Abstract
Objective
To investigate whether Tumor Necrosis Factor-alpha (TNFα) is capable of activating Rho kinase pathway which leads to smooth muscle cell proliferation and the intervention function of Rosuvastatin, and clarify the mechanism and intervention manner of anti-atherosclerosis by Rosuvastatin.
Methods
Wistar neonate rat smooth muscle cells were cultured, and the activity of cell proliferation was determined by methyl thiazolyl tetrazolium (MTT). The expression of Rho kinase genes after the stimulation of TNFα was evaluated by RT-PCR. Western blot method was used to measure the protein expression of proliferating cell nuclear antigen (PCNA) after TNFα stimulation and Rosuvastatin intervention in smooth muscle cell.
Results
The TNFα stimulation significantly enhanced the expression of Rho kinase and increased the expression of PCNA protein in smooth muscle cells (P < 0.05). These effects were positively correlated with prolonged treatment whereas additional Rosuvastatin administration inhibited the above-mentioned effects (P < 0.05).
Conclusions
The activation of TNFα mediated Rho kinase signaling pathway can significantly promote smooth muscle cell proliferation, and Rosuvastatin can not only inhibit this pathway but also the induced proliferation.
Keywords: Tumor necrosis factor-alpha, Rho kinase, Signaling transduction, Vascular smooth muscle cell
1. Introduction
Vascular smooth muscle cell proliferation is the main pathological characteristic of atherosclerosis. Recent studies showed a close relationship between the activation of Rho kinase signaling transduction pathway and cardiovascular diseases, which may participate in the occurrence and development of vascular smooth muscle cell proliferation. Rho kinase can phosphorylate myosin-binding subunit (MBS) and results in the inactivation of myosin light chains dephosphorylase (MLCPh). Accordingly, the vascular smooth muscle cell (SMC) cannot finish the constriction status, which is closely related to the occurrence and development of main cardiovascular diseases, such as myocardial ischemia, and atherosclerosis. Rosuvastatin, a commonly used hypolipidemic agent, also has anti-inflammatory effect and plays a role with multiple inflammatory factors. Rosuvastatin has been widely used in clinical treatment in recent years, and the effect in primary prevention has been identified.[1] In this study, we tried to explore the association between anti-atherosclerosis effect of Rosuvastatin and inhibition of the TNFα– Rho kinase signaling pathway, which might be the internal mechanism of the anti-proliferation effect of Rosuvastatin in smooth muscle cells. This study aimed to explore the specific regulatory mechanism of the anti-atherosclerosis effect of Rosuvastatin from the aspect of molecular biology, and provide more favorable evidence for the secondary prevention of coronary heart disease.
2. Methods
2.1. Reagents
Rho kinase, β-actin primer (Sangon Biotech (Shanghai) Co., Ltd.), proliferating cell nuclear antigen (PCNA) antibody (Wuhan Boster Biological Technology., Ltd.), DMEM-F12 culture medium, fetal calf serum (Hyclone, US), methyl thiazolyl tetrazolium (MTT) (Sigma, US), cell lysate (Shanghai Shenergy Company, Ltd.), chemoluminescence kit (Pierce, US).
2.2. Vascular smooth muscle cell culture
The Wister rat (provided by the Animal Center, China Medical University) within 24 hours after birth was sacrificed, the aorta was immediately collected when heart was still beating. The aorta tissue was carefully sheared, washed with ice-cold PBS, and further sheared into scraps of 0.5–1 mm3. Two to three drops of cell culture medium were added and slightly pipetted with pipette, and then the suspension of tissue pieces was assimilated in the fraction and arranged evenly in a 75 mL culture flask. The flask was slightly flipped, 3 mL of high glucose DMEM supplied with 15% fetal calf serum and 0.1 mmol/L of 5-Bromodeoxyuridine (Brdu) was added. The flask was placed in a constant 37°C incubator for 30–40 minutes, making sure the tissue pieces were slightly dried and could stick to the bottle wall, and then the flask was slowly and slightly turned around to enable the tissue pieces to become soaked in the culture medium for continuous static cultivation. The flask was observed under a phase contrast microscope every day. Cells were found stretched out around the tissue pieces two hours later, and more cells surroundings the tissue species were observed one day later. Cells got confluence in most areas three to four days later. The tissue pieces were removed by slightly pipetted with pipette, and one milliliter of 0.1% trypsinase was added. Cells were observed under an inverted microscope, some of the cells were found to turn round about 3 to 5 minutes later, and the areas were marked on the flask. The trypsinase solution was abandoned, 5–10 mL of culture medium containing 0.1mmol/L of Brdu was added and repeatedly blew and hit at the marked position. 5 × 105/mL cells were obtained and they were passaged to the culture plates for cultivation. Pyknotic monolayer would be formed for most cases 3 to 4 days later, which can be used for further experiments. The cultured smooth muscle cells were identified by α-actin immunohistochemisty.
