Abstract
Objective
Both decreased glomerular filtration rate (GFR) and arterial stiffness were considered as risk factors for atherosclerosis. Previous studies have suggested the association between central arterial stiffness and the degree of GFR loss. Whether decreased GFR contributes to peripheral artery stiffness remains controversial. Moreover, data analyzed from a cohort of Chinese women are rare. Our aim was to explore the relationship between GFR and regional arterial stiffness in Chinese women.
Methods
In this cross-sectional study, we randomly recruited 1131 adult women residents with GFR ≥ 60 mL/min per 1.73 m2 estimated by the Chinese Modification of Diet in Renal Disease equation from three large communities. Central and peripheral arterial stiffness were estimated simultaneously by measuring carotid-femoral pulse wave velocity (PWVcf) and carotid-radial PWV (PWVcr) using a validated automatic device. Augmentation Index at heart rate 75 beats/minutes (AIx-75) was measured by pulse wave analysis as a composite parameter reflecting both large and distal arterial properties.
Results
The mean estimated GFR (eGFR) of the study group was 100.05 ± 23.26 mL/minute per 1.73 m2. Subjects were grouped by tertiles of eGFR level. PWVcf and AIx-75 increased ongoing from the top to the bottom eGFR tertile, while the values of PWVcr were comparable. Both univariate Pearson correlations and multiple stepwise regression analyses showed that eGFR significantly correlated to PWVcf, but not to PWVcr and AIx-75.
Conclusions
In Chinese women with normal to mildly impaired renal function, decreased eGFR affected carotid-to-femoral rather than carotid-to-radial stiffening. This provides rational to conduct future prospective studies to investigate predictors of atherosclerosis in this population.
Keywords: Arterial stiffness, Augmentation index, Pulse wave velocity, Glomerular filtration rate, Chinese women
1. Introduction
Cardiovascular disease (CVD) is a common cause of death among women.[1] Though evidence-based guidelines offer clinicians recommendations for preventing CVD in women, high coronary risk in many women remains under- diagnosed and under-treated.[2]–[4] Hence, changing the burden of cardiovascular morbidity is based on early recognition and risk stratification for CVD.
In recent years, renal insufficiency is considered as an independent risk marker for CVD.[5] Even minor reductions in glomerular filtration rate (GFR) were associated with a higher cardiovascular (CV) mortality risk.[6] Renal insufficiency may cause premature and accelerated atherosclerosis, which is considered a strong predictor of CV mortality.[7] Traditional risk factors such as age, hypertension, glucose intolerance, or hypercholesterolemia, account partly for these processes. One possible critical mechanism for this association may be increased arterial stiffness.
Arterial stiffness can be systemic or regional, and has an independent predictive value for all-cause and CV mortality in patients with various levels of CV risk.[8]–[10] Several methods exist for evaluating arterial stiffness. Among them, pulse wave velocity (PWV) and the augmentation index (AIx) are practical and reproducible non-invasive indicators for early detection of arterial stiffness.[11] Carotid-femoral PWV (PWVcf) and carotid-radial PWV (PWVcr) are used to measure central and peripheral arterial stiffness, respectively.[10] The AIx was measured by pulse wave analysis as a composite parameter reflecting both large and distal arterial properties.
Past studies suggested the relationship between the degree of GFR loss and of pertinent alterations of PWV in patients with atherosclerosis.[10],[12],[13] However, whether GFR could serve as an independent predictor of arterial stiffness in subjects with normal renal function remains controversial, and whether decreased GFR contributes equally to central and peripheral arterial stiffness in women remains unclear. Given the large burden of cardiovascular disease in individuals with chronic kidney disease, assessing the relationship between estimated GRR (eGFR) and vascular stiffness parameters in its early stage is important. Hence, we conducted a cross- sectional study in 1,131 women with normal GFR and analyzed possible correlations between renal function, parameters of CVD and arterial stiffness in this population.
2. Methods
2.1. Design and participants
This cross-sectional study included female residents from three different communities of Beijing. These underwent a survey for Women's cardiovascular risks evaluation during the period from May 2007 to July 2009. A total of 1,340 women, over 20 years of age, were invited to participate in the program that consisted of an interview, anthropometric measurements, blood sampling, PWV and AIx measurements. All participants were ethnically homogeneous (100% Han Nationality). The study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Chinese PLA General Hospital. Each participant provided her written informed consent.
Among a total of 1,340 participants, those who had a clinical history of congestive heart failure, arrhythmia, valvular heart disease, eGFR less than 60 mL/min per 1.73 m2, took medications like ACE inhibitors and beta blockers for the treatment of hypertension, or those with incomplete data were excluded from the analysis. Consequently, 1,131 eligible individuals with a mean age of 55.4 ± 13.8 years were included.
