Abstract
Purpose
In the absence of evidence from large clinical trials, optimal therapy for localized prostate cancer remains unclear; however, treatment patterns continue to change. We examined changes in the management of patients with prostate cancer in the Medicare population.
Methods and Materials
We conducted a retrospective claims-based analysis of the use of radiation therapy, surgery, and androgen deprivation therapy in the 12 months after diagnosis of prostate cancer in a nationally representative 5% sample of Medicare claims. Patients were Medicare beneficiaries 67 years or older with incident prostate cancer diagnosed between 1999 and 2007.
Results
There were 20,918 incident cases of prostate cancer between 1999 and 2007. The proportion of patients receiving androgen deprivation therapy decreased from 55% to 36%, and the proportion of patients receiving no active therapy increased from 16% to 23%. Intensity-modulated radiation therapy replaced 3-dimensional conformal radiation therapy as the most common method of radiation therapy, accounting for 77% of external beam radiotherapy by 2007. Minimally invasive radical prostatectomy began to replace open surgical approaches, being used in 49% of radical prostatectomies by 2007.
Conclusions
Between 2002 and 2007, the use of androgen deprivation therapy decreased, open surgical approaches were largely replaced by minimally invasive radical prostatectomy, and intensity-modulated radiation therapy replaced 3-dimensional conformal radiation therapy as the predominant method of radiation therapy in the Medicare population. The aging of the population and the increasing use of newer, higher-cost technologies in the treatment of patients with prostate cancer may have important implications for nationwide health care costs.
Keywords: Prostatic Neoplasms, Prostatectomy, Radiotherapy, Intensity-Modulated, Androgen Antagonists, Medicare, Physician's Practice Patterns
Introduction
Prostate cancer is the most common type of cancer and is the second leading cause of cancer-related deaths among men in the United States. Management options include active surveillance, radical prostatectomy, external beam radiation therapy, and interstitial brachytherapy. A recent systematic review concluded that there is limited evidence concerning the relative effectiveness of localized treatment modalities.1 Previous studies have observed geographic variations and temporal changes in the treatment of patients with localized prostate cancer.2 In the absence of large comparative trials, the optimal treatment strategy for these patients remains unclear, leading some to suggest that changes in treatment patterns may reflect changes in reimbursement the availability of new technology, or the belief that new approaches will benefit patients even though evidence from randomized trials is not available.3
The relative impact of prostate cancer in the United States will likely grow in coming years as demographic trends lead to greater incidence of the disease. Costs associated with prostate cancer were an estimated $7 billion in 2005, placing it among the 4 most costly malignancies.4 Most patients with prostate cancer are enrolled in Medicare, which both directly and indirectly influences the coverage policies of private insurers and Medicaid programs.
The introduction of new technologies also drives increases in health care costs. In 2005 US dollars, the average cost of treating patients with prostate cancer increased from $8900 in 1993 to $10,700 in 2003, largely as a result of the expanded use of androgen deprivation therapy (ADT) and radiation therapy. Minimally invasive radical prostatectomy (MIRP) and intensity-modulated radiation therapy (IMRT) emerged in the early 2000s, with MIRP being used in one-quarter of radical prostatectomies by 2005.5 A recent study of the use of MIRP and IMRT using Surveillance Epidemiology and End Results (SEER)-Medicare linked data found rates of IMRT as high as 80% by 2005.6 The SEER-Medicare data come from a combination of cancer registries in 15 states that are disproportionately concentrated among urban, nonwhite, affluent populations with relatively high enrollment in health maintenance organizations and low cancer mortality.7 Significant geographic variation has been observed in the diagnosis, evaluation, and management of prostate cancer.2,8 In this study, we used Medicare claims data to examine changes in the management of prostate cancer in a nationally representative 5% sample of Medicare beneficiaries and extend this analysis to patients diagnosed through 2007.
