Abstract
Objective
To investigate the role of transforming growth factor-β1 (TGF-β1), Smad2/3 and Smad7 expressions in carotid artery remodeling in renovascular hypertensive rats, and also the therapeutic effect of Enalapril and Amlodipine.
Methods
The renovascular hypertensive rat (RHR) models with “two-kidney and one-clip” were established, including model group (n = 6), sham-operated group (n = 6), Enalapril group (10 mg/kg per day, n = 6), Amlodipine group (5 mg/kg per day, n = 6) and combination group (Amlodipine 2.5 mg/kg per day + Enalapril 5mg/kg per day, n = 6). The medication were continuous administrated for six weeks. Carotid artery morphological and structural changes in the media were observed by HE staining, Masson staining and immuno histochemical staining. Media thickness (MT), MT and lumen diameter ratio (MT/LD), and the expression levels of media α-smooth muscle actin (α-actin), proliferating cell nuclear antigen (PCNA), TGF-β1, phosphorylated Smad2/3 (p-Smad2/3) and Smad7 in carotid arteries were measured.
Results
The media of carotid arteries in RHR model group was significantly thickened, the volume of smooth muscle cell was increased, and the array was in disorder; MT, MT/LD, the proliferation index of smooth muscle cell and collagen fiber area percentage of carotid arteries in the model group were significantly higher than those in the sham-operated group (P < 0.01). Compared to sham-operated group, the model group had significantly higher expressions of TGF-β1 and p-Smad2/3 (P < 0.05) and lower Smad7 expression. Both Enalapril and Amlodipine improved smooth muscle hypertrophy and collagen deposition, reduced RHR carotid MT, MT/LD, proliferation index of smooth muscle cell, collagen fiber area percentage and the expressions of TGF-β1 and p-Smad2/3 (P < 0.05), increased Smad7 expression (P < 0.05). Moreover, the combination treatment of Enalapril and Amlodipine had significantly better effects than single Amlodipine group (P < 0.05), but not single Enalapril group.
Conclusions
TGF-β1/Smads pathway may participate in the mechanism of carotid artery remodeling in RHR; the role of Amlodipine and Enalapril in inversing carotid artery remodeling may be related to the change of TGF-β1/Smads pathway, the combination treatment of Amlodipine and Enalapril had better effects than single administration of Amlodipine.
Keywords: Hypertension, Vascular remodeling, Transforming growth factor-β1, p-Smad2/3, Smad7, Enalapril, Amlodipine
1. Introduction
Vascular remodeling is one of the important pathophysiological basis of primary hypertension. The clinical treatment are not only limited to the blood pressure level control, but also inverse vascular remodeling. Recent studies have shown that transforming growth factor–β (TGF-β) plays an important role in hypertension-induced vascular remodeling. However, the role of TGF-β1/Smads signal pathway in carotid artery remodeling of renovascular hypertension is still unclear. In this study, by taking renovascular hypertensive rat (RHR) as model and adopting 2 × 2 factor analysis, the expressions of TGF-β1, downstream signal protein Smad2/3 and Smd7, as well as the effects of single and combination treatment of Amlodipine and Enalapril in this signal pathway were investigated; so as to explore the possible mechanism of Amlodipine and Enalapril in improving hypertension-induced vascular remodeling and the action effects.
2. Methods
2.1. Experimental animals, drugs and reagents
Male Sprague-Dawley (SD) rats aged from 8 to 12 weeks, with a body weight of 180 g to 220 g, were provided by the Animal Center of Third Military Medical University, Certificate No.: SCXK – (military) 2002008. Enalapril and Irbesartan were kindly provided by Yangtze River Pharmaceutical Cooperation. Rat anti α-smooth muscle actin (α-actin) monoclonal antibody and rat anti proliferating cell nuclear antigen (PCNA) monoclonal antibody were purchased from Wuhan Boster Biotech Co., Ltd. Rabbit anti-TGF-β1 polyclonal antibody, goat anti-phosphorylated-Smad2/3 (p-Smad2/3) polyclonal antibody and rat anti-Smad7 monoclonal antibody were purchased from Santa Cruz Inc. Universal immunohistochemisty detection kit and concentrated DAB coloration kit were purchased from Zhongshan Goldbridge Biotechnology Co., Ltd.
