Abstract
Introduction
The perpetuating mechanisms for human AF remain undefined. Localized rotors and focal beat sources may sustain AF in elegant animal models, but there has been no direct evidence for localized sources in human AF using traditional methods. We developed a clinical computational mapping approach, guided by human atrial tissue physiology, to reveal sources of human AF.
Methods and Results
In 49 AF patients referred for ablation (62±9 years; 30 persistent), we defined repolarization dynamics using monophasic action potentials (MAP) and recorded AF activation from 64-pole basket catheters in left atrium and, in n=20 patients, in both atria. Careful positioning of basket catheters was required for optimal mapping. AF electrograms at 64-128 electrodes were combined with repolarization and conduction dynamics to construct spatiotemporal AF maps. We observed sustained sources in 47/49 patients, in the form of electrical rotors (n=57) and focal beats (n=11) that controlled local atrial activation with peripheral wavebreak (‘fibrillatory conduction’). Patients with persistent AF had more sources than those with paroxysmal AF (2.1±1.0 vs 1.5±0.8, p=0.02), related to shorter cycle length (163±19 vs 187±25 ms, p<0.001). Approximately one-quarter of sources lay in the right atrium.
Conclusions
Physiologically-guided computational mapping revealed sustained electrical rotors and repetitive focal beats during human AF for the first time. These localized sources were present in 96% of AF patients, and controlled AF activity. These results provide novel mechanistic insights into human AF and lay the foundation for mechanistically-tailored approaches to AF ablation.
Keywords: atrial fibrillation, catheter ablation, mapping, rotors, fibrillatory conduction
Introduction
Despite great strides in percutaneous and surgical therapy for atrial fibrillation (AF) 1-3, their efficacy remains suboptimal 2, 4. Seminal observations by Haïssaguerre 1 revealed that ectopic beats from the pulmonary veins (PVs) may trigger AF, establishing AF ablation therapy with PV isolation as its cornerstone 1, 2. However, the mechanisms that perpetuate AF once it has been triggered are not defined 5, 6. Historically, detailed mapping defined the patient-specific sustaining mechanisms that now serve as ablation targets for the routine cure of supraventricular or ventricular arrhythmias 7, yet the complex and highly variable cycle-to-cycle activation of AF makes traditional mapping difficult.
We hypothesized that a novel approach to mapping AF, that physiologically interprets fibrillatory wave activity by analyzing widely sampled simultaneous multisite electrograms in the context of rate-dependent atrial repolarization and conduction, may enable identification of AF-sustaining mechanisms. There are 2 prevailing hypotheses. The multiwavelet hypothesis proposes that continuously meandering electrical waves cause AF 8, 9. However, this hypothesis does not readily explain consistent spatial nonuniformities in AF 10, 11, while ablation designed to address this hypothesis rarely terminates AF and has modest success 2, 12. Alternatively, the localized source hypothesis is based on experimental models in which reentrant circuits (rotors) 6, 13, 14 or focal impulses 11, 15 activate rapidly enough to cause disorganized AF. However, there has until now been no 9 or little 16, 17 or evidence to support localized sources in human AF.
We tested our hypothesis by developing a computational mapping approach, employing careful wide-area mapping of the atria during AF and analysis of tissue conduction and repolarization, to create individualized spatiotemporal maps of AF in patients with paroxysmal and persistent AF referred for percutaneous ablation.
Methods
Patient Flow
We recruited patients with drug-refractory AF referred for ablation for standard indications 2 to the Veterans Affairs and University of California Medical Centers in San Diego, on a mapping protocol approved by our joint Institutional Review Board. Consecutive patients were approached, and all subjects provided written informed consent. The only exclusion criterion was refusal or inability to provide informed consent. These patients were used to report APD restitution 18, APD alternans 19 and conduction restitution 20 as underlying mechanisms for the dynamics of human AF. After the detailed electrophysiological mapping outlined below, all patients underwent conventional ablation for the endpoint of pulmonary vein isolation 2.
Electrophysiological Mapping
Electrophysiology study was performed > 5 half-lives after discontinuing antiarrhythmic medications, except for amiodarone (stopped for > 30 days) (Table 1). Using femoral venous access, a decapolar catheter was placed in the coronary sinus, and then a 7F monophasic action potential (MAP) catheter (Boston Scientific, Natick MA) was advanced to the right atrium then left atrium via transseptal puncture. Heparin was administered intravenously to maintain activated clotting time (ACT) > 350 seconds throughout mapping (and subsequent ablation).
Table I.
