Abstract
Objective
We performed experiments using Neuregulin-1β (NRG-1β) treatment to determine a mechanism for the protective role derived from its beneficial effects by remodeling gap junctions (GJs) during heart failure (HF).
Methods
Rat models of HF were established by aortocaval fistula. Forty-eight rats were divided randomly into the HF (HF, n = 16), NRG-1β treatment (NRG, n = 16), and sham operation (S, n = 16) group. The rats in the NRG group were administered NRG-1β (10 µg/kg per day) for 7 days via the tail vein, whereas the other groups were injected with the same doses of saline. Twelve weeks after operation, Connexin 43 (Cx43) expression in single myocytes obtained from the left ventricle was determined by immunocytochemistry. Total protein was extracted from frozen left ventricular tissues for immunoblotting assay, and the ultrastructure of myocytes was observed by transmission electron microscopy.
Results
Compared with the HF group, the cardiac function of rats in the NRG group was markedly improved, irregular distribution and deceased Cx43 expression were relieved. The ultrastructure of myocytes was seriously damaged in HF rats, and NRG-1β reduced these pathological damages.
Conclusions
Short-term NRG-1β treatment can rescue pump failure in experimental models of volume overload-induced HF, which is related to the recovery of GJs structure and the improvement of Cx43 expression.
Keywords: Neuregulin-1β, Cardiac function, Heart failure, Connexin 43, Gap junction, Remodeling
1. Introduction
Heart failure (HF) remains as a serious public health problem with high morbidity and mortality rates despite significant progress in drug development.[1] Thus, developing new therapeutic strategies and elucidating underlying mechanisms of HF are crucial.
Neuregulin-1(NRG-1), an activator of ErbB2, is characterized by the receptor tyrosine kinases of the ErbB family. The NRG-1/ErbB signal transduction pathway is activated when NRG-1 binds to the ErbB4 receptors on cardiomyocytes to form ErbB2/ErbB4 receptor dimers, subsequently activating downstream signaling cascades. NRG-1 influences a series of events in cardiomyocytes, including anti-apoptosis, promotion of cell survival, regulation of myofibrillar organization, and cell-to-cell contact.[2],[3]
The NRG-1/ErbB signaling system is not only involved in cardiac development as well as maintenance of structural and functional integrity, but is also closely related to the occurrence and development of HF.[4] Negro et al.[5] found that knockouts of the ErbB2 gene in mice lead to thinning of the heart wall, decreased contractility, and chamber dilatation similar to the pathological changes of dilated cardiomyopathy. This result was also confirmed by a follow-up study where NRG-1 gene conditional knockout mice exhibited dilated cardiomyopathy.[6] ErbB2/4 receptors and NRG-1 are essential for heart development, and mutations in NRG-1, ErbB2, or ErbB4 in mice result in impaired ventricular trabeculation, causing mid-gestation lethality.[7]
Gap junctions (GJs) play a critical role in cell-cell coupling and provide substrates for electrical and chemical communication between cells. Heterogeneous remodeling of GJs lead to loss of synchronous ventricular activation, which has been confirmed to directly depress cardiac performance.[8] Connexin is a constituent protein of GJs. The distribution and function of this protein are closely associated with the direction and velocity of myocardial conduction, and impairment of connexin leads to malignant ventricular arrhythmia, the main cause of death in HF.[9] Connexin 43 (Cx43) is, by far, the most predominant type in working myocytes and responsible for intercellular communication between ventricular myocytes.[10] Cx43 has been shown to be involved in cardiomyocyte functions other than electrical impulse propagation, including mitochondrial respiration and energy metabolism. Cx43 deficiency is associated with dilated cardiomyopathy with left ventricular dilatation and reduced systolic function, associated with depressed mitochondrial respiration, and manifested at advanced age.[11] Improvement of intercellular coupling derived from reduced GJ remodeling benefits synchronous contraction in heart and restores cardiac function. Inhibition of NADPH oxidase ameliorates electrical remodeling and exerts anti-arrhythmogenic effects through up-regulating Cx43 in rabbits with HF.[12] Losartan and Captopril treatment reduced ventricular tachycardia risk in cardiac-specific angiotensin-converting enzyme (ACE) overexpression (ACE 8/8) mice, which was associated with a recovery of gap junctional conductance by increasing Cx43 expression.[13]
Previous research have shown that recombinant NRG-1β could enhance the connection between cardiomyocytes by prompting Src/focal adhesion kinase (FAK) phosphorylation, which localizes to the intercalated disk (ID) and forms focal adhesion complexes in freshly isolated ventricular myocytes.[14] Hence, these findings might imply that NRG-1β may up-regulate Cx43 expression and effect on the remodeling of GJs that contribute to coupling between ventricular myocytes, consequently, rescuing cardiac performance.
