Abstract
BACKGROUND
Low-level mutations in clinical tumor samples often reside below mutation detection limits, thus leading to false negatives that may impact clinical diagnosis and patient management. COLD-PCR is a technology that magnifies unknown mutations during PCR, thus enabling downstream mutation detection. However, a practical difficulty in applying COLD-PCR has been the requirement for strict control of the denaturation temperature for a given sequence, to within ±0.3°C. This precludes simultaneous mutation enrichment in sequences of substantially different melting-temperature (Tm) and limits the technique to a single sequence at a time. We present a temperature-tolerant (TT-COLD-PCR) approach that reduces this obstacle.
METHODS
Thermo-cycling programs featuring a gradual increase of the denaturation temperature during COLD-PCR are described. This approach enables enrichment of mutations when the cycling achieves the appropriate critical denaturation temperature of each DNA amplicon that is being amplified. Validation is provided for KRAS and TP53 exons 6–9 using dilutions of mutated DNA, clinical cancer samples and plasma-circulating DNA.
RESULTS
A single thermocycling program with a denaturation-temperature window of 2.5–3.0°C enriches mutations in all DNA amplicons simultaneously, despite their different Tms. Mutation enrichments of 6–9-fold were obtained using TT-full-COLD-PCR. Higher mutation enrichments were obtained for the other two forms of COLD-PCR, fast-COLD-PCR and ice-COLD-PCR.
CONCLUSIONS
Low-level mutations in diverse amplicons with different Tm can be mutation-enriched via TT-COLD-PCR provided that their Tms fall within the denaturation-temperature window applied during amplification. This approach enables simultaneous enrichment of mutations in several amplicons, and increases significantly the versatility of COLD-PCR.
Keywords: COLD-PCR, mutation detection, mutation enrichment, low-abundance mutations, cancer
INTRODUCTION
In the area of personalized medicine for cancer, technologies that detect mutations and sequence DNA variants play an important role, as these alterations are related to therapeutic success, prognosis and detection of residual cancer following surgical resection. These technologies often face challenges in detecting minority alleles in clinical specimens, which can be masked by a high background of wild type DNA. To date, there are different methodologies that address this issue and are capable of identifying known and/or unknown low-abundance mutations with high sensitivity (1), including the recently described co-amplification at lower denaturation temperature, COLD-PCR (2–5). This novel form of PCR, developed by our group, enriches preferentially mutated DNA sequences over wild type by using a lower temperature denaturation step during the cycling protocol (critical denaturation temperature, Tc). The Tc is below the melting temperature (Tm) of the wild type amplicon and permits the selective denaturation of the mutated sequences in every round of PCR, while wild type sequences remain substantially double-stranded, thus amplifying less. Mutated DNA enrichment by COLD-PCR facilitates subsequent mutation detection. This method is applicable to both known or unknown mutation enrichment and can be integrated with downstream detection methodologies such as Sanger sequencing, high resolution melting (HRM) analysis, real-time PCR and next generation sequencing, among others (6–9). COLD-PCR has been used successfully by diverse groups for mutation detection in different targets such as KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) (2, 7, 10–15), GNAS1 (Guanine nucleotide binding protein-G protein- alpha stimulating activity polypeptide 1) (16), IDH1 (isocitrate dehydrogenase 1-NADP+) (17), BRAF (18), EGFR (11) fetal paternally inherited mutations of plasma (19, 20), peach floral genes (21).
The main challenge in the application of COLD-PCR is to establish precisely the correct Tc since a variation in critical temperature as small as ±0.2–0.3°C could make it difficult to obtain satisfactory mutation enrichment. A consequence of this requirement is the inability to amplify diverse sequences simultaneously with a single thermo-cycling program because in general each amplicon has a different Tc. As such, it would be highly desirable to enable enrichment of mutations in multiple sequences in existing PCR thermo-cyclers or in emerging, high-throughput PCR platforms (22, 23) prior to amplicon sequencing.
This manuscript presents a temperature-tolerant COLD-PCR approach (TT-COLD-PCR) that relaxes the stringency on the denaturation temperature and simultaneously amplifies different targets with diverse Tc using a single cycling program. We combine TT-COLD-PCR with high resolution melting analysis (HRM) (24, 25) providing a rapid, simple, and sensitive screening method to confirm mutation enrichment prior to Sanger sequencing or other complimentary assays.
MATERIAL AND METHODS
DNA and Tumor samples
Human male-genomic DNA, was obtained from Promega, Inc. (catalog number G1471, Madison, WI, USA), and used as a wild type control. Genomic DNA from cell lines containing TP53 mutations (Supplementary Table 1) was purchased from ATCC (Manassas, VA, USA). A549 lung carcinoma cell line with defined KRAS mutation (p.G12S, c.34G>A) was cultured in F12 medium supplemented with 10% FBS at 37°C with 5% CO2. Genomic DNA from A549 cells was extracted using DNeasy Blood & Tissue Kit (Qiagen Inc., Valencia, CA, USA). DNA from clinical colorectal (n=4), glioblastoma (n=5), and lung (n=1) cancer specimens, previously reported to have mutations at different abundances using COLD-PCR (8, 26) and other independent methods, were used to further validate the technology (listed in Supplementary Table 2). Additionally, we also tested plasma-circulating DNA (obtained from a radiation therapy patient following informed consent and IRB approval) that demonstrated a TP53 mutation. The plasma-circulating DNA was pre-amplified by ligation-mediated PCR as previously described (27), along with plasma-circulating DNA from a healthy donor, Supplementary Table 2.
Pre-amplification using conventional PCR from genomic DNA
A conventional-PCR pre-amplification step from genomic DNA was applied prior to testing temperature-tolerant (TT) approaches in a nested PCR format, for the three forms of COLD-PCR, (fast, full or ice-COLD-PCR). Primers for all forms of PCR were synthesized by Integrated DNA Technologies (IDT Inc., Coralville, IA, USA) and are listed in Supplementary Table 3. PCR was performed in 25 µL final volume for all PCR reactions, with the Phusion™ high fidelity DNA polymerase system (New England Biolabs Inc., Ipswich, MA).