Clinical Characteristics
| Characteristic | Paroxysmal AF | Persistent AF | P |
|---|---|---|---|
| Number | 19 | 30 | |
| Age /years (range) | 61±9 (35-77) | 62±9 (40-82) | 0.81 |
| History of AF / yrs (range) | 3.0±2.6 (0.4-10.2) | 6.2±6.3 (0.6-28.6) | 0.04 |
| LA Diameter /mm | 41±4 | 47±5 | 0.07 |
| LVEF /% (range) | 59±6 | 51±13 | 0.30 |
| Hypertension / n (%) | 16 (84) | 23 (77) | 0.52 |
| Coronary Disease / n (%) | 7 (37) | 10 (33) | 0.80 |
| Diabetes Mellitus /% | 9 (47) | 10 (33) | 0.33 |
| Medications / n (%) | |||
| ACEI/ARB | 12 (63) | 20 (67) | 0.80 |
| Statins | 15 (79) | 21 (70) | 0.49 |
| Failed Antiarrhythmics | |||
| Class I | 4 (21) | 7 (23) | 0.85 |
| Beta-Blockers | 14 (74) | 22 (73) | 0.98 |
| Amiodarone | 3 (16) | 16 (53) | 0.01 |
| Other Class III | 12 (63) | 14 (47) | 0.26 |
| Digoxin | 5 (26) | 10 (33) | 0.60 |
Simultaneous multisite recordings of the atria were achieved using 64 pole catheters (Constellation, Boston Scientific, MA) advanced transseptally to the left atrium in all patients, with a second basket placed simultaneously in the right atrium in n=20 patients (12 persistent, 8 paroxysmal; figure 1). Great care was taken to optimize electrode contact, to cover the majority of the atria and the ostia of the pulmonary veins and atrial appendages, maintaining relatively uniform interelectrode spacing (figure 1). Electrodes span the orifices of the thoracic veins and atrial appendages and so this approach can identify activation emanating from, versus progressing towards, these structures.
Figure 1. Atrial Basket Positioning for Computational AF Mapping.
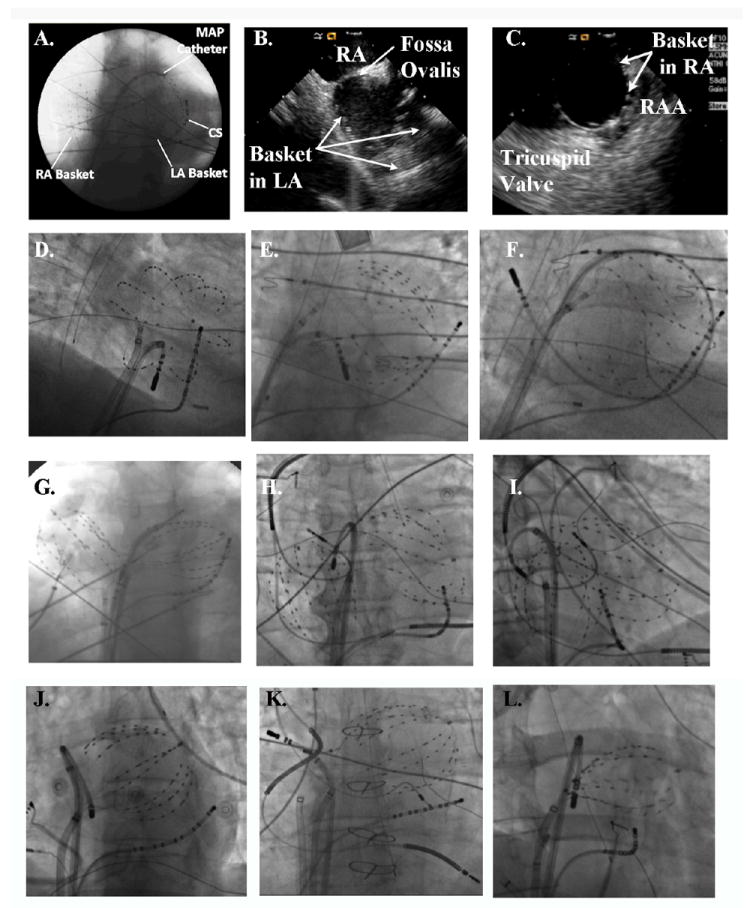
A. Good catheter placement in both atria (48 mm baskets, anteroposterior fluoroscopy), with MAP catheter at the left superior pulmonary vein antrum. B, C. Intracardiac Echocardiography confirms electrode opposition to walls of B. Left Atrium and C. Right Atrium. D. Good Electrode coverage of left atrium (60 mm Basket, RAO 30° fluoroscopy); E. Spline crowding detracts from otherwise good LA coverage (60 mm Basket, AP fluoroscopy); F. Large Left Atrium, With Poor Electrode Coverage of its Septum, outlined by ablation catheter loop with tip at right superior PV os (60 mm basket, LAO 30° fluoroscopy). G. Poor Coverage of Superior LA, indicated by wide separation of LA basket from the left main bronchus; the RA basket is well-deployed (60 mm baskets, AP fluoroscopy). H. Basket Placement With Pre-existing device leads. The RA basket is well positioned in this AP projection, yet the LA basket poorly covers the inferior LA. This appearance is consistent with basket displacement through the mitral apparatus. I. Anterior displacement of the LA basket through the mitral annulus, with the ablation catheter defining the true posterior border of the LA (same patient as panel H, in 30° RAO fluoroscopy). J. Undersized basket, despite an apparently good deployment, floating freely within the large left atrium that gave very poor signals and was replaced. K. Undersized basket in a patient with heart failure, a cardiac-resynchronization defibrillator and previously diagnosed ‘permanent AF’ now reclassified as longstanding persistent AF and undergoing ablation. L. Underexpanded basket, evident from the elliptical shape due to pinching at the interatrial septum. This was remedied by advancing the basket further into the left atrium with clockwise torque.