Methods and Materials
We obtained administrative claims data for a nationally representative 5% sample of Medicare beneficiaries from 1997 through 2008 from the US Centers for Medicare & Medicaid Services (CMS). The inpatient files include claims covered under Medicare Part A for institutional facility services. The outpatient files include claims covered under Medicare Part A for institutional outpatient providers such as hospital outpatient departments and ambulatory surgery centers. The carrier files contain provider claims for services covered under Medicare Part B. The denominator files contain beneficiary identifiers, sex, race/ethnicity, birth dates, dates of death, zip codes, and information about program eligibility and enrollment. Medicare beneficiaries report race/ethnicity at the time of enrollment. In this analysis, we used the categories “black” and “other.” The institutional review board of the Duke University Health System approved this study.
Study Population
Consistent with methods developed in previous research,9,10 the study population included Medicare beneficiaries living in the United States for whom a diagnosis of prostate cancer (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 185) was listed on an inpatient, outpatient, or carrier claim between 1999 and 2007. We used claims data from 2008 for ascertainment of initial therapy for prostate cancer only. We defined the date of disease onset as the date of the earliest observed cancer claim. To be considered a new-onset or incident case, we required beneficiaries to be 67 years or older, to be enrolled in fee-for-service Medicare for at least 2 years before the first diagnosis of prostate cancer, and to have no claims for any type of cancer during that 2-year period. In addition, we required that beneficiaries have at least 1 additional claim containing a diagnosis of prostate cancer within 60 days after the first claim and a prostate biopsy within 12 months after diagnosis. Using these criteria to identify diagnoses of prostate cancer, we selected the first diagnosis for each patient for the analysis. We applied these criteria to ensure that we selected incident cases rather than metastatic or recurrent disease. Inclusion in the analysis was conditional on survival for at least 60 days after the date of disease onset.
Initial Therapy
We identified the therapies for prostate cancer received by each patient by examining Current Procedural Terminology codes on claims in the year after the initial diagnosis. We organized claims for treatment into 3 non–mutually exclusive categories according to whether the patient received each treatment type: ADT, radiation therapy, and surgery. Androgen deprivation therapies included leuprolide and goserelin. Radiation therapies included conventional 2-dimensional radiation therapy, 3-dimensional conformal radiation therapy (3-D CRT), intensity-modulated radiation therapy (IMRT), brachytherapy (low or high dynamic range), stereotactic body radiation therapy (SBRT), and proton therapy. Surgical therapies included retropubic radical prostatectomy, radical perineal prostatectomy, and MIRP. Patients receiving neither ADT, radiation, nor surgery were categorized as “no active therapy,” which could include no therapy, active surveillance, or watchful waiting. We limited the analysis to treatments received (vs treatments planned) on the basis of Healthcare Common Procedure Coding System (HCPCS) codes. For patients who received radiation therapy, we did not distinguish among radiation alone, radiation in addition to surgery (ie, as adjuvant treatment), and radiation in combination with hormones.
Statistical Analysis
For characteristics of patients in the incident cohorts, we present categorical variables as frequencies with percentages. We identified comorbid conditions by using validated coding algorithms10 to search all inpatient, outpatient, and carrier claims for 365 days before the date of disease onset for cerebrovascular disease, chronic obstructive pulmonary disease, congestive heart failure, coronary heart disease, dementia, diabetes mellitus, hypertension, peripheral vascular disease, and renal disease.
We tested for associations between each categorical variable and year of diagnosis using Cochran-Mantel-Haenszel χ2 tests (row mean score statistic). We compared overall trends in treatment patterns by plotting the total proportion of patients receiving each treatment modality by year of diagnosis between 1998 and 2007. In a sensitivity analysis, we examined the effect of examining treatment using claims within 1 year vs 2 years after diagnosis. We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses, and we considered P < .001 to be statistically significant.
Results
There were 20,918 incident cases of prostate cancer between 1999 and 2007 that met the study criteria. Approximately 60% of the study population was aged 67 to 75 years. The percentage of black patients was approximately 10% throughout the study period. Rates of most comorbid conditions remained unchanged during the study period, including cerebrovascular disease, chronic obstructive pulmonary disease, congestive heart failure, and dementia. However, the rates of diabetes mellitus, hypertension, peripheral vascular disease, and renal disease increased substantially from 1999 to 2007 (Table 1). Median age in all years of the study was 74 years.
