Abstract
Background
Previous studies indicate that acute CD4 T-cell-mediated cardiac allograft rejection requires donor MHC class II expression and can be independent of ‘indirect’ antigen presentation. However, other studies suggest that indirect antigen presentation to CD4 T-cells may play a primary role in cellular xenograft immunity. Thus, the relative roles of direct/indirect CD4 T-cell reactivity against cardiac xenografts is unclear. We set out to determine the role for indirect CD4 T-cell reactivity in cardiac xenograft rejection.
Methods
Rat hearts were transplanted heterotopically into wild-type and immuno-deficient mice. Recipients were untreated, treated with depleting antibodies or reconstituted with wild-type cells.
Results
Antibody depletion confirmed that rat heart xenograft rejection in C57Bl/6 mice was CD4 T-cell-dependent. Also, heart xenografts survived long-term in B6 MHC class II (C2D) deficient mice. Graft acceptance in C2D mice was not secondary to CD4 T-cell deficiency alone, because transferred B6 CD4 T-cells failed to trigger rejection in C2D hosts. Furthermore, purified CD4 T-cells were sufficient for acute rejection of rat heart xenografts in immune-deficient B6rag1−/− recipients. Importantly, CD4 T-cells did not reject rat hearts in C2Drag1−/− hosts, contrasting results using cardiac allografts. ‘Direct’ xenoreactive CD4 T-cells were not sufficient to mediate rejection despite vigorous reactivity to rat stimulator cells in vitro.
Conclusion
Taken together, results show that CD4 T-cells are both necessary and sufficient for acute cardiac xenograft rejection and host MHC class II is critical in this process.
Keywords: T-cell, MHC, antigen presentation/processing, transplantation
Introduction
A major issue thwarting clinical transplantation remains the limited supply of human donor organs, therefore xenografts may be considered an alternative organ source. The first limitation of using xeno-organs is the phenomenon of hyperacute rejection, which can cause complete organ failure in minutes to hours due to pre-formed antibodies and complement activation [1]. Studies based on the removal or reduction of antibodies or antibody responses show promise [2–5]. However, even if hyperacute rejection can be prevented, adaptive T-cell dependent humoral and cellular mechanisms are capable of aggressive xenograft rejection [6, 7]. The role of CD4+ T-cells in the rejection of cardiac allografts and xenografts is well established. A variety of studies indicate that CD4+ T-cells can be necessary and often sufficient to trigger acute xenograft rejection [8–14]. It remains unclear, however, whether this CD4 response to cardiac grafts involves ‘direct’ (donor MHC restricted) or ‘indirect’ (host MHC restricted) antigen recognition, or both [15–20].
Early studies indicated that some inter-species T-cell responses, such as human-anti-mouse, could discriminate between individual mouse H-2 haplotypes, suggesting a direct recognition of xenogeneic MHC [18]. Later studies indicated that human responder-type APCs or human cytokines were important for this xenogeneic response, raising the prospect that ‘indirect’ (host APC-dependent) reactivity may be important for the xenogeneic T-cell response [19]. This was consistent with the concept that in a clinically-relevant xenograft scenario, such as pig to human, T-cell reactivity to donor APCs might be relatively weak [21]. That is, it was posited that the potential incompatibility of various T-cell/APC ligands and/or receptors that might prevent binding of donor MHC, might bias discordant xenograft rejection to rely heavily on antigen presentation via indirect antigen presentation [22]. However, later studies clearly demonstrated potent human T-cell ‘direct’ reactivity to porcine APCs [23–27] in addition to ‘indirect’ reactivity [24, 27]. Mouse CD4+ T-cells exhibit strong direct T-cell reactivity to rat APCs and yet surprisingly can still show a major reliance on indirect reactivity for the rejection of cellular islet xenografts [14].
The relative contribution of direct and indirect pathways of donor antigen presentation in T-cell dependent rejection of vascularized organ xenografts in mice remains controversial. A previous study indicates that treatment with mAbs against mouse MHC class II molecules post-transplant can significantly prolong rat heart xenograft survival [28], leading to the conclusion that a reactive CD4+ T-cell pathway likely requires indirect antigen recognition. This is in contrast to findings for acute cardiac allograft rejection, where CD4+ T-cell mediated rejection requires direct class II antigen presentation [29, 30]. Our previous findings analyzing the rejection of cellular islet transplants, allografts were rejected with no dependence on host class II while concordant rat xenografts show prolonged survival, but ultimate rejection, in class II deficient mouse recipients [14]. Neonatal porcine islets (discordant xenografts) survived long term in class II deficient mice, even if the recipients are reconstituted with purified wild type CD4+ T-cells, demonstrating that direct CD4 cells alone cannot mount a rejection response to islets in the absence of host class II. However, it is becoming increasingly obvious that the rules governing rejection in one transplant model do not necessarily apply for another. Therefore, despite our previous experience with islet xenograft studies, it is necessary to also explore the requirements for cardiac xenograft rejection. Consequently, the purpose of this study was to define the requirements for antigen presentation in CD4-mediated xeno-rejection. To our knowledge, this is the first study utilizing an MHC class II deficient mouse to define the contribution of host derived MHC.
