Abstract
Background
The protective effects against reperfusion injury of cardioprotective drugs have recently been evaluated and found to be inadequate. Guanxinshutong (GXST), a combination of the traditional herb and Mongolian medicine, is effective and safe in treating angina pectoris in clinical trials. We assess the cardioprotective effects of GXST against myocardial ischemia and reperfusion (MI/R) injury in rats and explore its possible mechanism.
Methods
Forty-five male Sprague Dawley rats were randomized into three groups: non-MI/R group (Sham, n = 15), MI/R group treated with vehicle (Control, n = 15) and MI/R group treated with GXST (Drug, n = 15). MI/R was induced by ligation of the left anterior descending coronary artery (LAD) for 30 minutes, followed by 2/24 hour reperfusion in the Control and Drug groups. In the Sham group, the LAD was exposed without occlusion. GXST powder (in the Drug group) or saline (in the Control and Sham groups) were administered via direct gastric gavage from 7 day prior to surgery. Blood samples were collected from the carotid artery (10 rats each group) after 2 hours of reperfusion, to determine the levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1) using enzyme-linked immunosorbent assays. The animals were then sacrificed and the hearts were harvested for histopathology and western blot analysis. Infarct size was measured in the remaining five rats in each group after 24 hours reperfusion.
Results
GXST significantly decreased levels of TNF-α, IL-1β, IL-6, ICAM-1, apoptosis index (AI) and infarct size. GXST also obviously inhibited nuclear factor kappa B (NF-κB) activity when compared with the Control group (all P < 0.05).
Conclusions
GXST is effective in protecting the myocardium against MI/R injury in rats. Its possible cardioprotective mechanism involves inhibition of the inflammatory response and apoptosis following MI/R injury.
Keywords: I/R injury, NF-κB, Inflammation, Apoptosis, Chinese herbal, Guanxinshutong
1. Introduction
Acute myocardial infarction (AMI) is the most common cause of cardiac death, and early reperfusion after coronary obstruction represents the most effective means of therapy. However, abundant evidence suggests that reperfusion may cause additional cell death. The innate immune and inflammatory responses play a critical role in the pathophysiology of myocardial ischemia/reperfusion (MI/R),[1] and repression of proinflammatory cytokine and chemokine synthesis can alleviate MI/R injury.[2]
Nuclear factor kappa B (NF-κB) is an important transcripttion factor, which plays a central role in regulating the expression of a variety of inflammatory genes that have been linked to MI/R injury.[3],[4] However, NF-κB controls not only inflammation, but also apoptosis in MI/R injury.[5] Thus, therapeutic interventions aimed at inhibiting NF-κB signaling may alleviate MI/R injury.[6]
Recent basic research into traditional Chinese herbs has shown that some may play important roles in regulating NF-κB signaling during MI/R.[7],[8] However, these studies have focused mainly on single medicines, or the active components of the medicine. Research into the applications of compounds of Chinese medicines is limited. The components of the traditional herb and Mongolian medicine, Guanxinshutong (GXST), are Choerospondias axillaris, Salvia miltiorrhiza, Syzygium aromaticum, borneol and concretio silicea bambusae. Clinical trials have shown that GXST is effective and safe for treating angina pectoris.[9] In a pharmacodynamic study, GXST was shown to inhibit the inflammatory response.[9] However, its cardioprotective effects against MI/R injury remain unclear.
This study investigated the effects of GXST in protecting against MI/R injury, and examined its functions to controlling important factors that play roles in aggravating MI/R injury.
2. METHODS
2.1. Experimental animals and protocol
Adult male Sprague-Dawley rats (250 ± 15 g body weight) were obtained from the Animal Experimental Center of Shenyang Northern Hospital. All animals received humane care in accordance with the “Guide for the Care and Use of Laboratory Animals”. All experimental procedures were approved by the Institutional Animal Ethics Committee of Shenyang Northern Hospital.
