Abstract
Objective
To describe the clinical characteristics of idiopathic ventricular fibrillation (IVF) with fragmented QRS complex (f-QRS) and J wave in resting electrocardiogram.
Methods
We reviewed data from 21 case subjects in our hospital who were resuscitated after cardiac arrest due to IVF and assessed the prevalence of f-QRS and J wave in resting electrocardiogram (ECG). All the case subjects were classified among three groups based on the electrocardiographic morphology: group I, both f-QRS and J wave were observed (n = 6), group II, only J wave was observed (n = 9), group III, neither f-QRS nor J wave was observed (n = 6). Population characteristics, history of syncope or sudden cardiac arrest, incidence of ventricular fibrillation (VF), and circumstance of VF were evaluated among the three groups.
Results
The incidence of index events (syncope, survived cardiac arrest and VF episodes recorded in implantable cardioverter defibrillator (ICD) or pacemakers) was 13.4 ± 5.6 per-year in group I, 10.8 ± 3.9 per-year in group II, and 9.8 ± 4.2 per-year in group III. There were significant differences in incidences among the three groups, the most frequent index events were observed in group I. The hazard ratio for incidence was 3.2 (95%CI, 1.1–7.9; P = 0.01). The history and circumstance of the index events were different among the groups. In group I, all the index events occurred during sleep in early morning. In group II, four subjects suffered VF during strenuous physical activities or agitation state, two during sleep in early morning, three in usual activity. In group III, one subject suffered VF during sleep in early morning, one in agitation state, four in usual activity.
Conclusions
This study suggests that the IVF patients with the combined appearance of f-QRS and J wave in the resting ECG suffer an increased risk of VF, this subgroup of IVF patients has a unique clinical feature.
Keywords: Idiopathic ventricular fibrillation, Electrocardiogram, Fragmented QRS, J wave
1. Introduction
Sudden cardiac death is one of the major public health problems and the majority of such cases relates to malignant ventricular arrhythmias.[1] Idiopathic ventricular fibrillation (IVF) is a cause of sudden cardiac death. Basic electrophysiological research has suggested a critical role of the J wave in the pathogenesis of IVF.[2],[3] Clinical evidence in support of an association between J wave and IVF was fully disclosed by Haïssaguerre et al.[4]
Previous studies had described the presence of a fragmented QRS complexes (f-QRS) on a routine 12-lead electrocardiogram (ECG) as an arrhythmogenic marker of depolarization abnormality.[5],[6] Notably, f-QRS is highly associated with increased mortality and arrhythmic events in patients, who were diagnosed with coronary cardiac disease, cardiomyopathy and congenital heart disease. They found the incidence of an arrhythmic event was significantly higher in the patients with f-QRS than those without f-QRS during mean follow-up of 17 months and the patients without f-QRS have a high survival probability for an arrhythmic event. f-QRS represents delayed activation in a larger ventricular mass that can cause multiple spikes within the QRS complex, which represented f-QRS in body surface ECG. In these patients, delayed activation causes delayed conduction, then arrhythmia.[6]–[11]
Intriguingly, we detected the f-QRS on resting ECGs in IVF patients. As we have known, f-QRS represents the depolarization abnormality which is different from J wave representing the repolarization abnormality. We want to determine if the marker of depolarization abnormality is related the IVF. Therefore, we followed those patients and summarized the results in this article attempting to better understand the relationship between the f-QRS and prognosis in IVF patients.
2. Methods
2.1. Patient population
The total patient population consisted of 171 cases (Age: 46.1 ± 29.6 years; range: 12–79 years; male: 68.2%) in Fuwai Cardiovascular Hospital (Beijing, China) from 2000 to 2008, who survived either an episode of cardiac arrest due to ventricular fibrillation (VF), or a syncope episode associated with documented self-terminating VF. Of these, 21 subjects (age: 38.5 ± 19.0 years); range: 12–69 years; male: 47.6 %) were diagnosed as IVF.
2.2. Definitions and investigation
Patients were diagnosed as having IVF if they had no detected structural heart disease by echocardiography, magnetic resonance imaging, and coronary angiography. We excluded other known causes of VT (ventricular tachycardia)/VF, such as congenital long- and short-QT syndrome, catecholamine- induced polymorphic ventricular tachycardia, and Brugada syndrome.