Panels A-L of figure 1 provide examples of optimal and suboptimal basket positions. Optimal positioning required selecting the basket size such that splines deformed slightly with cardiorespiratory motion on fluoroscopy or showed tissue opposition on intracardiac echocardiography (figure 1A-D). With currently available baskets (48 mm diameter, 4 mm electrode spacing; or 60 mm diameter, 5 mm electrode spacing), the LA was incompletely mapped in patients with LA diameter >≈ 60 mm in whom the septal LA and right pulmonary vein antra were typically under-represented (figure 1F).
Simultaneous biatrial basket recordings are described in this report, but the atria may also be sampled sequentially using a single basket to first analyze RA data while performing transseptal cannulation, then repeating this process for the LA.
Electrophysiological Data Acquisition
Computational mapping was applied to native AF when possible. Patients in sinus rhythm, or in whom AF terminated prior to mapping, were paced into AF using a decremental protocol via cycle lengths (CL) of 500 ms, 450 ms, 400 ms, 350 ms, 300 ms, then in 10 ms steps to AF. AF was mapped after it had sustained for at least 10 minutes, since our preliminary data suggest that spatial maps of induced AF converge with maps of native AF in the same patient over this timeframe.
Multisite electrograms were recorded as unipoles (e.g., A1, A2, A3, …) or as overlapping bipoles (i.e., A12, A23…) to reduce far-field artifact, and filtered at 0.05-500 Hz. AF data were exported digitally in 1-minute epochs.
Briefly, computational mapping is centrally based upon MAP data, that we used to define regional restitution of MAP duration as recently reported during pacing 18, 19 and in AF 21, and regional conduction restitution defined by conduction time to each basket electrode during progressively faster pacing to the onset of AF 20.
Nomenclature for Patient-Specific 3-Dimensional Spatiotemporal Maps of AF
Electrode positions were identified from fluoroscopy and used to identify regions of interest. Absolute electrode locations within the atria were not used for analysis but are illustrated using electroanatomic mapping with NavX (St. Jude, St Paul, MN) (figure 2A) or CARTO (Biosense-Webster, Minneapolis, MN). Figure 2B illustrates the nomenclature of 3D map projections. The left atrium was opened at its ‘equator’ with each half of the mitral annulus reflected superiorly and inferiorly, while the right atrium was opened between its poles and the tricuspid annulus halves reflected laterally and septally. For illustration, figure 2C shows sinus rhythm initiation at the high RA (red), proceeding to the LA over Bachmann’s bundle to finally activate the low lateral LA (blue), in relation to 128 bi-atrial electrodes (dots).
Figure 2. Anatomic Reference and Map Nomenclature.
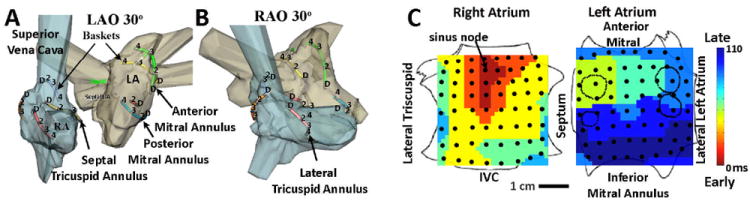
A, B. Electrodes Illustrated Within Patient-Specific Atria (NavX system, St Jude Medical, MN) in 30° LAO and 30° RAO projections, showing alternate splines and electrodes for clarity. C. Sinus Rhythm Map on Biatrial Schematic. Activation at basket electrodes, shown as dots, is displayed as a color-coded map from the sinus node to the lateral inferior LA. The RA is opened between its poles with tricuspid annulus opened laterally and medially; the LA is opened along its equator, with mitral annulus opened superiorly and inferiorly. The pulmonary vein ostia are indicated by dashed lines.
Physiologically-Directed Computational AF Mapping
Spatiotemporal analyses of AF were performed primarily by directly analyzing electrograms to construct movies of numerous AF activation cycles (figure 3A), and secondarily by constructing isochronal maps to illustrate single cycle snapshots of AF (figure 3B). Computational mapping is a physiologically guided approach that analyses AF in the context of regional variations in the minimum tissue wavelength for reentry, λ = refractory period × conduction velocity 22. We used MAP recordings to define APD restitution18, 19, and regional conduction restitution 20 to define regions of slow conduction.
Figure 3. Mapping Reveals Spiral Wave during AF in the Human Left Atrium.