Methods
Animals
Inbred female C57Bl/6J (B6, H-2b), BALB/cByJ (BALB/c H-2d), immune-deficient C57Bl/6-ragtm1/mom (B6rag1−/−, H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Female C57Bl/6 I-Ab-gene targeted MHC class II-deficient (I-Ab−/−) (C2D, H-2b) mice were obtained from Taconic Farms (Germantown, NY). Dark-Agouti (DA) rats and C2Drag1−/− mice were bred in-house. C2Drag1−/− mice were generated as previously described [30]. All animals were housed under pathogen-free conditions at the University of Colorado Barbara Davis Center Animal Facility under IACUC approval and cared for according to NIH guidelines.
Heterotopic Cardiac Transplantation
Hearts from DA rats (25–40grams) were transplanted heterotopically into B6, C2D, B6rag1−/−, or C2Drag1−/− mice. Vascularized grafts were transplanted according to standard microsurgical techniques [31]. Briefly the donor heart was placed in 4°C saline until transplantation. End-to-side anastomoses of the donor aorta to the recipient aorta and the donor pulmonary artery to the recipient IVC were made using running 10–0 nylon sutures. Graft survival was monitored daily by palpation with rejection defined as cessation of detectable beat and confirmed by laparotomy under anesthesia.
T-cell depletion in vivo
The rat anti-mouse CD4 mAb GK1.5 (IgG2b) [32] was produced for in vivo CD4 T-cell depletion. Rat anti-mouse CD8 mAb 2.43 (IgG2b) [33] was produced for in vivo CD8 T-cell depletion. The antibodies were produced as ascites in rag1−/− mice and quantitated by an isotype-specific ELISA. B6 recipients were left either untreated or were treated with GK1.5 antibody (10mg/kg) or with 2.43 antibody (20mg/kg) administered intraperitoneally on days −1, 0, 1 and 7 relative to transplant.
Adoptive transfer of purified CD4+ T-cells
Cervical, axillary and mesenteric lymph nodes (LNs) were harvested from B6 mice. Single cell suspensions of LN cells were enriched for CD4+ T-cells by negative selection of CD8+ T-cells and B-cells on an immunoaffinity column according to the manufacturer’s specifications (Cellect, Edmonton, Alberta, Canada) [30]. Cellular phenotyping of freshly purified cells was determined by flow cytometry. CD4-enriched T-cells contained less than 0.5% contaminating CD8+ T-cells or CD19+ cells. Ten million unfractionated LN cells or CD4-enriched T-cells were injected intraperitoneally into the indicated adoptive transfer recipients 3–7 days post cardiac transplant.
Histology
Transplanted and native hearts were removed and divided in half in the long axis perpendicular to the intraventricular septum. Halves were then placed in 10% formaldehyde. Sections were cut and stained with hematoxylin and eosin (H&E) and examined in a blinded fashion.
Flow cytometry
Freshly isolated lymphocytes were directly labeled with PerCP conjugated rat anti-mouse CD4, (clone RM 4–5), PE CD8a (clone Ly-2) and PE CD19 (clone 6D5) (Pharmingen, San Diego, CA). Approx. 5 × 105 cells were labeled for 20 min at 4°C with the indicated Abs. Frequency determinations were calculated from single-parameter and double-parameter fluorescence histograms on a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) after gating on viable lymphocytes. Cellquest software (BD Immunocytometry Systems) was used to analyze flow cytometry data.
Mixed lymphocyte reactions
Mixed lymphocyte reactions (MLR) of DA splenocyte-stimulator cells and Balb/c splenocyte-stimulator cells (allo control) with column purified CD4+ B6 responder lymph node cells were performed. Briefly, triple wells containing 2.0 × 105 responder cells were mixed with 3.0 × 105 irradiated (2500 Rads) stimulator cells in 96-well flat bottom plates. Cells, cultured in EMEM supplemented with 10% FCS, 10–5 M 2-Me, and antibiotics, were incubated at 37C in 10% CO2. Cultures were pulsed with 1.0 μCi thymidine for 6 hours on the indicated day of cell culture. Plates were harvested and counted on a Trilux 1450 micro beta scintillation counter (Wallac Inc., Gaithersburg, Maryland, USA).
Statistical analysis
The Mann-Whitney U test was used to determine significance of graft survival data. A p value of less than 0.05 was considered significant.
Results
CD4+ T-cells, but not CD8+ T-cells, are required for acute cardiac xeno-rejection
To confirm the requirement for CD4+ T-cells in acute rat-to-mouse heart xenograft rejection, DA rat hearts were transplanted into wild type B6 mice that were left untreated or treated with depleting anti-CD4 or anti-CD8 mAbs. Untreated mice rejected rat hearts acutely (7.6 +/−1.5 days, n=5). B6 mice treated with a course of depleting anti-CD4 mAb exhibited prolonged survival (79.8 +/−21.7 days, n=5, p<0.005), although all were eventually rejected when peripheral CD4+ T-cell numbers had returned to baseline as determined by peripheral blood flow cytometry. B6 recipients treated with depleting anti-CD8 mAb rejected their grafts acutely despite depletion of peripheral CD8+ T-cells to < 0.1% (10.5 +/−2.6 days, n=4, p=N.S. vs. control) (data not shown).