2.3. Experimental grouping
For both the non-drug intervention group (only treated with 10−7 mol/L TNFα, group A) and the drug intervention group (combined treatment with 10−7 mol/L TNFα and 10−5 mol/L Rosuvastatin, group B); the individual data was determined instantly, 12 hours, 24 hours and 48 hours after the treatments. Each experiment was repeated at least five times.
2.4. Detection of cell proliferation activity with MTT method
Cells were seeded in a 96-well plate at a concentration of l × 104/mL, 200 µL per well. Twenty four hours later, DMEM- F12 medium without serum was added into each well and inoculated for another 24 hours to synchronized cell cycle into G0 phase. Cells were cultured in a 5% CO2 incubator at a constant temperature of 37°C for 12 hours, 24 hours and 48 hours. Twenty microlitre of 5 g/L MTT was added to each well and continuously cultured for four hours. The supernatant of each well was inhaled and discarded, 150 µL of DMSO was added and agitated for 10 minutes. The absorbance (A) values were determined at the wavelength of 490 nm on the microplate reader. DMEM-F12 culture medium was used as blank control in the normal control group and for zero adjustment of the microplate reader. The rate of cell proliferation was calculated as follows: cell proliferation rate = (A value in test group – A value in normal control group)/A value in normal control group × 100%.
2.5. Detection of Rho kinase gene expression with RT-PCR method
Forty eight hours later, the primarily cultured 5 × 106 smooth muscle cells were switched to serum-free culture media and continuously cultured for 24 hours. Smooth muscle cells were stimulated with 10−7 mol/L TNFα or 10−7 mol/L TNFα and 10−5 mol/L Rosuvastatin, and collected at different time points. RNA was extracted by guanidinium isothiocyanate- phenol-chloroform one-step method, and the following reagents were added according to the instruction of MMLV one-step method RT-PCR kit (Shanghai Sangon Biological Engineering Technology and Service Co., Ltd.), primer, RNA sample, Taq enzyme, and sterile double distilled water until the final volume of 50 µL. The reverse transcription condition was 38°C, 30 minutes; PCR condition was 94°C initial denaturation for two minutes, 35 cycles of 94°C denature 15 seconds, 60°C anneal smooth muscle cell 30 seconds, 72°C extend 10 minutes, and then 72°C extended for 10 minutes. Each PCR reaction was repeated at least 3 times, the primer was synthesized by Shanghai Sangon Biological Engineering Technology and Service Co., Ltd., Rho kinase (512 bp), upper stream primer: 5′-GAG CAA CTA TGA TGT GCC TGA AAA AT-3′, downstream primer: 5′-GAT GTC GTT TGA TTT CTT CTA C-3′; β-actin (387 bp): upper stream primer: 5′-CGT AAA GAC CTC TAT GCC AA-3′, downstream primer: 5′-AGC CAT GCC AAA TGT GTC AT-3′. Two percent agarose gel electrophoresis was performed with 5 µL of PCR product and analyzed by gel imaging system, the gray scale ratio of Rho kinase mRNA and β-actin mRNA was calculated, and each group was detected for ten times.
2.6. Detection of PCNA protein expression in smooth muscle cells with Western blot
Smooth muscle cells were cultured and stimulated as mentioned above, the proteins were extracted at different time points and quantified. Forty micrograms of total proteins were separated by polyacrylamide denaturant gel, transferred to nitrocellulose membrane, blocked, incubated with primary antibody, then species specific HRP-conjugated secondary antibody, and finally developed with x-ray films with chemoluminescence reagent. The results were analyzed in gel imaging system; each group was repeated 5 times.
2.7. Statistical analysis
F test was used for all data; t test was used for inter- group comparison. The data were expressed as mean ± SD, and P < 0.05 indicated statistical significance.