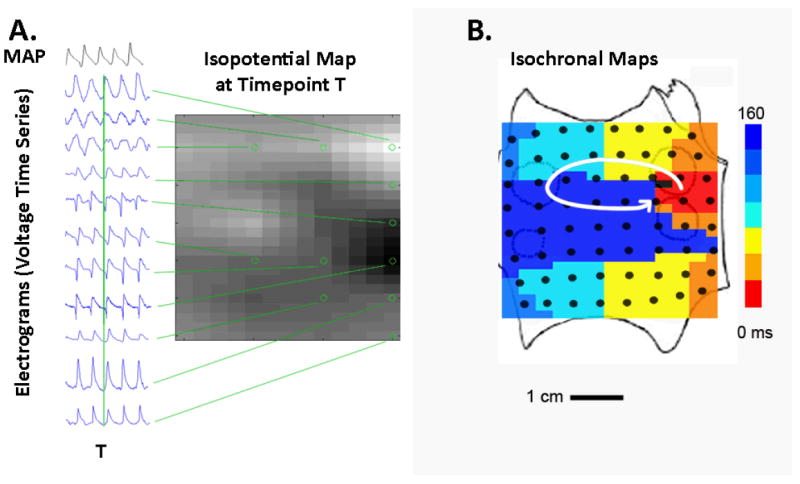
A. Raw electrograms used to create maps and movies. Unipolar electrograms (locations indicated by circles on grid) are used to construct an isopotential snapshot at any time point T (indicated by vertical green line). Monophasic action potentials (MAP) indicate repolarization and are used to calibrate unipolar electrograms. These isopotential maps are created successively for multiple time points T to create movies (see Supplemental movie). B. Isochronal snapshot of a LA rotor during one cycle of AF, created from activation times determined when each unipolar electrogram crosses a voltage threshold.
In figure 3A movies were constructed by plotting the electrogram voltage at each electrode location at a timepoint T (indicated by vertical green line), then repeating this process for multiple timepoints to create an animation (see Supplemental movie). To circumvent the difficulty of defining cycle length during AF 23, APD restitution defines minimum repolarization. Figure 3A illustrates a concurrent MAP tracing in AF used for this purpose. We recently reported that minimum APD and APD restitution differ markedly between patients with persistent and paroxysmal AF, yet for any one patient are consistent over time during pacing 18, 19 or in 24 AF. Conduction restitution is used to define physiologically plausible propagation paths 20. For instance, red and blue activation in figure 3B lie at adjacent electrodes (4 mm apart), yet their delay (≈160 ms) could not represent direct propagation because that would require a slower-than physiological CV 25 of 2.5 cm/sec (=0.4/0.16).
Figure 3B illustrates an isochronal snapshot of one cycle of persistent AF indicating a counterclockwise spiral wave (rotor, arrow) in the posterior left atrium, represented by color-coded activation from red to blue. The AF rotor indicates 1:1 activation within its proximal spiral arms, with wavebreak and collision (fibrillatory conduction) at the spiral arm periphery and beyond.
Definition of Localized Sources
Computational maps were analyzed to reveal sustained localized sources of human AF in the form of focal impulse 15, 26 or rotor 6 maps (FIRM) as shown in animal models but not previously in human AF 6, 27. Rotors were identified as rotational activity around a center (using isopotential movies and isochronal activation maps). Focal beats in AF were identified at their point of origin from surrounding diastole from isopotential movies and isochronal maps. We diagnosed rotors or focal beat sources only if sustained throughout several recording epochs (arbitrarily > 50 cycles), to exclude transient rotational and/or centrifugal activation that by definition cannot represent sources but likely represent wavebreak and/or passive activation that are highly variable in fibrillation.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD). The t-test was used to compare variables between 2 groups, such as LA area or AF cycle length. Paired continuous variables were compared using linear regression and the paired t-test. The chi-square test was applied to contingency tables. A probability of < 5 % was considered statistically significant.
Results
Table I indicates the clinical characteristics of our population.
Computational Mapping of Electrical Rotors During Human AF
Computational mapping revealed electrical rotors or focal beats during AF in 47 of 49 (95.9%) patients (table 1), that were sustained throughout multiple epochs over many minutes (i.e., thousands of cycles). These regions exhibited 1:1 activation of atrial activation within their spiral arms, with propagation to the remaining atria characterized by wavebreak or ‘fibrillatory conduction’ (disorganization of 1:1 activation) distally and in the contralateral atrium. Thus, rotors and focal beats were sources for AF activation rather than passively activated elements.
Figure 4A (50-year-old man with persistent AF and LA diameter 52 mm) shows a solitary rotor in the mid-posterior LA during AF with counterclockwise (CCW) activation represented by the red-to-blue color coded activation with cycle length 140 ms. This rotor accounted for 1:1 activation in ≈70% of the LA (its spiral arms), with fibrillatory conduction shown by collision between late activation from the rotor in the inferoposterior wall (blue) with early activation from asynchronous activation at the inferior mitral annulus (red/yellow) and to the coronary sinus (see electrograms). Figure 4B illustrates a clockwise (CW) rotor that controls a smaller region of the atrium during persistent AF in the anterior LA in a 62-year-old with LA diameter 52 mm and heart failure (LVEF 37%).