2.2. “Two-kidney and one-clip” model establishment, grouping and administration
Rats were anesthesia with 20% chloral hydrate (ip, 0.18 mg/kg), the left renal arteries were separated, and the silver brain clip was clipped at the crotch of left renal artery and aorta. In the sham-operated group, only the left renal artery was separated, the silver brain clip was not settled. The caudal arterial pressure of rats were measured by non-invasive pressure measurement device every two weeks after the operation, the rats with a caudal systolic arterial pressure of above 125 mmHg and more than 20 mmHg higher than preoperative blood pressure were considered as hypertensive rat.[1] The survival rats with successful model establishment were randomized into four groups: RHR model group, Amlodipine group, Enalapril group, Amlodipine + Enalapril combination treatment group, n = 6 in each group. In each group, the corresponding drugs were intragastrically administered once a day for 6 weeks: Amlodipine group (5 mg/kg per day), Enalapril group (10 mg/kg per day), combination treatment group (Amlodipine 2.5 mg/kg per day + Enalapril 5 mg/kg per day, n = 6). The model group and sham-operated group were intragastrically administered with same volume of distilled water.
The rats in each group were sacrificed at the 12th week after operation, the carotid arteries were separated, and clipped at about 0.5 cm away from the carotid bifurcation, rinsed with cold physiological saline, fixed in 4% paraformaldehyde, and then dehydrated, embedded, and made into paraffin slice with a thickness of 4 µm.
2.3. Observation indexes
2.3.1. HE staining
Tissue slices in each group were stained by HE staining, visualized and captured with a Leica DM2500 optical microscope. Image analysis was performed by BI2000 image analysis system, media thickness and lumen diameter were measured and the media thickness and lumen diameter ratio (MT/LD) was calculated.
2.3.2. Masson staining
Masson staining was performed for tissue slices in each group. Pictures captured in Leica DM2500 optical microscope was analyzed by Image-pro plus image analysis software, 6 visual fields were randomly selected for measuring the collagen fiber area percentage of carotid arteries.
2.3.3. Immunohistochemical staining
Immunohistochemical staining of α-actin, PCNA, TGF-β1, p-Smad2/3 and Smad7 was performed by Elivison two-step method; a brown staining indicates the positive expression. Leica DM2500 optical microscope was used to collect pictures. PCNA was expressed in nucleus, the percentage of positive nucleus in total media cellular score was calculated, i.e., the proliferation index of smooth muscle cell of carotid arteries media. TGF-β1 was expressed in cytosol, Smad7 was expressed in cytosol, nucleus and nucleus surroundings, the mean optical density (MOD) was measured by Image-pro plus image analysis software. P-Smad2/3 was expressed in nucleus, and the ratio of p-Smad2/3 positive nucleus in total media cell score was calculated.
2.4. Statistical analysis
SPSS13.0 software was used to perform analysis. All data were quantified approximate normal distribution data, expressed as mean ± SD. Factorial analysis of variance was used to perform difference comparison, for the interaction data, one drug was further fixed to compare the effect of the other drug.
