Abstract
Context:
Anterior cruciate ligament (ACL) reconstructions are common, especially in young, active people. The lower extremity neuromuscular adaptations seen after aerobic exercise provide information about how previously injured patients perform and highlight deficits and, hence, areas for focused treatment. Little information is available about neuromuscular performance after aerobic exercise in people with ACL reconstructions.
Objective:
To compare dynamic balance, gluteus medius muscle activation, vertical jump height, and hip muscle strength after aerobic exercise in people with ACL-reconstructed knees.
Design:
Case-control study.
Setting:
Research laboratory.
Patients or Other Participants:
Of 34 recreationally active volunteers, 17 had a unilateral primary ACL reconstruction at least 2 years earlier and 17 were matched controls.
Intervention(s):
All participants performed 20 minutes of aerobic exercise on a treadmill.
Main Outcome Measure(s):
We recorded dynamic, single-legged balance electromyographic gluteus medius muscle activation, single-legged vertical jump height, and maximum isometric strength for hip abduction, extension, and external rotation preexercise and postexercise.
Results:
Participants with ACL reconstructions exhibited shorter reach distances during dynamic balance tasks, indicating poorer dynamic balance, and less gluteus medius muscle electromyographic activation. Reductions in hip abduction and extension strength after exercise were noted in all participants; however, those with ACL reconstructions displayed greater hip extensor strength loss after aerobic exercise than did the control group.
Conclusions:
Neuromuscular changes after aerobic exercise exist in both patients with ACL reconstructions and controls. The former group may experience greater deficits in hip extensor strength after aerobic exercise. Reduced reach distances in people with ACL reconstructions may represent a protective mechanism against excessive tibiofemoral rotation during dynamic balance. Clinicians should identify weaknesses in the resting state and after aerobic exercise in recreationally active patients and those with ACL reconstructions.
Keywords: skeletal muscle adaptations, fatigue, strength, isometric activity, isokinetic activity
Key Points.
After aerobic exercise, both patients with anterior cruciate ligament–reconstructed knees and the control group demonstrated neuromuscular changes.
Compared with the control group, those with anterior cruciate ligament reconstructions displayed greater hip extensor strength deficits after exercise and shorter reach distances on the Star Excursion Balance Test.
Clinicians should consider neuromuscular performance both at rest and after exercise when assessing functional outcomes in patients with anterior cruciate ligament reconstructions.
Anterior cruciate ligament (ACL) injuries are common among young, active people,1,2 resulting in more than 100 000 estimated reconstructions in the United States annually.3 Despite excellent outcomes after surgery, active people with reconstructed knees remain at risk for graft failure4 and long-term degenerative joint conditions such as osteoarthritis.5,6 An important factor in postoperative rehabilitation and return-to-play decision making is to ensure appropriate restoration of lower extremity muscular strength and dynamic postural control.
Deficits after ACL injury and reconstruction have been observed in various measurements, including force output, balance, and neuromuscular control. Patients with ACL- reconstructed knees (ACLRs) have deficits in balance and quadriceps muscle strength.7 Postural control deficits measured during single-legged balance with eyes open and eyes closed have been observed in ACLRs and may be attributed to a decrease in mechanoreceptor information about joint position of the affected knee.8,9 Altered afferent information may result in compensatory gait patterns or lower extremity muscle activation strategies during aerobic exercise or sport and predispose an athlete to injury. Therefore, clinicians will benefit from information about exercise in ACLRs because compensations or deficits would be accentuated, thereby highlighting areas that may benefit from therapeutic intervention. Specific information about neuromuscular deficits that are accentuated after exercise may help to focus therapeutic interventions in patients with previous knee injuries.