The current study aimed to investigate whether NRG-1β benefited GJ and reversed cardiac remodeling in failing hearts, improving cardiac function in a novel manner.
2. Methods
2.1. Animal model preparation
Young male Sprague-Dawley rats weighing from 160 g to 180 g, obtained from the experimental animal center of Xi'an Jiaotong University (Xi'an, Shaanxi, China), were fed normal rat chow and allowed free access to tap water. The 48 rats were divided randomly into the HF (HF, n = 16), NRG-1β treatment (NRG, n = 16) and sham operation (S, n = 16). Volume-overloaded rat models were created using aortocaval fistula (ACF), according to the method of Garcia et al.,[15] with slight modifications. Briefly, the rats in the S group only received aortic puncture, instead of ACF.
2.2. Study protocol
Echocardiograms were performed at week eight, post- operation, to confirm the existence of HF and then rats in the NRG group were injected with NRG-1β (10 µg/kg per day, ProSpec-Tany Technogene Ltd., Israel) for 7 days via the tail vein. By contrast, rats in HF and S groups were injected with the same doses of saline for 7 days. All rats were continued to be raised on standard food and water until 12 week. At week 12, echocardiographic characteristics were re-evaluated. Some hearts were subsequently excised, and the left ventricular tissue was either stored at -80°C for molecular analyses or fixed in glutaraldehyde for observation of the ultrastructure. Remaining rats were ready for cell disaggregation. All procedures were in accordance with the guidelines of the Chinese Committee for Experiments on Animals.
2.3. Echocardiographic, hemodynamic and ECG measurement
Rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (300 mg/kg). Echocardiography was performed using the SONOS-2500 ultrasound system with an ultraband transducer of 7.5 MHz (HP, USA). Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) dimensions were measured, and the left ventricular ejection fraction (LVEF) and fractional shortening (LVFS) were calculated.
Hemodynamic determinations were made at the end of all tests. Invasive parameter was measured using a BL-420F Data Acquisition & Analysis System (Chengdu TME Technology Co, Ltd., China). A catheter (PE-50 tubing) was inserted in the right carotid artery of rats under chloral hydrate anesthesia (300 mg/kg) for measurement of the arterial pressure. The catheter was then advanced into the left ventricle to measure the end-diastolic pressure (LVEDP) and end-systolic pressure (LVESP). We evaluated the maximum velocity of the ascending and descending in intraventricular pressures (±dP/dtmax) as the indices of contractility and relaxation, respectively. The QT interval was measured using standard II lead ECG and was corrected (QTc).
2.4. Plasma concentration of brain natriuretic peptide and angiotensin II measurement
After hemodynamic measurement, blood (4 mL) was collected from carotid artery of the rat. Half of the blood sample (2 mL) was used to detect the plasma brain natriuretic peptide (BNP) concentration using the BNP ELISA Kit (96T AssayMax Rat BNP-32, Assaypro, America), according to specifications. The remaining 2 mL was used for plasma angiotensin II level measurement using the Iodine [125I] Angiotensin II Radioimmunoassay Kit (BNIBT, China), according to specifications.
2.5. Ventricular myocytes isolation
Single ventricular myocyte was obtained through enzymatic dissociation of the ventricle of rat heart using Langendorff retrograde perfusion via the aorta, as described by Isenberg and Klockner.[16] Briefly, the heart was resected quickly, immersed in Tyrode's solution at 4°C, and cannulated via the aorta. Then, the heart was perfused successively at 37°C with the following solution: (1) Ca2+-free Tyrode's solution for 10 minutes, at a velocity of 6 mL/minute; (2) digestion solution for 8–10 minutes, at a velocity of 5–6 mL/minute; (3) 0.06 mol/L Tyrode's solution for 5 minutes, at a velocity of 8 mL/minutes. Subsequently, the left ventricle (LV) was dissected from the heart, placed in Kraft-Brühe (KB) solution, and sheared with ophthalmic scissors. Isolated cells were released from the separated tissue blocks by mechanical agitation and stored at 4°C in KB solution. All solutions were equilibrated with 100% O2 at 37°C.