Host MHC class II is required for acute xeno-rejection
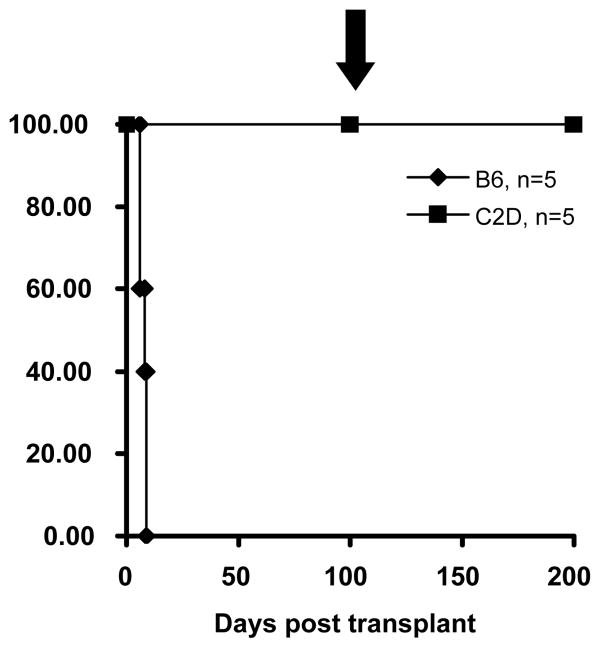
The requirement for an indirect MHC class II-restricted pathway of antigen presentation was investigated by transplanting rat hearts into C2D (MHC II−/−) mice. These mice did not reject DA hearts (>100 days) (Fig. 1) indicating that host MHC class II is indeed critical for acute xeno-rejection. As C2D mice are also CD4+ T-cell deficient [34], we adoptively transferred wild-type CD4+ T-cells into C2D recipients bearing long term surviving grafts (>100days). Despite successful engraftment of these cells (demonstrated by flow cytometry, DNS), they were still unable to reject their grafts (>100 days post CD4 transfer/>200 days post transplantation) demonstrating that CD4+ T-cells require host MHC class II restricted antigen presentation (Fig 1). Importantly, our previous studies demonstrated that CD4+ T-cells could mediate acute cardiac allograft rejection in C2D recipients, indicating that CD4+ T-cells can mediate rejection in the C2D environment [30].
Figure 1. Host MHC class II is required for acute xeno-rejection.
C2D mice tolerate rat cardiac grafts indefinitely (>100 days, n=5, p<0.005ν). Long-term surviving C2D hosts of rat cardiac grafts (>100 days) then received the adoptive transfer of 1×107 column-purified B6 CD4+ LNCs on day 100 post heart transplantation (see arrow: ↓). Despite this challenge, these grafts continued to survive indefinitely (> than an additional 100 days).
CD4+ T-cells are sufficient for acute xeno-rejection and require antigen presentation by host MHC class II
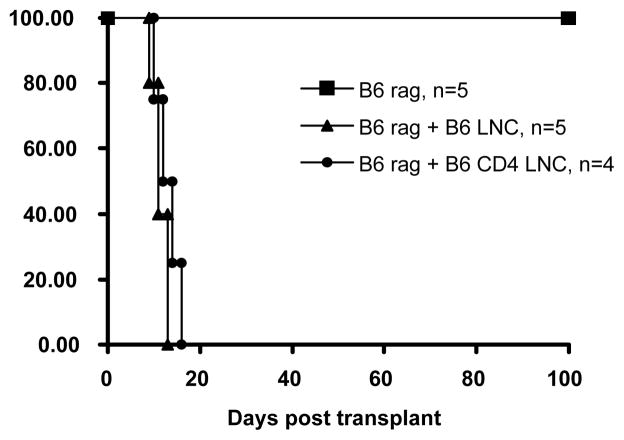
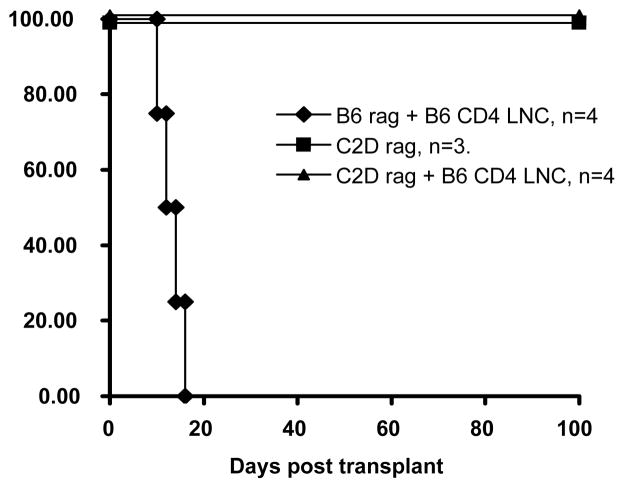
Having ascertained that CD4+ T-cells are necessary for graft rejection and that xeno-rejection requires host MHC class II, we sought to determine whether indirect CD4 cells are sufficient to cause rejection in the absence of other lymphocytes. To test this we transplanted rat hearts into immune-deficient C2Drag1−/− mice that were either untreated or were reconstituted with purified naive CD4+ T lymphocytes from B6 mice. This model effectively isolates antigen presentation to the CD4+ T-cells and to the direct pathway, as host derived MHC class II is absent. Additionally, this model allows for isolation of the response to the CD4+ T-cells as the host remains B-cell and T-cell deficient. B6rag1−/− recipients (intact MHC class II, but mature T-cell and B-cell deficient) were used as controls (Fig. 2). Cardiac xenografts survived indefinitely in unreconstituted B6rag1−/− and C2Drag1−/− mice. Importantly, while unfractionated lymph node cells and purified CD4+ cells were sufficient to reject cardiac xenografts in B6rag1−/− recipients (11.4 days, and 13.0 days respectively), column purified CD4+ cells were incapable of rejecting xenografts when adoptively transferred to C2Drag1−/− mice (>100 days) (Fig. 3). Taken together, these findings indicate that: 1) CD4+ T-cells are sufficient in the absence of CD8+ T-cells and B-cells to reject grafts, 2) direct CD4+ T-cells are not sufficient for xenograft rejection and 3) the indirect pathway of antigen presentation is required for concordant cardiac xenograft rejection.
