Abstract
Background
Hyperuricemia is frequently present in patients with heart failure. Many pathological conditions, such as tissue ischemia, renal function impairment, cardiac function impairment, metabolic syndrome, and inflammatory status, may impact uric acid (UA) metabolism. This study was to assess their potential relations to UA metabolism in heart failure.
Methods
We retrospectively assessed clinical characteristics, echocardiological, renal, metabolic and inflammatory variables selected on the basis of previous evidence of their involvement in cardiovascular diseases and UA metabolism in a large cohort of randomly selected adults with congestive heart failure (n = 553). By clustering of indices, those variables were explored using factor analysis.
Results
In factor analysis, serum uric acid (SUA) formed part of a principal cluster of renal functional variables which included serum creatinine (SCr) and blood urea nitrogen (BUN). Univariate correlation coefficients between variables of patients with congestive heart failure showed that the strongest correlations for SUA were with BUN (r = 0.48, P < 0.001) and SCr (r = 0.47, P < 0.001).
Conclusions
There was an inverse relationship between SUA levels and measures of renal function in patients with congestive heart failure. The strong correlation between SUA and SCr and BUN levels suggests that elevated SUA concentrations reflect an impairment of renal function in heart failure.
Keywords: Serum uric acid, Heart failure, Renal function impairment, Factor analysis
1. Introduction
Hyperuricemia is frequently present in patients with heart failure. Serum uric acid (SUA) level is a strong, independent predictor of mortality in patients with heart failure.[1],[2] Uric acid (UA) is the final product of purine degradation with xanthine oxidase, an enzyme implicated as a mechanistic participant in oxidative stress. Approximately 70% of the UA is excreted through the kidneys and 30% of the UA is excreted through the gastrointestinal tract. Accordingly, either overproduction or reduced excretion of UA can result in hyperuricemia. Tissue ischemia depletes adenosine triphosphate and activates the purine nucleotide degradation pathway to UA, resulting in UA overproduction in the heart, lungs, liver, and skeletal muscle.[3]–[6] Therefore, SUA elevation is related to the diseases with hypoxic states, such as heart failure,[3],[7] cyanotic congenital heart disease,[8] and pulmonary arterial diseases.[9] However, many other pathological conditions may impact UA metabolism as well. In congestive heart failure, venous congestion could worsen renal function, and impair glomerular filtration and tubular excretion of UA. Additionally, hyperuricemia is also associated with coronary artery disease and with its risk factors, such as obesity, hypertension, hypertriglyceridemia, dyslipidemias and diabetes mellitus,[10]–[12] which are also the risk factors for heart failure. Finally, diuretic treatment used in heart failure is another significant confounder impacting on SUA levels. Therefore, determining their relations to SUA in heart failure will improve our understanding of the role of UA in heart failure. Consequently, we designed this retrospective, observational study to assess the potential relations between metabolic factors, cardiac function, renal function, and inflammatory markers in heart failure with UA metabolism. Factor analysis was used to explore the possible interrelationships between the above factors and SUA. With this statistical technique, it is possible to address whether their relationships are multidimensional, or if they can be reduced to a single factor.
2. Methods
We reviewed the medical record of 553 patients with congestive heart failure from our heart center between January 2005, and March 2010. Patients were excluded from the analysis when variables (i.e., metabolic factors, cardiac function, renal function, and inflammatory markers) were not available from the same time point (defined as a maximum interval of 7 days), when the patients received treatment with the xanthine oxidase inhibitor, allopurinol, or an uricosuric agent, or when a coexisting renal disease was present, or when the kidney function was severely impaired (e.g., serum creatinine levels > 200 µmol/L). The detailed clinical characteristics and medications are listed in Table 1.