Table 1. Baseline Characteristics of the Study Population.
| Characteristics | Year of Diagnosis | P Value* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999 (n = 2151) | 2000 (n = 2106) | 2001 (n = 2150) | 2002 (n = 2448) | 2003 (n = 2345) | 2004 (n = 2349) | 2005 (n = 2380) | 2006 (n = 2538) | 2007 (n = 2451) | ||
| Age group, No. (%) | .004 | |||||||||
| 67-75 y | 1239 (57.6) | 1238 (58.8) | 1231 (57.3) | 1442 (58.9) | 1368 (58.3) | 1330 (56.6) | 1425 (59.9) | 1540 (60.7) | 1512 (61.7) | |
| 76-80 y | 539 (25.1) | 511 (24.3) | 541 (25.2) | 606 (24.8) | 550 (23.5) | 576 (24.5) | 572 (24.0) | 588 (23.2) | 538 (22.0) | |
| ≥ 81 y | 373 (17.3) | 357 (17.0) | 378 (17.6) | 400 (16.3) | 427 (18.2) | 443 (18.9) | 383 (16.1) | 410 (16.2) | 401 (16.4) | |
| Race, No. (%) | .10 | |||||||||
| Black | 230 (10.7) | 213 (10.1) | 205 (9.5) | 233 (9.5) | 250 (10.7) | 212 (9.0) | 261 (11.0) | 227 (8.9) | 221 (9.0) | |
| Other | 1921 (89.3) | 1893 (89.9) | 1945 (90.5) | 2215 (90.5) | 2095 (89.3) | 2137 (91.0) | 2119 (89.0) | 2311 (91.1) | 2230 (91.0) | |
| Comorbid conditions, No. (%) | ||||||||||
| Cerebrovascular disease | 262 (12.2) | 250 (11.9) | 255 (11.9) | 281 (11.5) | 319 (13.6) | 314 (13.4) | 277 (11.6) | 338 (13.3) | 278 (11.3) | .66 |
| COPD | 406 (18.9) | 390 (18.5) | 413 (19.2) | 479 (19.6) | 482 (20.6) | 453 (19.3) | 446 (18.7) | 486 (19.1) | 433 (17.7) | .46 |
| Coronary heart disease | 673 (31.3) | 663 (31.5) | 653 (30.4) | 788 (32.2) | 737 (31.4) | 804 (34.2) | 736 (30.9) | 830 (32.7) | 746 (30.4) | .69 |
| Congestive heart failure | 216 (10) | 195 (9.3) | 213 (9.9) | 233 (9.5) | 253 (10.8) | 223 (9.5) | 221 (9.3) | 215(8.5) | 203 (8.3) | .02 |
| Dementia | 27 (1.3) | 28 (1.3) | 45 2.1) | 43 (1.8) | 40 (1.7) | 46 (2) | 42 (1.8) | 48 (1.9) | 38 (1.6) | .28 |
| Diabetes mellitus | 418 (19.4) | 436 (20.7) | 437 (20.3) | 499 (20.4) | 502 (21.4) | 565 (24.1) | 576 (24.2) | 632 (24.9) | 625 (25.5) | <.001 |
| Hypertension | 1136 (52.8) | 1219 (57.9) | 1238 (57.6) | 1532 (62.6) | 1472 (62.8) | 1567 (66.7) | 1638 (68.8) | 1781 (70.2) | 1749 (71.4) | <.001 |
| Peripheral vascular disease | 221 (10.3) | 211 (10) | 219 (10.2) | 290 (11.8) | 311 (13.3) | 334 (14.2) | 286 (12) | 345 (13.6) | 334 (13.6) | <.001 |
| Renal disease | 61 (2.8) | 53 (2.5) | 82 (3.8) | 64 (2.6) | 88 (3.8) | 107 (4.6) | 113 (4.7) | 131 (5.2) | 161 (6.6) | <.001 |
Abbreviation: COPD, chronic obstructive pulmonary disease.