A total of 45 rats were randomized into three groups: non-MI/R group (Sham, n = 15), MI/R group treated with vehicle (Control, n = 15) and MI/R group treated with GXST (Drug, n = 15). After 30 minutes, ischemia by ligation of the left anterior descending coronary artery (LAD), the myocardium was reperfused for 2/24 hours after knot release in the Control and Drug groups. In the Sham group, the LAD was exposed without occlusion. GXST powder (BuChang, Shanxi, batch number: 100519) at a dose of 1.5 g/kg mixed with 2 mL water (in the Drug group) or saline (in the Control and Sham groups) was administered via direct gastric gavage twice daily, 7 days prior to surgery. Blood samples were collected from the carotid artery after two hours of reperfusion (10 rats from each group) to determine the levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1) using enzyme- linked immunosorbent assays (ELISAs, Nanjing Jiancheng Bioengineering Institute). The animals were then sacrificed and the hearts were harvested for histopathology and western blot analysis. Infarct size was measured in the remaining five rats in each group after 24 hours reperfusion.
2.2. MI/R model
Rats were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal injection) and ventilated with room air by using a rodent ventilator. After performing left thoracotomy and exposure of the hearts, a 6-0 silk suture was passed underneath the LAD (2–3 mm inferior to the left auricle) and a slipknot was tied. MI/R was induced by 30 minutes of ischemia followed by 2/24 hours of reperfusion. Significant changes, including widening of the QRS complex and elevation of ST segment detected by electroencephalography, were indicators of successful coronary occlusion. The chest was closed in layers, and animals were weaned from the ventilator when they resumed spontaneous breathing.
2.3. Assessment of myocardial infarct size
At the end of 24-hour reperfusion, the ligature around the LAD was retied and 2 mL of 2% Evans blue dye (Solarbio, China) was injected into the left ventricular cavity. The dye was circulated and uniformly distributed, except in the portion of the heart previously perfused by the occluded coronary artery (area at risk, AAR). The heart was quickly removed, frozen at -20°C, and sliced horizontally to yield four slices. The slices were incubated in 1% triphenyltetrazolium chloride (TTC) (Solarbio, China) prepared with phosphate buffer (pH 7.8) for 15 minutes at 37°C, and photographed with a digital camera. The areas stained with Evans blue (area not at risk, ANAR), TTC (red staining, ischemic but viable myocardium), and TTC-negative area (white area, infarct size) was measured digitally using Image Pro-plus (version 6.0). The myocardial infarct size was measured and expressed as a percentage of infarct size over total AAR.
2.4. Determination of serum levels of TNF-α, IL-1β, IL-6 and ICAM-1
Blood samples were collected 2 hours after MI/R. Serum levels of TNF-α, IL-1β, IL-6 and ICAM-1 were measured using ELISA kits, according to the manufacturer's instructtions. Serum levels of TNF-α, IL-1β, and IL-6 were calculated from the kit standards and expressed in pg/mL, while ICAM-1 was expressed in ng/mL.
2.5. Western blotting
At 2-hour after MI/R, five rats from each group were sacrificed immediately after collection of blood samples. A sample of the left ventricular anterior wall was clipped on ice and weighed using an electronic weighing device. Aliquots of 30 µL were refrigerated at -20°C, according to the instructions for the Nc-nuclear/plasma protein extraction kit (CoWin, China). Protein concentrations were determined using the BCA protein assay. Aliquots from extractions were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electro-transferred to a cellulose acetate membrane, and blocked with 5% nonfat milk in buffer (20 mmol/L Tris HCl, pH7.4, 135 mmol/L NaCl, 0.1% Tween) for two hours at room temperature. They were then incubated overnight with mouse NF-κB p65 and rabbit histone H1 (P13) antibodies (1: 1000 dilution, Santa Cruz, USA) at 4°C, before conjugation with anti-mouse or anti-rabbit secondary antibodies (1: 1000 dilution) for one hour at room temperature. After extensive washing, immunoreactive proteins were detected using an enhanced chemiluminescence detection system. Autoradiographic images were analyzed using Quantity One software.
2.6. Immunofluorescence and immunohistochemical staining
Five rats in each group were sacrificed after blood collection at two hour after MI/R. Their hearts were removed, fixed in 4% paraformaldehyde for four hours, dehydrated in 30% sucrose (4°C overnight), embedded in tissue freezing medium (Leica Microsystems, Germany) and cut into 5 µm frozen sections. NF-kB translocation from the cytoplasm to the nucleus in area at risk was evaluated by immunofluorescence using mouse NF-κB p65 (1: 100 dilution, Santa Cruz). Caspase-3 activity was examined by immunohistochemistry using mouse caspase-3 antibody (1: 100 dilution, Imgenex, USA) and treated using an enhanced horseradish peroxidase-3, 3′-diaminobenzidine (DAB) chromogenic substrate kit (Tiangen, China). Four different areas at risk of each section were observed by light microscopy.