2.6. Solutions and drugs
Ca2+-free Tyrode's solution consisted of the following: NaCl 140 mmol/L, KCl 5.4 mmol/L, MgCl2 1.8 mmol/L, Glucose 10 mmol/L, HEPES 10 mmol/L, and CaCl2, 1.8 mmol/L, pH 7.35–7.40 (with NaOH). KB solution contained the following: KCl 25 mmol/L; KH2PO4 10 mmol/L; MgCl2 3 mmol/L; Taurine 20 mmol/L; L-glutamic acid 70 mmol/L; EGTA 0.5 mmol/L; HEPES 10 mmol/L; and glucose 10 mmol/L, pH 7.35–7.40 (with KOH). Tyrode's solution (0.06 mmol/L) contained 100 mL Ca2+-free Tyrode's solution, supplemented with 60 µL CaCl2 (0.1 mol/L). The digestion solution consisted of 30 mg BSA, 24mg collagenase II, and 30 mL Ca2+-free Tyrode's solution. Collagenase II was purchased from Worthington (USA). The other reagents were purchased from Sigma (USA).
2.7. Immunofluorescence
Cell suspensions from four rats in each group were dropped onto glass slides and fixed in 4% paraformaldehyde in 0.1 mol phosphate buffer. Cells were incubated with 0.1% Triton and 10% goat serum in phosphate-buffered saline, then with the primary antibody Cx43 (1: 50; Cell Signaling Technology, America), following by the appropriate secondary antibody (goat anti-Rabbit IgG-FIFC, 1: 100; ZSGB- BIO, China). Finally, cells were stained with DAPI (Southern Biotech, USA) to label the nucleus. The glass slides were covered and observed under a confocal microscope.
2.8. Western blot analysis of Cx43 protein expression
Proteins were initially extracted from LV tissues by disruption of cellular structures and removal of non-protein components, then immunoblotting was performed to analyze Cx43 protein level.
Briefly, myocardium tissues were homogenized in radioimmunoprecipitation lysis buffer with a protease-inhibitor and centrifuged at 4°C. Protein concentrations were measured using the Bradford assay. Protein samples (30 µg per lane) were separated on 10% polyacrylamide-SDS gels and transferred onto nitrocellulose membranes. For Western blotting, membranes were first incubated with an antibody directed against Cx43 (1: 200), then with the horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (PIERCE, USA). Immunoreactive bands were visualized on X-ray film using the enhanced chemiluminescence method. Band densities were quantified by densitometry, and standardized by the integrated density of β-actin of the lane used as an internal control. Each sample has three replicates. Finally, the amount of Cx43 was normalized to β-actin.
2.9. Transmission electron microscopy (TEM)
The LV anterior wall tissue from four rats in each group was cut rapidly into small 1 mm3 pieces, and immersed in glutaraldehyde. The ultrastructure in the different groups of cardiac tissue was observed using a Hitachi H-7650 transmission electron microscope.
2.10. Statistical analysis
All data were expressed as means ± SE. Statistical analysis was performed using one-way ANOVA with a post-hoc Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
3. Results
3.1. Effect of NRG-1β on cardiac function
At eight weeks post operation, the first echocardiographic measurement showed that the HF and NRG rats had significant left ventricular hypertrophy and systolic dysfunction compared with the S rats, which indicated the successful establishment of volume-overloaded HF. Repeated echocardiography were performed at 12 week post operation. Compared with the HF group, LVEF and LVFS significantly increased in the NRG group (P < 0.01), whereas, LVEDD and LVESD were smaller in NRG group than that in HF group rats (P < 0.01), (Table 1).
Table 1. Echo-cardiographic measurement results.
| Before treatment (BT) |
After treatment (AT) |
|||||
| S (n = 16) | HF (n = 16) | NRG (n = 16) | S (n = 16) | HF (n = 16) | NRG (n = 16) | |
| HR (bpm) | 411.30 ± 39.51 | 392.33 ± 28.52 | 396.13 ± 20.84 | 417.03 ± 40.60 | 424.01 ± 30.92 | 390.23 ± 30.52 |
| LVESD (mm) | 3.50 ± 0.40 | 6.26 ± 0.59** | 5.95 ± 0.45** | 3.05 ± 0.41 | 6.02 ± 0.53## | 4.21 ± 0.55##††§§ |
| LVEDD (mm) | 6.00 ± 0.45 | 9.01 ± 0.69** | 8.91 ± 0.55** | 6.19 ± 0.28 | 8.95 ± 0.68## | 7.22 ± 0.67##††§§ |
| LVEF (%) | 84.06 ± 4.45 | 63.38 ± 4.67** | 60.91 ± 7.05** | 82.64 ± 2.11 | 61.53 ± 4.45## | 78.28 ± 4.04#††§§ |
| LVFS (%) | 46.29 ± 2.52 | 30.53 ± 2.62** | 32.38 ± 2.50** | 51.87 ± 5.57 | 31.32 ± 7.19## | 46.78 ± 4.53##††§§ |
**P < 0.01 vs. S BT; ##P < 0.01 vs. S AT; ††P < 0.01 vs. HF AT; §§P < 0.01 vs. NRG BT (self control ). HF: heart failure; HR: heart rate; LVEDD: left ventricular end-diastolic dimension; LVEF: left ejection fraction; LVESD: left ventricular end-systolic dimension; LVFS: fraction shortening; NRG: neuregulin-1β; S: sham operation.