Prior to performing nested TT-fast and TT-ice-COLD-PCR, DNA pre-amplification was conducted by amplifying individual amplicons from genomic DNA to build enough template for subsequent COLD-PCR reactions. Prior to performing TT-full-COLD-PCR, genomic DNA pre-amplification of all TP53 exons simultaneously was conducted using a single-tube multiplex-PCR approach as reported previously (26), with minor modifications. Reactions were carried out in 15 µl reaction volume; 1× Phusion™ high-fidelity (HF) buffer, 0.2 mM each dNTPs, 0.1 µM (each) primer and 0.6 U Phusion™ DNA polymerase. The resulting PCR product was then diluted 200-fold and used as a template for TT-full-COLD-PCR reactions which were performed in separate tubes for each target amplicon. For comparison to COLD-PCR formats, a nested conventional PCR was always performed in parallel with TT-COLD-PCR.
Critical denaturation temperature (Tc) window for temperature-tolerant-full-COLD-PCR
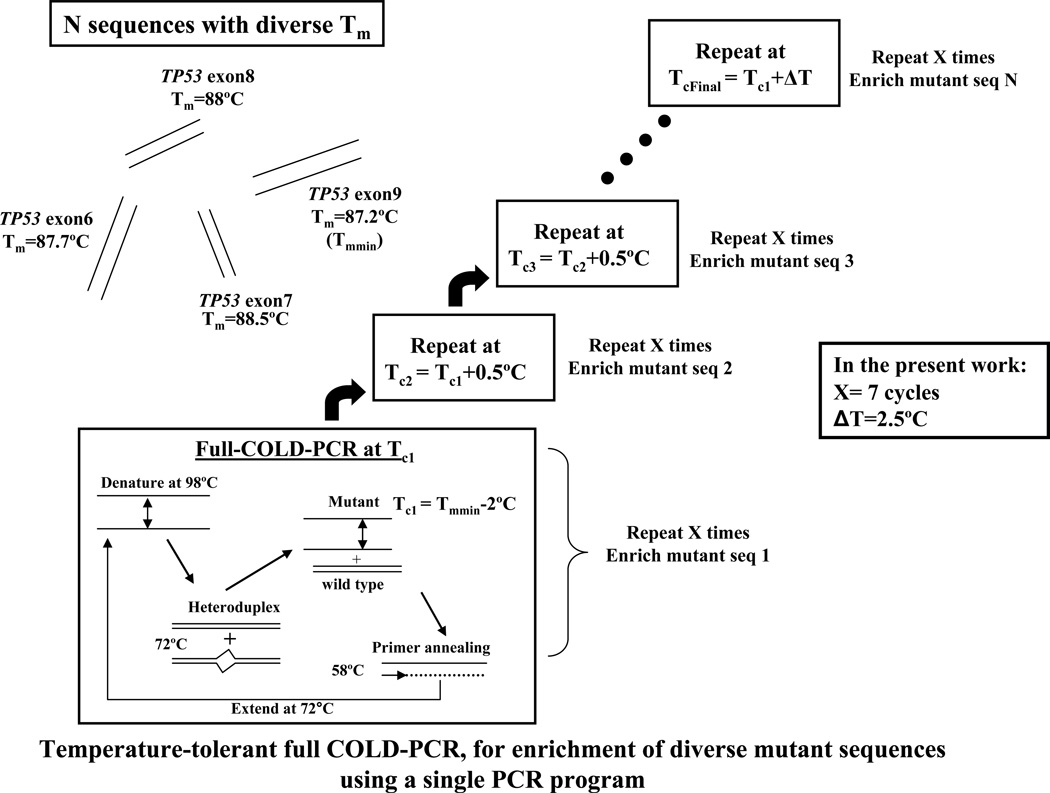
The critical denaturation temperature (Tc) is the temperature(s) at which the majority of the major alleles are inhibited while the minor alleles are selectively amplified. While in previous COLD-PCR reports a discrete temperature for a given amplicon was required for robust enrichment, we present here an alternative that eases the requirement for stringency on the denaturation temperature while also offers satisfactory enrichment. The aim is to increase incrementally the denaturation temperature such that the mutant alleles are preferentially denatured and amplified before the WT alleles, even if they are present in amplicons with diverse Tc. To achieve this, melting temperatures (Tm) of the wild type target DNA sequences are experimentally determined using a conventional PCR in the presence of 1X LCGreen dye (Idaho Technologies Inc., Salt Lake City, UT), in order to define the initial Tc. Alternatively, the amplicon Tm can simply be predicted using bioinformatic tools (28). The initial Tc applied for TT-full-COLD-PCR is one or more degrees (°C) below the minimum Tm (Tmmin) among all target amplicons tested, so that when the temperature increases gradually it first encounters the Tc of the amplicon with lowest Tm. Thus, for TT-full-COLD-PCR we adopted (initial Tc = Tmmin −2 °C). The TT-full-COLD-PCR protocol applied here consists of successive steps, with seven cycles each step, where the Tc is incremented at 0.5°C intervals between successive steps, Figure 1.
Figure 1.
Temperature tolerant-full-COLD PCR (TT-full-COLD PCR) principle. Each TT-full-COLD-PCR step is repeated X times (X=7 herein), following which a new step at a critical denaturation temperature (Tc) incremented by 0.5°C is applied. In the present work, there are a total of 5 steps corresponding to a combined Tc window of 2.5°C.
Temperature tolerant-full-COLD-PCR reactions
PCR thermocycling conditions are described in Supplementary Tables 4 and 6. Reaction conditions were the same for all cases except that for KRAS the concentration of primers was 0.075 µM each.