2.7. Determination of myocardial apoptosis
Frozen sections were produced as described above. Myocardial apoptosis was determined by terminal deoxynucleotidyl transferase (TdT enzyme)-mediated dUTP nick end labeling (TUNEL) (Roche, Switzerland). In brief, tissue slides (4 slides/heart sample) were photographed digitally (× 400) using a QICAM-Fast Digital Camera mounted onto an Olympus BX51 fluorescence microscope (Olympus, Japan) to cover the entire area. Total and TUNEL-positive nuclei were counted using IP Lab Imaging Analysis Software (Version 3.5; Scanalytics, Fairfax, VA). The apoptotic index (AI) (myocardial apoptosis index = number of apoptotic myocardial cells/ total number of myocardial nuclei × 100%) was calculated automatically and exported to Microsoft Excel for further analysis. Results from different fields taken from the same animal were averaged and counted as one sample.
2.8. Statistical analysis
All values are presented as means ± SE of independent experiments. All data were subjected to analysis of variance (ANOVA) followed by the Scheffe's correction for post hoc t-test comparison. P ≤ 0.05 was considered statistically significant.
3. RESULTS
3.1. GXST reduced myocardial infarct size following MI/R injury
At the end of 24-hour reperfusion, myocardial infarct size was assessed using the Evans blue/TTC staining method. As illustrated in Figure 1, ischemia followed by reperfusion resulted in development of substantial myocardial infarcts, which were significantly attenuated by GXST treatment (22.8% ± 6.2% vs. 50.4% ± 7.0%, P < 0.05).
Figure 1. Myocardial infarct size in the different treatment groups.
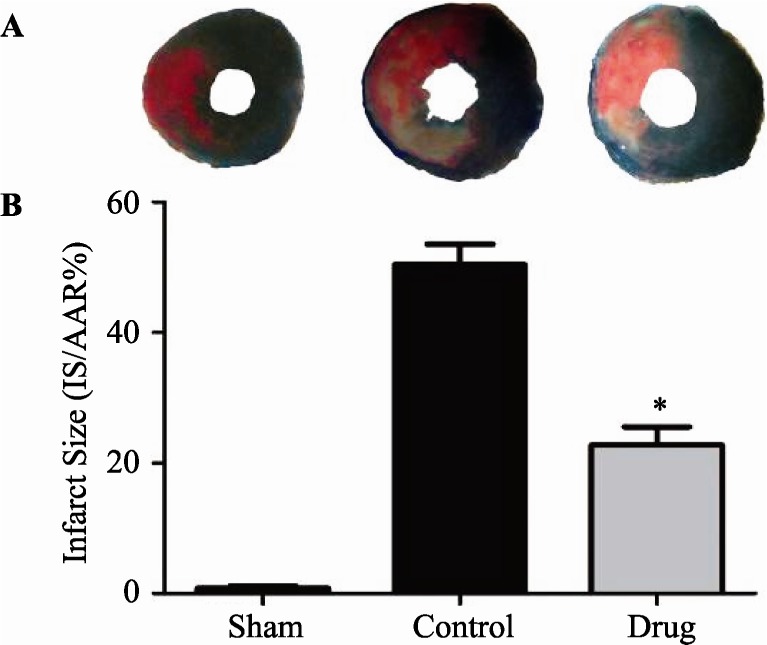
(A): Photomicrographs (× 10) of heart sections obtained from rats subjected to sham myocardial ischemia, ischemia/reperfusion (30 minutes/24 hours) treated with vehicle, or ischemia/reperfusion (30 minutes/24 hours) treated with GXST. Blue-stained portion: non- ischemic, normal region; red-stained portion: ischemic/reperfused, but not infarcted region; unstained portion (white area): ischemic/ reperfused, infarcted region. (B): Myocardial infarct sizes (IS) expressed as percent of total ischemic-reperfused area (area-at-risk, AAR). Data are presented as mean ± SE, *P < 0.05 vs. Control, n = 5.