From Cochran-Mantel-Haenszel tests for associations between characteristic and year of diagnosis.
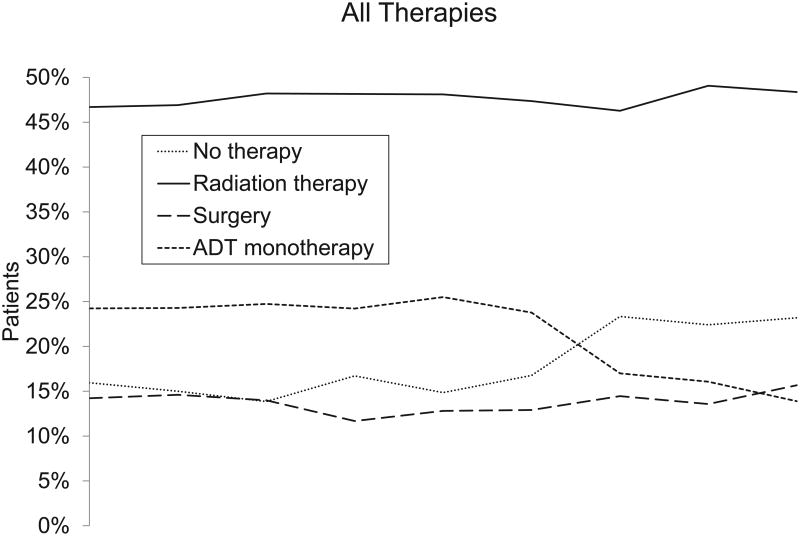
The proportion of patients who received ADT alone or in combination with other therapies decreased from 55% to 36% between 1999 and 2007 (Figure 1 and Table 2). During the same period, ADT monotherapy decreased from 25% to 14%, and the proportion of patients who received no active therapy increased from 16% to 23%. The proportion of patients who underwent definitive surgical or radiation-based therapy remained at roughly 60% during the study period.
Figure. Changes in the Treatment of Patients With Prostate Cancer in the Medicare Population, 1999-2007.
The vertical axes indicate the percentage of patients who had 1 or more claim for the procedure within 1 year after diagnosis. The horizontal axes indicate the year of diagnosis. Abbreviation: ADT, androgen deprivation therapy.
Table 2. Selected Treatments for Prostate Cancer With 1 Year After Diagnosis*.
| Characteristics | Year of Diagnosis | P Value† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999 (n = 2151) | 2000 (n = 2106) | 2001 (n = 2150) | 2002 (n = 2448) | 2003 (n = 2345) | 2004 (n = 2349) | 2005 (n = 2380) | 2006 (n = 2538) | 2007 (n = 2451) | ||
| No active therapy, No. (%) | 345 (16.0) | 313 (14.9) | 296 (13.8) | 394 (16.1) | 344 (14.7) | 382 (16.3) | 534 (22.4) | 544 (21.4) | 551 (22.5) | <.001 |