2.2. Questionnaire and anthropometric measurements
A questionnaire was completed for each subject at inclusion using a face-to-face interview method. The survey assessed traditional cardiovascular risk factors, including age, family history of premature cardiovascular events, cigarette smoking and history of hypertension, CVD and diabetes mellitus. Subjects were considered as non-smokers if they have never smoked or if they have ceased smoking for at least three consecutive years. The investigation was completed by physicians in the Department of Geriatric Cardiology of the PLA General Hospital who were trained by the research team.
Physical examinations, including anthropometry and blood pressure (BP) measurements, were performed in the morning following an overnight fast in the supine position for each patient. Brachial BP was measured with a mercury sphygmomanometer (Yuyue, Armamentarium Limited Company, Jiangsu, China) after 15 minutes of recumbent rest. Phases I and V of the Korotkoff sounds were used as the systolic BP (SBP) and diastolic BP (DBP), respectively. Pulse pressure (PP) is the difference between systolic blood pressure and diastolic blood pressure. The mean blood pressure (MBP) was calculated from the following formula: MBP = DBP + PP/3. Two measurements at an interval of 3 minutes were averaged. Anthropometric measures (height, body weight, and waist and hip circumferences) were recorded by a standardized protocol. Body mass index (BMI) was calculated as weight (kg)/height (m2).
2.3. Laboratory measurements
All subjects underwent full laboratory evaluation (lipid profile, glucose and kidney function indices). Venous blood samples were obtained between 8 a.m. and 10 a.m. from fasting participants after they had been resting for 10 minutes to 15 minutes, routinely stored at 4°C, and delivered to the Department of Biochemistry, Chinese PLA General Hospital on the same day. The biochemical variables were measured by a qualified technician using enzymatic assays (Roche Products Ltd., Switzerland) on a fully automatic biochemical autoanalyzer (COBAS c6000, Roche Products Ltd., Switzerland). Renal function was quantified as eGFR that was calculated according to the Chinese modified MDRD equations.[14]
2.4. Pulse wave reflections and PWV evaluation
Pulse wave analysis was used to determine aortic AIx, defined below. Subjects remained in a seated position, and measurements were taken immediately following determination of brachial BP.[12],[15] The right radial artery was gently compressed with the tip of a tonometer at the site of maximal pulsation. The tonometer contains a micromanometer which provides a very accurate recording of the pressure within the artery. A generalized transfer function was applied to the radial artery waveform in order to derive the aortic pressure waveform. From this aortic pressure waveform, the augmentation pressure (AG) and AIx were calculated. The AG is defined as the height of the late systolic peak above the inflection point on the waveform and may be positive or negative depending on the relative heights of the two peaks. The AIx is defined as AG expressed as a percentage of the aortic pulse pressure. As there is a linear relationship between heart rate and augmentation index, the AIx was standardized to a steady heart rate of 75 beats/min (AIx-75). Data from the mean of two central aortic pressure waveforms were taken for each subject. The Sphygmocor® (Atcor Medical, West Ryde, Australia) system was used for analysis of the radial pressure wave contour.
PWVcf and PWVcr pulse wave velocity were then obtained using validated non-invasive devices (Complior Colson device, Createch Industrie, France). Three different pressure waveforms were obtained simultaneously at three sites: the right carotid, radial and femoral arteries. Transit distances were assessed between each pulse-recording site. PWVcf and PWVcr were then automatically calculated from measurements of pulse transit time and the distance between the two sites from tonometry waveforms and body surface measurements as previously described.[12],[15] The mean PWV of at least 10 consecutive pressure waveforms was calculated for further analysis.
The same observer, blinded to the subject's identity, performed all the measurements. The interclass correlation coefficients between the first and second measurements were 0.95 for the AIx-75 and 0.87 for PWV. The coefficients of variation for the AIx-75 and PWV were less than 5%.
2.5. Statistical analysis
Statistical analyses were performed with SPSS 11.0 software (Statistical Package for the Social Sciences, Inc., Chicago, IL, USA). The data are presented as mean ± SD or percentages. Student's t-test or one-way analysis of variance (ANOVA) was used to compare continuous variables for groups and the chi-square test to compare categorical variables. Uni-variate linear regression analysis was performed to investigate correlations between PWV, or AIx-75, and the variables of interest in the study population. Multiple stepwise regression analyses were performed using PWV or AIx-75 as a dependent variable to evaluate the effect of risk factors, including eGFR, to alterations of arterial stiffness. All tests were two-tailed and P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics categorized by tertiles of eGFR
A total of 1,131 Chinese women (mean age 55.4 ± 13.8 years; range 20–85 years) completed all the procedures. General characteristics of all the subjects are shown in Table 1. The mean eGFR of the study group was 100.05 ± 23.26 mL/min per 1.73 m2. The mean PWVcf, PWVcr and AIx-75 were 10.7 ± 2.73 m/s, 8.97 ± 1.28 m/s and 27.87% ± 9.74%, respectively.