A summary of the terminal hemodynamics was shown in Table 2. Systolic dysfunction was evident in HF rats in the fact that +dP/dtmax was reduced by 35% compared with the S rats (P < 0.01), similar to the reduction in -dP/dtmax, which is an index of diastolic dysfunction (P < 0.01). By contrast, rats treated with NRG-1β preserved both ±dP/dtmax (P < 0.01 vs. HF). LVEDP, as another index of systolic dysfunction, was increased from 3.2 ± 0.5 mmHg to 12.2 ± 0.7 mmHg in HF rats versus S rats (P < 0.01). In contrast to HF rats, NRG rats were reduced to 7.5 ± 0.8 mmHg (P < 0.05 vs. HF).
Table 2. Echo-cardiographic measurement results.
| S (n = 6) | HF (n = 7) | NRG (n = 6) | |
| HR (bmp) | 410.00 ± 15.76 | 439.43 ± 29.22 | 397.33 ± 41.69 |
| LVESP (mmHg) | 127.04 ± 5.92 | 89.27 ± 6.89** | 111.04 ± 6.51 ** ## |
| LVEDP (mmHg) | 3.20 ± 1.13 | 12.17 ± 1.91** | 7.47 ± 1.78 ** ## |
| +dP/dtmax (mmHg/S) | 5468.16 ± 345.28 | 3573.60 ± 511.22** | 4968.64 ± 141.74 * ## |
| -dP/dtmax (mmHg/S) | 4163.79 ± 155.51 | 2446.42 ± 540.17** | 3773.14 ± 269.52 ## |
| QT interval (ms) | 61.21 ± 6.31 | 84.72 ± 7.85 ** | 76.51 ± 6.73** # |
| QTc (ms) | 156.63 ± 12.32 | 206.7 ± 27.81** | 187.85 ± 18.92** # |
*P < 0.05 vs. S; **P < 0.01 vs. S; #P < 0.05 vs. HF; # #P < 0.01 vs. HF. -dP/dtmax: maximum velocity of ascending in intraventricular pressure; -dP/dtmax: minimum velocity of descending in intraventricular pressure; HF: heart failure; HR: heart rate; LVESP: left ventricular end-systolic pressure; LVEDP: left ventricular end-diastolic pressure; NRG: neuregulin-1β; S: sham operation; QTc: corrected QT interval.
3.2. ECG changes
In the HF group, QT and QTc increased to 84.72 ± 7.85 ms and 206.7± 27.8 ms, respectively; however, increased to 76.51 ± 6.73 ms and 187.85 ± 18.92 ms, respectively, in NRG group.
3.3. Plasma concentration of BNP and angiotensin II
Data showed that the plasma concentration of BNP in the HF group (0.80 ± 0.05) was higher than in the S group (0.05 ± 0.09), (P < 0.01), whereas the BNP level in NRG group (0.27 ± 0.02) was reduced by 67% when compared with the HF (0.80 ± 0.05) (P < 0.01). NRG-1β reduced the plasma concentration of BNP in rats with HF (Figure 1A). Similarly, elevated plasma angiotensin II level of rats in the HF group (0.68 ± 0.03) was remarkably lowered through NRG-1β treatment (0.45 ± 0.040), (P < 0.05), as shown in Figure 1B.
Figure 1. Plasma concentrations of BNP and AngII.
(A): Plasma concentrations of BNP; (B): Plasma concentrationin of AngII. *P < 0.05 vs. S; **P < 0.01 vs. S; #P < 0.05 vs. HF; # #P < 0.01 vs. HF. BNP: brain natriuretic peptide; HF: heart failure; NRG: neuregulin-1β; S: sham operation.