| Radiation therapy, No. (%) | 998 (46.4) | 982 (46.6) | 1037 (48.2) | 1185 (48.4) | 1130 (48.2) | 1114 (47.4) | 1112 (46.7) | 1266 (49.9) | 1197 (48.8) | .05 |
| Brachytherapy | 380 (17.7) | 388 (18.4) | 428 (19.9) | 491 (20.1) | 464 (19.8) | 467 (19.9) | 438 (18.4) | 471 (18.6) | 425 (17.3) | .43 |
| External beam | ||||||||||
| 2-D | 52 (2.4) | 38 (1.8) | 36 (1.7) | 26 (1.1) | 24 (1.0) | 12 (0.5) | 14 (0.6) | 11 (0.4) | <.001 | |
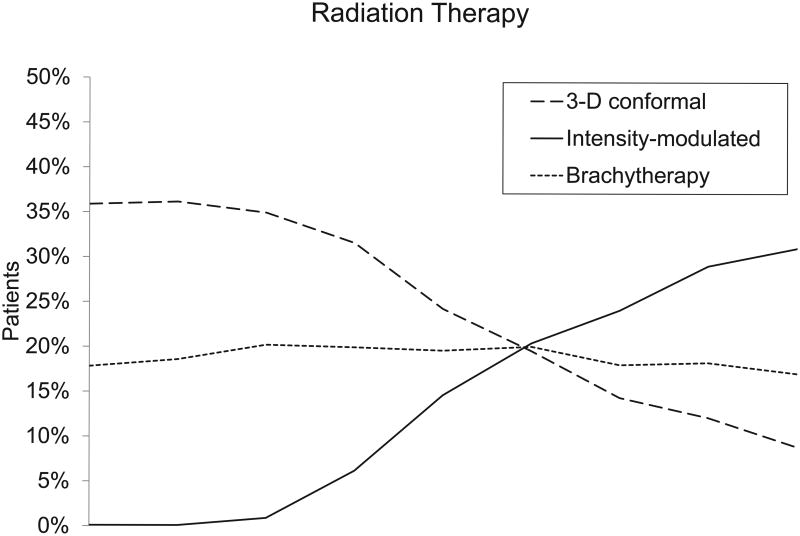
| 3-D conformal | 767 (35.7) | 755 (35.8) | 756 (35.2) | 775 (31.7) | 561 (23.9) | 466 (19.8) | 354 (14.9) | 319 (12.6) | 221 (9) | <.001 |
| IMRT | 20 (0.9) | 151 (6.2) | 345 (14.7) | 482 (20.5) | 574 (24.1) | 747 (29.4) | 761 (31.0) | <.001 | ||
| Surgery, No. (%) | <310 (<13.0) | <310 (<13.0) | <310 (<13.0) | <310 (<13.0) | 298 (12.7) | 304 (12.9) | 339 (14.2) | 343 (13.5) | 386 (15.7) | .16 |
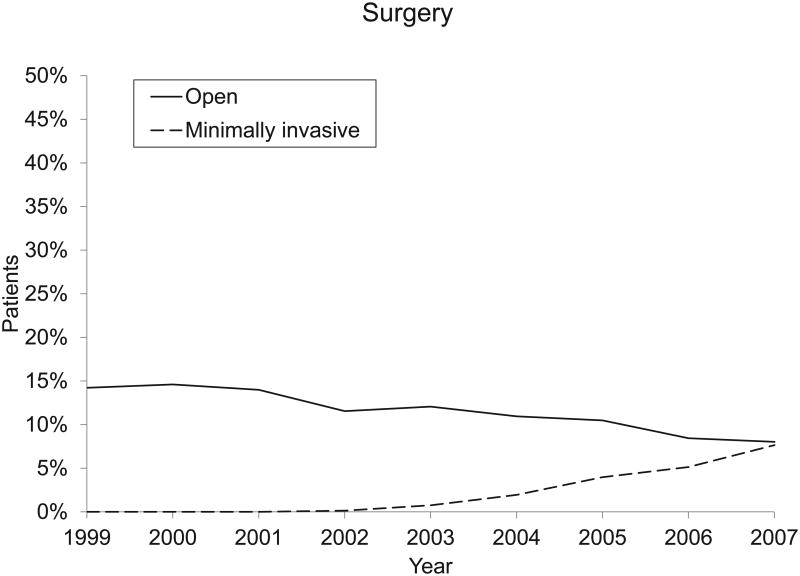
| Open | 299 (13.9) | 305 (14.5) | 295 (13.7) | 283 (11.6) | 281 (12.0) | 259 (11.0) | 246 (10.3) | 214 (8.4) | 197 (8.0) | <.001 |
| Minimally invasive | 17 (0.7) | 45 (1.9) | 93 (3.9) | 129 (5.1) | 189 (7.7) | <.001 | ||||
| ADT, No. (%) | 1187 (55.2) | 1140 (54.1) | 1184 (55.1) | 1345 (54.9) | 1265 (53.9) | 1267 (53.9) | 1030 (43.3) | 1093 (43.1) | 880 (35.9) | <.001 |
| ADT monotherapy, No. (%) | 531 (24.7) | 522 (24.8) | 539 (25.1) | 602 (24.6) | 601 (25.6) | 567 (24.1) | 419 (17.6) | 414 (16.3) | 345 (14.1) | <.001 |
Abbreviation: ADT, androgen deprivation therapy; IMRT, intensity-modulated radiation therapy.