Temperature tolerant-fast-COLD-PCR reactions
PCR products from the genomic DNA pre-amplification were diluted 500–1000-fold and used as a template for nested TT-fast-COLD-PCR reactions. Nested TT-fast-COLD-PCR was performed on an Eppendorf Mastercycler EP machine (Eppendorf Inc., Haupage, NY) using the “plate” block setting and “safe” temperature mode to ensure better well-to-well uniformity and to prevent over-heating above the target temperatures (Supplementary Tables 4 and 5). A single TT-fast-COLD-PCR program was used to amplify TP53 exons 6–9. For TT-fast-COLD-PCR we adopted (initial Tc = Tmmin −1.3 °C, Supplementary Table 5. Melting temperatures for exon 7 and 8 amplicons were effectively reduced by adding 4% and 2% DMSO, so that amplicon Tm falls within the TT-fast-COLD-PCR temperature window applied in this investigation.
Temperature-tolerant-ice-COLD PCR reactions
Amplification via ice-COLD-PCR inhibits preferentially the amplification of one DNA strand in WT DNA (5), while the second strand remains un-inhibited. In view of this, and to inhibit excessive amplification of the second strand, the nested amplification reaction was performed in an asymmetric-PCR mode, wherein the reverse primer was present at five times the concentration of the forward primer. Conventional PCR was performed in asymmetric mode as well, for comparative analysis. Reagent conditions for the conventional PCR were the same as reported for genomic amplification except for primer concentration, with 0.2 µM forward primer and 1.0 µM reverse primer, and 1 µl of the diluted (1:5000-fold) amplicon from the genomic DNA pre-amplification. Reagent conditions for the ice-COLD-PCR reactions were the same as in the conventional assays but with the additional incorporation of a reference sequence (RS) at a 25 nM concentration. The RS used was the same as previously described (5). For TT-ice-COLD-PCR, a range of Tc values below the melting temperature of the amplicon tested were examined prior to determining the optimal initial Tc of 83.7°C that generates maximum enrichment for the temperature window applied (Supplementary Tables 7 and 8).
High resolution melt (HRM) analysis and Sanger sequencing
TT-full-COLD-PCR and conventional amplicons were directly analyzed using HRM on a Light Scanner HR96 system (Idaho Technologies, Inc.) prior to sequencing. For Sanger sequencing, PCR products were digested by Exonuclease I/shrimp alkaline phosphatase and sequenced at Eton Bioscience, Inc. (Cambridge, MA, USA). Due to the short amplicon length, a 30-T tail was added to the 5' end of the sequencing primer as reported previously (29). Chromatograms were then analyzed with BioEdit v7.1.3 (Ibis Biosciences, Abbott Laboratories, USA). Mutant allele abundances relative to wild type were estimated from the peak height values of the Sanger sequencing chromatograms as previously reported (5). However, as Sanger sequencing is not quantitatively accurate, these estimations may not be precise.
RESULTS
TT-full-COLD-PCR versus conventional PCR or traditional full-COLD-PCR
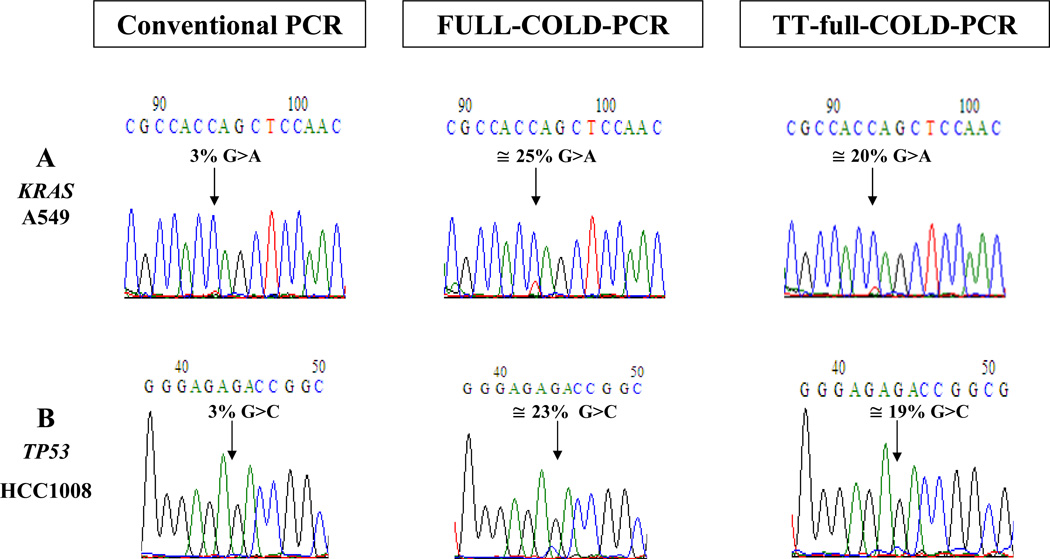
We evaluated the mutation enrichment obtained via TT-full-COLD-PCR compared to other forms of PCR. For this purpose, we amplified serial dilutions of DNA from mutation-containing cell lines for KRAS (c.34G>A mutation, p.G12S) and TP53 exon 8 (c.841G>C mutation, p. D281H) by conventional PCR, TT-full-COLD-PCR and traditional, single-Tc full-COLD PCR prior to Sanger-sequencing, Figure 2. In both cases, a 3% mutant content was not visible in the chromatogram of the conventional amplification, but was visible and enriched to ~19–25% mutant content for KRAS and TP53 by traditional full-COLD PCR and TT-full-COLD-PCR. The mutation enrichment, ~6–8-fold, was evident for both types of COLD-PCR. The enrichment obtained from TT-full-COLD-PCR was somewhat less than that of single-Tc COLD-PCR.
Figure 2.
TT-full-COLD PCR evaluation for a 3% mutation abundance following dilution of the KRAS codon 12 G>A mutation (A) and TP53 exon 8 G>C mutation (B) into wild-type DNA. TT-full-COLD PCR is compared to conventional PCR and to traditional full-COLD PCR performed at a single Tc, and followed by Sanger sequencing.