Figure 2. CD4+ T-cells are sufficient for acute xeno-rejection.
B6 rag1−/− mouse recipients tolerate rat cardiac grafts indefinitely (>100 days, n=5, p<0.005) but can acutely reject grafts after reconstitution with unfractionated or column purified CD4+ LNCsλ.
Figure 3. CD4+ T-cells require host MHC class II for acute xeno-rejection.
Unfractionated lymph node cells and purified CD4+ cells were sufficient to reject cardiac xenografts in B6 rag1−/− recipients (11.4 +\− 1.7 days, n=5 and 13.0 +/− 2.6 days, n=4 respectively). Column purified CD4+ cells were incapable of rejecting xenografts when adoptively transferred to C2D rag1−/− mice (>100 days, n=5, p<0.005). Unreconstituted C2D rag1−/− mouse recipients cannot reject rat cardiac grafts (>100 days, n=3). They are still incapable of rejecting rat hearts after reconstitution with column purified B6 CD4+ LNCs. Although we did not expect an antibody involvement in our rag−/− recipients, we did perform flow cytometry for IgG, IgG1 and IgG2a to confirm that our column purified T-cell preparations were not contaminated. These immunoglobulins were all evident in wild type mice that had rejected rat grafts, but totally absent in B6rag−/− and C2Drag−/− recipients (data not shown).
‘Direct’ B6 CD4+ T cells respond vigorously to DA stimulator cells
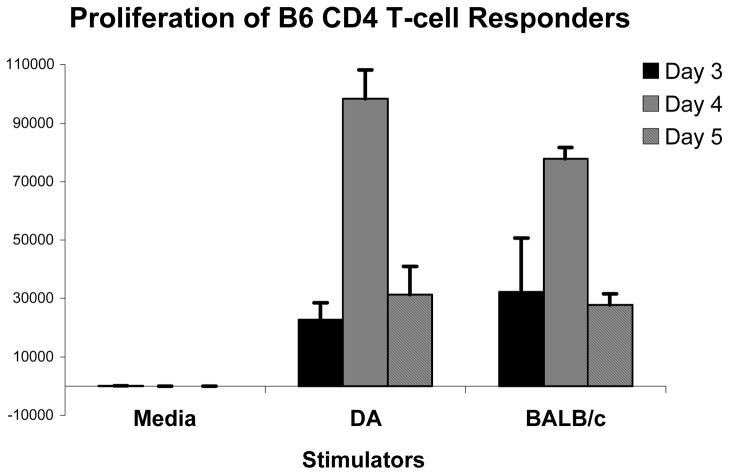
The main hypothesis of this study was to ascertain the requirement for indirect T-cell reactivity in xenograft rejection. However, results suggested that xenograft MHC expression was not sufficient for triggering rejection in the absence of host MHC class II. This result was in stark contrast to our previous finding that donor MHC class II expression was sufficient for ‘direct’ CD4+ T-cell mediated cardiac allograft rejection [30, 35]. Therefore, we set out to confirm that B6 mouse CD4+ T-cells could respond directly to DA rat xenogeneic APCs in vitro. CD4+ column purified naive B6 lymphocytes proliferated vigorously to DA stimulators to levels comparable to BALB/c allogeneic APCs (Fig. 4). Thus, although direct B6 CD4+ T-cells have the intrinsic capacity to respond strongly to rat APCs, this reactivity is not sufficient to trigger acute cardiac xenograft rejection in vivo.
Figure 4. Dark Agouti xeno-APCs are capable of directly stimulating B6 CD4+ T-cells.
MLRs were performed utilizing B6 CD4+ T-cells as responders with BALB/c γ-irradiated splenocyte allo-stimulators (positive control) or DA γ-irradiated splenocyte xeno-stimulators or media alone (control). Error bars represent the standard deviation from quadruplicate wells in the MLR assay. Each panel is representative of 3 experiments. Results show significant B6 CD4+ T-cell proliferation to both the BALB/c allo-control stimulators and the DA xeno-stimulators, thus demonstrating that xeno APCs are capable of directly stimulating CD4+ T-cells. The media controls did not elicit a significant response from B6 responders.
