingly, we have previously reported that mast cells within the ASM-bundle are activated with increased expression of important Th2 cytokines including IL-13.35 One possible explanation for this upregulated IL-13 expression by mast cells is that ASM-derived TSLP in the asthmatic airway may promote mast cell activation. Indeed, we confirmed that TSLP modulated mast cell production of IL-13 and in coculture, this is in part dependent upon ASM-derived TSLP.15 Therefore, TSLP may present a novel target for asthma that may exert effects beyond epithelial repair and Th2 polarization to include the potential to modulate mast cell-ASM cell interactions.
Possible criticisms of this study are that the in vivo findings are cross-sectional and the mechanistic studies are ex vivo. We are, therefore, unable to determine whether modulating TSLP expression by mast cells or ASM within the asthmatic airway would lead to important clinical outcomes. We were unable to demonstrate a relationship between airflow obstruction and TSLP expression questioning the role of this axis in airway dysfunction. Interestingly, we did not find differences in TSLP expression in vitro in primary cells from health and disease, suggesting that in vivo either upregulation of TSLP in asthma or downregulation in health is a consequence of differences in the microenvironment.
In conclusion, we report here that both mast cells and ASM express TSLP and TSLPR. Mast cell-ASM interactions are important in the pathogenesis of asthma and coculture of these cells leads to reciprocal activation and differentiation. We found that TSLP potently activates mast cells, but we were unable to demonstrate further roles for TSLP in mast cell-ASM crosstalk. The mechanisms leading to mast cell activation within the ASM bundle and the consequences of this activation are likely to be manifold and, thus, the relative importance of the TSLP axis in these interactions requires further investigation. The results from specific TSLP therapy in humans are eagerly awaited.
Acknowledgments
Author contributions: Dr Brightling guarantees the integrity of the work.
Dr Kaur: contributed to study design and culture of all ex vivo cell experiments, studied TSLP and TSLPR expression and function, conducted the statistical analysis, ran the gene array system, contributed to the writing of the manuscript, and approved the final decision for submission.
Ms Doe: conducted all immunohistochemistry, contributed to the writing of the manuscript, and approved the final decision for submission.
Dr Woodman: ran the electrochemiluminescence arrays (Mesoscale Discovery), contributed to the writing of the manuscript, and approved the final decision for submission.
Ms Wan: ran the electrochemiluminescence arrays (Mesoscale Discovery), contributed to the writing of the manuscript, and approved the final decision for submission.
Ms Sutcliffe: acquired the statistical analysis, ran the gene array system, contributed to the writing of the manuscript, and approved the final decision for submission.
Dr Hollins: conducted the collagen gel contraction assay, contributed to the writing of the manuscript, and approved the final decision for submission.
Dr Brightling: was involved in the recruitment of volunteers and isolation of tissue, supervised all experimental design, contributed to the writing of the manuscript, and approved the final decision for submission.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Brightling has received consultancy fees and research funding from AstraZeneca, MedImmune LLC, GlaxoSmithKline, Chiesi Ltd, Hoffmann-La Roche Inc, and Novartis AG. The remaining authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank B. Hargadon, RGN, and S. McKenna, RGN, for assistance in clinical characterization of subjects that underwent bronchoscopy. We also thank Roberta Milone, BSc, and Rick Williamson, PhD (GlaxoSmithKline, Stevenage, England) for providing access to a Mesoscale Discovery Electrochemiluminescence reader and assay plates and for their advice, and to Neeta Kulkarni, MD, for assistance on hue thresholding.
Abbreviations
- ASM
airway smooth muscle
- CFSE
carboxyfluorescein succinimidyl ester
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- GINA
Global Initiative for Asthma
- GMFI
geometric mean fluorescence intensity
- HLMC
human lung mast cell
- HMC-1
human mastocytoma cell line
- IQR
interquartile range
- ITS
insulin transferrin sodium selenite
- mRNA
messenger RNA
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium
- rh-TSLP
recombinant human thymic stromal lymphopoietin
- Th2
T-helper cell type 2
- TNF
tumor necrosis factor
- TSLP
thymic stromal lymphopoietin
- TSLPR
thymic stromal lymphopoietin receptor
Footnotes
For editorial comment see page 11
Funding/Support: This work was supported by GlaxoSmithKline. Dr Brightling was supported by a Wellcome Senior Clinical Fellowship [082265].