Table 1. Selected clinical and demographical characteristics categorized by tertiles of eGFR.
| eGFR tertiles | Total | 1st tertile | 2nd tertile | 3rd tertile | P |
| Age (year) | 55.4 ± 13.8 | 65.4 ± 9.3 | 52.9 ± 12.1* | 48 ± 13.4*,# | < 0.001 |
| Current smoking, n (%) | 74 (6.5%) | 41 (10.9%) | 16 (4.2%) | 17 (4.5%) | < 0.001 |
| Hypertension, n (%) | 528 (46.7%) | 220 (58.4%) | 165 (43.8%) | 143 (37.9%) | < 0.001 |
| CVD, n (%) | 172 (15.2%) | 81 (21.5%) | 56 (14.9%) | 35 (9.3%) | < 0.001 |
| Type 2 diabetes, n (%) | 183 (16.2%) | 76 (20.2%) | 48 (12.7%) | 59 (15.6%) | 0.02 |
| BMI (kg/m2) | 25.1 ± 3.8 | 24.7 ± 3.7 | 25 ± 3.8 | 25.5 ± 3.8*,# | 0.013 |
| Height (cm) | 157.9 ± 5.8 | 156.4 ± 5.7 | 158.4 ± 5.8* | 158.9 ± 5.5* | < 0.001 |
| Weight (kg) | 62.5 ± 9.8 | 60.5 ± 9.4 | 62.6 ± 9.7* | 64.4 ± 10*,# | < 0.001 |
| SBP (mmHg) | 124.9 ± 18.6 | 130.8 ± 18.4 | 122.7 ± 17.3* | 121.0 ± 18.5*,# | < 0.001 |
| DBP (mmHg) | 74.4 ± 10.0 | 73.9 ± 9.7 | 74.6 ± 9.7 | 74.8 ± 10.5 | 0.393 |
| MBP (mmHg) | 91.2 ± 11.5 | 92.9 ± 10.9 | 90.6 ± 11.2* | 90.2 ± 12.2* | 0.003 |
| PP (mmHg) | 50.5 ± 14.9 | 57.0 ± 16.0 | 48.2 ± 13.0* | 46.2 ± 13.2* | < 0.001 |
| HR (bpm) | 77.3 ± 9.9 | 76.8 ± 9.8 | 77.0 ± 10.1 | 78.1 ± 9.8 | 0.443 |
| TC (mmol/L) | 5.02 ± 0.94 | 5.29 ± 0.99 | 5.03 ± 0.89* | 4.74 ± 0.86*,# | < 0.001 |
| TG (mmol/L) | 1.62 ± 1.05 | 1.77 ± 1.13 | 1.60 ± 1.11* | 1.50 ± 0.89* | 0.002 |
| HDL-C (mmol/L) | 1.48 ± 0.38 | 1.48 ± 0.41 | 1.50 ± 0.37 | 1.47 ± 0.35 | 0.423 |
| LDL-C (mmol/L) | 2.91 ± 0.79 | 3.15 ± 0.79 | 2.88 ± 0.77* | 2.71 ± 0.73* | < 0.001 |
| Creatinine (µmol/L) | 56.16 ± 9.93 | 63.59 ± 8.19 | 57.09 ± 6.83* | 47.80 ± 7.51*,# | < 0.001 |
| UA (µmol/L) | 256.5 ± 60.9 | 277.4 ± 63.5 | 253.8 ± 58.4 | 238.2 ± 54.3*,# | < 0.001 |
| FBG (mmol/L) | 5.29 ± 1.60 | 5.33 ± 1.51 | 5.18 ± 1.22 | 5.36 ± 1.98 | 0.246 |
| eGFR (mL/min per 1.73 m2) | 100.05 ± 23.26 | 75.16 ± 7.69 | 98.26 ± 6.14* | 126.75 ± 13.78*,# | < 0.001 |
| PWVcf (m/s) | 10.70 ± 2.73 | 12.10 ± 3.19 | 10.28 ± 2.25* | 9.73 ± 2.04*,# | < 0.001 |
| PWVcr (m/s) | 8.97 ± 1.28 | 9.05 ± 1.34 | 8.95 ± 1.27 | 8.91 ± 1.23 | 0.309 |
| AIx-75 (%) | 27.87 ± 9.74 | 29.72 ± 8.73 | 27.28 ± 10.53* | 26.6 ± 9.61* | < 0.001 |
| AG (mmHg) | 11.71 ± 8.25 | 14.03 ± 9.02 | 10.56 ± 7.42 | 9.73 ± 7.03 | < 0.001 |
| Tr (s) | 75.95 ± 65.37 | 84.88 ± 64.67 | 74.29 ± 68.58 | 75.05 ± 69.31 | 0.119 |