Table 1. Clinical characteristics of patients with congestive heart failure.
| Variables | Value |
| Age (year), n = 553 | 63.26 ± 14.49 |
| Female, n = 553 | 223 (40.33%) |
| BMI, n = 553 | 24.93 ± 3.62 |
| MI | 123 (22.24%) |
| AF | 174 (31.46%) |
| DM | 116 (20.98%) |
| Etiology of heart failure | |
| IHD | 248 (44.85%) |
| IDC | 93 (16.82%) |
| VHD | 110 (19.89%) |
| HTN | 94 (17.00%) |
| Others | 8 (1.45) |
| NYHA functional class | |
| I | 47 (8.50%) |
| II | 118 (21.34%) |
| III | 170 (30.74%) |
| IV | 218 (39.42%) |
| LVEF (%), n = 451 | 45.28 ± 16.33 |
| Laboratory test | |
| SUA (µmol/L), n = 553 | 420.31 ± 162.23 |
| SCr (µmol/L), n = 553 | 92.95 ± 28.28 |
| BUN (mmol/L), n = 553 | 7.31 ± 4.69 |
| FG (mmol/L), n = 498 | 6.32 ± 3.34 |
| TCL (mmol/L), n = 541 | 4.20 ± 1.26 |
| TG (mmol/L), n = 541 | 1.34 ± 0.93 |
| LDL-C (mmol/L), n = 541 | 2.53 ± 1.03 |
| HDL-C (mmol/L), n = 541 | 1.07 ± 0.49 |
| Neutrophils (109/L), n = 553 | 5.42 ± 3.47 |
| Monocytes (109/L), n = 553 | 0.60 ± 1.34 |
| Medication | |
| Furosemide | 299 (54.07%) |
| HCTZ | 298 (53.88%) |
| Spironolactone | 457 (82.64%) |
| Digitalis | 230 (41.59%) |
| ACE I | 357 (64.56%) |
| ARB | 74 (13.38%) |
| CCB | 114 (20.61%) |
| β-blocker | 339 (61.30%) |
| Warfarin | 112 (20.25%) |
| Statin | 205 (37.07%) |
Data are expressed as mean ± SD. ACE I: angiotensin-converting enzyme inhibitors; AF: atrial fibrillation/flutter; ARB: angiotensin II receptor blockers; BMI: body mass index; BUN: blood urea nitrogen; MI: myocardial infarction; CCB: calcium channel blocker; CHL: total cholesterol; DM: diabetes mellitus; FG: fasting glucose; HCTZ: hydrochlorothiazide; HDL-C: high-density lipoprotein cholesterol; IDC: idiopathic dilated cardiomyopathy; IHD: ischemic heart disease; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SCr: serum creatinine; SUA: serum uric acid; TCL: total cholesterol level, TG: triglyceride; VHD: valvular heart disease.
Cardiac functional, renal, metabolic variables, and inflammatory markers (such as leukocyte profile) were selected on the basis of previous evidence of their involvement in heart failure and/or UA metabolism in cardiovascular diseases.[10]–[20] The following variables were finally included in the analysis: body mass index (BMI), New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), arterial pressure, fasting glucose (FG), lipid profile (including total cholesterol level (TCL), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG), serum creatinine (SCr), blood urea nitrogen (BUN), leukocyte profile (including neutrophils and monocytes), and diuretic dose. Statistical analyses were carried out using the SPSS statistical package (16.0). To avoid the emergence of a separate factor consisting solely of the two blood pressure measurements in the factor analysis, a single measurement of mean arterial pressure (diastolic + systolic/3) was employed. University Pearson correlation coefficients were derived. Significant clustering of correlated renal function was identified by factor analysis. Factor analysis assumes inter-correlations between observed variables are influenced by a smaller number of hypothetical underlying variables, termed factors. Factors are characterized by the variables which, according to their so-called factor loadings, most strongly correlate with the concerned factor. Principal components analysis was used to extract the initial components. The varimax method of rotation was used to obtain the final factors and their loadings for each variable. Variables with factor loadings equal to, or greater than, 0.40 were primarily used for interpretation of the obtained factors. We used three alternatives (i.e., exclude cases listwise, exclude cases pairwise, or replace with mean) to handle the missing values, and compared their impact on the final results.