3. Results
3.1. Comparison of carotid arteries morphology in different groups
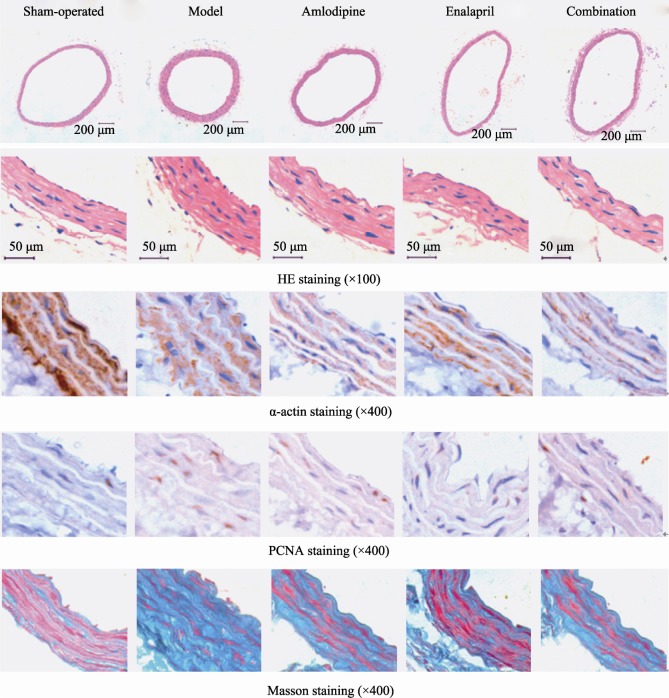
As shown in Figure 1 and Table 1 & 2, HE staining results indicated that the tunica media of carotid arteries in model group was significantly thickened, carotid arteries MT and MT/LD were increased compared to sham-operated group (MT: t = -6.588, P < 0.01; MT/LD: t = -7.063, P < 0.01). Factor analysis indicated there is correlation between Amlodipine and Enalapril (MT: F = 7.176, P = 0.016; MT/LD: F = 6.715, P = 0.02). With single administration of Enalapril, the carotid arteries MT and MT/LD were lower than model group (MT: t = 4.934, P < 0.01; MT/LD: t = 4.779, P < 0.01), but higher than Amlodipine + Enalapril group (MT: t = 3.552, P < 0.01; MT/LD: t = 2.699, P = 0.027); With single administration of Amlodipine, the carotid arteries MT and MT/LD were decreased compared to model group (MT: t = 3.246, P = 0.012; MT/LD: t = 3.843, P = 0.012), and there was no statistical difference compare to Amlodipine + Enalapril group (MT: t = 0.501, P = 0.63; MT/LD: t = 0.656, P = 0.53).
Figure 1. HE staining, α-actin staining, PCNA staining and Masson staining of rats' carotid arteries in sham-operated group, RHR model group, Amlodipine group, Enalapril group and Amlodipine + Enalapril treatment group, respectively.
PCNA: proliferating cell nuclear antigen; RHR: renovascular hypertensive rat.
Table 1. MT, LD and MT/LD comparisons of rats in each group (n = 6, mean ± SD).
| Grouping | MT(µm) | LD (µm ) | MT/LD(%) |
| Sham-operated | 52.15 ± 7.18 | 525.72 ± 18.58 | 9.94 ± 1.517 |
| RHR Model | 91.28 ± 11.17a | 441.38 ± 14.13 | 20.75 ± 3.068a |
| Amlodipine | 72.57 ± 6.42b | 478.10 ± 27.45 | 15.18 ± 1.051b |
| Enalapril | 63.23 ± 6.07c | 481.76 ± 62.37 | 13.26 ± 1.700c |
| Combination | 61.79 ± 2.20d,f | 481.76 ± 62.38 | 12.50 ± 1.959d,e |
aP < 0.01 vs. sham-operated group; bP < 0.05, cP < 0.01 vs. model group; dP > 0.05 vs. Enalapril group; eP < 0.05, fP < 0.01 vs. Amlodipine group. MT: media thickness; LD: lumen diameter; RHR: renovascular hypertensive rat.
Table 2. Collagen fiber area percentage and smooth muscle cell proliferation index of tunica media of carotid arteries of rats in each group (n = 6, mean ± SD).
| Grouping | Collagen fiber area percentage (%) | Smooth muscle cell proliferation index (%) |
| Sham-operated | 15.35 ± 4.446 | 1.84 ± 0.519 |
| Model | 67.53 ± 7.684a | 35.31 ± 12.969a |
| Amlodipine | 48.62 ± 10.175b | 4.79 ± 1.184c |
| Enalapril | 30.69 ± 9.140c | 3.73 ± 0.423c |
| Combination | 32.23 ± 12.008d,e | 3.15 ± 0.543d,e |
aP < 0.01 vs. sham-operated group; bP < 0.05, cP < 0.01, vs. model group; dP > 0.05 vs. Enalapril group; eP < 0.05 vs. Amlodipine group. MT: media thickness; LD: lumen diameter; RHR: renovascular hypertensive rat.
α-actin immunohistochemisty staining showed positive signal in the cytosol smooth muscle cell of carotid artery tunica media, the carotid artery tunica media in model group was significantly thickened, the volume of smooth muscle cell was increased, and arranged in disorder. The treatment of Amlodipine and Enalapril improved artery smooth muscle hypertrophy and disordered arrangement.