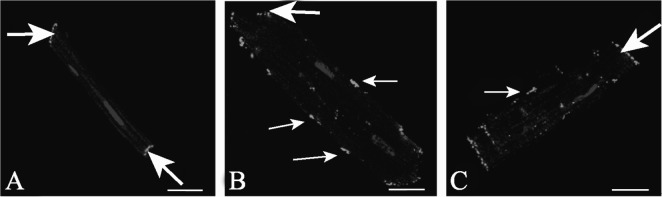
3.4. Patterns of Cx43 distribution
In isolated LV myocytes from normal hearts, Cx43 was located mainly at both ends of cells, which localizes to the IDs, with low expression at the lateral sarcolemma (Figure 3A). In the HF group, Cx43 labeling was distributed not only at the IDs, but also at the lateral sarcolemma (Figure 3B). Lateralization of Cx43 was less in the NRG group, with the amount of Cx43 at sides of the cells obviously attenuated (Figure 3C).
Figure 3. Confocal images of Cx43 (green signal) labeling in LV isolated myocytes in S group, HF group and NRG group.
(A): S group (control), Cx43 labeling confined to the IDs; (B): HF group, Cx43 staining appears at cardiomyocyte lateral membrane; (C): NRG group, Cx43 staining appears mostly at ends. In all micrographs, nuclei are stained blue with DAPI. Variations in the pattern of organization of GJ were seen. Thick arrows indicate Cx43 labeling confined to the IDs, thin arrows point to the lateral Cx43. Scale bars = 20 µm. Cx43: Connexin 43; HF: heart failure; LV: left ventricle; NRG: neuregulin-1β; S: sham operation.
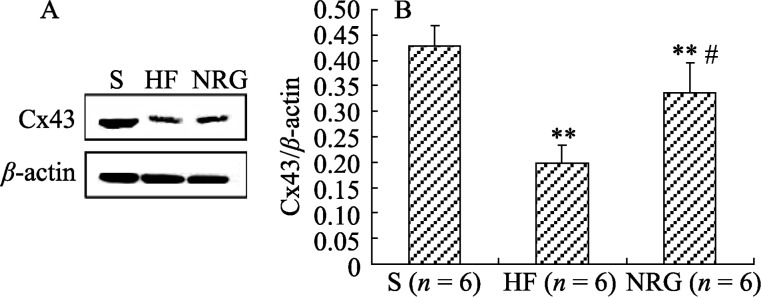
3.5. Cx43 expression
The amount of Cx43 in LV tissue was markedly decreased in the HF group (0.13 ± 0.02) compared with the S group (0.43 ± 0.05) (P = 0.001). This change was inhibited by NRG-1β treatment (NRG 0.34 ± 0.07 vs. HF 0.13 ± 0.02; P = 0.015). Data are illustrated in Figure 2.
Figure 2. Western blot results of Cx43.
(A): Representative western blots; (B): The amount of Cx43 normalized to β-actin. **P < 0.01 vs. S; #P < 0.05 vs. HF. BNP: brain natriuretic peptide; Cx43: Connexin 43; HF: heart failure; NRG: neuregulin-1β; S: sham operation.
3.6. Ultrastructure
TEM revealed regular myofibrils in S group, characterized by their regular arrangement, clear Z lines, intact sarcomeres, dense mitochondrial crest, normal spaces between cardiac cells, well-shaped IDs (Figure 4A), and well-structured GJs with strong electrical density (Figure 4 A1 ). Swollen cardiac myocytes can be seen in the HF group with abnormal myofibril arrangement, obscure sarcomeres and Z lines, reduced mitochondrial size, and increased electronic density. The continuity of the ID structure is interrupted (Figure 4B), and the GJ structures were vague, with dissolved or disappeared portions. Electronic density was also lowered (Figure 4B1). In contrast to the HF group, the pathological damage was obviously attenuated in the NRG group (Figures 4C and 4C1).
Figure 4. TEM of cardiac tissue sections in 3 groups, IDs were clearly seen (thick arrow).