3. Results
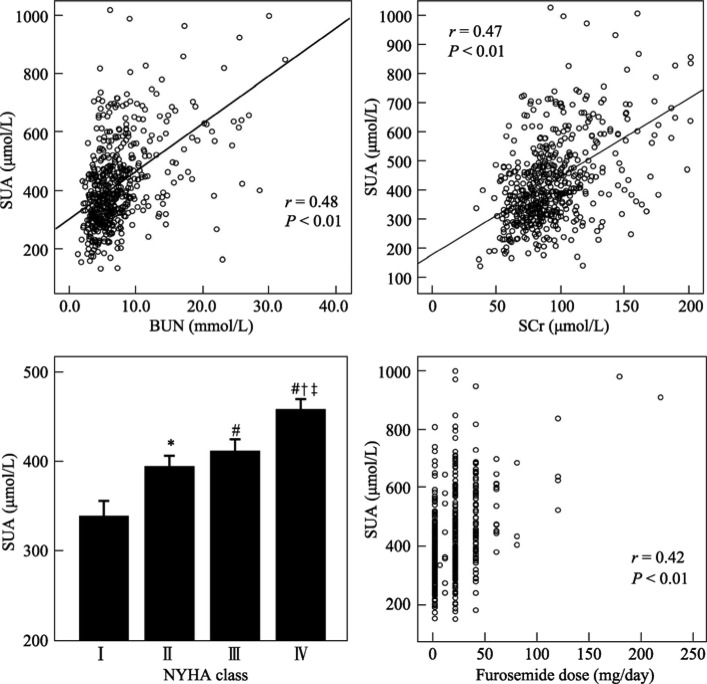
Univariate correlation coefficients between variables of patients with congestive heart failure are shown in Table 2. The strongest correlations for UA were with BUN (r = 0.48), SCr (r = 0.47), dosage of furosemide (r = 0.43), and NYHA functional class (r = 0.216) (both P < 001) (Figure 1). Loop diuretic dose, entered as the furosemide-equivalent dose (one milligram of bumetanide was taken as equivalent to 40 mg of furosemide), correlated positively to BUN (r = 0.45, P < 0.001), but to a lesser degree with NYHA class (0.31, P < 0.001). When the heart failure group was divided into those who were taking thiazide diuretics (n = 297), and those who were not (n = 255), there was no significant difference in SUA levels between the two groups (418.47 ± 143.71 µmol/L in thiazide group vs. 420.73 ± 179.90 µmol/L in those not given thiazide, P = 0.055).
Table 2. Univariate pearson correlation coefficients between variables of patients with heart failure.
| BMI | BP | NYHA | LVEF | DIU | SUA | SCr | BUN | FG | TCL | TG | LDL | HDL | Neutrophils | Monocytes | |
| Age | 0.017 | 0.163*** | 0.103** | 0.350*** | −0.106** | −0.094* | 0.223*** | 0.131* | 0.040 | 0.040* | −0.079 | 0.017 | 0.101* | 0.089* | −0.043 |
| BMI | 0.143*** | −0.143*** | 0.095* | −0.145*** | 0.013 | 0.085* | −0.112** | 0.084 | 0.126** | 0.197 | 0.104** | −0.042 | −0.057 | −0.016 | |
| BP | 0.060 | 0.179*** | −0.104** | −0.025 | 0.093* | −0.042 | 0.037 | 0.169*** | 0.087* | 0.124** | 0.055 | 0.040 | −0.038 | ||
| NYHA | −0.109* | 0.306*** | 0.216*** | 0.168*** | 0.248*** | 0.039 | −0.026 | −0.094* | 0.054 | −0.082* | 0.227*** | 0.009 | |||
| LVEF | −0.258*** | −0.200*** | 0.047 | 0.007 | 0.021 | 0.095* | 0.022 | 0.056 | 0.102* | 0.012 | −0.018 | ||||
| DIU | 0.426*** | 0.265*** | 0.452*** | 0.013 | −0.159*** | −0.128** | −0.065 | −0.102** | 0.097* | 0.058 | |||||
| SUA | 0.474*** | 0.478*** | -0.071 | −0.102** | −0.039 | −0.002 | −0.215*** | −0.018 | 0.030 | ||||||
| SCr | 0.632*** | 0.106** | −0.008 | −0.021 | 0.054 | −0.138** | 0.170*** | −0.042 | |||||||
| BUN | 0.131** | −0.095* | −0.106** | −0.030 | −0.086 | 0.246*** | −0.021 | ||||||||
| FG | 0.090* | 0.083* | 0.055 | 0.049 | 0.200*** | −0.017 | |||||||||
| TCL | 0.356*** | 0.875*** | 0.315*** | −0.079* | −0.045 | ||||||||||
| TG | 0.149* | −0.018 | −0.081* | −0.008 | |||||||||||
| LDL | 0.055 | −0.047 | −0.048 | ||||||||||||
| HDL | 0.000 | −0.007 | |||||||||||||
| Neutrophils | 0.005 |
*P < 0.05; **P < 0.01; ***P < 0.001. BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; DIU: Diuretic; FG: fasting glucose; HDL: high-density lipoprotein; LDL-C: low-density lipoprotein; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SCr: serum creatinine; SUA: serum uric acid; TCL: total cholesterol level; TG: triglyceride.