Where cells had 10 or fewer observations, data have been suppressed to protect patient confidentiality.
From Cochran-Mantel-Haenszel tests for associations between characteristic and year of diagnosis.
Although overall rates of surgery and radiation therapy did not change, the types of surgery and radiation therapy changed beginning in 2002. The overall proportion of patients who underwent radical prostatectomy remained steady at 12% to 16% between 1999 and 2007. A shift from open surgical techniques to MIRP began in 2002 and continued through 2007. In 2001, all radical prostectomies were performed using open approaches. By 2007, half of all radical prostatectomies were perfomed using minimally invasive techniques (all P < .001; Figure 1 and Table 2).
Among patients who underwent radiation therapy, IMRT largely replaced 3-D CRT by 2007. In 1999, 36% of patients underwent 3-D CRT and none underwent IMRT. By 2007, 9% of patients underwent 3-D CRT and 31% underwent IMRT. Proton therapy remained a rare treatment modality, used in less than 1% of patients in 2007. Stereotactic body radiation therapy for prostate cancer was essentially unused in the Medicare population in 2007.
Patients aged 67 to 76 years were more likely to undergo definitive treatment (75% vs 40%), including surgery (22% vs 1%), brachytherapy (24% vs 12%), or external beam radiation therapy (40% vs 32%), compared with older patients, who were instead more likely to undergo ADT monotherapy (37% vs 11%) or no active therapy (24% vs 13% ; all P < .001). The use of ADT monotherapy persisted among older beneficiaries, with 39% of beneficiaries older than 80 years receiving ADT monotherapy as late as 2007, compared with less than 7% of beneficiaries aged 67 to 75 years. Patients with multiple comorbid conditions were slightly less likely to undergo any treatment, compared with patients who had no comorbid conditions (18.6% vs 16.2%; P < .001), but were much less likely to undergo surgery (10.4% vs 19.1%; P < .001).
In a sensitivity analysis, we examined the effect of analyzing treatment using 1 year vs 2 years of claims data after a diagnosis of prostate cancer. The effect of examining treatment out to 2 years varied by treatment modality but increased the proportion of patients treated by less than 10% regardless of modality. The proportion of patients who underwent no active therapy during the entire study period was 19% vs 17%, respectively, when we used 1 year vs 2 years of follow-up data. Claims for surgery and brachytherapy occurred within the first 12 months in more than 95% of patients who received either treatment. Patterns of use were qualitatively and quantitatively similar in the primary and sensitivity analyses.