(A): S group: myofibril arranged regularly, sarcomeres and Z lines were distinct, the structure of intercalated discs and gap junction were normal; (B): Micrographs of HF group: the structure of myocyte was disrupted, GJ was vague, part of them dissolved and disappeared; (C): Micrographs of NRG group, GJ recovered to some extent, the structure was integrate. A1, B1, C1, Magnified and extended view of the boxed region (thin arrow) in A, B, C, respectively. GJs were clearly seen (arrow head). Scale bars: A, B, C = 1 µm; A1, B1, C1 =100 nm. HF: heart failure; LV: left ventricle; NRG: neuregulin-1β; S: sham operation; TEM: Transmission electron microscope
4. Discussion
Chronic heart failure (CHF) is a common outcome of most cardiovascular diseases, with high morbidity, poor prognosis, tendency towards malignant arrhythmia, and sudden death. Pump failure is an independent predictor of death. Short- term improvement of cardiac pump function can reduce HF mortality.[17]
Our data demonstrated short-term intravenous administration of NRG-1β into rats with HF can obviously ameliorate all malignant changes in the parameters of cardiac function, such as elevated ±dP/dtmax, increased EF and lowered LVEDP. Echocardiography showed that NRG-1β can reverse general cardiac remodeling by reducing LVEDD and LVESD. The hemodynamic effects of NRG-1β have been well-documented. NRG-1/ErbB-activation improves cardiac function in models of ischemic, dilated, and viral cardiomyopathy.[18] Fukazawa et al.[19] found that NRG-1 protects ventricular myocytes from anthracycline-induced apoptosis via ErbB4-dependent activation of PI3-kinase/Akt. Jabbour et al.[20] first applied recombinant human NRG-1(rhNRG-1) to patients with stable CHF, and found that rhNRG-1 produced favorable acute and chronic hemodynamic effects in patients with stable CHF. In recent years, studies have shown that the level of plasma BNP is an important biomarker for the diagnosis and prognosis in chronic HF.[21],[22] Our data revealed that NRG-1β can reduce the plasma concentration of BNP in rats with HF, further confirming the efficacy of NRG-1β treatment.
Heart rate corrected QT interval (QTc) is a crucial and critical factor in the assessment of repolarization changes considering safety of drugs and cardiac disorders.[23] HF is characterized by prolonged QTc accompanying arrhythmia susceptibility. Prolonged QTc is closely related to malignant ventricular arrhythmia in HF, as well as a negative correlation with declined index of the left ventricular systolic function.[24]
It is reported that the decrease of QTc was correlated with the increase of LVEF and the therapy of shortening QTc improved cardiac function,[25] which is consistent with our findings. The current study revealed that NRG rats QTc was shorter than HF rats with the improvement of cardiac function. Kirchhoff et al.[26] reported that Cx43-heterozygous knockout mice exhibited cardiac conduction abnormalities with QT prolongation.
GJs are mainly confined to IDs in normal myocardium. Lateralized and internalized GJs are remarkable features in HF and also serve as the pathological basis of functional disorder.[27] We also observed the ultrastructure of LV tissues by TEM, revealing that myofibrils, sarcomeres, Z lines, IDs and GJs were all injured in HF group. Fortunately, the pathological damages were obviously diminished by NRG-1β treatment. Impressively, we found, for the first time, that NRG-1β can restore GJ structure in failing hearts. Other protective myocardial roles of NRG-1β have also been reported. Sawyer et al.[28] reported that doxorubicin- induced myofibrillar disarray in cultured adult rat ventricular myocytes (ARVMs) were significantly reduced after the ErbB2 receptor of ARVMs was activated by NRG-1β.
Cardiac myocytes suffering from injury were related to increased oxidative stress in HF.[29] There is substantial evidence that oxidative stress can activate many cellular responses in HF, including cellular hypertrophy, changes in gene expression, ultrastructural abnormality, and cell death.[30],[31] NRG-1β can rapidly modulate nitric oxide synthesis to enhance antioxidant stress.[32] NRG-1, as a survival factor for adult cardiocytes, enhances levels of redox regulators and the cell cycle regulator, cyclin D1, as well as protects cardiocytes against injuries.[33] These mechanisms are apparently used by NRG-1β for restoring organelles, including the remodeling of GJs.
In myocardial tissue, Cx43 constitutes the GJs located at the IDs, which are mainly limited to the ends of a cell. Prominent features of structural heart disease are the redistribution of Cx43 to the lateral surface and decreased Cx43 expression levels.[27] We found that, using immunofluorescence staining in freshly isolated ventricular myocyte, the lateral and dispersed distribution of Cx43 was increased in the HF group, whereas, this kind of disorder was alleviated in the NRG group.