Figure 1. Plots of SUA concentrations against BUN, SCr, NYHA class and furosemide dose.
For NYHA functional class, SUA values are plotted as mean ± SD. *P < 0.05 compared with NYHA functional class I. # P < 0.01 compared with NYHA functional class I. † P < 0.01 compared with NYHA functional class II. ‡ P < 0.01 compared with NYHA functional class III. BUN: blood urea nitrogen; NYHA: New York Heart Association; SCr: serum creatinine; SUA: serum uric acid.
In factor analysis of the congestive heart failure group (the cases with missing values were excluded listwise), 15 inter-correlated variables were reduced to six uncorrelated factors (Table 3). The factor which accounted for most of the variance in the dataset (factor 1, 17.64 % of the variance) comprised, in order of factor loading, SUA, SCr, BUN, DIU, and NYHA functional class. Taking the highest loading in each factor, factor 1 is interpreted as the high SUA/SCr; factor 2 as the cholesterol profile factor; factor 3 as the impaired cardiac function factor; factor 4 as the high BMI/ TG factor; factor 5 as the high FG/ neutrophils; and factor 6 as the monocytes factor. These factors accounted for 63.89% of the total variance in the dataset. We also excluded cases pairwise, or replace the missing values with mean to handle missing values. There was little impact on the results of factor analysis.
Table 3. Results of factor analysis.
| Factor | 1 | 2 | 3 | 4 | 5 | 6 |
| BMI | 0.033 | 0.051 | 0.707 | 0.206 | 0.048 | −0.005 |
| BP | 0.149 | 0.157 | 0.041 | 0.675 | −0.049 | 0.102 |
| NYHA | 0.423 | 0.181 | −0.389 | −0.045 | 0.179 | 0.274 |
| SUA | 0.763 | −0.044 | 0.060 | −0.130 | −0.255 | 0.058 |
| SCr | 0.750 | 0.014 | 0.084 | 0.258 | 0.183 | −0.170 |
| BUN | 0.722 | −0.023 | −0.079 | 0.027 | 0.330 | −0.150 |
| FG | 0.019 | 0.036 | 0.357 | −0.146 | 0.720 | −0.069 |
| TCL | −0.092 | 0.949 | 0.207 | 0.107 | 0.056 | −0.050 |
| TG | −0.014 | 0.232 | 0.675 | −0.057 | −0.003 | 0.075 |
| LDL | 0.034 | 0.935 | 0.077 | 0.066 | −0.035 | −0.017 |
| HDL | −0.396 | 0.254 | −0.194 | 0.105 | 0.339 | −0.166 |
| Neutrophils | 0.161 | −0.040 | −0.175 | 0.106 | 0.682 | 0.143 |
| Monocytes | −0.061 | −0.066 | 0.045 | 0.019 | 0.031 | 0.896 |
| LVEF | −0.171 | −0.004 | 0.061 | 0.760 | 0.077 | −0.072 |
| DIU | 0.552 | −0.024 | −0.242 | −0.393 | 0.085 | 0.195 |
| Total variance explained, % | 17.64 | 14.69 | 8.83 | 8.49 | 7.52 | 6.72 |
Expressed in terms of loadings following rotation of principal components. Rotated loadings greater than 0.40 are shown in bold. Factor 1: high SUA/SCr; factor 2: cholesterol profile; factor 3: impaired cardiac function; factor 4: high BMI/TG; factor 5: high FG/ neutrophils; factor 6: monocytes. BMI: body mass index; BP: blood pressure; BUN: blood urea nitrogen; DIU: Diuretic; FG: fasting glucose; HDL: high-density lipoprotein; LDL-C: low-density lipoprotein; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; SCr: serum creatinine; SUA: serum uric acid; TCL: total cholesterol level; TG: triglyceride.