As shown by PCNA immunohistochemisty staining, the smooth muscle cell proliferation of carotid artery tunica media in model group was increased compared to sham- operated group (t = -5.766, P < 0.01). Factor analysis showed correlation between Amlodipine and Enalapril (F = 26.364, P < 0.01). With single administration of Enalapril, the proliferation index of smooth muscle cell was decreased compared to model group (t = 5.442, P < 0.01), but higher than Amlodipine + Enalapril group (t = 2.805, P = 0.023); With single administration of Amlodipine, the proliferation index of smooth muscle cell was decreased compared to model group (t = 5.24, P < 0.01), and no statistical difference was found comparing with Amlodipine + Enalapril group (t = 1.858, P = 0.1).
The cytosol of smooth muscle cell of carotid artery tunica media was stained with red by Masson staining method, and the collagen fiber was blue. Results showed that the collagen fiber content of carotid artery tunica media was significantly increased in model group, and the collagen fiber area percentage were also higher than the sham-operated group (t = -13.13, P < 0.01). Factor analysis indicated there was correlation between Amlodipine and Enalapril (F = 5.361, P = 0.034). With single administration of Enalapril, the collagen fiber area percentage of carotid artery tunica media was decreased compared to model group (t = 6.9, P < 0.01), but higher than Amlodipine + Enalapril group (t = 2.328, P = 0.048); With single administration of Amlodipine, the collagen fiber area percentage of carotid artery tunica media was decreased compared to model group (t = 3.317, P = 0.011), and there was no significant difference compare to Amlodipine + Enalapril group (t = -0.229, P = 0.825).
3.2. Comparisons of TGF-β1, p-Smad2/3 and Smad7 expressions in tunica media of caroid arteris in different groups
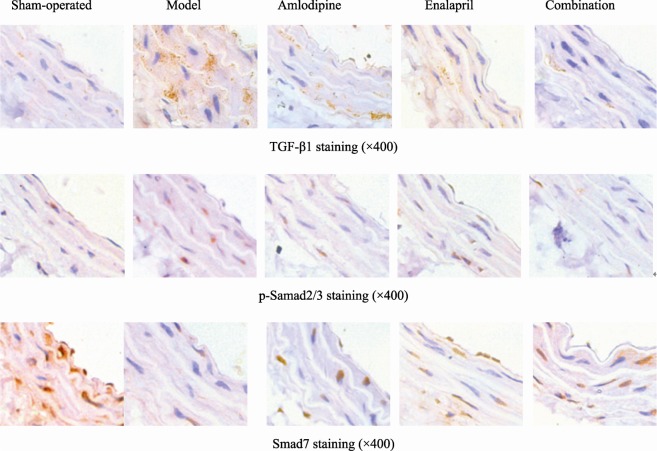
As shown in Figure 2 and Table 3, the expressions of TGF-β1 and p-Smad2/3 in carotid artery tunica media in model group was higher than sham-operated group (TGF-β1: t = -3.305, P = 0.03; p-Smad2/3: t = -10.85, P < 0.01). Factor analysis indicated the correlation between Amlodipine and Enalapril (TGF-β1: F = 5.651, P = 0.03; p-Smad2/3: F = 8.241, P = 0.011). With single administration of Enalapril, the expressions of TGF-β1 and p-Smad2/3 were decreased compared to model group (TGF-β1: t = 2.97, P = 0.018; p-Smad2/3: t = 8.444, P < 0.01), but higher than Amlodipine + Enalapril group (TGF-β1: t = 4.278, P = 0.01; p-Smad2/3: t = 3.734, P < 0.01); With single administration of Amlodipine, the expressions of TGF-β1 and p-Smad2/3 were lower compared to model group (TGF-β1: t = 2.467, P = 0.039; p-Smad2/3: t = 6.025, P < 0.01), and there was no significant difference compare to combination group (TGF-β1: t = 0.114, P = 0.613; p-Smad2/3: t = 1.871, P = 0.098).
Figure 2. Immunohistochemisty staining of TGF-β1, p-Smad2/3 and Smad7 in carotid arteries of rats in sham-operated group, RHR model group, Amlodipine group, Enalapril group and Amlodipine + Enalapril treatment group, respectively.