Histology
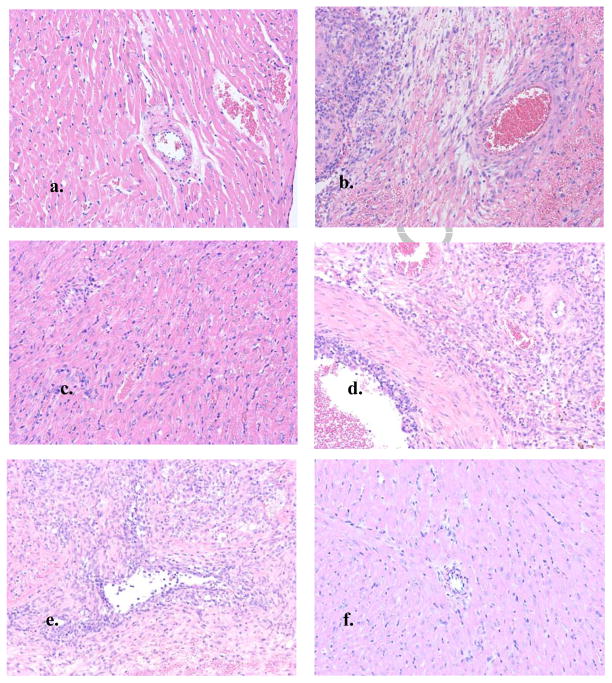
Compared to an untransplanted DA rat heart, a rat heart rejected by a B6 recipient shows extensive mononuclear infiltrate, intimal thickening, cardiomyocyte necrosis and some interstitial hemorrhage (Fig. 5a & b). A xenograft surviving >100 days in an untreated B6rag1−/− recipient (Fig. 5c) has virtually intact cellular architecture. A B6rag1−/− reconstituted with unfractionated LNCs (Fig. 5d) demonstrates extensive infiltrate, severe vasculopathy and some necrosis. A B6rag1−/− recipient reconstituted with purified CD4+ T-cells has extensive mononuclear infiltrating cells and some interstitial hemorrhage Fig. 5e). A DA heart which survived in a C2Drag1−/− recipient for >100d post reconstitution with B6 CD4+ LNCs (Fig. 5f) displays pristine cellular architecture.
Figure 5. Histology of cardiac xenografts.
a: Naive DA rat heart; b: DA rat heart in a B6 recipient, rejected 6 days; c: DA rat heart from a B6 rag1−/− recipient, >100 days; d: DA rat heart from a B6 rag1−/− recipient reconstituted with 107 B6 lymph node cells, rejected 9 days; e: DA rat heart from a B6 rag1−/− recipient reconstituted with 107 B6 CD4+ lymph node cells, rejected 14 days; f: DA rat heart from a C2D rag1−/− recipient reconstituted with 107 B6 CD4+ lymph node cells, >100 days (all 10x magnification)
Discussion
While fully immune competent transplant recipients possess a broad repertoire of pathways available to mount a response to non-self tissue, it is becoming increasingly apparent that the characteristics of xenograft immunity depend both on the tissue/organ grafted and the specific type of phylogeneic difference between donor and recipient [6, 14]. Therefore, one must examine the requirements for T-cell-dependent xenograft rejection using defined tissues/organs of interest and particular inter-species transplant combinations. It is now possible to genetically manipulate porcine donors such that they become less vulnerable to hyperacute rejection by naturally occurring anti-porcine antibodies and complement [36–38]. As such, the problem of T-cell-dependent xenograft immunity has become a more pressing obstacle to overcome. Like the human anti-porcine response [23–27], the rat-to-mouse concordant model demonstrates both potent ‘direct’ and ‘indirect’ CD4 T-cell reactivity [14]. In light of this, we chose a model utilizing B6rag−/− and C2Drag−/− recipients of cardiac xenografts to determine the relative importance of host MHC class II expression for rejection. Importantly the B6rag is capable of mounting either a direct or an indirect CD4 T-cell response via MHC class II, whilst the C2Drag, by virtue of its lack of class II expression, is only capable of mounting a direct response. Thus, this straightforward model allows the analysis of T-cell-dependent rejection of primarily vascularized cardiac xenografts.
Interestingly, despite the capacity of mouse T-cells to respond directly to rat APCs in vitro [14], MHC class II deficient mice are incapable of rejecting rat hearts. This suggests that while rat APCs can stimulate T-cells from C2D mice in vitro, directly stimulated T-cells are not critical or even required for in vivo concordant xeno-rejection. Importantly, because C2D mice have a corresponding deficiency in CD4+ T-cells [34] the lack of rejection in these animals could be due to down-regulation of MHC class II, CD4 cells, or both. To clarify this issue, C2D recipients of rat hearts were challenged with adoptively transferred CD4+ T-cells. While our previous studies indicate that CD4+ T-cells can reject cardiac allografts independently of host MHC class II [30], cardiac xenografts fail to reject despite engraftment of the CD4+ T-cells in the C2D environment. We conclude that host class II indirect antigen presentation is essential for CD4 T-cell-mediated cardiac xenograft rejection, in stark contrast to allograft rejection that relies on direct CD4 reactivity [28, 30]. It is important to note that results suggest that APCs from the Rag-deficient host are competent to mediate productive indirect xenoantigen presentation. Since lymphoid organ architecture is disrupted due to the lack of T and B cells in such immune-deficient animals, it is conceivable that indirect antigen presentation by the Rag−/− animal is aberrant. For example, the intrinsic functional capacity of APCs in immune-deficient mice is somewhat controversial [39, 40]. The implication of our current study is that Rag−/− derived APCs can readily present xenoantigens to CD4+ T-cells since the tempo of rejection in CD4+ T-cell reconstituted Rag−/− is essentially the same as primary acute rejection in wild-type animals.