Data are presented as mean ± SD or percentages, *P < 0.05 compared with the 1st tertile; #P < 0.05 compared with the 2nd tertile. AG: augmentation pressure; AIx-75: augmentation Index at heart rate 75 beats/min; BMI: body mass index; CVD: cardiovascular disease; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; HDL-C: high-density lipoprotein cholesterol; HR: heart rate; LDL-C: low-density lipoprotein cholesterol; MBP: mean blood pressure; PP: pulse pressure; PWVcf: carotid-femoral pulse wave velocity; PWVcr: carotid-radial pulse wave velocity; SBP: systolic blood pressure; TC: total cholesterol; TG: triglyceride; UA: uric acid; Tr: transit time.
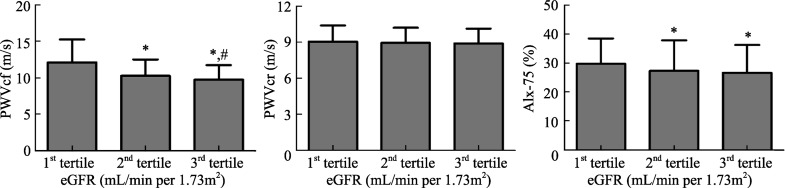
The subjects were grouped by tertiles of estimated GFR level; partition values among tertiles were 87.34 and 108.82 mL/min per 1.73 m2 (Table 1). Subjects in the bottom eGFR tertile were older, had higher serum total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), and uric acid (UA) values. Moreover, SBP and PP increased with decreasing eGFR. In contrast, DBP values were not significantly different among the three groups. The groups did not differ by HR, serum HDL-C and fasting blood glucose (FBG) levels. As shown in Table 1 and Figure 1, PWVcf and AIx-75 increased from the top to the bottom in the eGFR tertile. On the contrary, PWVcr showed no significant difference among the three groups (Figure 1).
Figure 1. PWVcf, PWVcr and AIx-75 indices according to tertiles of eGFR.
One-way analysis of variance (ANOVA) was used to compare PWVcf, PWVcr and AIx-75 indices grouped by eGFR value. X-axis: tertiles of eGFR (mL/min per 1.73 m2); Y-axis: the value of PWVcf (m/s), PWVcf (m/s), or AIx-75 (%); *P < 0.05 when compared with 1st tertile of eGFR group; #P < 0.05 when compared with 2nd tertile of eGFR group. AIx-75: Augmentation index at heart rate 75/min; eGFR: estimated glomerular filtration rate; PWVcf: carotid-femoral pulse wave velocity; PWVcr: carotid-radialpulse wave velocity.
3.2. Association of eGFR with arterial stiffness
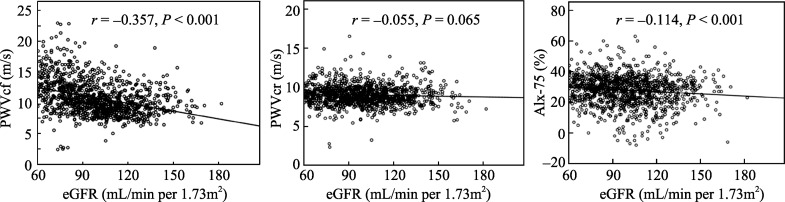
As depicted in Figure 2, the values of eGFR had a significant inverse relation with PWVcf (r = -0.357; P < 0.001), as well as with AIx-75 (r = -0.114; P < 0.001), but not with PWVcr (r = -0.055; P = 0.065). As reported in Table 2, PWVcf, PWVcr and AIx-75 significantly correlated to each other (r = 0.097–0.362; P < 0.001, for all). All measured parameters of arterial stiffness were correlated with BP and PP. PWVcf increased steeply with age and had a significant relation to body height, HR, lipid profile, serum UA and FBG levels. Only BP positively correlated to PWVcr.
Figure 2. Bivariate relations of eGFR with PWVcf, PWVcr and AIx-75.