TGF-β: transforming growth factor-β1; RHR: renovascular hypertensive rat.
Table 3. Comparisons of TGF-β1, p-Smad2/3 and Smad7 expressions in tunica media of carotid arteries of rats in each group (mean ± SD, n = 6).
| Grouping | TGF-β1 (MOD) | p-Smad2/3 | Smad7 (MOD) |
| Sham-operated | 0.0001 ± 0.00006 | 0.0658 ± 0.02117 | 0.0121 ± 0.00302 |
| Model | 0.0040 ± 0.00259a | 0.3721 ± 0.05947b | 0.0013 ± 0.00116b |
| Amlodipine | 0.0011 ± 0.00034c | 0.1987 ± 0.02294d | 0.0035 ± 0.00035c |
| Enalapril | 0.0005 ± 0.00033c | 0.1400 ± 0.01556d | 0.0045 ± 0.00161d |
| Combination | 0.0004 ± 0.00008e,f | 0.0846 ± 0.06438e,g | 0.0046 ± 0.00058e,g |
TGF-β1: transforming growth factor -β1, p-Smad2/3: phosphorylated Smad2/3. aP < 0.05, bP < 0.01 vs. sham-operated group; cP < 0.05, dP < 0.01 vs. model group; eP > 0.05 vs. Enalapril group; fP < 0.05, gP < 0.01 vs. Amlodipine group. MOD: mean optical density; TGF-β: transforming growth factor-β1; RHR: renovascular hypertensive rat.
The Smad7 expression in tunica media of carotid arteries in model group was decreased compared to sham-operarted group (t = 7.477, P < 0.01). Factor analysis indicated the correlation between Amlodipine and Enalapril (F = 4.776, P = 0.044). With single administration of Enalapril, the Smad7 expression was higher than model group (t = -3.607, P < 0.01), but lower than Amlodipine + Enalapril group (t = -3.824, P < 0.01); with single administration of Amlodipine, the Smad7 expression was higher than model group (t = -4.008, P = 0.012), and there was no statistical difference compare to combination group (t = -0.163, P = 0.877).
4. Discussion
TGF-β is a kind of multifunctional cytokine that synthesized by variety of tissue cells, it not only can regulate the cell cycle but also affect the proliferation, differentiation, adhesion, migration and apoptosis of cells. TGF-β includes 3 subtypes, i.e., TGF-β1, TGF-β2 and TGF-β3. TGF-β1 is expressed in vascular endothelial cells, vascular smooth muscle cells (VSMC), myofibroblast and hematopoietic cells. Smad protein is a downstream target of TGF-β1 signaling pathway, which serves as a media molecule to transmit extracellular TGF signal into nucleus.[2] Smads protein family member Smad2 and Smad3 can combine with TGF receptor and generate phosphorylation, and then form heterogenic oligomerization complex with Smad4, and transferred into nucleus to initiate target gene of TGF-β1. Smad7 can inhibit the signal transduction process by interfering the phosphorylation of Smad2 and Smad3.[3] Recent studies confirmed that TGF-β1 can directly stimulate the synthesis of extracellular matrix, reduce degradation, and simultaneously induce VSMC hyperplasia and hypertrophy, stimulate fibroblast hyperplasia and the transformation of fibroblast to myofibroblast, resulting in vascular remodeling at the hypertensive vascular injury site.[4]–[8] Agrotis et al.[9] reported that TGF-β1 can reinforce epidermal growth factor, platelet growth factor, fibroblast growth factor to promote VSMC proliferation of spontaneously hypertensive rats (SHR); while regarding WKY rats, TGFβl can inhibit the function of these growth factors on VSMC proliferation. Cheng et al.[10] found that the transfection of antisense Smad3 adenovirus vector can block TGF-β signal transduction and therefore inhibit the proliferation of vascular smooth muscle cells. After stimulating with TGF-β1, Smad2 in vitro cultured rat VSMC was phosphorylated. After the transfection of Smad7 viral vector, the TGF-β1-mediated Smad2 phosphorylation was inhibited in dose-dependent manner. In this study, the tunica media of carotid arteries in RHR model group was thickened, the volume of smooth muscle cell was increased and the arrangement was in disorder, the collagen fiber content was significantly increased, the MT, MT/LD and collagen fiber area percentage of carotid artery tunica media were all increased compared to sham-operated group (P < 0.01), which was similar to the SHR carotid artery remodeling reported by Chen et al.[11] However, in this study, the proliferation index of smooth muscle cell of RHR carotid tunica media was increased, suggesting that the smooth muscle cell of RHR carotid tunica media was not only with hypertrophy, but also accompanied with hyperplasia; the TGF-β1 expression in RHR carotid tunica media was increased compared to sham-operated group, accompanied with increased p-Smad2/3 and reduced Smad7 expression, which suggesting that RHR carotid vascular remodeling was relevant to TGF-β1/ Smads signal pathway expression.