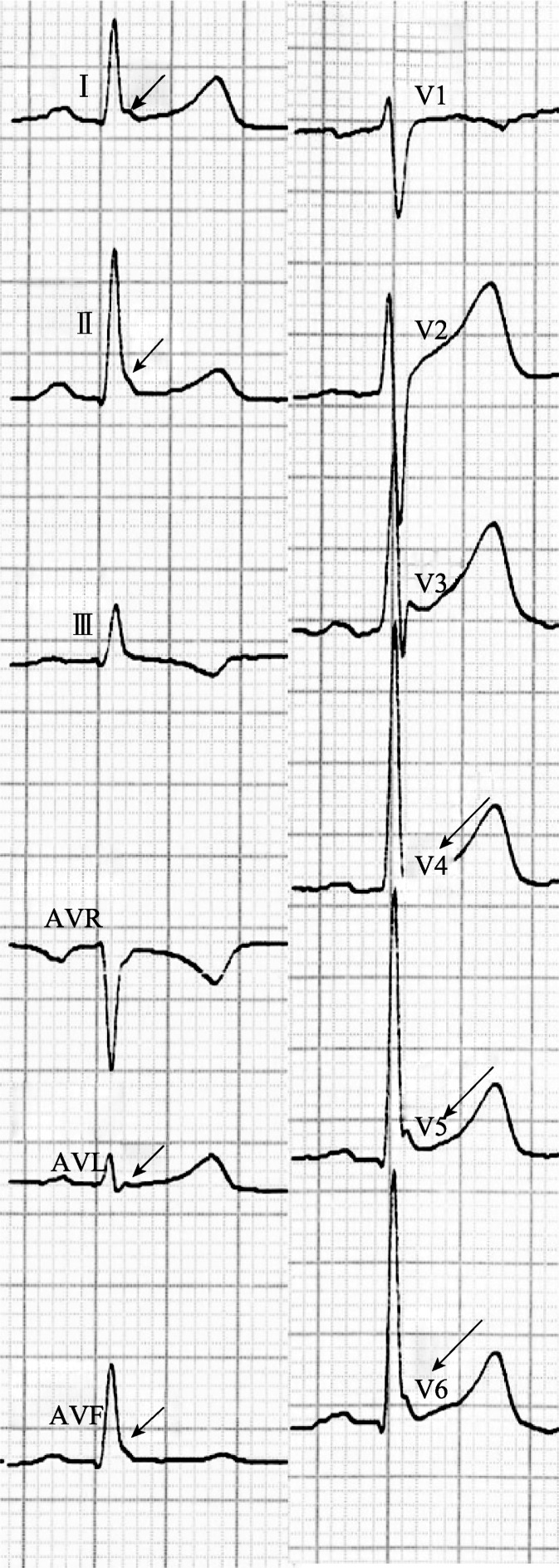
The electrocardiograms were evaluated for the presence of J wave, and f-QRS. We defined J wave as an elevation of the QRS–ST junction (J point) in at least two leads on the resting 12-lead ECG (filter range, 0.15 to 150 Hz; AC filter, 60 Hz, 25 mm/s, 10 mm/mV). The amplitude of J-point elevation had to be at least 1 mm (0.1 mV) above the baseline level, either as QRS slurring (a smooth transition from the QRS segment to the ST segment), or notching (a positive J deflection inscribed on the S wave) in the inferior lead (II, III, and aVF), lateral lead (I, aVL, and V4 to V6), or both (Figure 1).
Figure 1. The 12-lead electrocardiogram showed J wave in the leads I, avL, V4-6, leads II and avF.
The arrows indicate J wave that was slurring shape with amplitude of J-point elevation over 0.1 mV above the baseline level.