Discussion
The treatment of patients with prostate cancer in the Medicare population changed dramatically between 1999 and 2007. We observed a significant decrease in the use of ADT early in the study period. Although overall rates of surgery and radiation therapy remained constant, MIRP became the dominant form of radical prostatectomy, and IMRT largely replaced 3-D CRT. These rapid changes in clinical practice occurred during a period when the evidence available from large clinical trials and clinical practice guidelines did not change.1
Our observation of decreased use of ADT from 2003 through 2007 extends previous studies that observed decreases through 2005.5 The use of ADT monotherapy persisted for a subgroup of patients. Although wait times and access to intravenous chemotherapy did not change after 2003,10 previous reports have speculated that reduced rates of overall ADT use in prostate cancer occurred in response to the Medicare Modernization Act of 2003 (MMA), which reduced Medicare reimbursement rates for chemotherapy.11 Another possibility is that the results of randomized trials have led to restricting ADT to patients who are most likely to benefit.12 Taken as a group, prostate cancer nationwide is a relatively low-risk disease for which decreased use of ADT may have decreased over time as knowledge regarding the natural course of early-stage prostate cancer has evolved. The decreased use of ADT has important financial implications, because the average annual cost of ADT was $7200 in 2005.13
The increasing use of MIRP has been previously observed between 2003 and 2005.5,6 Our study extends these observations through 2007, at which time the use of MIRP captured almost 50% of the surgery market. Compared with estimates from the predominantly urban SEER-Medicare population, we found substantially lower rates of IMRT adoption in the general Medicare population. Medicare payment for radical prostatectomy does not vary by surgical method, and evaluations of robotic prostatectomy have demonstrated decreased hospital profit margins without clear evidence of improved clinical outcomes.14 In the context of negative financial incentives and unclear clinical benefit, the increasing use of these technologies may be driven instead by patient and physician demand.15
Our study demonstrates that IMRT has largely replaced external beam radiation therapy for the initial treatment of patients with prostate cancer in the Medicare population. This change has significant financial implications. Medicare reimburses more than twice as much for IMRT than for 3-D CRT.16 As with MIRP, the adoption of IMRT has occurred in the absence of large randomized trials comparing IMRT to conventional therapy.2 High-level evidence supports decreased toxicity with IMRT compared with 3-D CRT in patients with head and neck cancer17; however, no large trial has definitively examined the safety or efficacy of these therapies in patients with prostate cancer. The accuracy of the estimates presented here are supported by a previous analysis using SEER data that found rates of approximately 30% for external beam radiation therapy and 50% overall for radiation therapy in 2004, similar to the rates we observed.18
The results of our study may have profound implications for US health care policy. Our finding that IMRT and MIRP have replaced older treatment modalities contrasts with findings from other areas of developing medical technology, such as imaging and diagnostic testing, where increasing use is typically additive rather than a substitute for conventional methods.9 Although the relative proportion of patients undergoing surgical or radiation-based therapy remained unchanged, a higher incidence of prostate cancer in the later years of our study translated to higher overall rates of patients undergoing therapy. We found that patients were only slightly less likely to undergo therapy if they had multiple vs zero comorbid conditions, despite recommendations to avoid aggressive treatment in these populations.19 Unlike reimbursement for other emerging technologies, Medicare reimbursement for radiation therapy is not guided by national coverage determinations and is instead guided by region-specific local coverage determinations. The current local coverage determinations date back to 2002 for IMRT, 2007 for SBRT, and 2009 for proton therapy. In this study, the use of both IMRT and MIRP increased through the last year of available data, suggesting that their use in the Medicare population had not plateaued by 2007.
Our study has some limitations. First, some cases we identified in the claims data as incident may have been recurrent cases. To mitigate this concern, we required patients in the analysis to have had a recent biopsy and no diagnosis of prostate cancer in the preceding 2 years. Second, Current Procedural Terminology codes for radiation therapy are not specific to cancer site, so some radiation therapy procedures included in the analysis may represent uses for indications other than prostate cancer. These cases likely represent a small proportion of study population, because we excluded patients who had claims for any other cancer in the 2 years before their diagnosis of prostate cancer. Alternative treatments, including orchiectomy or chemotherapy, were not included in this study but were likely infrequent. Third, only treatments reimbursed by Medicare are represented in the study; treatment in the Veteran's Administration system and treatments covered entirely by private insurance or the patient were not included. Finally, the data lacked clinical information, such as disease severity, that would have allowed assessment of outcomes. Clinical information, including disease severity, is available through the SEER-Medicare linked data. However, by using Medicare claims data exclusively, we were able to extend our analysis to patients diagnosed through 2007. Stage migration during the era of prostate-specific antigen screening may have affected clinical decisions whether or not to treat early-stage disease.20 However, no guidelines or recommendations currently exist that would suggest a change in MIRP or IMRT use as a result of stage migration. Recent work by Nguyen et al6 also found little impact of disease stage on the use IMRT vs 3-D CRT. Nevertheless, it is possible that the changes in treatment patterns we observed were influenced by unmeasured changes in disease severity. Finally, in this study, we were unable to differentiate between no therapy, active surveillance, and watchful waiting.