The activities of arterial smooth muscle cell membrane sodium pump and calcium pump were inhibited in intracellular hyalomitomes, which increased the free calcium ion concentration,[12],[13] and further affected arterial smooth muscle proliferation and hypertrophy, resulting in arterial remodeling.[13]–[15] Calcium channel blockers (CCB) can reduce intracellular calcium level, release pressure and improve vascular remodeling by blocking the extracellular calcium influx, but there is rare publication discussing about whether the effect of improving vascular remodeling is related to the expressions of TGF-β1 and Smads proteins. In this study, Amlodipine, a selective blocking cell membrane L-type CCB, was chose as therapeutic drug, and the results indicated that the carotid tunica media remodeling after therapy was improved, the expressions of TGF-β1 and p-Smad2/3 were decreased, Smad7 expression was increased, suggesting that Amlodipine can play a role in anti-RHR carotid artery remodeling by reducing TGF-β1 expression. The mechanism is still unclear, some studies reported that TGF-β1 can reduce the expression of myocardial cells L-type calcium channels Cav3.1 (α1D).[16] On the other hand, researchers observed that Amlodipine can result in reduced local AngII level in plasma and kidney of SHR,[17] and speculated that down regulation of TGF-β1 expression by Amlodipine may be related to the fact that TGF-β1 can reduce vascular calcium channel expression and Amlodipine can also reduce AngII level in renovascular hypertensive rats.
Studies have confirmed that AngII was an important factor that leading to vascular remodeling and its effect was partially dependent on TGF-β. Wang et al.[18] stimulated vascular smooth muscle cells by AngII for 24 hours, and found that the activation of Smad2/3 signal transduction pathway was dependent on TGF-β as the effect can be blocked by TGF-β neutralization antibody. Angiotensin converting enzyme inhibitor (ACEI) improve large-artery compliance, preventing aortic collagen accumulation independently of blood pressure changes.[19] ACEI can reduce AngII level in plasma and tissue by inhibiting the activity of ACE. In this study, inversion of carotid tunica media and decreased TGF-β1 were observed in Enalapril (a kind of ACEI drug) intervention group, the expression of downstream protein Smad2/3 was correspondingly decreased, the Smad7 expression was increased, indicating that the improvement of RHR carotid vascular remodeling by Enalapril was also related to the down regulation of TGF-β1 expression.
In the aspect of combination treatment, the results of this experiment suggested that the combination of Amlodipine and Enalapril was more effective in improving RHR carotid vascular remodeling than single administration of Amlodipine, and simultaneously reduced the expressions of TGF-β1 and p-Smad2/3, increased Smad7 expression, while there was no statistical difference between combination treatment and single administration of Enalapril.
Taken together, TGF-β1 participated in RHR carotid artery remodeling, displayed as the up-regulation of TGF-β1 and its downstream protein p-Smad2/3 and down-regulation of Smad7. Amlodipine and Enalapril can both reverse RHR carotid artery remodeling, which may be related to TGF-β1/ Smads signal pathway regulation, and the combination treatment of these two drugs was more effective than single administration of Amlodipine.
Acknowledgments
The authors give their sincere thanks to Prof. Zhou Yan for his kind help in experimental data analysis. This work were funded by Social Development Research Project in Guizhou Province [QKH SY No: (2011)3047] and fund of Affiliated Hospital of Zunyi Medical College.