Thus, while CD4 T-cells are required for acute cellular heart rejection of both allografts and rat xenografts, the recognition requirements (i.e. host versus donor) for rejection are markedly distinct. As such, results are similar to those found for CD4 T-cell-mediated islet xenograft rejection in which rejection was highly dependent on host MHC class II expression [14]. However, this result is rather surprising in that mouse CD4 T-cells mount a formidable ‘direct’ response to rat APCs in vitro [14]. Given that such ‘direct’ CD4 T-cells are sufficient for acute cardiac allograft rejection [29, 30], one might have also assumed that such CD4 T-cells could readily reject rat cardiac xenografts. The fact that potential anti-rat CD4 T-cells accumulate within the xenograft but fail to acutely reject MHC class II-bearing rat xenografts in vivo may be related to the issue of how graft-reactive CD4 T-cells actually mediate graft rejection. It is possible that while mouse CD4 T-cells respond to rat APCs, there may nevertheless be species-specific cytokine and/or accessory molecule interaction(s) that make a direct interaction with the xenogeneic rat target inefficient in vivo.
Since indirect CD4 T-cell reactivity – independent of either CD8 T-cells or B-cells – can account for primary cellular cardiac xenograft rejection, a key ongoing issue will center on the effector mechanism(s) of graft destruction. Strong indirect CD4 reactivity may promote the activation of host inflammatory cells that have been implicated in other models of T-cell-dependent xenograft immunity [35, 41–43]. Because indirect T-cells are restricted to host MHC molecules, they are unable to initiate a direct, cognate T-cell receptor engagement of the xenogeneic target cells [44]. As such, the primary route of xenograft rejection in this case would presumably be through ‘bystander’ inflammatory tissue injury as previously shown in the CD4 T-cell-dependent rejection of cellular transplants [44, 45]. Major molecular and cellular candidates for mediating this type of graft destruction would include inflammatory cytokines [44, 45], death-inducing receptors such as Fas ligand (CD95L), or the contribution of other innate cells such as activated macrophages. Also, complement activation by T-cell-dependent inflammation, independent of donor-reactive antibody reactivity, may be an additional innate response that might contribute to xenograft injury [46]. Clarifying the relative role of a variety of innate mediators triggered by CD4 T-cell-dependent graft injury will be an important goal in understanding both xenograft rejection and general transplant immunity.
Another intriguing possibility is that a host MHC-restricted (indirect) response can, by chance, generate a small population of cross-reactive T-cells recognizing native allogeneic or perhaps xenogeneic MHC molecules. This phenomenon of ‘heterologous’ immunity is well appreciated in the setting of allograft immunity [47] and could be responsible for generating CD4 T-cells directly reactive to native xenogeneic MHC despite originating from a self MHC-restricted T-cell repertoire. Such T-cells could conceivably be responsible for xenograft rejection. However, we would posit that even if this type of heterologous direct reactivity did occur, it would presumably not be sufficient to account for acute cardiac xenograft rejection. That is, although rat APCs are able to directly stimulate mouse CD4 T-cells, this ‘direct’ pathway is not sufficient to permit xenograft rejection conditions when host mouse MHC II is absent and rat MHC II is present. By extrapolation – under conditions whereby indirectly activated CD4 T-cells are present – if direct engagement of the donor (via donor xeno MHC II) were critical one would also expect rejection to occur under conditions of isolated direct xeno-antigen presentation. Therefore, our data suggest that although direct engagement of rat donor targets is possible, it is not sufficient for acute rejection to occur. Therefore, by inference, it is likely that indirect CD4 T-cells ‘kill’ xeno-targets via a non-cognate bystander effector mechanism.
The role of B-cells, and therefore xenoreactive antibody production, remains to be fully investigated. However, our study clearly demonstrates that CD4+ T-cell mediated xeno-rejection can occur in the absence of B-cells. Thus, B-cells are not required as primary APCs for xenoreactive CD4 T-cells for initiating primary acute rejection. Certainly, such results should not be construed as implying that humoral immunity is irrelevant in xenotransplantation. Antibody dependent reactivity clearly can contribute to xenograft injury [48, 49]. Rather, results support the contention that vigorous CD4 T-cell-dependent immunity can result in cardiac xenograft rejection without a requirement for donor-specific antibody production.
In summary, this study indicates CD4+ T-cells are both necessary and sufficient for acute concordant cardiac xenograft rejection and that intact host MHC class II is required for CD4+ T-cell mediated concordant cardiac xenograft rejection.
Acknowledgments
Funding
This work was supported by NIH Grants R01 HL64976–02 (BAP) and RO1 DK 45773 (RGG).