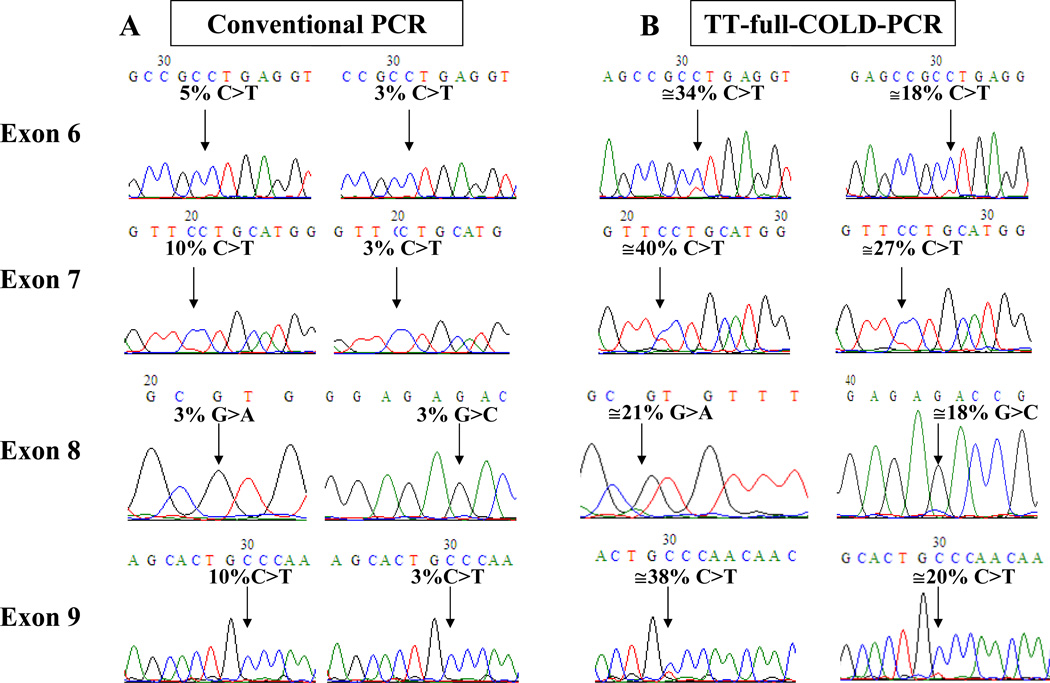
TT-full-COLD-PCR: a single thermocycling program for diverse TP53 amplicons
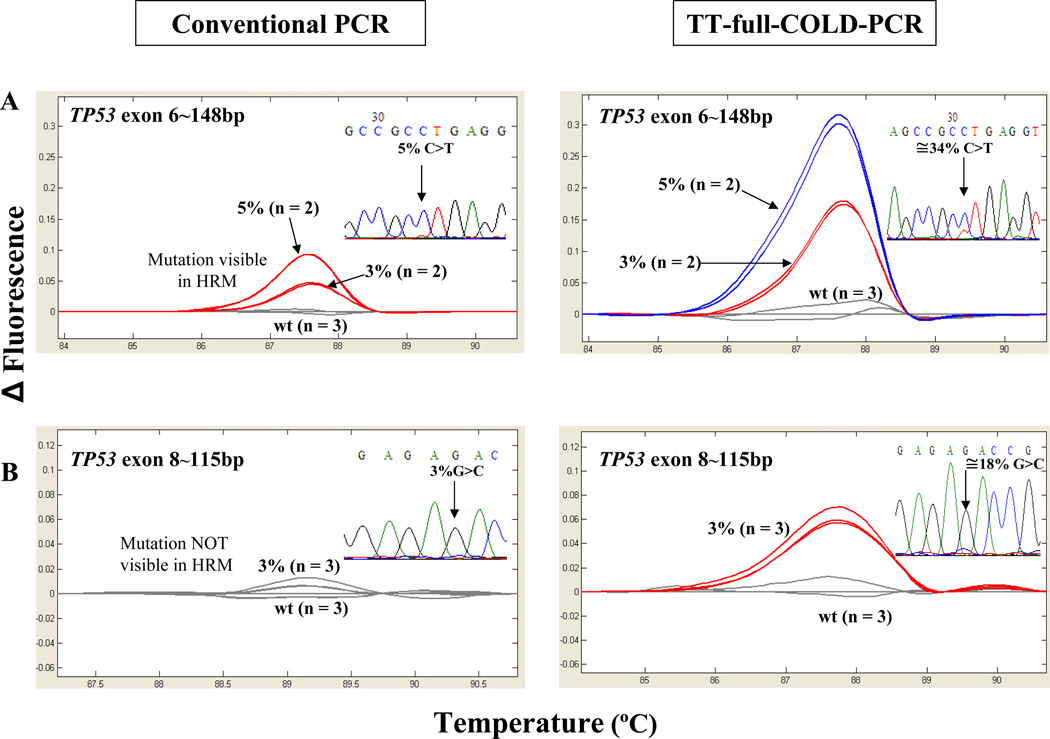
Using the scheme of Figure 1, universal thermo-cycling conditions spanning a window of denaturation temperatures from 85.2°C–87.7 °C were applied to simultaneously amplify and enrich for mutations in four different regions of TP53 gene, exons 6–9. The protocol applied was capable of enriching mutation abundances by approximately 4–9-fold in all examined exons simultaneously, based on the results from Sanger sequencing, Figure 3, frames A and B. TT-full- COLD-PCR amplicons were also examined by HRM prior to sequencing and compared to HRM from the conventional-PCR protocol. Figure 4 shows an example of two of the amplicons tested via HRM; 148 bp of TP53 exon 6 (c.668C>T, Tm-reducing mutation) and 115 bp of TP53 exon 8 (c.841G>C, Tm-equivalent mutation) are presented. The exon 6 mutation is evident via HRM but cannot be confirmed by sequencing, while the exon 8 mutation cannot be confirmed by either HRM or sequencing following conventional PCR. In contrast, following TT-full-COLD-PCR the 3% mutation abundance is detectable via both HRM and sequencing. Additional HRM testing of DNA from cell lines with Tm-reducing mutations in TP53 exon 8 (c.818G>A) and exon 9 (c.925C>T) are presented in Supplementary Figure 1. Based on the magnitude of the HRM curve differentiation from wild-type DNA, mutation detection sensitivity was notably higher in all TT-full-COLD-PCR amplicons compared to that obtained with conventional PCR amplicons. Finally, DNA from a cell line with a Tm-increasing mutation (PFSK-1, c.823T>G, p.C275G) for TP53 exon 8 was diluted into wild type DNA to a final 4% or 2% mutation abundance and tested with TT-full-COLD-PCR or conventional amplification strategies. The data indicate that a mutation enrichment of ~7–14-fold was obtained, based on the sequencing chromatograms (Supplementary Figure 2).