References
- 1.Sun JF. Animal Experiment Methodology. Beijing: People's Medical Publishing House; 2001. p. 564. [Google Scholar]
- 2.Schultz JJ, Witt SA, Glascock BJ, et al. TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goumans MJ, Liu Z, Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 4.O'laghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-β1. Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 5.Bray P, Agrotis A, Bobik A, et al. Transforming growth factor-β and receptor tyrosine-activating growth factors negatively regulate collagen genes in smooth muscle of hypertension rats. Hypertension. 1998;31:986–994. doi: 10.1161/01.hyp.31.4.986. [DOI] [PubMed] [Google Scholar]
- 6.Laviades C, Varo N, Diez J, et al. Transforming growth factor-β in hypertensive with cardiorenal damage. Hypertension. 2000;36:517–525. doi: 10.1161/01.hyp.36.4.517. [DOI] [PubMed] [Google Scholar]
- 7.Cheng ZH, Sheng J. Effect of transforming growth factor β1 on vascular smooth muscle cell behavior. Foreign Med Sci (Geriatrics) 2008;29:252–256. [Google Scholar]
- 8.Xing JY, Wang SJ, Lu FH. Progress in the relationship on transforming growth factor-β1 and hypertension and hypertensive target organ injury. Res Prog. 2010;17:11–14. [Google Scholar]
- 9.Agrotis J, Saltis A, Bobik A, et al. Transforming growth factor-beta1 gene activation and growth of smooth muscle from hypertensive rats. Hypertension. 1994;23:593–599. doi: 10.1161/01.hyp.23.5.593. [DOI] [PubMed] [Google Scholar]
- 10.Cheng ZH, Cai WW, Lu P, et al. Effects of signal transduction interruption of transforming growth factor β by anti- Smad3 on proliferation of vascular smooth muscle cells. Journal of Shanghai Jiaotong University (Medical) 2009;29:935–938. [Google Scholar]
- 11.Chen H, Hong HS, Jiang Q. Effects of telmisartan on carotid artery remodeling in rats with spontaneous hypertension. J Pract Med. 2007;23:3665–3667. [Google Scholar]
- 12.Guo YH, Shang QH, Wu Q, et al. Effects of Lisinopril on the Activities and mRNA Expression of Ion Pumps in Aortic Smooth Muscle Cells from Spontaneously Hypertensive Rats. Chin J Arteriosclerosis. 2010;18:441–444. [Google Scholar]
- 13.Shang QH, Liu XP, Fang N, et al. Calcium overload and adenosine triphosphatase in artery smooth muscle cells in spontaneously hypertensive rats. Chin J Hypertens. 2006;5:379–384. [Google Scholar]
- 14.Su CJ. Pathologic mechanism and treatment strategy of hypertensive vascular remodeling. Guangdong Med J. 1999;20:458–486. [Google Scholar]
- 15.Zheng HH, Li YH, Luo DS, et al. Effects of tetramethylphrazine on calcinerin and proliferating cell nuclear antigen gene expression in the proliferation of vascular smooth muscle cells. Chin J Phathophysiology. 2006;22:154–154. [Google Scholar]
- 16.Avila T, Arturo Andrade A, Felix R. Transforming growth factor-β1 and bone morphogenetic protein-2 downregulate CaV3.1 channel expression in mouse C2C12 myoblasts. J Cell Physiol. 2006;209:448–456. doi: 10.1002/jcp.20743. [DOI] [PubMed] [Google Scholar]
- 17.Ning ZQ, Mou SC, Wang XQ, et al. Effects of Amlodipine and enalapril on renal mitogen-activated protein kinases, angiotonin II and endothelin from spontaneously hypertensive rats. Medical Journal of Chinese People's Liberation Army. 2000;25:296–298. [Google Scholar]
- 18.Wang W, Huang XR, Canlas E, et al. Essential role of Smad3 in angiotensin II-induced vascular Fibrosis. Cire Res. 2006;98:1032–1039. doi: 10.1161/01.RES.0000218782.52610.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng K, Hildreth CM, Avolio AP, et al. Angiotensin-converting enzyme inhibitor limits pulse-wave velocity and aortic calcification in a rat model of cystic renal disease. Am J Physiol Renal Physiol. 2011;301:959–966. doi: 10.1152/ajprenal.00393.2011. [DOI] [PubMed] [Google Scholar]