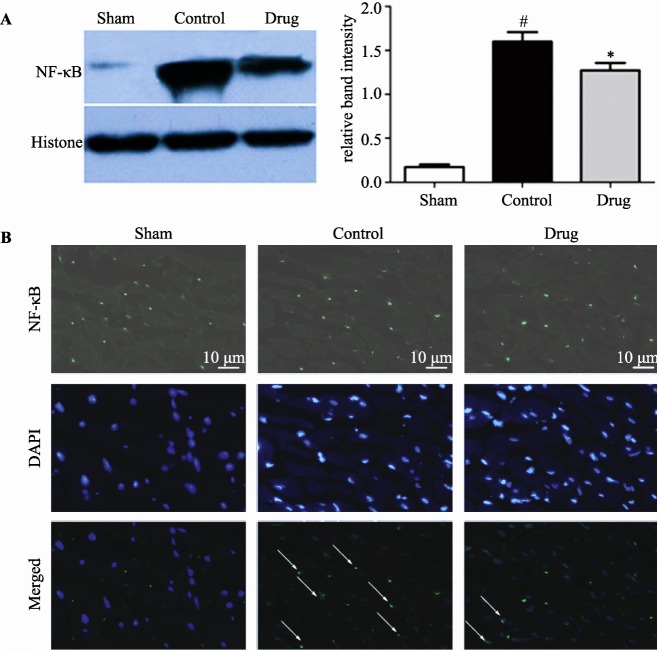
3.2. GXST reduced expression of nuclear NF-κB in the myocardium following MI/R injury
At the end of 2-hour reperfusion, nuclear expression of NF-κB was detected in myocardial tissue by western blotting. As shown in Figure 2A, nuclear NF-κB expression following MI/R injury was increased, 9.24-fold compared with the Sham group (P < 0.05), while nuclear NF-κB expression in the Drug group was reduced by 20% compared with the Control group (P < 0.05). Immunofluorescence examination showed that MI/R injury increased NF-κB translocation from the cytoplasm to the nucleus compared with the Sham group, while GXST reduced NF-κB nuclear translocation caused by MI/R injury (Figure 2B).
Figure 2. Expression of nuclear NF-κB in myocardial tissue from rats subjected to sham myocardial ischemia, ischemia/ reperfusion (30 minutes/2 hours) treated with vehicle, or ischemia/reperfusion (30 minutes/2 hours) treated with GXST.
(A): Nuclear expression of NF-κB was examined by western blotting. Data are presented as mean ± SE, #P < 0.05 vs. Sham, n = 5; *P < 0.05 vs. Control, n = 5; (B): Immunofluorescence evaluation of NF-κB nuclear translocation following MRI injury. Representative photomicrographs of sections stained for NF-kB translocation from the cytoplasm to the nucleus are shown (× 400). Green fluorescence indicates the presence of NF-κB; DAPI counterstaining (blue) indicates total nuclei. The green fluorescence embedded within the blue fluorescence represents NF-κB nuclear translocation as arrows indicated. GXST: Guanxinshutong.
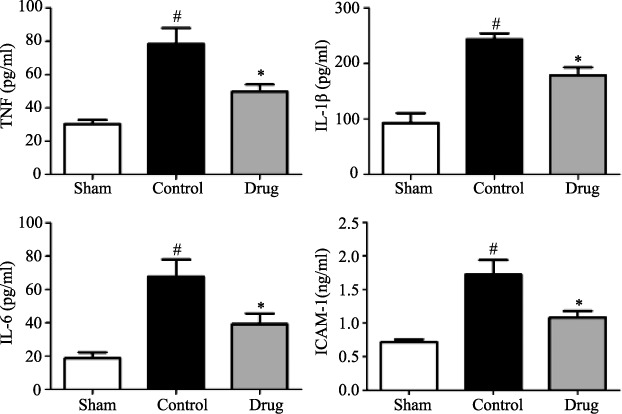
3.3. GXST capsule reduced TNF-α, IL-1β, IL-6, and ICAM-1 levels following MI/R injury
Serum levels of inflammatory cytokines and adhesion molecules following MI/R were analyzed by ELISA. Figure 3 shows that MI/R injury increased the levels of serum TNF-α by 2.59-fold (78.26 ± 21.43 vs. 30.24 ± 5.43 pg/mL), IL-1β by 2.63-fold (243.64 ± 23.39 vs. 92.80 ± 39.37 pg/mL), IL-6 by 2.91-fold (67.64 ± 23.24 vs. 18.57 ± 8.22 pg/mL), and ICAM-1 by 3.53-fold (1.720 ± 0.487 vs. 0.715 ± 0.092 ng/mL), respectively, compared with the Sham group (P < 0.05). In the GXST treatment group, serum levels of TNF-α (49.64 ± 9.67 vs. 78.26 ± 21.43 pg/mL), IL-1β (178.10 ± 33.07 vs. 243.64 ± 23.39 pg/mL), IL-6 (39.13 ± 14.25 vs. 67.64 ± 23.24 pg/mL), and ICAM-1 (1.082 ± 0.218 vs. 1.720 ± 0.487 ng/mL) were reduced by 36.5%, 26.9%, 42.1%, and 37.1%, respectively, compared with the Control group (P < 0.05).