The Pearson's correlation was used to describe the relationships of eGFR with PWVcf, PWVcr and AIx-75. eGFR had a significant inverse relation with PWVcf and AIx-75, but not with PWVcr in 1131 women subjects. X-axis: the value of eGFR (mL/min per 1.73 m2); Y-axis: the value of PWVcf (m/s), PWVcf(m/s), or AIx-75 (%), respectively. P < 0.05 compared with statistical, significance. AIx-75: Augmentation Index at heart rate 75 beats/min; eGFR: estimated glomerular filtration rate; PWVcf: carotid-femoral pulse wave velocity; PWVcr: carotid-radial (PWVcr) pulse wave velocity; r: coefficient of Pearson's correlation.
Table 2. Univariate Pearson correlations between risk factors and arterial stiffness measures.
| Univariate | PWVcf |
PWVcr |
AIx-75 |
|||
| r | P | r | P | r | P | |
| Age (years) | 0.629 | < 0.001 | 0.049 | 0.102 | 0.321 | < 0.001 |
| Height (cm) | −0.237 | < 0.001 | 0.021 | 0.476 | −0.229 | < 0.001 |
| BMI (kg/m2) | 0.193 | < 0.001 | −0.019 | 0.515 | 0.132 | < 0.001 |
| HR (beats/min) | 0.135 | < 0.001 | −0.018 | 0.702 | 0.068 | 0.154 |
| TC (mmol/L) | 0.169 | < 0.001 | 0.016 | 0.593 | 0.170 | < 0.001 |
| TG (mmol/L) | 0.245 | < 0.001 | −0.003 | 0.908 | 0.117 | < 0.001 |
| HDL-C (mmol/L) | −0.189 | < 0.001 | −0.009 | 0.760 | −0.040 | 0.178 |
| LDL-C (mmol/L) | 0.253 | < 0.001 | 0.007 | 0.816 | 0.204 | < 0.001 |
| UA (µmol/L) | 0.228 | < 0.001 | 0.034 | 0.252 | 0.081 | < 0.001 |
| FBG (mmol/L) | 0.168 | < 0.001 | 0.026 | 0.391 | 0.070 | 0.019 |
| Creatinine (µmol/L) | −0.009 | 0.767 | 0.018 | 0.543 | −0.079 | < 0.001 |
| SBP (mmHg) | 0.486 | < 0.001 | 0.175 | < 0.001 | 0.257 | < 0.001 |
| DBP (mmHg) | 0.145 | < 0.001 | 0.181 | < 0.001 | 0.230 | < 0.001 |
| PP (mmHg) | 0.509 | < 0.001 | 0.097 | < 0.001 | 0.167 | < 0.001 |
| MBP (mmHg) | 0.322 | < 0.001 | 0.186 | < 0.001 | 0.241 | < 0.001 |
| eGFR (mL/min per 1.73m2) | −0.357 | < 0.001 | −0.055 | 0.065 | −0.114 | <0.001 |
| PWVcf (m/s) | 1 | − | 0.362 | <0.001 | 0.203 | < 0.001 |
| PWVcr (m/s) | 0.362 | < 0.001 | 1 | − | 0.097 | 0.001 |
| AIx-75 (%) | 0.203 | < 0.001 | 0.097 | < 0.001 | 1 | − |
| AG (mmHg) | 0.347 | < 0.001 | 0.084 | < 0.001 | 0.585 | < 0.001 |
| Tr (s) | 0.162 | < 0.001 | 0.078 | < 0.001 | −0.149 | < 0.001 |
AG: augmentation pressure; AIx-75: Augmentation Index at heart rate 75/min; BMI: body mass index; DBP: diastolic blood pressure; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; HDL-C: high-density lipoprotein cholesterol; HR: heart rate; LDL-C: low-density lipoprotein cholesterol; MBP: mean blood pressure; PP: pulse pressure; PWVcf: carotid-femoral pulse wave velocity; PWVcr: carotid-radial pulse wave velocity; SBP: systolic blood pressure; TC: total cholesterol; TG: triglyceride; Tr: transit time; UA: uric acid.
In a stepwise multiple regression model in which eGFR, age, smoking habits, BMI, MBP, serum LDL-C, TG, HDL-C, UA and FBG concentration served as independent variables, it was found that eGFR was significantly associated with PWVcf, along with age, MBP, HDL-C and FBG (R2 = 0.479, F = 156.89, P < 0.001). However, only MBP was the significant predictor for PWVcr (R2 = 0.058, F = 32.703, P < 0.001). MBP, age and BMI were significantly correlated to AIx-75 (R2 = 0.493, F = 109.781, P < 0.001). All the explanatory variables were shown in Table 3.