Figure 4. Localized Electrical Rotors (Spiral Waves) in Human AF, Revealed by Computational Mapping.
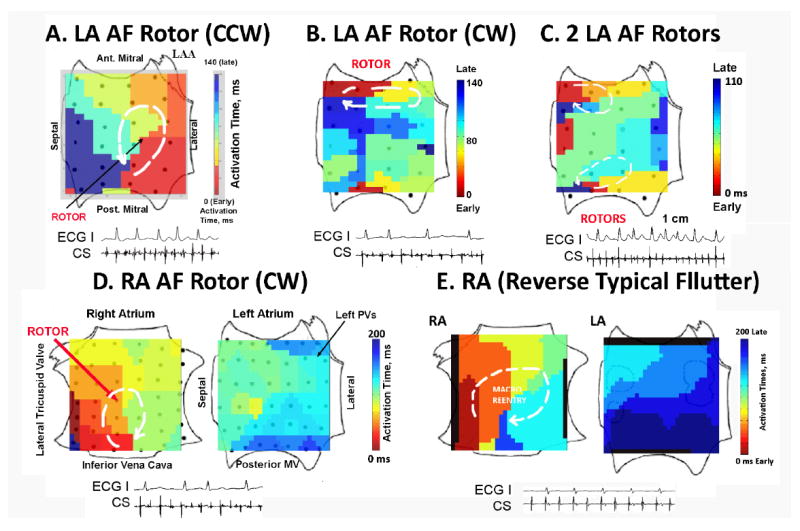
A. Solitary Counterclockwise (CCW) Rotor During Persistent AF in the posterior LA; B. CW Rotor in Persistent AF in the anterior LA; C. Two Concurrent Rotors During Paroxysmal AF in the anterior LA (CW) and inferior LA (CCW); D. Right Atrial Rotor (CW) During Paroxysmal AF in the mid-posterior wall, with fibrillatory conduction to the LA. By Contrast, E. Clockwise Rotor of Reverse Typical Atrial Flutter differs from AF, with 1:1 activation throughout the RA that engages Bachmann’s bundle to activate the LA and no fibrillatory conduction. Key: ECG lead I, CS=coronary sinus electrogram.
Table 2 summarizes that most patients had multiple concurrent sources, typically in the left and right atrium. Figure 4C shows 2 concurrent rotors in the left atrium during paroxysmal AF in the anterior LA (CW) and inferior LA (CCW). Figure 4D shows biatrial maps illustrating a CW rotor in the mid-posterior RA during paroxysmal AF, with fibrillatory conduction to the LA in a 66-year-old Native American with LA diameter of 42 mm.
Table 2.
Characteristics of Localized AF Sources
| Characteristic | Paroxysmal AF (n=19) |
Persistent AF (n=30) |
P |
|---|---|---|---|
| AF CL /ms | 187±25 | 163±19 | <0.001 |
| No. Patients with Sources | 19 (100%) | 28 (94%) | |
| No. Sources/Pt (Biatrial Cases) | 1.5±0.8 | 2.1±1.0 | 0.017 |
| No. Rotors/Focal Beats (Biatrial) | 16/6 | 41/5 | 0.09 |
| LA Recordings, n | 19 | 30 | |
| Rotors/Focal Beats (LA), n | 12/4 | 29/4 | |
| RA Recordings, n | 8 | 12 | |
| Rotors/Focal Beats (RA), n | 4/2 | 12/1 |
Although some AF rotors appeared relatively organized in single isochronal snapshots, static images do not reflect rotor precession (limited movement) seen in multi-cycle animations (Supplemental Movie) and may not emphasize peripheral breakdown of 1:1 conduction. In contrast, figure 4E illustrates true 1:1 biatrial activation (with no fibrillatory conduction) from clockwise macroreentry in the right atrium (reverse typical atrial flutter) and activation to the left atrium over Bachman’s bundle.
Demonstration of Focal Beats during Human AF
Repetitive focal beats were visualized represented 11 of 68 (16.2%) of sources in patients during paroxysmal and persistent AF (table 2). The FIRM map of AF in figure 5A shows earliest biatrial activation emanating from a repetitive focal beat (CL 104 ms) in the inferoseptal LA in a 67-year-old man with persistent AF, moderately dilated LA and left ventricular ejection fraction 45%. Notably, activation emanates radially but nonuniformly to surrounding LA (arrows), with wavebreak in the peripheral LA then contralateral RA. In contrast, figure 5B shows a FIRM map of focal atrial tachycardia (CL 290 ms) located in the mid-posterior LA, with organized and uniform centrifugal activation throughout the ipsilateral LA to the contralateral RA.
Figure 5. Repetitive Focal Beats revealed by Computational Mapping.