Figure 3. Serum levels of TNF-α, IL-1β, IL-6 and ICAM-1 in rats subjected to sham myocardial ischemia, ischemia/reperfusion (30 minutes/2 hours) treated with vehicle, or ischemia/reperfusion (30 minutes/2 hours) treated with GXST.
Data are presented as mean ± SE, #P < 0.05 vs. Sham, *P < 0.05 vs. Control, n = 10. ICAM-1: intercellular adhesion molecule-1; IL: interleukin; TNF: tumor necrosis factor.
3.4. GXST capsule alleviated myocardial apoptosis following MI/R injury
Substantial evidence suggests apoptosis plays a critical role in cardiomyocyte loss and subsequent development of cardiac dysfunction after MI/R injury.[10] TUNEL and caspase-3 immunohistochemical stainings were used to determine if the observed protective effect of GXST against MI/R injury in rats was associated with decreased apoptosis. Figure 4 shows that MR/I injury increased AI by 12.7-fold compared with the Sham group (28.0 ± 4.9% vs. 2.2 ± 0.8%, P < 0.05), while AI was reduced by 38.6% in the GXST-treated group compared with the Control group (17.2 ± 7.0% vs. 28.0 ± 4.9%, P < 0.05). Immunohistochemical examination also showed that MI/R injury increased caspase-3 expression compared with the Sham group, while GXST inhibited MI/R induced caspase-3 expression (Figure 5).
Figure 4. Myocardial apoptosis determined by TUNEL staining.
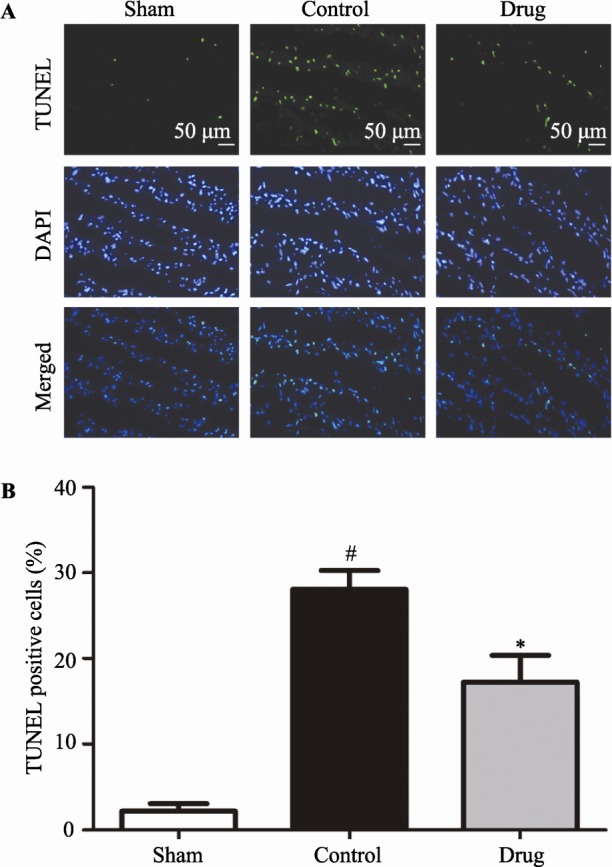
(A): Representative photomicrographs (× 200) of heart sections from rats subjected to sham myocardial ischemia, ischemia/reperfusion (30 minutes/2 hours) treated with vehicle, or ischemia/reperfusion (30 minutes/2 hours) treated with GXST are shown. TUNEL staining (green) indicates apoptotic nuclei; DAPI counterstaining (blue) indicates total nuclei. (B): Quantification of apoptotic nuclei (n = 5). Data are presented as mean ± SE. TUNEL-positive nuclei are expressed as a percentage of the total number of nuclei, automatically counted and calculated using Image-Pro Plus software. #P < 0.05 vs. Sham, *P < 0.05 vs. Control.