Table 3. Relationship between various risk factors including eGFR and arterial stiffness.
| Predictors | B | SE | β | t | P |
| PWVcf (R2 = 0.479, F = 156.89, P < 0.001) | |||||
| Constant | 3.671 | 0.675 | − | 5.436 | < 0.001 |
| Age (years) | 0.102 | 0.005 | 0.553 | 19.842 | < 0.001 |
| MBP (mmHg) | 0.031 | 0.005 | 0.148 | 6.189 | < 0.001 |
| HDL-C (mmol/L) | −0.655 | 0.171 | 0.09 | −3.824 | < 0.001 |
| eGFR(mL/min per 1.73m2) | −0.008 | −0.002 | 0.104 | −3.864 | < 0.001 |
| FBG (mmol/L) | 0.123 | 0.039 | 0.072 | 3.132 | 0.002 |
| PWVcr (R2 = 0.058, F = 32.703, P < 0.001) | |||||
| Constant | 7. 647 | 0.302 | − | 25.358 | < 0.001 |
| MBP (mmHg) | 0.025 | 0.003 | 0.264 | 8.076 | < 0.001 |
| AIx-75 (R2 = 0.493, F = 109.781, P < 0.001) | |||||
| Constant | −1.999 | 2.192 | − | −0.912 | 0.362 |
| MBP (mmHg) | 0.295 | 0.022 | 0.391 | 13.21 | < 0.001 |
| Age (years) | 0.184 | 0.019 | 0.274 | 9.506 | < 0.001 |
| BMI (kg/m2) | −0.254 | 0.078 | −0.099 | −3.273 | 0.001 |
R2: coefficient of determination; B: Unstandardized coefficients; SE: standard error of B; β, standardized coefficients; MBP: mean blood pressure; HDL-C: high-density lipoprotein cholesterol; FBG: fasting blood glucose; eGFR: estimated glomerular filtration rate; BMI: body mass index; PWVcf: carotid-femoral pulse wave velocity; PWVcr: carotid-radial pulse wave velocity; AIx-75: Augmentation Index at heart rate 75 beats/min.
4. Discussion
This study underlined the correlation between the decreased eGFR and increased arterial stiffness in Chinese women. We found that the decreased eGFR exhibited a significant reverse association with PWVcf in women with normal to mildly impaired renal function, and thus may play a role in the process of central arterial stiffening.
Although some population-based studies indicated a highly significant relationship between artery stiffness and decreased GFR in subjects with mild to severe renal insufficiency, there were less extensive data about subjects with normal to mildly impaired renal function. In agreement with our results, Kawamoto et al.[16] showed the decreased eGFR had an independent, inverse association with arterial stiffness, as reflected by increased heart to carotid, heart to brachial, and heart to ankle PWV in general residents. Yoshida et al.[17] showed the degree of GFR loss had a weak, but significant, relationship with arterial stiffness (measured by brachial- ankle PWV) independent of the conventional atherosclerotic risk factors in middle-aged Japanese men with mildly impaired eGFR (60–89 mL/min per 1.73 m2). In contrast, Schillaci et al.[10] reported the decreased eGFR (using Mayo clinic equation) was a major determinant of both central and peripheral arterial stiffness in hypertensive patients with normal renal function. Thus, different sample sizes, variety of indices used to assess arterial stiffness, different arteries studied, different equation for the evaluation of GFR and different characteristics of the monitored study population may at least partially explain discrepancies in the results of the above mentioned studies. Our study, to the best of our knowledge, demonstrated for the first time in women with normal to mildly impaired renal function, decreased eGFR values were correlated to parallel increments of central arterial stiffness (PWVcf). In this study, subjects with lower eGFR had advanced age, altered lipid profiles and hypertension as risk factors; hence the former could not be identified as an independent predictor of arterial stiffness.
The aging process affects kidney function as well as arterial stiffness. However, the arterial system is heterogeneous, and the aging processes of structural and functional changes differ markedly in central capacitive arteries and more peripheral conduit arteries. It has been reported that PWV increases with age and that this increase was higher in the PWVcf than in the carotid-brachial PWV.[18] The accelerated stiffening of central over peripheral arteries was also observed in end stage renal disease patients[8] and in patients with impaired glucose metabolism or type 2 diabetes.[19] Herein, PWVcf is independently associated with impaired kidney function along with other traditional CVD risk factors, such as age, MBP and hyperglycemia, whereas PWVcr and AIx-75 is principally dependent on MBP.