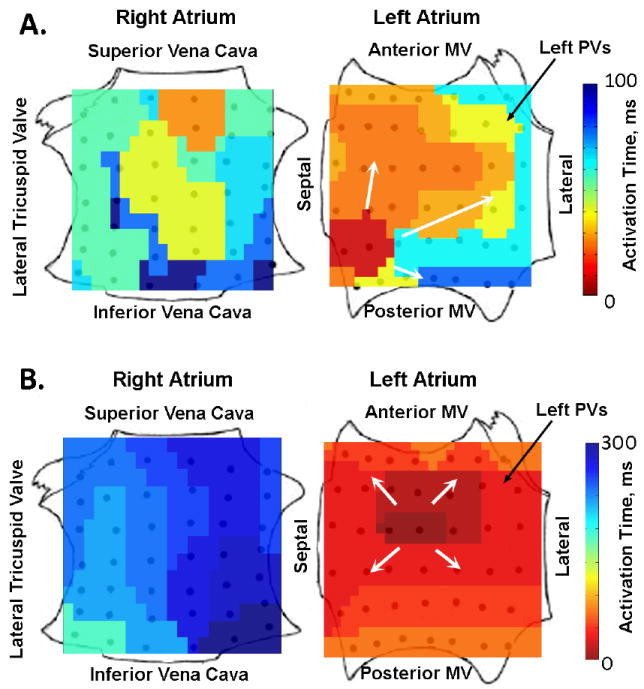
A. Repetitive Focal Beat during Paroxysmal AF (in low septal LA), with activation to remaining LA and fibrillatory conduction to the RA (CL≈100 ms). In contrast, B. Focal Atrial Tachycardia (non-fibrillatory) from the high posterior LA differs from AF by showing 1:1 activation centrifugally to the ipsilateral then contralateral atria (CL 300 ms).
Localized Sources were Prevalent in Human AF
Computational mapping revealed localized sources in nearly all patients, with most exhibiting multiple sources (table 2). The number of concurrent AF sources was higher for patients with persistent than paroxysmal AF (p=0.017; table 2) and, surprisingly, 27% lay in the right atrium. The number of rotors was greater than the number of focal beats, and this ratio trended higher for patients with persistent AF (41:5) than paroxysmal AF (16:6; p=0.088).
There were no complications during mapping.
Discussion
We report a novel physiological mapping approach for human AF that reveals, for the first time, sustained localized electrical rotors and repetitive focal beat sources for human AF. We identified localized sources in nearly all AF patients, who had an average of 1.5-2 sources that directly controlled local activation with breakdown of 1:1 conduction (wavebreak, or ‘fibrillatory conduction’) to surrounding regions. Persistent AF exhibited a higher number of concurrent rotors or focal beat sources than paroxysmal AF, and that may explain the greater apparent complexity and difficulty of treatment of this AF phenotype. These findings shed novel insights into the mechanisms of human AF, and provide the foundation for a patient-tailored mechanistic approach to AF ablation.
Computational Mapping Compared to Prior Mapping of Human AF
This study reveals that human AF is predominantly caused by localized sources that control surrounding fibrillation and, for each patient, lie at patient-specific locations that remain stable for at least tens of minutes (thousands of cycles). Although localized AF sources have been shown in elegant animal models in the laboratories of Jalife 6, 28 and Waldo 15, 29, recent human studies have reported that rotors are rare 16 or do not exist 8, 27, 30 in human AF. Methodologic differences may explain our divergence from those data.
Allessie et al., in pioneering human AF mapping, initially showed complex right atrial activation consistent with multiple wavelets during electrically induced AF in patients undergoing open-chest ablation for accessory pathways 8, 30. Those findings were recently extended to the left atrium and persistent AF patients 27. However, while those studies provide high spatial resolution, they sampled relatively narrow and fixed areas using plaques in the anterolateral right atrium and posterior left atrium, where sources may not exist in all patients. In contrast, wide-area mapping in computational mapping makes no assumptions about the spatial uniformity of human AF. The spatial resolution of baskets (4-5 mm interelectrode distance along a spline) provides sufficient spatial resolution to resolve small human reentry circuits (≈2cm2) 22, while regions that as sources (reentrant or focal) should be detectable at a greater distance 31. Finally, if ablation is the clinical goal, then higher resolution may be moot given that the diameter of a single ablation lesion is ≈ 7 mm. Computational mapping also benefited from our recent studies documenting, for the first time, differences in human biatrial repolarization and conduction dynamics from animal studies, and between patients with paroxysmal and persistent AF 18, 19.
Notably, other studies provide indirect evidence for the existence of localized sources in human AF. Sahadevan et al reported n=9 patients with persistent AF at open-chest surgery, in whom gradients of rate, organization and spectral DF 11 were consistent with localized sources, although spatial AF activation maps were not created and interventions were not performed. Using noncontact maps, Lin et al. revealed regions of high dominant frequency during macroreentry and imminent disorganization to AF 32. We recently used LA baskets to show gradients in spectral DF and organization at specific sites, of which some corresponded to sites of AF termination during extensive ablation 33. Studies using lower resolution mapping also confirm consistent gradients in rate, consistent with regional ‘driver regions’, during human AF 10, 34. In addition, Ravelli et al. used RA baskets to show consistent sites of electrogram organization in human AF 35. Unlike the present report, however, prior reports did not reveal rotors or repetitive focal beat sources, did not identify nor locate AF sources, nor did they demonstrate using activation mapping that any specific region determines activation in surrounding tissue during human AF.