Figure 3.
Application of a single TT-full-COLD PCR thermocycling program to enrich mutations in exons 6–9 in TP53, using known serial dilutions into wild-type DNA. Sanger sequencing chromatograms depict results from conventional PCR (A) and TT-full-COLD-PCR amplification (B). The left and right columns in frame A correspond to the left and right columns in frame B.
Figure 4.
High resolution melt analysis (HRM) and sequencing after conventional PCR and TT-full- COLD PCR for TP53 exon 6 (C>T mutation) and exon 8 (G>C mutation), using known serial dilutions into wild-type DNA. Following conventional PCR, the 3% exon 6 mutation is detected via HRM but not via sequencing; the 3% exon 8 mutation is detected via neither method. Following TT-full-COLD these mutations are detected with both HRM and sequencing, although in exon8 the enrichment is marginally discriminated from background.
TT-full COLD-PCR-HRM -testing of clinical samples with low-level mutations
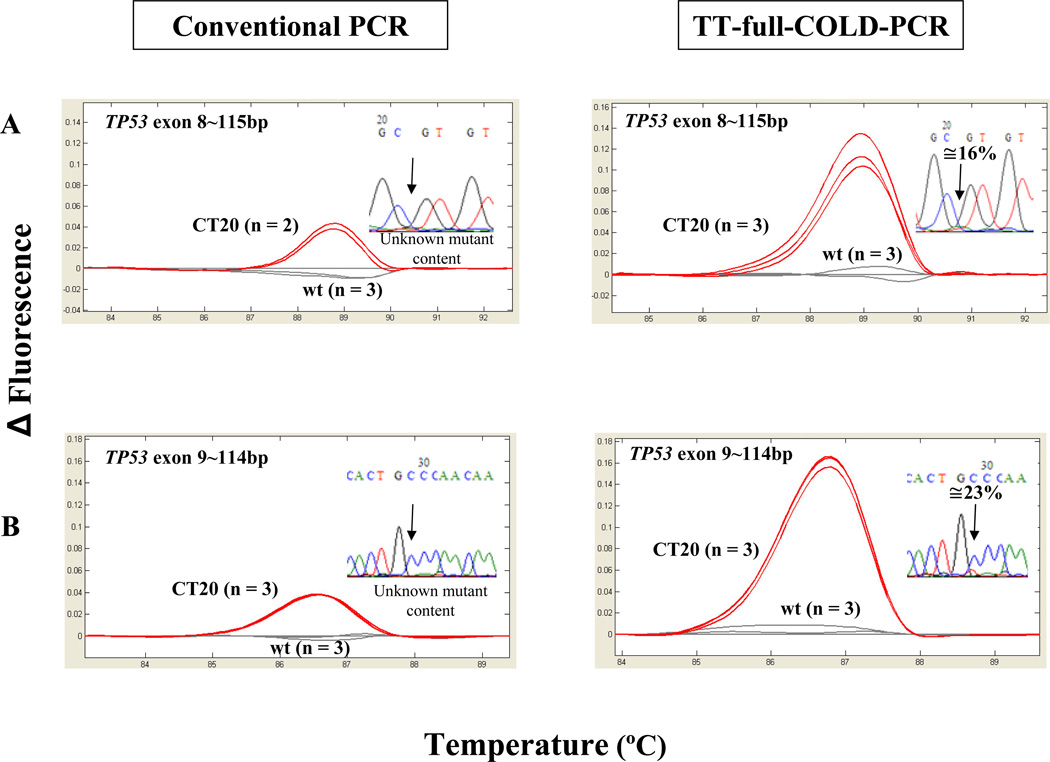
To evaluate further TT-full-COLD-PCR, we screened DNA from previously tested lung, colorectal and glioblastoma tumor specimens (26) containing high-, mid-, and low-abundance mutations (Supplementary Table 2). Clinical samples were amplified by TT-full-COLD-PCR and conventional PCR, evaluated by HRM and validated by Sanger sequencing (Figure 5 and Supplementary Figure 3). Figure 5 depicts HRM profiles from a clinical sample, CT20, containing low-level mutations in both TP53 exons 8 and 9 following amplification with TT-full-COLD-PCR or alternatively via conventional-PCR. Mutations are evident via HRM in both cases, but are only visible in the TT-full-COLD-PCR Sanger chromatograms – although the mutation in the exon8 chromatogram is close to the limit of detection. Clinical samples containing mid to high-abundance mutations were also examined by HRM and sequencing. These mutations were detectable via both conventional and TT-full-COLD-PCR (not shown).
Figure 5.
Analysis of a colorectal cancer sample CT20 with low abundance mutations that were detectable via HRM but not detectable via Sanger sequencing following conventional PCR. The mutations in TP53 exons 8 and 9 are detectable with both methods following TT-full-COLD.