Abbreviations
- APC
Antigen-presenting cell
- C2D
MHC class II-deficient
- LN
lymph node
Footnotes
Disclosures:
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rose AG. Understanding the pathogenesis and the pathology of hyperacute cardiac rejection. Cardiovasc Pathol. 2002;11(3):171–6. doi: 10.1016/s1054-8807(01)00097-7. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DK, et al. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. Journal of Heart Lung Transplantation. 2000;19(12):1125–65. doi: 10.1016/s1053-2498(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 3.Knosalla Non-Specific Removal of Antibodies in Patients With Idiopathic Dilated Cardiomyopathy: Implications for Xenotransplantation. Journal of Heart and Lung Transplantation. 2004;23(5):623–6. doi: 10.1016/S1053-2498(03)00229-8. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Stawinski Rituximab as Monotherapy for Elicited Xenoreactive Antibody Responses. Journal of Heart and Lung Transplantation. 2006;25(12):1462–6. doi: 10.1016/j.healun.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.McGregor Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. The Journal of Thoracic and Cardiovascular Surgery. 2005;130(3):844–51. doi: 10.1016/j.jtcvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Rayat GR, Gill RG. Pancreatic islet xenotransplantation: barriers and prospects. Curr Diab Rep. 2003;3(4):336–43. doi: 10.1007/s11892-003-0027-8. [DOI] [PubMed] [Google Scholar]
- 7.Ogata Potential Applications and Prospects for Cardiac Xenotransplantation. Journal of Heart and Lung Transplantation. 2004;23(5):515–26. doi: 10.1016/j.healun.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Friedman T, et al. A critical role for human CD4+ T-cells in rejection of porcine islet cell xenografts. Diabetes. 1999;48(12):2340–8. doi: 10.2337/diabetes.48.12.2340. [DOI] [PubMed] [Google Scholar]
- 9.Gill RG, et al. CD4+ T cells are both necessary and sufficient for islet xenograft rejection. Transplant Proc. 1994;26(3):1203. [PubMed] [Google Scholar]
- 10.Olack BJ, et al. Rejection of porcine islet xenografts mediated by CD4+ T cells activated through the indirect antigen recognition pathway. Xenotransplantation. 2002;9(6):393–401. doi: 10.1034/j.1399-3089.2002.01070.x. [DOI] [PubMed] [Google Scholar]
- 11.Wecker H, Winn H, Auchincloss HJ. CD4+ T cells, without CD8+ or B lymphocytes, can reject xenogeneic skin grafts. Xenotransplantation. 1994;(1):8–16. [Google Scholar]
- 12.Wolf LA, Coulombe M, Gill RG. Donor antigen-presenting cell-independent rejection of islet xenografts. Transplantation. 1995;60(10):1164–70. doi: 10.1097/00007890-199511270-00018. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, et al. The critical role of mouse CD4+ cells in the rejection of highly disparate xenogeneic pig thymus grafts. Xenotransplantation. 2000;7(2):129–37. doi: 10.1034/j.1399-3089.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 14.Rayat GR, et al. The degree of phylogenetic disparity of islet grafts dictates the reliance on indirect CD4 T-cell antigen recognition for rejection. Diabetes. 2003;52(6):1433–40. doi: 10.2337/diabetes.52.6.1433. [DOI] [PubMed] [Google Scholar]
- 15.Gill RG. The role of direct and indirect antigen presentation in the response to islet xenografts. Transplantation Proceedings. 1992;24(2):642–3. [PubMed] [Google Scholar]
- 16.Auchincloss H, Jr, Moses R, Conti D, et al. Xenograft rejection of class I-expressing transgenic skin is CD4-dependent and CD8-independent. Tranplantation Proceedings. 1990;22(5):2335–6. [PubMed] [Google Scholar]
- 17.Murphy B, Auchincloss H, Jr, Carpenter CB, Sayegh MH. T cell recognition of xeno-MHC peptides during concordant xenograft rejection. Transplantation. 1996;61:1133–7. doi: 10.1097/00007890-199604270-00001. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl KF, Bach FH. Genetic and Cellular Aspects of Xenogeneic Mixed Leukocyte Culture Reaction. J Ex Med. 1976;144(2):305–18. doi: 10.1084/jem.144.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alter BJ, Bach FH. Cellular basis of the proliferative response of human T cells to mouse xenoantigens. J Ex Med. 1990;171(1):333–8. doi: 10.1084/jem.171.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stock PG, Ascher NL, Chen S, Field J, Bach FH, Sutherland DE. Evidence for Direct and Indirect Pathways in the Generation of the Alloimmune Response Against Pancreatic Islets. Transplantation. 1991;52(4):704–9. doi: 10.1097/00007890-199110000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Auchincloss H, Jr, Sachs DH. Xenogeneic transplantation. Annu Rev Immunol. 1998;16:433–70. doi: 10.1146/annurev.immunol.16.1.433. [DOI] [PubMed] [Google Scholar]
- 22.Auchincloss H., Jr Cell-mediated xenoresponses: strong or weak? Clin Transplant. 1994;8(2 Pt 2):155–9. [PubMed] [Google Scholar]
- 23.Bravery CA, et al. Direct recognition of SLA- and HLA-like class II antigens on porcine endothelium by human T cells results in T cell activation and release of interleukin-2. Transplantation. 1995;60(9):1024–33. [PubMed] [Google Scholar]
- 24.Dorling A, et al. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur J Immunol. 1996;26(6):1378–87. doi: 10.1002/eji.1830260630. [DOI] [PubMed] [Google Scholar]