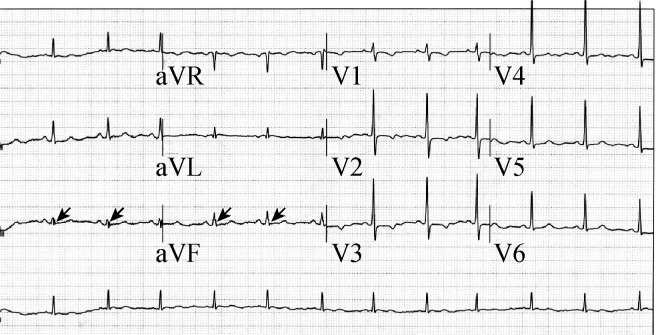
f-QRS includes the presence of an additional R wave (R′), or notching in the nadir of the R wave, or the S wave, or the presence of 1 R′, or spikes (fragmentation) in two contiguous leads, corresponding to a major coronary artery territory (Figure 2). Excluded from the definition of f-QRS,[5] were the typical bundle branch block (BBB) pattern (QRS > 120 ms) and incomplete right BBB.
Figure 2. The 12-lead electrocardiogram showed fragmented QRS complex in the leads III and avF.
The arrows indicate f-QRS that was spike shape on the up-strike of QRS complex.
The following clinical data were collected: a history of unexplained syncope, circumstances of sudden cardiac arrest, incidence rate of recurrence of VF or syncope, a family history of unexplained sudden death, and the physical activity (strenuous, usual, and sleep).
2.3. Patient groups
We classified these patients among three groups: (1) f-QRS-J wave group (group I), both f-QRS and J wave were observed in resting ECG; (2) J wave group (group II), only J wave was observed; and (3) ‘normal ECG’ group (group III), neither f-QRS nor J wave was observed.
2.4. Therapy and follow up
All patients took antiarrhythmia medications. Ten patients received an implantable defibrillator. All patients were followed in the outpatient clinic every 3 to 6 months before February, 2010. History, physical examination, a 12-lead ECG, chest roentgenograms and echocardiography were repeated to exclude or detect the development of features of underlying structural heart disease that might not have been manifested previously.
2.5. Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are presented as percentage in each group. Comparison of continuous variables was performed with Student's t-test or the non-parametric Wilcoxon rank- sum test. Categorical variables were compared with Fisher's exact test. Hazard ratios from Cox proportional-hazards models were used to estimate the relative risk associated with J wave, and f-QRS-J wave. All tests were two-tailed, and P < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Population characteristics
The group I (both f-QRS and J wave were positive) included three men and three women with a mean age of 43.2 ± 26.7 years. The group II (only J wave was positive) included three men and six women with a mean age of 37.8 ± 15.8 years. The group III (both f-QRS and J wave were negative) included five men and one women with a mean age of 43.3 ± 10.2 years. There were some differences in the characteristics among the three groups, female was majority gender in the group II, male was majority in the group III, while the patients of group II were the youngest (Table 1).
Table 1. Clinical characteristics of recruited IVF patients.
| Characteristics | Group I (n = 6) | Group II (n = 9) | Group III (n = 6) |
| Gender (Male) | 3 (50%) | 3 (33.3%) | 5 (83.3%) |
| Age (years) | 43.2 ± 26.7 | 37.8 ± 15.8 | 43.3 ± 10.2 |
| Positive family history of SCD | 1 (16.7%) | 0 | 0 |
| ICD therapy | 5 (83.3%) | 2 (22.2%) | 3 (50%) |
| Pacemaker implanted | 1 (16.7%) | 0 | 3 (50%) |
| Antiarrhythmia agent | |||
| Amiodarone | 2 | 2 | 0 |
| Amiodarone + β-blocker | 2 | 1 | 1 |
| β-blocker | 1 | 2 | 1 |
| Mexiletine | 1 | 3 | 1 |
| Calcium channel bloker | 0 | 1 | 0 |
| None | 0 | 0 | 3 |
| Events recurrence* | 13.4 ± 5.6 | 10.8 ± 3.9 | 9.8 ± 4.2 |
| Death event | 1 | 1 | 1# |
*included syncope, survived cardiac arrest and VF episodes recorded in ICD or pacemakers; #dead from stroke. ICD: implantable cardioverter defibrillator; IVF: idiopathic ventricular fibrillation.
3.2. Prevalence of f-QRS and J wave
Group I: The incidence of f-QRS in inferior leads, lateral leads, and inferior-lateral leads, was two (33.3%), one (16.7%), and three (50%), respectively (Table 1). The different morphologies of f-QRS were summarized in Table 1. The J wave was present in the inferior leads in five subjects (83.3%), in the lateral leads in one subject (16.7%), and none in the inferior-lateral leads (Table 1).