TT-fast-COLD-PCR: testing of serial dilutions and plasma-circulating DNA
Fast-COLD-PCR is useful for enriching the subset of mutations that decrease the melting temperature of the amplicon, G:C>A:T or G:C>T:A, which comprise the majority of somatic mutations in human cancer (7). Fast-COLD-PCR is a rapid protocol that generally results in higher enrichments than full-COLD-PCR. Thus, temperature tolerant thermo-cycling was also adapted for fast-COLD-PCR format, for KRAS and TP53 exons 6–9 mutations, in an Eppendorf thermo-cycler. Cycling conditions for KRAS spanned a temperature window from 82.7°C to 85.3°C, and for TP53 a temperature window from 85.5°C to 88.5°C (Supplementary Tables 4 and 5).
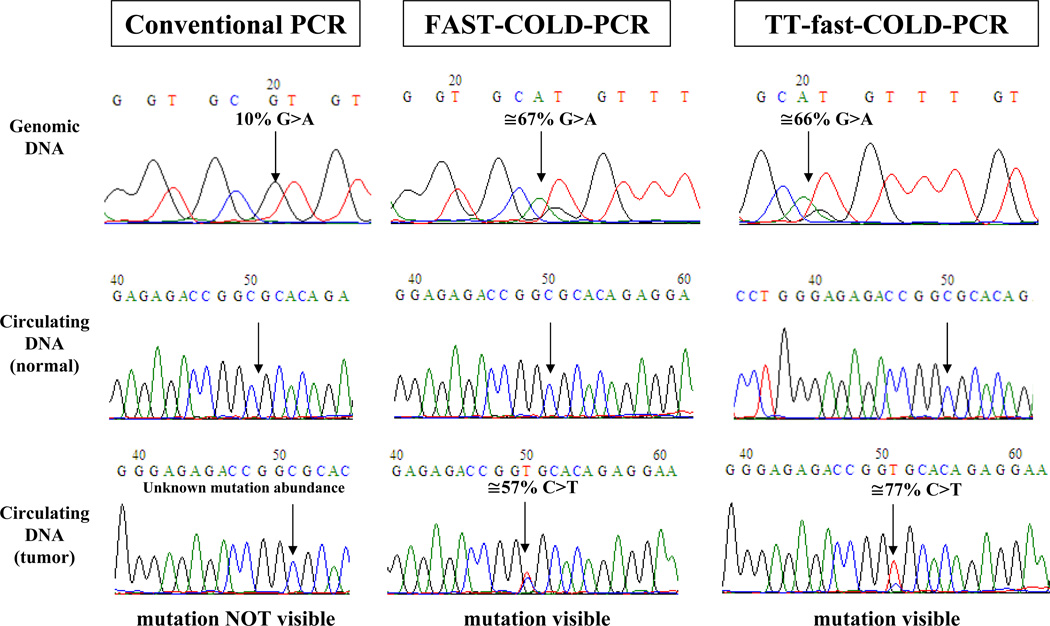
We tested for KRAS mutation enrichment, by diluting A549 cell line (mutant in c.34G>A, p.G12S) into wild type DNA, amplifying by TT-fast-COLD-PCR or conventional PCR and applying Sanger sequencing. Supplementary Figure 4 demonstrates that, following TT-fast-COLD-PCR 3% and 10% mutation abundances increased to ~50% and 87% respectively. Similarly, we applied a single TT-fast-COLD-PCR protocol for simultaneous enrichment of DNA with TP53 mutations in exon 6 (SNU-182, p.S215I), exon 7 (HCC2157, p.R248W), exon 8 (SW480, p.R273H) and exon 9 (SW480, p.P309S). Subsequent Sanger sequencing revealed mutation enrichment in all exons, Supplementary Figure 5. To also demonstrate the application of TT-fast-COLD-PCR in the enrichment of low-level mutations in a clinical sample, we tested plasma-circulating DNA from a radiation therapy patient where a low-level mutation in TP53 exon 8 (c.847C>T, p.R823C) had been identified via traditional fast-COLD-PCR. Plasma-circulating DNA isolated from a normal volunteer was tested in parallel. The pair of plasma-circulating DNA was pre-amplified via LM-PCR using the method we described (27). To exclude the possibility of a polymerase-introduced error during the LM-PCR pre-amplification step, the mutation was also validated using fast-COLD-PCR directly from unamplified DNA isolated from plasma (not shown). Sanger sequencing of the conventional-PCR product revealed no mutation (Figure 6). In contrast, TT-fast-COLD-PCR product revealed an enrichment of the mutation to an abundance of ~77% in the patient circulating DNA sample (Figure 6).
Figure 6.
TT-fast-COLD-PCR applied to circulating DNA: Circulating tumor DNA isolated from plasma of a radiation therapy patient with low-abundance mutation (c.847C>T, R283C) was analyzed using conventional PCR, fast-COLD PCR at a single Tc and TT-fast-COLD-PCR. The mutational enrichment was compared to that obtained for a 10% mutation abundanceand also with circulating DNA obtained from a normal volunteer.
TT-ice-COLD-PCR
We also adapted the temperature-tolerant COLD-PCR to ice-COLD-PCR and compared the mutation enrichment obtained via conventional-PCR, TT-ice-COLD-PCR and ice-COLD-PCR at a single Tc. We used DNA from a cell line with a Tm-equivalent mutation (HCC1008, p.841G>C, p.D281H) diluted into wild type DNA to a final 3% mutation abundance. Following evaluation of a series of critical denaturation temperatures (Supplementary Table 8) it was shown that optimal mutation enrichment was observed when the initial Tc was set ~5°C below the melting temperature of the amplicon: RS90 duplex, which was subsequently increased by 0.5°C every 7 cycles, to a final Tc 2.5°C below the melting temperature of the amplicon: RS90 duplex. The Sanger sequencing results demonstrated that both TT-ice-COLD-PCR and ice-COLD-PCR at a single Tc resulted in similar mutation enrichment, i.e. 3% mutation abundance was enriched to ~36%, Supplementary Figure 6.