3. Results
3.1. Comparison of cell proliferation activity between two groups
Non-drug intervention group: the TNFα (10−7 mol/L) stimulation significantly increased the proliferation rate of smooth muscle cell, and the cell proliferation rates at different time points were 0.053 ± 0.014 at 0 hour; 0.132 ± 0.006 at 12 hour; 0.167 ± 0.004 at 24 hour; and 0.234 ± 0.067 at 48 hour; respectively (24 hour vs. 0 hour, P < 0.01; 48 hour vs. 0 hour, P < 0.01). Drug intervention group: Rosuvastatin (10−5 mol/L) intervention inhibited the TNFα-induced smooth muscle cell proliferation, and the cell proliferation rates at different time points were, 0 hour: 0.052 ± 0.018; 12 hour: 0.055 ± 0.005; 24 hour: 0.059 ± 0.006; 48 hour: 0.054 ± 0.004, respectively. No significance was found among the individual time points (P > 0.05), as show in Table 1.
Table 1. The different results of cell proliferation activity, the ratio of the gene expression of Rho kinase and the protein expresion of PCNA in Non-drug intervention group (Group A) and drug intervention group (Group B).
| 0 hour |
12 hour |
24 hour |
48 hour |
|||||
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | |
| Cell proliferation activity | 0.053 ± 0.014 | 0.052 ± 0.018 | 0.132 ± 0.006 | 0.055 ± 0.005 | 0.167 ± 0.004* | 0.059 ± 0.006 | 0.234 ± 0.067* | 0.054 ± 0.004 |
| Rho kinase | 0.490 ± 0.002 | 0.549 ± 0.048 | 0.506 ± 0.001 | 0.555 ± 0.055 | 0.632 ± 0.001* | 0.559 ± 0.055 | 1.053 ± 0.007* | 0.540 ± 0.104 |
| PCNA | 5.100 ± 0.870 | 5.060 ± 0.713 | 11.760 ± 4.280* | 4.800 ± 0.515 | 25.300 ± 9.702* | 5.080 ± 0.801 | 50.200 ± 14.991* | 4.920 ± 0.249 |
Data are expressed as mean ± SD. *P < 0.01 compared with 0 hour. PCNA: proliferating cell nuclear antigen.
3.2. Comparison of Rho kinase expression between two groups
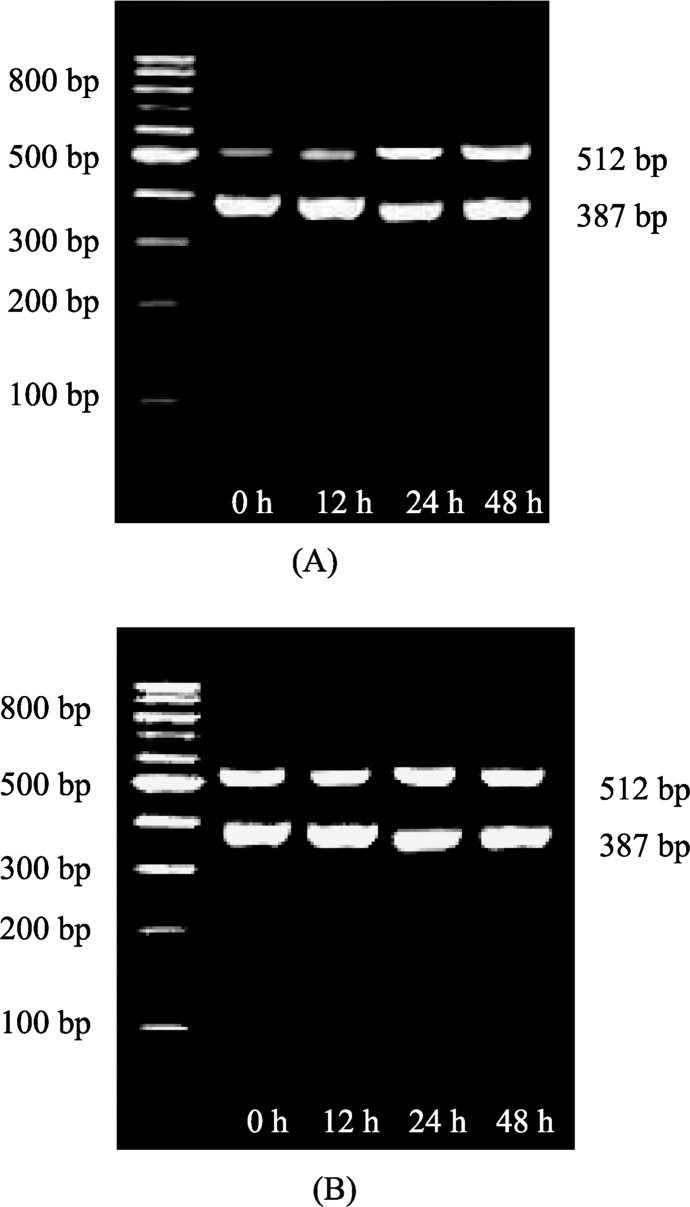
Non-drug intervention group: TNFα administration significantly increased the expression of Rho kinase gene in smooth muscle cell (Figure 1A). The ratios of gray scale at different time points were 0 hour: 0.490 ± 0.002; 12 hour: 0.506 ± 0.001; 24 hour: 0.632 ± 0.001; 48 hour: 1.053 ± 0.007 (24 hour vs. 0 hour, P < 0.01; 48 hour vs. 0 hour, P < 0.01), respectively. Drug intervention group: there was no significantly increase of Rho kinase gene expression after Rosuvastatin intervention (Figure 1B). The ratios of gray scale at different time points were, 0 hour: 0.549 ± 0.048; 12 hour: 0.555 ± 0.055; 24 hour: 0.559 ± 0.055; 48 hour: 0.540 ± 0.104, respectively. No significance was found among the individual time points (P > 0.05), as show in Table 1.