- 25.Kirk AD, et al. The human antiporcine cellular repertoire. In vitro studies of acquired and innate cellular responsiveness. Transplantation. 1993;55(4):924–31. [PubMed] [Google Scholar]
- 26.Rollins SA, et al. Evidence that activation of human T cells by porcine endothelium involves direct recognition of porcine SLA and costimulation by porcine ligands for LFA-1 and CD2. Transplantation. 1994;57(12):1709–16. [PubMed] [Google Scholar]
- 27.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155(11):5249–56. [PubMed] [Google Scholar]
- 28.Singh NP, et al. Blockade of indirect recognition mediated by CD4+ T cells leads to prolonged cardiac xenograft survival. Xenotransplantation. 2004;11(1):33–42. doi: 10.1111/j.1399-3089.2004.00079.x. [DOI] [PubMed] [Google Scholar]
- 29.Grazia TJ, et al. A two-step model of acute CD4 T-cell mediated cardiac allograft rejection. J Immunol. 2004;172(12):7451–8. doi: 10.4049/jimmunol.172.12.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietra BA, et al. CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. J Clin Invest. 2000;106(8):1003–10. doi: 10.1172/JCI10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–50. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Wilde DB, et al. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J Immunol. 1983;131(5):2178–83. [PubMed] [Google Scholar]
- 33.Sarmiento M, et al. Cloned T lymphocytes and monoclonal antibodies as probes for cell surface molecules active in T cell-mediated cytolysis. Immunol Rev. 1982;68:135–69. doi: 10.1111/j.1600-065x.1982.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 34.Grusby MJ, et al. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253(5026):1417–20. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 35.Yi S, et al. CD4+ T cells initiate pancreatic islet xenograft rejection via an interferon-gamma-dependent recruitment of macrophages and natural killer cells. Transplantation. 2002;73(3):437–46. doi: 10.1097/00007890-200202150-00019. [DOI] [PubMed] [Google Scholar]
- 36.Harrison S, et al. An efficient method for producing alpha(1,3)-galactosyltransferase gene knockout pigs. Cloning Stem Cells. 2004;6(4):327–31. doi: 10.1089/clo.2004.6.327. [DOI] [PubMed] [Google Scholar]
- 37.Zhou CY, et al. Transgenic pigs expressing human CD59, in combination with human membrane cofactor protein and human decay-accelerating factor. Xenotransplantation. 2005;12(2):142–8. doi: 10.1111/j.1399-3089.2005.00209.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Pig-to-Non-human Primate Heart Transplantation: Immunologic Progress Over 20 Years. The Journal of Heart and Lung Transplantation. 2007;26(3):210–218. doi: 10.1016/j.healun.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 39.De Creus A, Van Beneden K, Taghon T, Stolz F, Debacker V, Plum J, Leclercq G. Langerhans cells that have matured in vivo in the absence of T cells are fully capable of inducing a helper CD4 as well as a cytotoxic CD8 response. J Immunol. 2000;165(2):645–53. doi: 10.4049/jimmunol.165.2.645. [DOI] [PubMed] [Google Scholar]
- 40.Shreedhar V, Moodycliffe AM, Ullrich SE, Bucana C, Kripke ML, Flores-Romo L. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11(5):625–36. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 41.Andres A, et al. Macrophage depletion prolongs discordant but not concordant islet xenograft survival. Transplantation. 2005;79(5):543–9. doi: 10.1097/01.tp.0000151764.39095.ca. [DOI] [PubMed] [Google Scholar]
- 42.Itescu S, et al. Role of natural killer cells, macrophages, and accessory molecule interactions in the rejection of pig-to-primate xenografts beyond the hyperacute period. Hum Immunol. 1998;59(5):275–86. doi: 10.1016/s0198-8859(98)00026-3. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt P, et al. A new murine model of islet xenograft rejection: graft destruction is dependent on a major histocompatibility-specific interaction between T-cells and macrophages. Diabetes. 2003;52(5):1111–8. doi: 10.2337/diabetes.52.5.1111. [DOI] [PubMed] [Google Scholar]
- 44.Kupfer TM, Crawford ML, Pham K, Gill RG. MHC-mismatched islet allografts are vulnerable to autoimmune recognition in vivo. J Immunol. 2005;175(4):2309–16. doi: 10.4049/jimmunol.175.4.2309. [DOI] [PubMed] [Google Scholar]
- 45.Angstetra E, Graham KL, Emmett S, et al. In vivo effects of cytokines on pancreatic beta-cells in models of type 1 diabetes dependent on CD4+ T lymphocytes. Immunol Cell Biol. 2009;87(2):178–85. doi: 10.1038/icb.2008.81. [DOI] [PubMed] [Google Scholar]
- 46.Heeger PS, Kemper C. Novel rolesof complement in T effector cell regulation. Immunobiology. 2012;217(2):216–24. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Adams AB, Williams MA, Jones TR, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–95. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daniels LJ, Platt JL. Hyperacute xenograft rejection as an immunologic barrier to xenotransplantation. Kidney Int Suppl. 1997;58:S28–35. [PubMed] [Google Scholar]
- 49.McKenzie IF, et al. Pig islet xenografts are susceptible to “anti-pig” but not Gal alpha(1,3)Gal antibody plus complement in Gal o/o mice. J Immunol. 1998;161(10):5116–9. [PubMed] [Google Scholar]