Conclusion
Management of patients with prostate cancer in the Medicare population has changed dramatically in recent years. Between 2002 and 2007, the use of ADT decreased, open surgical approaches were largely replaced by MIRP, and IMRT replaced 3-D CRT as the predominant method of radiation therapy. The aging of the population together with the increasing use of newer, higher-cost technologies in the treatment of patients with prostate cancer may have significant implications for nationwide health care costs.
Footnotes
Conflict of Interest Notification: Dr Lee reported being employed by the American Society of Radiation Oncology as the editor-in-chief of Practical Radiation Oncology. Dr Schulman reported receiving personal income < $10,000 for consulting from Blue Cross and Blue Shield of North Carolina; and owning stock > $10,000 in the General Electric Company. Drs Curtis, Reed, and Schulman have made available online detailed listings of financial disclosures (https://www.dcri.duke.edu/about-us/conflict-of-interest/). No other disclosures were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 2.Krupski TL, Kwan L, Afifi AA, Litwin MS. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol. 2005;23(31):7881–7888. doi: 10.1200/JCO.2005.08.755. [DOI] [PubMed] [Google Scholar]
- 3.Furlow B. US urology clinics overprescribe prostate radiotherapy. Lancet Oncol. 2011;12:112. doi: 10.1016/s1470-2045(11)70022-5. [DOI] [PubMed] [Google Scholar]
- 4.Roehrig C, Miller G, Lake C, Bryant J. National health spending by medical condition, 1996-2005. Health Aff (Millwood) 2009;28(2):w358–w367. doi: 10.1377/hlthaff.28.2.w358. [DOI] [PubMed] [Google Scholar]
- 5.Hu JC, Wang Q, Pashos CL, Lipsitz SR, Keating NL. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008;26(14):2278–2284. doi: 10.1200/JCO.2007.13.4528. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 8.Hu JC, Hevelone ND, Ferreira MD, et al. Patterns of care for radical prostatectomy in the United States from 2003 to 2005. J Urol. 2008;180(5):1969–1974. doi: 10.1016/j.juro.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 9.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999-2006. JAMA. 2010;303(16):1625–1631. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 10.Shea AM, Curtis LH, Hammill BG, DiMartino LD, Abernethy AP, Schulman KA. Association between the Medicare Modernization Act of 2003 and patient wait times and travel distance for chemotherapy. JAMA. 2008;300(2):189–196. doi: 10.1001/jama.300.2.189. [DOI] [PubMed] [Google Scholar]
- 11.Shahinian VB, Kuo YF, Gilbert SM. Reimbursement policy and androgen-deprivation therapy for prostate cancer. N Engl J Med. 2010;363(19):1822–1832. doi: 10.1056/NEJMsa0910784. [DOI] [PubMed] [Google Scholar]
- 12.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25(24):3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 14.Bolenz C, Gupta A, Hotze T, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. 2010;57(3):453–458. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med. 2010;363(8):701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 16.Konski A, Watkins-Bruner D, Feigenberg S, et al. Using decision analysis to determine the cost-effectiveness of intensity-modulated radiation therapy in the treatment of intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):408–415. doi: 10.1016/j.ijrobp.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terakedis BE, Rossi PJ, Liauw SL, Johnstone PA, Jani AB. A surveillance, epidemiology, and end results registry analysis of prostate cancer modality time trends by age. Am J Clin Oncol. 2010;33(6):619–623. doi: 10.1097/COC.0b013e3181c4c6e1. [DOI] [PubMed] [Google Scholar]
- 19.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 20.Catalona WJ, Smith DS, Ratliff TL, Basler JW. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;270(8):948–954. [PubMed] [Google Scholar]