Reductions in adduction strength,10 hip extensor strength,11 and hamstring strength12 have been reported in patients after ACL reconstruction. Altered neuromuscular function during aerobic exercise may prevent normal joint function during exercise, thus increasing risk of injury. Little information is available about hip abduction strength after ACL injury, and none describes lower extremity muscle strength or muscle activation during aerobic exercise in the ACLRs. The gluteus medius muscle is a primary abductor of the hip joint and contributes to pelvic control during the midstance of gait.13 The gluteus medius plays an important role during the stance phase of gait by assisting with eccentric control of femoral internal rotation. The combination of femoral internal rotation and adduction, which may be resisted to a lesser extent in people with weakness or fatigability (or both) of the hip joint musculature, contributes to a condition of dynamic valgus, which is a risk factor for non-contact ACL injury.14
Deficits in hip muscle function10–12 have been identified in ACLRs. These deficits may be magnified during exercise, potentially because of different rates of muscular fatigue or an alternative coping strategy, resulting in altered performance. Clinicians should be aware of exercise-related neuromuscular changes that may place active people with a history of major knee joint injury at risk for further injury.
Exercise testing provides a global evaluation of tolerance to exertion, including responses from the pulmonary, cardiovascular, and musculoskeletal systems15,16 that may not be adequately characterized by resting measurements. The Balke15,16 protocol has been described as a simple exertional exercise test that involves treadmill walking at a constant speed; exercise intensity is incrementally increased via treadmill incline. In the sports medicine clinic and laboratory setting, standardized, perception-regulated exertional exercise protocols can be used to induce a level of subexhaustive muscular fatigue that will help us to understand how patients respond during simulated and controlled tasks that closely resemble activities of daily living. In using graded exercise protocols, the goal is for people to exert. On the continuum of exertion leading to total exhaustion, muscular function may become altered by fatigue or other neuromuscular responses. In the presence of hip muscle dys-function, ACLRs may experience greater responses involving hip muscle strength, jump height, and dynamic single-legged balance compared with healthy, uninjured control participants after exertion.
The purpose of our study was to compare the change in hip muscle force output (primary outcome of interest), vertical jump height (VJH), dynamic balance, and gluteus medius surface electromyography (EMG) activation after aerobic exercise in ACLRs with a healthy control population. As an exploratory aim, we performed a subgroup analysis based on graft type in our ACLRs.
METHODS
We used a case-control, pretest-posttest design with 2 independent variables: group (ACLRs, healthy controls) and time (before and after aerobic exercise). The dependent variables were (1) dynamic balance, measured as normalized reach distance in the 3 directions of the Star Excursion Balance Test (SEBT), anterior, posteromedial, and posterolateral; (2) average root mean square of the gluteus medius EMG signal measured at the point of maximal reach during the SEBT; (3) maximum single-legged VJH; and (4) maximum isometric force output of the hip extensors, abductors, and external rotators.
Participants
A total of 17 ACLRs (6 men: age = 26.8 ± 4.6 years, height = 176.1 ± 10.6 cm, mass = 86.7 ± 15.8 kg; 11 women: age = 23.2 ± 3.4 years, height = 168.8 ± 7.7 cm, mass = 64.7 ± 5.5 kg) who had undergone primary, unilateral ACL reconstruction (10 semitendinosis-gracilis autografts, 7 bone-tendon-bone autografts) an average of 3.3 ± 1.66 years earlier were recruited for this study. The ACLRs were matched with healthy controls (6 men: age = 25.5 ± 2.9 years, height = 176.1 ± 5.7 cm, mass = 78.6 ± 7.0 kg; 11 women: age = 21.9 ± 1.5 years, height = 165.7 ± 5.4 cm, mass = 62.8 ± 5.9 kg) based on sex and age. Volunteers in both groups were recreationally active (ie, they exercised 3 or more days per week). Activity levels between the 2 groups were similar: control participants reported exercising on average 3.9 ± 0.9 times per week and 1.2 ± 0.5 hours per session. The ACLRs exercised 3.7 ± 1.4 times per week and 1.0 ± 0.3 hours per session. Most participants reported gym-based exercise regimens that included a combination of weight lifting, aerobic activity, and recreational sport involvement. One female ACLR was an out-of-season collegiate soccer player. Volunteers were excluded from the study if they had incurred a lower extremity injury within the last 6 months. Each participant provided informed consent before testing occurred, and the study was approved by the university's institutional review board.