Clinical Implications
These mechanistic findings provide potential insights into the results of AF ablation. The greater number of sources in patients with persistent versus paroxysmal AF is consistent with more difficult ablation in the former group. Patient-specific source locations may explain why extensive ablation outside of the pulmonary veins is often required to eliminate AF 2. The presence of right atrial sources in one-quarter of patients may explain the 70-80% ‘ceiling’ in AF ablation success in many current studies, even after 3 or more (predominantly left atrial) procedures 2. Future studies should apply computational mapping prospectively and target electrical rotors and focal beats for ablation or other therapeutic modalities, such as pharmaceutical or regenerative therapy, to terminate and eliminate AF. Other applications of computational mapping are to track patient-specific AF mechanisms longitudinally over time, to assess the progression of atrial remodeling or possible reverse remodeling from antiarrhythmic medications, gene therapy, stem cell therapy or pacing.
Limitations
In this series, ablation was not performed prospectively at localized sources because early iterations of computational mapping required hours to days of processing time. Prospective mapping studies with targeted ablation of sources (Focal Impulse and Rotor Modulation, FIRM) are underway. Although the population is relatively small, it represents a typical tertiary care referral group including patients with and without heart failure, of young and advanced age, with short to very long AF durations, and normal-sized to very enlarged atria. Thus, we feel that our description of rotors and focal beat sources is robust and likely applies to the vast majority of patients referred for AF ablation. Patients with persistent AF had a higher historical usage of amiodarone than those with paroxysmal AF, but the drug was discontinued > 60 days prior to the procedure in all cases (often more than a year before enrollment). Although epicardial and endocardial signals may differ in regions of thick atrial musculature 9, we did not perform epicardial mapping in this series. We did not exhaustively analyze regions of complex fractionated atrial electrograms in the present report, and also did not evaluate the impact of autonomic function on localized sources. Patients in this series were predominantly male, but more recent studies have already extended these observations to women. As shown in figure 1, technical factors such as LA diameter > 60 mm may prevent adequate mapping in patients with very advanced structural remodeling, although this may be mitigated if larger baskets become available.
Conclusions
Physiologically-guided computational mapping revealed localized electrical rotors and repetitive focal beat sources for human AF for the first time. The vast majority of AF patients showed localized sources, for an average of 1.5-2 concurrent rotors and/or focal beats, that controlled local AF activation and caused peripheral wavebreak. One-quarter lay in the right atrium. These results shed novel mechanistic insights into human AF, and offer a foundation for mechanistically targeted patient-tailored approaches to AF ablation.
Supplementary Material
Acknowledgments
This work was supported by grants to Dr Narayan from the NIH (HL70529, HL83359, HL83359-S1) and Doris Duke Charitable Foundation. Dr. Krummen’s work is supported by NIH grant HL83359.
Footnotes
Drs. Narayan and Rappel are authors of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Drs. Narayan and Rappel own equity shares in Topera. Dr. Narayan reports having received honoraria from Medtronic, St. Jude Medical, and Biotronik. Dr. Krummen has no disclosures.
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Dimarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012 doi: 10.1007/s10840-012-9672-7. [DOI] [PubMed] [Google Scholar]
- 3.Weimar T, Bailey MS, Watanabe Y, Marin D, Maniar HS, Schuessler RB, Damiano RJ., Jr The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol. 2011 doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 6.Vaquero M, Calvo D, Jalife J. Cardiac fibrillation: From ion channels to rotors in the human heart. Heart Rhythm. 2008;5:872–879. doi: 10.1016/j.hrthm.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipes DP, Jalife J. Cardiac Electrophysiology: From Cell to Bedside. Saunders,Elsevier; 2009. [Google Scholar]
- 8.Konings K, Kirchhof C, Smeets J, Wellens H, Penn O, Allessie M. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 9.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. The ElectroPathological Substrate of Longstanding Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Longitudinal Dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 10.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of Left-to-Right Atrial Frequency Gradient in Paroxysmal but Not Persistent Atrial Fibrillation in Humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 11.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial Mapping of Chronic Atrial Fibrillation in Patients: Preliminary Observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 12.Beukema WP, Sie HT, Misier AR, Delnoy PP, Wellens HJ, Elvan A. Predictive factors of sustained sinus rhythm and recurrent atrial fibrillation after a radiofrequency modified Maze procedure. Eur J Cardiothorac Surg. 2008;34:771–775. doi: 10.1016/j.ejcts.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992;355:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- 14.Ideker RE, Rogers JM. Human ventricular fibrillation: wandering wavelets, mother rotors, or both? Circulation. 2006;114:530–532. doi: 10.1161/CIRCULATIONAHA.106.644765. [DOI] [PubMed] [Google Scholar]