Immunoblotting data showed that an increase in Cx43 level in the NRG group with NRG-1β, in contrast to the HF group. Down-regulation of Cx43 is a typical feature of myocardial remodeling in heart failure regardless of its origin.[10],[34],[35] To our knowledge, the confirmed mechanism for the up−regulation of Cx43 by NRG−1β is poorly known. Activated renin−angiotensin− aldosterone system (RAAS) may lead to an increase in angiotensin II concentration during pump failure, consequently accelerating the degradation of the Cx43 protein.[36] The present study found that angiotensin II levels in the NRG group were reduced, compared with that of the HF group. In addition, angiotensin II weakened NRG−1 mRNA synthesis and resulted in decreased levels of endogenous NRG−1.[37] NRG−1β treatment corrected the NRG−1 down−regulation and increased Cx43 content by lowering serum angiotensin II levels that resulted from the inhibition of RAAS,[18] and by compensating for the endogenous deficiency in NRG−1. By contrast, increased oxidative stress during pump failure was involved in altered Cx43 expression. Oxidative stress inhibited intercellular communication and decreased Cx43 expression. Liu Y et al.[12] reported that the antioxidants prevented oxidative stress−induced inhibition of Cx43, ameliorating electrical remodeling and exerting anti−arrhythmogenic effect in rabbits with HF. NRG protected cells from injury by balancing redox states, prompting the antioxidant system, and inhibiting oxidative stress. NRG−1, similar to the antioxidant N−acetylcysteine, can protect cardiac myocytes against injury of oxidative stress using the PI3−kinase/Akt pathway via the ErbB2 receptor.[38] NRG was shown to decrease reactive oxygen species in PC12 cells transfected with ErbB4 dependent on the PI3K pathway.[39] Endothelial nitric oxide synthase (eNOS), as a mediator of oxidative stress, reduced in HF.[40] NRG−1− activated Akt, a serine/threonine kinase, was involved in the phosphorylation of eNOS,[41] this phenomenon enhances the production of eNOS and NO to stimulate antioxidant action and inhibit reduction of Cx43 expression.
In summary, the present study reveals that NRG-1β restored heterogeneous remodeling of GJ in the failing heart and improves cardiac function of general animal. Because GJ abnormalities exist in many cardiovascular diseases, restoration of normal intercellular coupling may well serve as a novel approach to diminish lethal arrhythmias and improve cardiac function. Strategies to improve intercellular coupling in the diseased heart could include methods designed to up-regulate Cx43 or other connexins.
5. Conclusion
Short-term intravenous injection of NRG-1β improves cardiac function in rats with volume-overloaded HF. NRG-1β may benefit heart performance through reducing GJ pathological damage and increasing Cx43 expression. NRG-1β is proven effective in treatment for HF.
Acknowledgments
We are grateful Dr. Shao-Li Cheng for his help in revision of the manuscript. This research was supported by the Key Program (No.30380051) of the National Natural Science Foundation of China.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;17:E25–E146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- 3.Freedman NJ, Ginsburg GS. Novel—and “Neu”—Therapeutic possibilities for heart failure. J Am Coll Cardiol. 2006;48:1448–1450. doi: 10.1016/j.jacc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- 5.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res. 2004;59:1–12. doi: 10.1210/rp.59.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Liu FF, Stone JR, Schuldt AJ, et al. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J PhysiolHeart Circ Physiol. 2005;289:H660–H666. doi: 10.1152/ajpheart.00268.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lee KF, Simon H, Chen H, et al. Requirement for neu- regulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 8.Gutstein DE, Morley GE, Vaidya D, et al. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 9.Akar FG, Nass RD, Hahn S, et al. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H1223–H1230. doi: 10.1152/ajpheart.00079.2007. [DOI] [PubMed] [Google Scholar]
- 10.Akar FG, Spragg DD, Tunin RS, et al. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 11.Esperanza A, Marisol RM, Rio A, et al. Cx43 deficiency is associated with dilated cardiomyopathy in old mice that can be rescued by genetic ablation of its c-terminal domain [Abstract] Circulation. 2011;124:A12556. [Google Scholar]