Figure 1. The mRNA expression of Rho kinase of smooth muscle cells treated with (A) TNFα, and (B) TNFα + Rosuvastatin (RT-PCR).
3.3. Comparison of PCNA protein expression between two groups
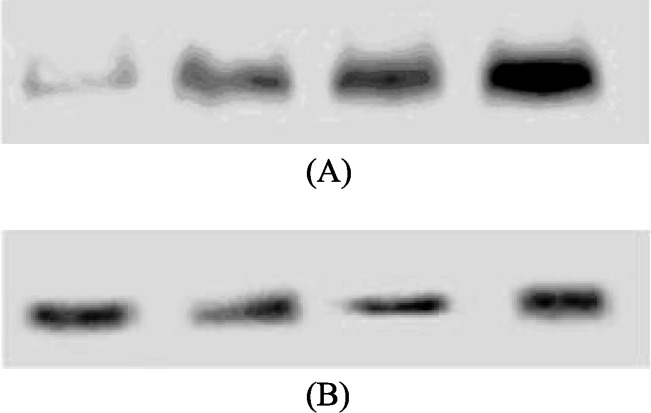
Non-drug intervention group: the administration of TNFα (1.0 × 10−7 mol/L) significantly increased the PCNA expression (Figure 2A). The ratios of gray scale at different time points were 0 hour: 5.100 ± 0.870; 12 hour: 11.760 ± 4.280; 24 hour: 25.300 ± 9.702; 48 hour: 50.200 ± 14.991 (12 hour vs. 0 hour, P < 0.01; 24 hour vs. 0 hour, P < 0.01; 48 hour vs. 0 hour, P < 0.01), respectively. Drug intervention group: Rosuvastatin (1.0 × 10−5 mol/L) intervention inhibited PCNA expression, and the ratios of gray scale at different time points were 0 hour: 5.060 ± 0.713; 12 hour: 4.800 ± 0.515; 24 hour: 5.080 ± 0.801; 48 hour: 4.920 ± 0.249, respectively (Figure 2B). There was no significance among the individual time points (P > 0.05), as show in Table 1.
Figure 2. The expression of proliferating cell nuclear antigen protein of smooth muscle cells treated with (A) TNFα and (B) TNFα + Rosuvastatin.
4. Discussion
One of the pathological characteristics of atherosclerosis is the excessive proliferation and migration of vascular smooth muscle cells, which results in intima thickening.[2] The molecular regulation effect of TNFα in smooth muscle cell is yet still unclear.[3] Recent studies indicated that Rho kinase pathway was associated to smooth muscle proliferation, and some other studies also suggested Rosuvastatin can significantly inhibit the growth of smooth muscle cell.[4] Therefore, this study investigated the effect of direct activation of Rho kinase by TNFα on vascular smooth muscle proliferation and also the intervention effect of Rosuvastatin.