- 15.Ryu K, Shroff SC, Sahadevan J, Martovitz NL, Khrestian CM, Stambler BS. Mapping of Atrial Activation During Sustained Atrial Fibrillation in Dogs with Rapid Ventricular Pacing Induced Heart Failure: Evidence for a Role of Driver Regions. J Cardiovasc Electrophysiol. 2005;16:1348–1358. doi: 10.1111/j.1540-8167.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 16.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, Li L, Rudy Y. Noninvasive Characterization of Epicardial Activation in Humans With Diverse Atrial Fibrillation Patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martinez-Alzamora N, Gonzalez-Torrecilla E, Arenal A, Fernandez-Aviles F, Berenfeld O. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and Activation Restitution Near Human Pulmonary Veins and Atrial Fibrillation Initiation: A Mechanism for the Initiation of Atrial Fibrillation by Premature Beats. J Am Coll Cardiol. 2008;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization Alternans Reveals Vulnerability to Human Atrial Fibrillation. Circulation. 2011;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalani G, Schricker A, Gibson M, Rostamanian A, Krummen DE, Narayan SM. Dynamic Conduction Slowing Precedes Human Atrial Fibrillation Initiation: Insights from Bi-Atrial Basket Mapping On Transitions to Atrial Fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narayan SM, Franz MR. Quantifying Fractionation and Rate in Human Atrial Fibrillation Using Monophasic Action Potentials: Implications for Substrate Mapping. Europace. 2007e;9:vi89–vi95. doi: 10.1093/europace/eum212. [DOI] [PubMed] [Google Scholar]
- 22.Rensma P, Allessie M, Lammers W, Bonke F, Schalij M. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circulation Research. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 23.Elvan A, Linnenbank A, van Bemmel M, Misier A, Delnoy P, Beukema W, de Bakker J. Dominant Frequency of Atrial Fibrillation Correlates Poorly with Atrial Fibrillation Cycle Length. Circulation: Arrhythmia and Electrophysiology. 2009;2:634–644. doi: 10.1161/CIRCEP.108.843284. [DOI] [PubMed] [Google Scholar]
- 24.Narayan SM, Krummen DE, Kahn AM, Karasik PL, Franz MR. Evaluating Fluctuations in Human Atrial Fibrillatory Cycle Length Using Monophasic Action Potentials. Pacing Clin Electrophysiol. 2006;29:1209–1218. doi: 10.1111/j.1540-8159.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 25.Harrild DM, Henriquez CS. A computer model of normal conduction in the human atria. Circulation Research. 2000;87:e25–e36. doi: 10.1161/01.res.87.7.e25. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki M, Vaquero LM, Hou L, Campbell K, Zlochiver S, Klos M, Mironov S, Berenfeld O, Honjo H, Kodama I, Jalife J, Kalifa J. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: interplay between rotors and focal discharges. Heart Rhythm. 2009;6:1009–1017. doi: 10.1016/j.hrthm.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 28.Skanes AC, Mandapati R, Berenfeld O, Davidenko JM, Jalife J. Spatiotemporal Periodicity During Atrial Fibrillation in the Isolated Sheep Heart. Circulation. 1998;98:1236–1248. doi: 10.1161/01.cir.98.12.1236. [DOI] [PubMed] [Google Scholar]
- 29.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 30.Konings K, Smeets J, Penn O, Wellens H, Allessie M. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 31.Ideker RE, Rogers JM, Fast V, Li L, Kay GN, Pogwizd SM. Can mapping differentiate microreentry from a focus in the ventricle? Heart Rhythm. 2009;6:1666–1669. doi: 10.1016/j.hrthm.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y-J, Tai C-T, Kao T, Tso H-W, Huang J-L, Higa S, Yuniadi Y, Huang B-H, Liu T-Y, Lee P-C, Hsieh M-H, Chen S-A. Electrophysiological Characteristics and Catheter Ablation in Patients With Paroxysmal Right Atrial Fibrillation. Circulation. 2005;112:1692–1700. doi: 10.1161/CIRCULATIONAHA.104.512731. [DOI] [PubMed] [Google Scholar]
- 33.Krummen DE, Peng KA, Bullinga JR, Narayan SM. Centrifugal Gradients of Rate and Organization in Human Atrial Fibrillation. Pacing Clin Electrophysiol. 2009;32:1366–1378. doi: 10.1111/j.1540-8159.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemola K, Ting M, Gupta P, Anker JN, Chugh A, Good E, Reich S, Tschopp D, Igic P, Elmouchi D, Jongnarangsin K, Bogun F, Pelosi F, Morady F, Oral H. Effects of Two Different Catheter Ablation Techniques on Spectral Characteristics of Atrial Fibrillation. Journal of the American College of Cardiology. 2006;48:340–348. doi: 10.1016/j.jacc.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 35.Ravelli F, Faes L, Sandrini L, Gaita F, Antolini R, Scaglione M, Nollo G. Wave Similarity mapping shows the Spatiotemporal Distribution of Fibrillatory Wave Complexity In the Human Right Atrium During Paroxysmal and Chronic Atrial Fibrillation. J Cardiovasc Electrophysiol. 2005;16:1071–1076. doi: 10.1111/j.1540-8167.2005.50008.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