DISCUSSION
COLD-PCR (3, 4), is inexpensive and relatively easy to implement because the primary required modification relative to conventional PCR lies in the cycling protocol and the denaturation temperature. However the requirement for strict reproducibility and precision (±0.3°C) in controlling the critical denaturation temperature can introduce significant variations between different laboratories, equipment or experiments. Factors such as PCR buffer composition and lot may generate differences in amplicon Tm and Tc. Additionally, differences in temperature ramping speed or in actual temperature between wells in a single PCR machine, may also influence the mutation enrichment obtained during individual COLD-PCR reactions. To address such issues, in preliminary work, Candau et al (30) reported that gradual increase of denaturation temperature within a fine interval during fast-COLD-PCR ('touch-up' COLD-PCR) removes the well-to-well temperature inconsistencies that introduce variations to the mutation enrichment. This concept was expanded herein, to encompass a broader window of temperatures spanning 2.5–3°C, in order to achieve simultaneous mutation enrichment in diverse amplicons of substantially different Tm using a single thermo-cycling program. Temperature-tolerant conditions can be defined for all forms of COLD-PCR, full, fast and ice-COLD-PCR. Using TT-full-COLD-PCR we demonstrated simultaneous mutation enrichment in four TP53 exons whose Tm spanned a 2.5°C temperature window using a single thermo-cycling program. It can be thus reasonably expected that mutations in any other amplicon whose Tm falls within the same temperature window would also be enriched; thereby enabling diverse PCR sequences to be enriched on a conventional PCR machine with a single thermo-cycling program in a high throughput format. Furthermore, amplicons with a Tm exceeding the applied temperature window can also be included via amplification in presence of DMSO or other modifiers that lower the Tm. It is possible to broaden the temperature window to encompass a wider range of Tm or to apply a finer 'step' between temperatures, i.e. 0.3°C instead of 0.5°C. This may boost further the capabilities of TT-COLD-PCR however caution is required to avoid primer dimers resulting from increasing PCR cycles Additional considerations include polymerase inactivation resulting from repeated heating, or reaching the maximum number of PCR cycles allowed by the thermo-cycler software. In practice, a balance among all these factors should be accomplished to define optimal conditions for TT-COLD-PCR.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Innovative Molecular Analysis Technologies Program of the NCI, grants CA-111994 and CA-151164 (GMM). The contents of this manuscript do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. Angela Brisci participated in this study as partial fulfillment of her PhD in Molecular Medicine, Program in Predictive and Preventive Medicine, San Raffaele University, Milan, Italy.
Footnotes
Publisher's Disclaimer: This is an un-copyedited authored manuscript copyrighted by the American Association for Clinical Chemistry (AACC). This may not be duplicated or reproduced, other than for personal use or within the rule of 'Fair Use of Copyrighted Materials' (section 107, Title 17, U.S. Code) without permission of the copyright owner, AACC. The AACC disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The final publisher-authenticated version of the article will be made available at http://www.clinchem.org 12 months after its publication in Clinical Chemistry.”
CONFLICT OF INTEREST STATEMENT: COLD-PCR is a technology owned by the Dana-Farber Cancer Institute and has been commercially licensed. The manuscript has not been published previously and is not being considered concurrently by another journal. All authors and acknowledged contributors have read and approved the manuscript.
REFERENCES
- 1.Milbury CA, Li J, Makrigiorgos GM. Pcr-based methods for the enrichment of minority alleles and mutations. Clin Chem. 2009;55:632–640. doi: 10.1373/clinchem.2008.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Wang L, Mamon H, Kulke MH, Berbeco R, Makrigiorgos GM. Replacing pcr with cold-pcr enriches variant DNA sequences and redefines the sensitivity of genetic testing. Nat Med. 2008;14:579–584. doi: 10.1038/nm1708. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Milbury CA, Li C, Makrigiorgos GM. Two-round coamplification at lower denaturation temperature-pcr (cold-pcr)-based sanger sequencing identifies a novel spectrum of low-level mutations in lung adenocarcinoma. Hum Mutat. 2009;30:1583–1590. doi: 10.1002/humu.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Makrigiorgos GM. Cold-pcr: A new platform for highly improved mutation detection in cancer and genetic testing. Biochem Soc Trans. 2009;37:427–432. doi: 10.1042/BST0370427. [DOI] [PubMed] [Google Scholar]
- 5.Milbury CA, Li J, Makrigiorgos GM. Ice-cold-pcr enables rapid amplification and robust enrichment for low-abundance unknown DNA mutations. Nucleic Acids Res. 2011;39:e2. doi: 10.1093/nar/gkq899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milbury CA, Li J, Liu P, Makrigiorgos GM. Cold-pcr: Improving the sensitivity of molecular diagnostics assays. Expert Rev Mol Diagn. 2011;11:159–169. doi: 10.1586/erm.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song C, Milbury CA, Li J, Liu P, Zhao M, Makrigiorgos GM. Rapid and sensitive detection of kras mutation after fast-cold-pcr enrichment and high-resolution melting analysis. Diagn Mol Pathol. 2011;20:81–89. doi: 10.1097/PDM.0b013e3181fde92f. [DOI] [PubMed] [Google Scholar]