Figure 5. Immunohistochemical evaluation of caspase-3 expression following MI/R injury.
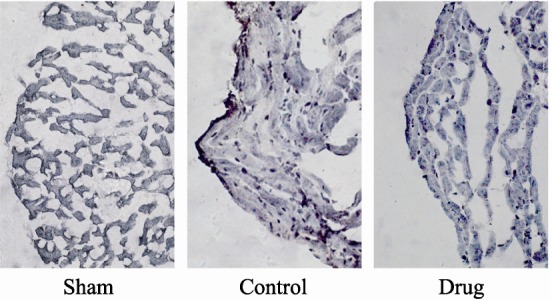
Representative photomicrographs of sections (× 400) from rats subjected to sham myocardial ischemia, ischemia/ reperfusion (30 minutes/2 hours) treated with vehicle, or ischemia/ reperfusion (30 minutes/2 hours) treated with GXST are shown. Dark staining indicates the presence of caspase-3.
4. Discussion
This is the first study to investigate the cardioprotective effects of GXST against MI/R injury in rats. The results demonstrated that GXST could reduce ischemia-reperfusion-induced myocardial injury, inhibit NF-κB activity and nuclear translocation in ischemic-reperfused myocardial tissue, decrease serum inflammatory cytokine levels following MI/R, and inhibit apoptosis in ischemic-reperfused myocardium.
Reperfusion injury is associated with an inflammatory cascade that perpetuates further damage to cardiac tissue after ischemia. One of the major factors upregulated in this process is the transcription factor NF-κB.[3],[4],[11] NF-κB nuclear translocation upregulates the expression of numerous inflammatory mediators, including interleukins, cytokines and cell adhesion molecules, resulting in injury of vascular endothelial and myocardial cells.[12] The central role of NF-κB makes it an appealing focus for intervention by many drugs commonly used in cardiovascular disease, such as aspirin and statins, which have intrinsic anti-NF-κB properties.[13] Researchers have found that some traditional Chinese medicines such as Cardiotonic pills, Tanshinone IIA, Sini decoction, and Atractylodes macrocephala can protect against MI/R injury in myocardial or hepatic tissue through the inhibition of NF-κB nuclear translocation.[14]–[17] The current study demonstrated that GXST significantly reduced levels of NF-κB in the nucleus through inhibition of NF-κB activity and nuclear translocation. GXST also decreased serum levels of TNF-α, IL-1β, IL-6 and ICAM-1 following MI/R injury. Studies showed that these inflammatory cytokines dramatically increased 2–4 hours after MI/R.[6],[18] These interleukins and cytokines play an important role in the activation and induction of neutrophil infiltration during MRI injury.[19] The cell adhesion molecule ICAM-1 mediates the early interaction and adhesion of neutrophils to coronary endothelial cells and cardiomyocytes following MI/R.[19],[20] Myocardial injury caused by MI/R has been reported to be attenuated in ICAM-1-deficient mice.[21] Overall, these data suggest that the NF-κB activation pathway contributes to MI/R injury, and that inhibition of this signaling pathway by GXST could, therefore, protect the myocardium against such injury.