The present study was in line with previous findings that BP is a very strong determinant of arterial stiffness. SBP and PP abet age-related changes in vessel stiffness by enhancing the magnitude of the pulse related component of the stress and strain placed on the vessel with each heartbeat.[20] In addition, the level of BP creates an initial “loading” condition that regulates pressure wave conductance in the aorta. Increasing the BP in an individual will increase the PWV.[21]
Tomiyama et al.[22] determined the age-related increase of brachial-ankle PWV was increased according to the severity of hypertension. It is clear that the systolic pressure varies in different arterial beds within the same person depending on the interaction of the forward and backward traveling waves, which is related to vessel stiffness. Thus, direct measurement of stiffness, such as PWV, provides the best estimate of aortic stiffness other than brachial pressure and PP.
Estimated GFR showed significant associations with BP in addition to PWV, indicating that hypertension was the associated factor underlying the presence of decreased eGFR. The present results are in agreement with the hypothesis that mild impairment of renal function might increase arterial stiffness. Schillaci et al.[10] found that the decreased eGFR was a major determinant of accelerated progression of central and peripheral arterial stiffness in hypertensive patients with normal renal function. However, most of our subjects were selected from middle to elderly community based samples and were considered to have been in a more advanced atherosclerotic group.
Several studies have found that diabetic subjects have higher PWV. Stehouwer et al.[23] reviewed studies in which regional stiffness estimates were compared in different arterial segments, results showed that diabetes preferentially affected the central, rather than peripheral, portion of the arterial tree or had a similar impact on the stiffness of central and peripheral segments. In contrast, in studies where stiffness estimates had been assessed locally at different (mainly peripheral) arterial sites, the deleterious effects of diabetes were stronger at the more muscular (i.e., radial, brachial, and femoral) rather than the more elastic (i.e., carotid) arteries. However, preferential stiffness of elastic over muscular arteries had also been shown.[23] In the present study, we found that FBG significantly correlatesd to, and also served as, a major contributor for PWVcf, which indicates a higher glucose level associated with higher central arterial stiffness. The mechanism might involve increased sympathetic and renin-angiotension-aldosterone system stimulation, endothelial dysfunction, as well as increased renal sodium absorption and advanced glycation end products resulting in vascular remodeling.
AIx is a composite parameter reflecting both large and distal arterial properties. Higher values of AIx are accompanied with stiffer vessels due to greater reflection of the pulse wave distally. Several studies also found that central PWV were closely related to AIx. Their correlation coefficient varies from 0.29 to 0.66, with women's correlation rate higher than men's.[24]–[27] The study here confirms the observation above. Furthermore, although the simple correlation analysis showed that AIx-75 was negatively associated with eGFR, this relation became blunt in the multivariate stepwise analysis, indicating the impaired eGFR only partly affected the systemic arterial stiffening. Some researchers consider that the AIx gives a better reflection of systemic vascular stiffness than PWV because AIx is affected by the amplitude of the reflected wave in addition to its velocity. Despite these potential confounders, AIx has been shown to predict cardiovascular mortality in end-stage renal failure,[12] but, to date, has not been seen to be predictive of outcome in any other patient group.
The underlying mechanism of renal functional alterations on arterial stiffening may involve the sodium and water balance, the renin–angiotensin–aldosterone system, the calcium– phosphate metabolism, or even vasoactive factors, such as nitric oxide and endothelin, or other compounds of endothelial origin.[28],[29] Moreover, the mean age of our study population is greater than the median menopause age of Chinese women.[30] This indicated that post-menopause might have also been involved in the promotion of atherosclerosis.[31]
There are several limitations in our study. First, our study has inherent limitations due to its cross-sectional nature. Second, since the majority of the study population had several risk factors, including hypertension and dyslipidemia, we could not eliminate the possible effect of underlying diseases and related medications on the present findings, although we ruled out those using the medications like ACE inhibitors and beta blockers, which may affects the measurements of PWV and AI.[32]
In conclusion, our study showed that the decreased GFR is significantly inversely related to increments of arterial stiffness in a large cohort of Chinese women with normal renal function. Notably, GFR alterations were not independent predictors of changes in pertinent arterial stiffness due to the combined effect of other important co-factors, such as aging, hypertension and glucose intolerance. However, the present findings provide rational to conduct future prospective studies to investigate predictors of atherosclerosis in this population.
Acknowledgments
The authors gratefully acknowledge the voluntary collaboration of the study participants and the support of the local public health authorities of Pingguoyuan communities, Beijing, China. This study was financially supported by Capital Medical Development Fund (2009-2038) of Beijing.