- 8.Milbury CA, Correll M, Quackenbush J, Rubio R, Makrigiorgos GM. Cold-pcr enrichment of rare cancer mutations prior to targeted amplicon resequencing. Clin Chem. doi: 10.1373/clinchem.2011.176198. Epub Dec 2011 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Wang L, Janne PA, Makrigiorgos GM. Coamplification at lower denaturation temperature-pcr increases mutation-detection selectivity of taqman-based real-time pcr. Clin Chem. 2009;55:748–756. doi: 10.1373/clinchem.2008.113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carotenuto P, Roma C, Cozzolino S, Fenizia F, Rachiglio AM, Tatangelo F, et al. Detection of kras mutations in colorectal cancer with fast cold-pcr. Int J Oncol. 2012;40:378–384. doi: 10.3892/ijo.2011.1221. [DOI] [PubMed] [Google Scholar]
- 11.Santis G, Angell R, Nickless G, Quinn A, Herbert A, Cane P, et al. Screening for egfr and kras mutations in endobronchial ultrasound derived transbronchial needle aspirates in nonsmall cell lung cancer using cold-pcr. PLoS One. 2011;6:e25191. doi: 10.1371/journal.pone.0025191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pritchard CC, Akagi L, Reddy PL, Joseph L, Tait JF. Cold-pcr enhanced melting curve analysis improves diagnostic accuracy for kras mutations in colorectal carcinoma. BMC Clin Pathol. 2010;10:6. doi: 10.1186/1472-6890-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen LS, Daugaard IL, Christensen M, Hamilton-Dutoit S, Hager H, Hansen LL. Increased sensitivity of kras mutation detection by high-resolution melting analysis of cold-pcr products. Hum Mutat. 2010;31:1366–1373. doi: 10.1002/humu.21358. [DOI] [PubMed] [Google Scholar]
- 14.Mancini I, Santucci C, Sestini R, Simi L, Pratesi N, Cianchi F, et al. The use of cold-pcr and high-resolution melting analysis improves the limit of detection of kras and braf mutations in colorectal cancer. J Mol Diagn. 2010;12:705–711. doi: 10.2353/jmoldx.2010.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuo Z, Chen SS, Chandra PK, Galbincea JM, Soape M, Doan S, et al. Application of cold-pcr for improved detection of kras mutations in clinical samples. Mod Pathol. 2009;22:1023–1031. doi: 10.1038/modpathol.2009.59. [DOI] [PubMed] [Google Scholar]
- 16.Delaney D, Diss TC, Presneau N, Hing S, Berisha F, Idowu BD, et al. Gnas1 mutations occur more commonly than previously thought in intramuscular myxoma. Mod Pathol. 2009;22:718–724. doi: 10.1038/modpathol.2009.32. [DOI] [PubMed] [Google Scholar]
- 17.Boisselier B, Marie Y, Labussiere M, Ciccarino P, Desestret V, Wang X, et al. Cold pcr hrm: A highly sensitive detection method for idh1 mutations. Hum Mutat. 2010;31:1360–1365. doi: 10.1002/humu.21365. [DOI] [PubMed] [Google Scholar]
- 18.Pinzani P, Santucci C, Mancini I, Simi L, Salvianti F, Pratesi N, et al. Brafv600e detection in melanoma is highly improved by cold-pcr. Clin Chim Acta. 2011;412:901–905. doi: 10.1016/j.cca.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Galbiati S, Brisci A, Lalatta F, Seia M, Makrigiorgos GM, Ferrari M, Cremonesi L. Full cold-pcr protocol for noninvasive prenatal diagnosis of genetic diseases. Clin Chem. 2011;57:136–138. doi: 10.1373/clinchem.2010.155671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du J, Zou X, Pan Y, Li SF, Lu GX. Non-invasive prenatal molecular detection of a fetal point mutation for congenital adrenal hyperplasia using co-amplification at lower denaturation temperature pcr. Chin Med J (Engl) 2010;123:3343–3346. [PubMed] [Google Scholar]
- 21.Chen Y, Wilde HD. Mutation scanning of peach floral genes. BMC Plant Biol. 2011;11:96. doi: 10.1186/1471-2229-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan M, Chan MW, Loh TW, Law HY, Yoon CS, Than SS, et al. Evaluation of nanofluidics technology for high-throughput snp genotyping in a clinical setting. J Mol Diagn. 2011;13:305–312. doi: 10.1016/j.jmoldx.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tewhey R, Warner JB, Nakano M, Libby B, Medkova M, David PH, et al. Microdropletbased pcr enrichment for large-scale targeted sequencing. Nat Biotechnol. 2009;27:1025–1031. doi: 10.1038/nbt.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gundry CN, Vandersteen JG, Reed GH, Pryor RJ, Chen J, Wittwer CT. Amplicon melting analysis with labeled primers: A closed-tube method for differentiating homozygotes and heterozygotes. Clin Chem. 2003;49:396–406. doi: 10.1373/49.3.396. [DOI] [PubMed] [Google Scholar]
- 25.Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ. High-resolution genotyping by amplicon melting analysis using lcgreen. Clin Chem. 2003;49:853–860. doi: 10.1373/49.6.853. [DOI] [PubMed] [Google Scholar]
- 26.Milbury CA, Chen CC, Mamon H, Liu P, Santagata S, Makrigiorgos GM. Multiplex amplification coupled with cold-pcr and high resolution melting enables identification of lowabundance mutations in cancer samples with low DNA content. J Mol Diagn. 2011;13:220–232. doi: 10.1016/j.jmoldx.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mamon H, Hader C, Li J, Wang L, Kulke M, Amicarelli G, et al. Preferential amplification of apoptotic DNA from plasma: Potential for enhancing detection of minor DNA alterations in circulating DNA. Clinical Chemistry. 2008;54:1582–1584. doi: 10.1373/clinchem.2008.104612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dwight Z, Palais R, Wittwer CT. Umelt: Prediction of high-resolution melting curves and dynamic melting profiles of pcr products in a rich web application. Bioinformatics. 2011;27:1019–1020. doi: 10.1093/bioinformatics/btr065. [DOI] [PubMed] [Google Scholar]
- 29.Binladen J, Gilbert MT, Bollback JP, Panitz F, Bendixen C, Nielsen R, Willerslev E. The use of coded pcr primers enables high-throughput sequencing of multiple homolog amplification products by 454 parallel sequencing. PLoS One. 2007;2:e197. doi: 10.1371/journal.pone.0000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Candau RSH, Han Y, Eastlake P, Kaldjian E, Makrigiorgos GM, Gerard GF. Very high sensitivity detection of k-ras exon 2 mutations using fast cold-pcr. AACR, Vol. Washington DC, USA: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.