4. Discussion
To our knowledge, this is the first study to analyze the interrelationships between SUA, clinical status, cardiac function, renal function, metabolic variables, and leukocyte profile. We found a significant inverse relationship between SUA levels and renal function that is independent of BMI, mean arterial pressure, fasting glucose level, lipid profile, cardiac function, and serum leukocyte profile, suggesting impaired renal function might be the primary reason for hyperuricemia in congestive heart failure. This finding is further supported by our previous observations that improvement in renal function was associated with a remarkable SUA reduction.[21]–[23]
It is well recognized that gout and hyperuricemia are associated with coronary artery disease and metabolic syndrome.[13] However, the present multivariate analysis demonstrated that SUA levels did not independently correlate with arterial pressure, TCL, TG, or FG in patients with heart failure. Hyperuricemia has also been linked to multiple pro-atherogenic processes, such as leukocyte activation in patients with coronary artery disease.[14],[15] However, our data did not show there was a significant correlation between leukocyte profile and SUA levels even after the patients were stratified to ischemic or non-ischemic, suggesting it contributes little to SUA elevation in the congestive heart failure.
Diuretic therapy is another possible confounder in UA metabolism. It is documented that loop diuretics produce potent diuretic effects at the cost of worsening of renal function in heart failure.[24] Additionally, thiazide and loop diuretics cause a dose-dependent elevation of SUA by increasing its tubular re-absorption in the context of volume depletion. In the present study, the strongest univariate correlations detected for diuretic dose were with BUN, SUA, and NYHA functional class. Despite the fact that diuretic dose influenced significantly the first factor together with SUA, SCr, BUN and NYHA class, diuretic dose failed to emerge as the strongest predictor of SUA in multiple regression analyses (data were not shown), and when it was entered in factor analysis, indicating increased diuretic dose may indirectly reflect the deterioration of renal function and clinical status.
A relationship between LVEF and SUA is expected because xanthine oxidase inhibition by allopurinol may improve cardiac function in cardiac injuries.[16]–[18] However, LVEF had no significant effect on the same factor as SUA. LVEF had significant effect on the cardiac function (factor 3). Since, by definition, factors isolated in factor analysis are uncorrelated, LVEF may have little effect on SUA in congestive heart failure.
It is well documented that hypoxia leads to accumulation of the precursors of UA (hypoxanthine and xanthine) and activation of xanthine oxidase/dehydrogenase.[25] Elevation in SUA is expected, since patients with heart failure have an impaired uptake of oxygen at rest and during exercise. Accordingly, elevated SUA is expected to reflect the metabolic effects of hypoxia on the microvasculature. It is supported by our finding that there is a weak, but statistically significant, correlation between SUA and NYHA functional class. However, impaired renal function remains the main contributor to SUA elevation in congestive heart failure.
The present study has several limitations. The most important one is the fact that it is a retrospective, observational study. The results, however, are hypothesis generating, and offer potential explanations for a hypothesis regarding the way SUA is elevated in congestive heart failure.
In conclusion, a strong inverse relationship between SUA levels and renal function in patients with heart failure suggests that impaired renal function may be the primary reason for hyperuricemia in congestive heart failure. Further studies are warranted to verify our findings.
Acknowledgments
The research was funded by Health Bureau of Hebei Province (GL200937).