Group II: The J wave was observed in the inferior leads in seven subjects (77.8%), in the lateral leads in one subject (11.1%), and in the inferior-lateral leads in one subject (11.1%). There was no difference between groups I and II (Table 1).
3.3. Therapy and follow up
All patients took oral antiarrhythmia agents during the entire follow-up period, including amiodarone, verapamil, mexiletine, and beta-blockers (Table 1). Ten patients received the implantable cardioverter defibrillator (ICD) implantation, five in the group I, two in the group II, and three in the group III. The other patients refused the implantation of ICD due to economical problems. Four patients received the implantation of pacemakers due to bradycardia caused by antiarrhythmia medications, one in the group I, three in the group III.
All patients had finished follow-up. During the mean follow-up of 4.0 ± 2.5 years (1.2–8.8 years), three subjects (3/21, 7.48%) died. Of these deaths, one in the group I was from cardiac causes, one in the group II was from cardiac causes, one in the group III was from stroke.
The incidence of index events (syncope, survived cardiac arrest and VF episodes recorded in ICD or pacemakers) was 13.4 ± 5.6 per-year in group I, 10.8 ± 3.9 per year in group II, and 9.8 ± 4.2 per year in group III. There were significant differences in incidences among the 3 groups, the most frequent index events were observed in group I. The hazard ratio for incidence was 3.2 (95%CI, 1.1–7.9; P = 0.01).
3.4. Circumstance of index events
Surprisingly, the circumstances of index events were quite different among the three groups. In group I, all the index events occurred during sleep in early morning. In group II, four subjects suffered VF during strenuous physical activities or agitation state, two during sleep in early morning, three in usual activity. In group III, one subject suffered VF during sleep in early morning, one in agitation state, four in usual activity.
4. Discussion
This study firstly presents new information on the clinical characteristics of IVF. Of IVF patients, the cases with f-QRS and J wave characterize the following features, compared to other cases with only J wave, or without f-QRS and J wave: (1) these cases suffer the most frequent VF episodes; (2) these cases suffer sudden cardiac arrest in specific physiologic state, during sleep in early morning. The other cases suffer the VF episodes in variant states, such as strenuous physical activity, agitation, sleep, or usual activity.
For nearly 50 years, the J wave was once considered to be a benign finding. Recently, J-point elevation, or called J wave, is found more frequently among patients with IVF than among healthy control subjects and considered as a predictor for VF recurrence in those patients.[4],[12] In 2008, Rosso et al.[13] suggested that J wave presented in the inferior leads represents a moderately arrhythmogenic substrate and facilitates fatal ventricular arrhythmias. In this study, we detected the J wave in 71.43% (15/21, six in group I, nine in group II) of the IVF patients, and the incidence of J wave in inferior leads was 86.7% (13/15), which is similar to results in previous studies.[4],[12],[13]
Yan et al.[14] elucidated the mechanism of the J wave. The heterogeneous distribution of a transient, outward current mediated, spike-and-dome morphology of the action potential across the ventricular wall underlies the manifestation of the electrocardiographic J wave.[14] However, direct evidence of the accurate mechanism between J wave and the onset of VF in IVF patients has been scarce.
The f-QRS represents in-homogeneous activation of the ventricles because of myocardial scar and/or ischemia, and then causes delayed myocardial activation and the arrhythmia. It is of value in risk stratification of sudden cardiac death. f-QRS have been noticed in various structural heart disorders, coronary artery disease, Brugada syndrome, and congenital heart disease. Even in the patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy, f-QRS has been noted to have similar diagnostic efficacy as the recording of traditionally diagnostic ϵ potential in the right precordial leads with a highly amplified, and modified, ECG recording technique, so as to increase the detection rate of the ϵ potentials.[6]–[11] A recent article has also linked f-QRS with suspected cardiac involvement of sarcoidosis. Presence of the f-QRS has been shown to indicate the presence of greater infiltrative myocardial disease as corroborated by gadolinium delayed enhancement cardiac magnetic resonance imaging.[15] So formal reports have clarified the relationship between the f-QRS and structural heart diseases, but had no study on the patients diagnosed IVF.