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Noncommercial License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Information for commercial entities is available online.
References
- 1.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926-938 [DOI] [PubMed] [Google Scholar]
- 2.Hartert TV, Peebles RS., Jr Epidemiology of asthma: the year in review. Curr Opin Pulm Med. 2000;6(1):4-9 [DOI] [PubMed] [Google Scholar]
- 3.Braman SS. The global burden of asthma. Chest. 2006;130(1suppl4S-12S [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui S, Hollins F, Saha S, Brightling CE. Inflammatory cell microlocalisation and airway dysfunction: cause and effect? Eur Respir J. 2007;30(6):1043-1056 [DOI] [PubMed] [Google Scholar]
- 5.Kaur D, Brightling CE. OX40/OX40 ligand interactions in T-cell regulation and asthma. Chest. 2012;141(2):494-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, Pavord ID. New insights into the relationship between airway inflammation and asthma. Clin Sci (Lond). 2002;103(2):201-211 [DOI] [PubMed] [Google Scholar]
- 7.Wang YH, Liu YJ. OX40-OX40L interactions: a promising therapeutic target for allergic diseases? J Clin Invest. 2007;117(12):3655-3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39(6):798-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui S, Mistry V, Doe C, Stinson S, Foster M, Brightling C. Airway wall expression of OX40/OX40L and interleukin-4 in asthma. Chest. 2010;137(4):797-804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117(12):3868-3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okayama Y, Okumura S, Sagara H, et al. FcepisolonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J. 2009;34(2):425-435 [DOI] [PubMed] [Google Scholar]
- 13.Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790-2798 [DOI] [PubMed] [Google Scholar]
- 14.Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L375-L382 [DOI] [PubMed] [Google Scholar]
- 15.Allakhverdi Z, Comeau MR, Jessup HK, Delespesse G. Thymic stromal lymphopoietin as a mediator of crosstalk between bronchial smooth muscles and mast cells. J Allergy Clin Immunol. 2009;123(4):958-60.e2 [DOI] [PubMed] [Google Scholar]
- 16.Brightling CE, Ammit AJ, Kaur D, et al. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2005;171(10):1103-1108 [DOI] [PubMed] [Google Scholar]
- 17.Global Initiative for Asthma guidelines. GINA website. http://www.ginasthma.com. Accessed May 9, 2011
- 18.Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52(6):498-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699-1705 [DOI] [PubMed] [Google Scholar]
- 20.Kaur D, Saunders R, Berger P, et al. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med. 2006;174(11):1179-1188 [DOI] [PubMed] [Google Scholar]
- 21.Saunders R, Sutcliffe A, Woodman L, et al. The airway smooth muscle CCR3/CCL11 axis is inhibited by mast cells. Allergy. 2008;63(9):1148-1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni NS, Hollins F, Sutcliffe A, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126(1):61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202(6):829-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182(3):1641-1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41(3):342-347 [DOI] [PubMed] [Google Scholar]
- 26.He JQ, Hallstrand TS, Knight D, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol. 2009;124(2):222-229 [DOI] [PubMed] [Google Scholar]
- 27.Hunninghake GM, Lasky-Su J, Soto-Quirós ME, et al. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med. 2008;177(8):830-836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179(2):1080-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A. 2007;104(3):914-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol. 2010;185(5):3035-3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollins F, Kaur D, Yang W, et al. Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6. J Immunol. 2008;181(4):2772-2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur D, Saunders R, Hollins F, et al. Mast cell fibroblastoid differentiation mediated by airway smooth muscle in asthma. J Immunol. 2010;185(10):6105-6114 [DOI] [PubMed] [Google Scholar]
- 33.Woodman L, Siddiqui S, Cruse G, et al. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol. 2008;181(7):5001-5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allakhverdi Z, Comeau MR, Smith DE, et al. CD341 hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123(2):472-478 [DOI] [PubMed] [Google Scholar]
- 35.Brightling CE, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID, Bradding P. Interleukin-4 and -13 expression is co-localized to mast cells within the airway smooth muscle in asthma. Clin Exp Allergy. 2003;33(12):1711-1716 [DOI] [PubMed] [Google Scholar]