References
- 1.Wenger NK. Coronary heart disease: the female heart is vulnerable. Prog Cardiovasc Dis. 2003;46:199–229. doi: 10.1016/j.pcad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Gu D, Gupta A, Muntner P, et al. Prevalence of cardiovascular disease risk factor clustering among the adult population of China: results from the International Collaborative Study of Cardiovascular Disease in Asia (Inter Asia) Circulation. 2005;112:658–665. doi: 10.1161/CIRCULATIONAHA.104.515072. [DOI] [PubMed] [Google Scholar]
- 3.Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. doi: 10.1161/01.CIR.0000154568.43333.82. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 6.Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney Int. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 7.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 8.Pannier B, Guerin AP, Marchais SJ, et al. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 10.Schillaci G, Pirro M, Mannarino MR, et al. Relation between renal function within the normal range and central and peripheral arterial stiffness in hypertension. Hypertension. 2006;48:616–621. doi: 10.1161/01.HYP.0000240346.42873.f6. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Boutouyrie P. Arterial stiffness: a new surrogate end point for cardiovascular disease? J Nephrol. 2007;20(Suppl. 12):S45–S50. [PubMed] [Google Scholar]
- 12.London GM, Blacher J, Pannier B, et al. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 13.Ohya Y, Iseki K, Iseki C, et al. Increased pulse wave velocity is associated with low creatinine clearance and proteinuria in a screened cohort. Am J Kidney Dis. 2006;47:790–797. doi: 10.1053/j.ajkd.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 14.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 15.Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 16.Kawamoto R, Kohara K, Tabara Y, et al. An association between decreased estimated glomerular filtration rate and arterial stiffness. Intern Med. 2008;47:593–598. doi: 10.2169/internalmedicine.47.0825. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida M, Tomiyama H, Yamada J, et al. Relationships among renal function loss within the normal to mildly impaired range, arterial stiffness, inflammation, and oxidative stress. Clin J Am Soc Nephrol. 2007;2:1118–1124. doi: 10.2215/CJN.01880507. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 19.Kimoto E, Shoji T, Shinohara K, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52:448–452. doi: 10.2337/diabetes.52.2.448. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension. 2007;49:1202–1206. doi: 10.1161/HYPERTENSIONAHA.106.076166. [DOI] [PubMed] [Google Scholar]
- 21.Harrison MR, Clifton GD, Berk MR, et al. Effect of blood pressure and afterload on Doppler echocardiographic measurements of left ventricular systolic function in normal subjects. Am J Cardiol. 1989;64:905–908. doi: 10.1016/0002-9149(89)90840-0. [DOI] [PubMed] [Google Scholar]
- 22.Tomiyama H, Arai T, Koji Y, et al. The age-related increase in arterial stiffness is augmented in phases according to the severity of hypertension. Hypertens Res. 2004;27:465–470. doi: 10.1291/hypres.27.465. [DOI] [PubMed] [Google Scholar]
- 23.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 24.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 25.Wimmer NJ, Townsend RR, Joffe MM, et al. Correlation between pulse wave velocity and other measures of arterial stiffness in chronic kidney disease. Clin Nephrol. 2007;68:133–143. [PubMed] [Google Scholar]
- 26.Woodman RJ, Kingwell BA, Beilin LJ, et al. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens. 2005;18:249–260. doi: 10.1016/j.amjhyper.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 27.Obara S, Hayashi S, Hazama A, et al. Correlation between augmentation index and pulse wave velocity in rabbits. J Hypertens. 2009;27:332–340. doi: 10.1097/HJH.0b013e32831ac951. [DOI] [PubMed] [Google Scholar]
- 28.Dubey RK, Jackson EK, Rupprecht HD, et al. Factors controlling growth and matrix production in vascular smooth muscle and glomerular mesangial cells. Curr Opin Nephrol Hypertens. 1997;6:88–105. doi: 10.1097/00041552-199701000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. 2004;43:163–168. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- 30.Chang C, Chow SN, Hu Y. Age of menopause of Chinese women in Taiwan. Int J Gynaecol Obstet. 1995;49:191–192. doi: 10.1016/0020-7292(95)02354-f. [DOI] [PubMed] [Google Scholar]
- 31.Rexrode KM, Manson JE, Lee IM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 32.Patrianakos AP, Parthenakis FI, Karakitsos D, et al. Relation of proximal aorta stiffness to left ventricular diastolic function in patients with end-stage renal disease. J Am Soc Echocardiogr. 2007;20:314–323. doi: 10.1016/j.echo.2006.08.034. [DOI] [PubMed] [Google Scholar]