Apoptosis plays an important role in ischemia-reperfusion- induced myocardial injury.[22] However, the interaction between NF-κB nuclear translocation and myocardial apoptosis has been controversial. Sasaki et al.[23] and Maulik et al.[24] found that ischemia/reperfusion increased the nuclear binding of NF- κB, but down regulated the induction of Bcl-2 mRNA expression, which was associated with an increased number of apoptotic cells. Other results have suggested that basal expression of NF-κB is required to prevent apoptosis of cardiomyocytes following ischemic insults.[25] Some traditional Chinese medicines, such as Bu-yang Huan-wu decoction and tetramethylpyrazine, can reduce apoptosis induced by cerebral ischemia-reperfusion.[26],[27] In the present study, GXST not only inhibited NF-κB activation, but also inhibited myocardial apoptosis and caspase-3 expression following MI/R injury. Apoptosis represents a potentially preventable form of cell death, and understanding this genetic cell death pathway may lead to the improvement in therapeutic strategies for alleviating myocardial injury through inhibition of apoptosis.
The protective effects against reperfusion injury of several cardioprotective drugs, including reactive oxygen species scavengers, calcium channel blockers, adenosine, and nicorandil, have recently been evaluated in clinical trials and found to be inadequate.[28] One of the reasons for these unsatisfactory results may be the multi-factorial mechanisms of reperfusion injury and the lack of therapies able to simultaneously target multiple pathways. As a combination of the traditional herb and Mongolian medicine, GXST may overcome the deficiencies of these single-target drugs in protecting against MI/R injury. Some studies showed that Choerospondias axillaris, the major component of GXST, can protect against myocardial infarction by increasing serum levels of TNOS and NO. Borneol was also considered to be effective in protecting functions of endothelial cells after myocardial infarction.
Acute ischemia manifests as “blood stasis”, and the intracellular water and electrolyte balances become suddenly disordered after reperfusion, which is referred to as “phlegm” in traditional Chinese medicine. “Stasis” and “phlegm” together obstruct the system of meridians and collaterals in traditional Chinese medicine theory. GXST plays an important role in promoting blood and QI (a fundamental matter in traditional Chinese medicine theory, which pushes the flow of blood and other fluid of body) circulation, removing blood stasis and activating the meridians and collaterals, resulting in its treatment of MI/R injury according to traditional Chinese medicine theory.
This study had several limitations. First, although the protective effects of GXST on MI/R injury and its inhibition of NF-κB activation and apoptosis were demonstrated, further studies are needed to elucidate its effects on upstream regulation of NF-κB activation and the relationship between NF-κB inhibition by GXST and apoptosis. Second, the components of GXST responsible for the observed protective effects remain unknown. Finally, further research is required to identify other possible pathways through which GXST may target MI/R injury.
In summary, pretreatment with GXST can limit myocardial infarct size, inhibit NF-κB activation and apoptosis in myocardial tissue, and decrease levels of inflammatory cytokines and adhesion molecules in serum following MI/R injury. These observations suggest the cardioprotective mechanism of GXST involves inhibition of the inflammatory response and apoptosis. These findings could help to provide some insight into protective potential of GXST in MI/R injury.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 2.Gao F, Yue TL, Shi DW, et al. p38 MAPK inhibition reduces myocardial reperfusion injury via inhibition of endothelial adhesion molecule expression and blockade of PMN accumulation. Cardiovasc Res. 2002;53:414–422. doi: 10.1016/s0008-6363(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 3.Clermont G, Vergely C, de Girard C, et al. Cellular injury associated with extracorporeal circulation. Ann Cardiol Angiol. 2002;51:38–43. doi: 10.1016/s0003-3928(01)00062-2. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Fan H, Honda T, et al. Activation of NF kappa B and expression of ICAM-1 in ischemic-reperfused canine myocardium. J Mol Cell Cardiol. 2001;33:109–119. doi: 10.1006/jmcc.2000.1280. [DOI] [PubMed] [Google Scholar]