The present study firstly described a subgroup of IVF patients, in which f-QRS was detected in resting ECGs. We provide the following hypothesis to explain the ECG marker of f-QRS in this subgroup of IVF patients: (1) sporadic scars in ventricular myocardium which could not be detected with the current techniques of echocardiography or magnetic resonance imaging and (2) a special physiological phenomenon, functional modulation of conduction, rather than a fixed scarred myocardium, such as caused by autonomic nerve activity, aging, temperature, or heart rate, produces such fragmentation, which has been proved in patients with Brugada syndrome.[7],[16],[17]
As noted, f-QRS represents depolarization abnormality, while J wave indicates the defect of repolarization. This might be the reason IVF patients with f-QRS and J wave suffer the most frequent VF episodes compared to those with only J wave, or without f-QRS and J wave.
In the present study, all the IVF patients with f-QRS and J wave suffered the VF episodes during sleep in early morning, which showed a prominent circadian pattern. Circadian variation of sympatho-vagal balance, hormones, and other metabolic factors are likely to contribute to this feature. It has been demonstrated that the transient Ito and the rapid inward sodium current (INa) plays a key role in the formation of J wave and f-QRS, respectively,[3],[7] and both can be affect by imbalance of autonomic nervous system, and then serve as the substrates for VF.[18],[19] However, the accurate mechanism of the circadian onset of the IVF is poorly understood, and needs further study.
There are some limitations in this study. First, this study included a small number of patients, so we are not sure whether there are other IVF subjects in which f-QRS was positive, J wave was negative, and whether these subjects have special clinical characteristics. Further research is necessary to clarify these questions. Second, only 10 patients (47.6%, 10/21) received the implantation of ICD due to the high cost of the device as well as the social and cultural barriers in China.
In conclusion, our study suggests IVF patients with the combined appearance of f-QRS and J wave in the resting ECG suffer an increased risk of VF and that this subgroup of IVF patients present a unique clinical feature. With regards to these features, the physicians could clarify the most dangerous patients who suffered pulseless ventricular tachycardia, or VF with “normal” heart structure, and make available ICD implantation to prevent the ventricular tachycardia, or VF recurrence.
References
- 1.Zipes DP, Wellens HJJ. Sudden cardiac death. Circulation. 1998:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 2.Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000;11:95–98. doi: 10.1111/j.1540-8167.2000.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 3.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 4.Haïssaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 5.Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 6.Peters S, Trümmel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm. 2008;5:1417–1421. doi: 10.1016/j.hrthm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Morita H, Fukushima K, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 8.Das M, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythmia Electrophysiol. 2008;1:258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 9.Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Maskoun W, Suradi H, Mahenthiran J, et al. Fragmented QRS complexes on a 12-lead ECG predict arrhythmic events in patients with ischemic cardiomyopathy who receive an ICD for primary prophylaxis. Heart Rhythm. 2007;4(Suppl.):S211–S212. [Google Scholar]
- 11.Michael M, Das M. Fragmented QRS (fQRS) on 12-lead EKG is a predictor of arrhythmic events and mortality in patients with dilated cardiomyopathy. Heart Rhythm. 2006;3(Suppl.):S103. [Google Scholar]
- 12.Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 13.Rosso R, Kogan E, Belhassen B, et al. J-Point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J Wave. Circulation. 1996;96:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Homsi M, Alsayed L, Vaz D, et al. 2064 fragmented QRS complexes on 12-lead ECG as a marker of greater myocardial infiltration by cardiacmagnetic resonance gadolinium delayed enhancement images in patients with sarcoidosis. J Cardio Mag Res. 2008;10(Suppl 1):A333. [Google Scholar]
- 16.Coronel R, Casini S, Koopmann TT, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 17.Frustaci A, Priori SG, Pieroni M, et al. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005;112:3680–3687. doi: 10.1161/CIRCULATIONAHA.105.520999. [DOI] [PubMed] [Google Scholar]
- 18.Shamsuzzaman ASM, Ackerman MJ, Kara T, et al. Sympathetic nerve activity in the congenital long-QT syndrome. Circulation. 2003;107:1844–1847. doi: 10.1161/01.CIR.0000066284.34258.59. [DOI] [PubMed] [Google Scholar]
- 19.Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]