Rho/Rho kinase is widely distributed in mammalian cells, and serves as a signal polypeptide with the functions of information transduction and molecular switch.[5] Rho kinase is serine/threonine protein kinase, and the MBS of MLCPh is the main action substrate.[6] MLCPh binds to phosphorylated myosin light chain (MLC) through MBS, and dephosphorylates the protein which leads to the relaxation of constricted smooth muscle. Rho kinase can phosphorylate MBS which results in the inactivation of MLCPh and, accordingly, the vascular SMC cannot finish the constriction status. Moreover, Rho kinase can also directly phosphorylate MLC to make the smooth muscle contracted. Therefore, Rho kinase can enhance the phosphorylation level of MLC through double pathways to promote the contraction of SMC, including direct phosphorylation of MLC and induction of MLCPh inactivation.[7],[8] It is closely related to the occurrence and the development of main cardiovascular diseases, such as myocardial ischemia, atherosclerosis, etc. [9],[10]
By using the MTT method, we found that the stimulation of TNFα in cultured smooth muscle cells can promote the cell proliferation in a time-dependent manner. The mechanism of TNFα induced smooth muscle cell proliferation was further analyzed, and we found that it was related to activation of Rho kinase pathway. TNFα can significantly promote the PCNA expression in smooth muscle cells, and the effect became more significant with the prolongation of treatment. It was possible that TNFα directly promoted Rho kinase gene expression, and therefore triggers the proliferation relevant gene and protein expression in smooth muscle cells. PCNA is a nucleoprotein required for DNA synthesis of eukaryotic cells,[11] which can well reflect the cell proliferation activity. In this study, TNFα showed a significant promotion effect on PCNA in a time-dependent manner.
In addition, this study confirmed that the smooth muscle cell proliferation, Rho kinase gene expression, and PCNA protein expression induced by TNFα were impaired by Rosuvastatin intervention, which indicated Rosuvastatin can inhibit the activation of TNFα mediated Rho kinase pathway and reduce the TNFα induced smooth muscle cell proliferation. Our results suggested Rosuvastatin had a well-defined intervention effect on smooth muscle cell proliferation, and inhibition of TNFα-Rho kinase pathway may be the internal mechanism for Rosuvastatin's effect of anti- atherosclerosis.
This study confirmed that Rosuvastatin can play an interventional role in smooth muscle proliferation through inhibiting TNFα-Rho kinase pathway. This finding further indicates that TNFα plays an important role in smooth muscle proliferation, and also provides a new target for the treatment of smooth muscle proliferation and arteriosclerosis with Rosuvastatin in clinical practice.
Acknowledgments
The Project is sponsored by Liaoning Province Natural Science Fund (20092073, 20102220), “Liaoning BaiQianWan Talents Program” and Shenyang Science Planning Program (1091138-9-00).
References
- 1.Wu YG, Chun Liang. Advance on inflammation and atherosclerosis research. 1st Edition. People's Military District Press; Beijing, China: 2007. pp. 58–84. [Google Scholar]
- 2.Duffy D, Rader DJ. Emerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transport. Circulation. 2006;113:1140–1150. doi: 10.1161/CIRCULATIONAHA.105.593855. [DOI] [PubMed] [Google Scholar]
- 3.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li XL, Guan RJ, Xu QH, et al. Effects of rosuvastatin on monocrotaline-induced pulmonary artery hypertension in rats. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:247–253. [PubMed] [Google Scholar]
- 5.Makrodouli E, Oikonomou E, Koc M, et al. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Mol Cancer. 2011;10:118. doi: 10.1186/1476-4598-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu B, Hua J, Zhang YW, et al. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse Ovaries. PLoS One. 2011;6:E16046. doi: 10.1371/journal.pone.0016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S, Del Re DP, Xiang SY, et al. Revisited and revised: Is RhoA always a villain in cardiac pathophysiology? J Cardiovasc Transl Res. 2010;3:330–343. doi: 10.1007/s12265-010-9192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Qu C, Zeng DY. The expression of Rho/Rho kinase of cardiac muscle in heart failure rats caused by pressure overload and the intervention of fasudil. Chin J Cardio. 2005;33:36–39. [PubMed] [Google Scholar]
- 10.Zhang M, Wang WG, Zeng DY. Effects of different dose fasudil on [Ca2+]i of cardiac myocytes of pressure over1oad heart failure rat model. Chin J New Drugs Clin Rem. 2006;25:33–36. [Google Scholar]
- 11.Yu Y, Cai JP, Tu B, et al. Proliferating cell nuclear antigen is protected from degradation by forming a complex with MutT Homolog2. J Biol Chem. 2009;284:19310–19320. doi: 10.1074/jbc.M109.015289. [DOI] [PMC free article] [PubMed] [Google Scholar]