References
- 1.Anker SD, Doehner W, Rauchhaus M, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 2.Cengel A, Turkoglu S, Turfan M, et al. Serum uric acid levels as a predictor of in-hospital death in patients hospitalized for decompensated heart failure. Acta Cardiol. 2005;60:489–492. doi: 10.2143/AC.60.5.2004969. [DOI] [PubMed] [Google Scholar]
- 3.Anker SD, Leyva F, Poole-Wilson PA, et al. Relation between serum uric acid and lower limb blood flow in patients with chronic heart failure. Heart. 1997;78:39–43. doi: 10.1136/hrt.78.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huizer T, de Jong JW, Nelson JA, et al. Urate production by human heart. J Mol Cell Cardiol. 1989;21:691–695. doi: 10.1016/0022-2828(89)90610-x. [DOI] [PubMed] [Google Scholar]
- 5.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 6.Elsayed NM, Nakashima JM, Postlethwait EM. Measurement of uric acid as a marker of oxygen tension in the lung. Arch Biochem Biophys. 1993;302:228–232. doi: 10.1006/abbi.1993.1204. [DOI] [PubMed] [Google Scholar]
- 7.Leyva F, Anker S, Swan JW, et al. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J. 1997;18:858–865. doi: 10.1093/oxfordjournals.eurheartj.a015352. [DOI] [PubMed] [Google Scholar]
- 8.Young D. Hyperuricemia in cyanotic congenital heart disease. Am J Dis Child. 1980;134:902–903. doi: 10.1001/archpedi.1980.02130210078028. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Hohlfeld JM, Fabel H. Hyperuricaemia in patients with right or left heart failure. Eur Respir J. 1999;13:682–685. doi: 10.1183/09031936.99.13368299. [DOI] [PubMed] [Google Scholar]
- 10.Zoccali C, Maio R, Mallamaci F, et al. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17:1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G, Montagnana M, Luca Salvagno G, et al. Epidemiological association between uric acid concentration in plasma, lipoprotein(a), and the traditional lipid profile. Clin Cardiol. 2009:E76–E80. doi: 10.1002/clc.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman JB, Goldbourt U. Uric acid and diabetes: observations in a population study. Lancet. 1982;2:240–243. doi: 10.1016/s0140-6736(82)90324-5. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 15.Kocaman SA, Sahinarslan A, Cemri M, et al. Independent relationship of serum uric acid levels with leukocytes and coronary atherosclerotic burden. Nutr Metab Cardiovasc Dis. 2009;19:729–735. doi: 10.1016/j.numecd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Grum CM, Ketai LH, Myers CL, et al. Purine efflux after cardiac ischemia: relevance to allopurinol cardioprotection. Am J Physiol. 1987;252(2 Pt 2):H368–H373. doi: 10.1152/ajpheart.1987.252.2.H368. [DOI] [PubMed] [Google Scholar]
- 17.Tabayashi K, Suzuki Y, Nagamine S, et al. A clinical trial of allopurinol (Zyloric) for myocardial protection. J Thorac Cardiovasc Surg. 1991;10:713–718. [PubMed] [Google Scholar]
- 18.Motoe M, Yoshida S. A useful canine model of ischemic myocardium with coronary retrograde flow diversion, and its application for the study of allopurinol on myocardial infarct size. Jpn Circ J. 1991;55:490–499. doi: 10.1253/jcj.55.490. [DOI] [PubMed] [Google Scholar]
- 19.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinelli M, Bindi M, Filardo FP, et al. Serum uric acid levels correlate with left ventricular ejection fraction and systolic pulmonary artery pressure in patients with heart failure. Recenti Prog Med. 2007;98:619–623. [PubMed] [Google Scholar]
- 21.Zhang H, Liu C, Ji Z, et al. Prednisone adding to usual care treatment for refractory decompensated congestive heart failure. Int Heart J. 2008;49:587–595. doi: 10.1536/ihj.49.587. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Liu G, Zhou C, et al. Potent diuretic effects of prednisone in heart failure patients with refractory diuretic resistance. Can J Cardiol. 2007;23:865–868. doi: 10.1016/s0828-282x(07)70840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C, Chen H, Zhou C, et al. Potent potentiating diuretic effects of prednisone in congestive heart failure. J Cardiovasc Pharmacol. 2006;48:173–176. doi: 10.1097/01.fjc.0000245242.57088.5b. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Neyses L. Diuretic usage in heart failure: a continuing conundrum in 2005. Eur Heart J. 2005;26:644–649. doi: 10.1093/eurheartj/ehi176. [DOI] [PubMed] [Google Scholar]
- 25.Hassoun PM, Shedd AL, Lanzillo JJ, et al. Inhibition of pulmonary artery smooth muscle cell growth by hypoxanthine, xanthine, and uric acid. Am J Respir Cell Mol Biol. 1992;6:617–624. doi: 10.1165/ajrcmb/6.6.617. [DOI] [PubMed] [Google Scholar]