- 5.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Hu Y, Wu W, et al. The TIR/BB-loop mimetic AS-1 protects the myocardium from ischaemia/reperfusion injury. Cardiovasc Res. 2009;84:442–451. doi: 10.1093/cvr/cvp234. [DOI] [PubMed] [Google Scholar]
- 7.Liu JX, Han X, Ma XB. Effect of Shuangshen Tongguan Recipe on nuclear factor-kappa B signal pathway and myocardial junction-mediated intercellular communication in acute myocardial ischemia/reperfusion injured control rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:228–230. [PubMed] [Google Scholar]
- 8.Zhang BJ, Wang YL, Wang CY. Effect of Shenfu injection on nuclear factor-kappaB during myocardial ischemia/reperfusion injury in rats. Chin J Traumatol. 2005;8:200–204. [PubMed] [Google Scholar]
- 9.Sun Z, Wuliji O. The observation on clinical effects of Guanxin Shutong capsule treating coronary heart disease and angina pectoris. Journal of Inner Mongolia University for Nationalities. 2006;21:422–424. [Google Scholar]
- 10.Eefting F, Rensing B, Wigman J, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Nichols TC. NF-kappaB and reperfusion injury. Drug News Perspect. 2004;17:99–104. doi: 10.1358/dnp.2004.17.2.829042. [DOI] [PubMed] [Google Scholar]
- 12.Valen G, Yan ZQ, Hansson GK. Nuclear factor kappa-B and the heart. J Am Coll Cardiol. 2001;38:307–314. doi: 10.1016/s0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin AS., Jr Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin C, Zhang PJ, Bao CQ, et al. Protective effects of Atractylodes macrocephala polysaccharide on liver ischemia reperfusion injury and its possible mechanism in rats. Am J Chin Med. 2011;39:489–502. doi: 10.1142/S0192415X11008981. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Wei L, Sun D, et al. Tanshinone IIA pretreatment protects myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Diabetes Obes Metab. 2010;12:316–322. doi: 10.1111/j.1463-1326.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhao N, Liu YY, Wang F, et al. Cardiotonic pills, a compound Chinese medicine, protects ischemia-reperfusion induced microcirculatory disturbance and myocardial damage in rats. Am J Physiol Heart Circ Physiol. 2010;298:H1166–H1176. doi: 10.1152/ajpheart.01186.2009. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Wu WK, Chen C, et al. Role of NF-kappaB in the induction of delayed preconditioning in rat heart by pretreatment with Sini decoction. Zhongguo Zhong Yao Za Zhi. 2005;30:453–456. [PubMed] [Google Scholar]
- 18.Yang Jian, Jiang Hong, Yang Jun, et al. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-κB signaling pathway. Mol Cell Biochem. 2009;330:39–46. doi: 10.1007/s11010-009-0098-1. [DOI] [PubMed] [Google Scholar]
- 19.Han X, Liu JX, Ma MB, et al. Effects of shuangshen tongguan (SSTG) on TNF-alpha, ICAM-1 during myocardial ischemia-reperfusion injury. Zhongguo Zhong Yao Za Zhi. 2004;29:1073–1075. [PubMed] [Google Scholar]
- 20.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar AK, Lefer DJ. Leukocyte and endothelial adhesion molecule studies in knockout mice. Curr Opin Pharmacol. 2004;4:154–158. doi: 10.1016/j.coph.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Eefting F, Rensing B, Wigman J, et al. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki H, Galang N, Maulik N. Redox regulation of NF- kappaB and AP-1 in ischemic reperfused heart. Antioxid Redox Signal. 1999;1:317–324. doi: 10.1089/ars.1999.1.3-317. [DOI] [PubMed] [Google Scholar]
- 24.Maulik N, Goswami S, Galang N, et al. Differential regulation of Bcl-2, AP-1 and NF-kappaB on cardiomyocyte apoptosis during myocardial ischemic stress adaptation. FEBS Lett. 1999;443:331–336. doi: 10.1016/s0014-5793(98)01719-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Kim JS, Kwon JS, et al. BAY 11-7082, a nuclear factor-kappaB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia-reperfusion injury model. Int Heart J. 2010;51:348–353. doi: 10.1536/ihj.51.348. [DOI] [PubMed] [Google Scholar]
- 26.Wang HW, Liou KT, Wang YH, et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011;138:22–33. doi: 10.1016/j.jep.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Kao TK, Ou YC, Kuo JS, et al. Neuroprotection by tetramethylpyrazine against ischemic brain injury in rats. Neurochem Int. 2006;48:166–176. doi: 10.1016/j.neuint.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Monassier JP. Reperfusion injury in acute myocardial infarction: from bench to cath lab. II. Clinical issues and therapeutic options. Arch Cardiovasc Dis. 2008;101:565–575. doi: 10.1016/j.acvd.2008.06.013. [DOI] [PubMed] [Google Scholar]