- 12.Liu Y, Huang H, Xia WF, et al. Inhibition of NADPH oxidase up-regulates connexin 43 and ameliorates electrical remodeling in rabbits with heart failure. Biomed Pharmacother. 2011;1:33–38. doi: 10.1016/j.biopha.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Iravanian S, Sovari AA, Lardin HA, et al. Inhibition of renin-angiotensin system (RAS) reduces ventricular tachycardia risk by altering connexin43. J Mol Med. 2011;89:677–687. doi: 10.1007/s00109-011-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuramochi Y, Guo X, Sawyer DB. Neuregulin activates erbB2- dependent src/FAK signaling and cytoskeletal remodeling in isolated adult rat cardiac myocytes. J Mol Cell Cardiol. 2006;41:228–235. doi: 10.1016/j.yjmcc.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res. 1990;24:430–432. doi: 10.1093/cvr/24.5.430. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg G, Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB” medium. Pflugers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- 17.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Gu X, Li Z, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 19.Fukazawa R, Miller TA, Kuramochi Y, et al. Neuregulin-1 protects ventricular myocytes from anthracycline -induced apoptosis via erbB4-dependent activation of P13-kinase/Akt. J Mol Cell Card. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour A, Hayward CS, Keogh AM, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 21.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Felker GM, Petersen JW, Mark DB. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ. 2006;175:611–617. doi: 10.1503/cmaj.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol. 2010;641:187–192. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Davey P. QT interval lengthening in cardiac disease relates more to left ventricular systolic dysfunction than to autonomic function. Eur J Heart Fail. 2000;2:265–271. doi: 10.1016/s1388-9842(00)00065-9. [DOI] [PubMed] [Google Scholar]
- 25.Xie RQ, Cui W, Liu F, et al. Statin therapy shortens QTc, QTcd, and improves cardiac function in patients with chronic heart failure. Int J Cardiol. 2010;140:255–257. doi: 10.1016/j.ijcard.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 26.Kirchhoff S, Kim JS, Hagendorff A, et al. Abnormal cardiac conduction and morphogenesis in Connexin40 and Connexin43 double-deficient mice. Circ Res. 2000;87:399–405. doi: 10.1161/01.res.87.5.399. [DOI] [PubMed] [Google Scholar]
- 27.Hesketh GG, Shah MH, Halperin VL, et al. Ultrastructure and regulation of lateralized connexin43 in the failing heart. Circ Res. 2010;106:1153–1163. doi: 10.1161/CIRCRESAHA.108.182147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawyer DB, Zuppinger C, Miller TA, et al. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1β and Anti-erbB2. Circulation. 2002;205:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 29.Sawyer DB. Oxidative stress in heart failure: What are we missing? Am J Med Sci. 2011;342:120–124. doi: 10.1097/MAJ.0b013e3182249fcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 31.Siwik DA, Tzortzis JD, Pimental DR, et al. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, anapoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- 32.Brero A, Ramella R, Fitou A, et al. Neuregulin-1β rapidly modulates nitric oxide synthesis and calcium handling in rat cardiomyocytes. Cardiovasc Res. 2010;88:443–452. doi: 10.1093/cvr/cvq238. [DOI] [PubMed] [Google Scholar]
- 33.Giraud MN, Fluck M, Zuppinger C, et al. Expressional reprogramming of survival pathways in rat cardiocytes by neuregulin-1β. J Appl Physiol. 2005;99:313–322. doi: 10.1152/japplphysiol.00609.2004. [DOI] [PubMed] [Google Scholar]
- 34.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–H1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 35.Peters NS, Green CR, Wilson PA, et al. Reduced content of connexin43 gap junction in ventricular myocardium from hypertrophied and ischaemic human hearts. Circulation. 1993;88:864–871. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 36.Severs NJ, Bruce AF, Dupont E, et al. Remodeling of gap junctions and connexin expression in diseased myoeardium. Cardiovase Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemmens K, Segers VF, Demolder M, et al. Role of neuregulin-1/ ERBB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;28:19469–19477. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 38.Timolati F, Ott D, Pentassuglia L, et al. Neuregulin-1 beta attenuates doxorubicin-induced alterations of excitation-contraction coupling and reduces oxidative stress in adult rat cardiomyocytes. J Mol Cell Cardiol. 2006;41:845–854. doi: 10.1016/j.yjmcc.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Goldshmidt Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescue PC12-ErbB4 cells from cell death induced by H2O2: regulation of reactive oxygen species levels by PI3K. J Biol Chem. 2001;49:6379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 40.Feng QP, Song W, Lu XR, et al. Development of heart failure and congenital septal defects in mice lacking endothelial nitric oxide synthase. Circulation. 2002;106:873–879. doi: 10.1161/01.cir.0000024114.82981.ea. [DOI] [PubMed] [Google Scholar]
- 41.Lemmens K, Fransen P, Sys SU, et al. Neuregulin-1 induces a negative inotropic effect in cardiac muscle:role of nitric oxide synthase. Circulation. 2004;109:324–326. doi: 10.1161/01.CIR.0000114521.88547.5E. [DOI] [PubMed] [Google Scholar]