Instruments
Maximum isometric hip external rotation, abduction, and extension force outputs were measured with a handheld dynamometer (model microFET2; Hoggan Health Industries, Draper, UT). A treadmill (model Q65 series 90; Quinton Instrument Company, Bothell, WA) was used for the aerobic exercise protocol. Gluteus medius muscle activity was measured with surface EMG through a 16-bit data acquisition system (model MP150; BIOPAC Systems, Inc, Goleta, CA). Signals were amplified (model EMG100C; BIOPAC Systems, Inc) using a gain of 1000 from disposable, round, pregelled 10-mm silver chloride electrodes (model EL503; BIOPAC Systems, Inc). The EMG measurements were sampled at 1000 Hz. The input impedance of the amplifier was 1 MΩ, with a common mode rejection ratio of 110 dB and a signal-to-noise ratio of 70 dB. AcqKnowledge Software (version 3.7.3; BIOPAC Systems, Inc) was used for data processing. Time off the ground during the vertical jump trials was measured using digitized signal from a pressure mat (imported through a universal interface module on our BIOPAC system). We subjectively monitored each participant's level of fatigue with the Borg Rate of Perceived Exertion (RPE) Scale.
Testing Procedures
All testing orders were counterbalanced using a Latin square in order to reduce the influence of multiple tests before and after exercise. The order of testing was the same during preexercise and postexercise measurements for each participant. The SEBT is a reliable test17 that requires single-legged dynamic balance while the participant performs a maximal reach task with the contralateral limb.18 While the participant stood on the reconstructed or the dominant limb (control group), he or she was instructed to maximally reach the contralateral limb along a straight line on the floor in 3 directions17,19: the anterior direction (12:00 on a clock face), posterolateral direction (4:30 on a clock face), and posteromedial (7:30 on a clock face) (Figure 1). Three practice trials were allowed in each direction for acclimation to testing procedures. We recorded the mean of 3 test trials for analysis. Trials were excluded if the volunteer was unable to maintain balance on the stance leg while performing maximal reach and return to the starting position.
Figure 1.
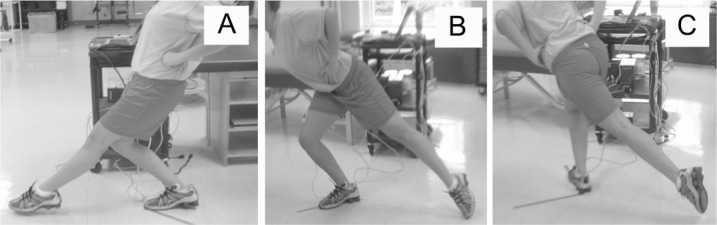
Performance of the Star Excursion Balance Test. A, Anterior. B, Posteromedial. C, Posterolateral. This image is not of an actual patient.
To record the EMG signal, electrodes were placed over the gluteus medius between the iliac crest and the greater trochanter20 of the injured or dominant leg and over active muscle tissue, as verified with palpation during an isolated contraction. Electrodes were placed parallel to muscle fiber orientation, with a standard interelectrode distance of 2 cm. The EMG signal was recorded while the participant performed the SEBT. We manually identified toe touchdown with a digital event marker inserted into the EMG data stream when the volunteer achieved maximum reach distance during the SEBT.
For VJH, participants were instructed to stand on the pressure mat on the reconstructed or dominant leg and jump vertically as high as possible, taking off only on the injured limb (ie, single-legged jump). They were allowed to perform a counter-movement jump and told to land on both legs. For the pretest, volunteers were given 3 practice trials to ensure comprehension. They then rested for approximately 30 seconds or until they felt adequately recovered from the practice trials followed by 3 test trials.
Maximal isometric hip force output was collected using a handheld dynamometer for 3 muscle groups: the abductors, extensors, and external rotators. To quantify force output, participants performed 2 practice trials (1 submaximal, 1 maximal) followed by 3 maximal test trials, each lasting 3 seconds. All contractions were performed in the same order to minimize the setup time during posttesting. The testing order went as follows: side-lying hip abduction, prone hip extension, and seated hip external rotation. Maximal isometric hip abduction force output was measured with the volunteer side lying and the contralateral limb positioned with the hip flexed and the knee bent to 90°. Hip extensor strength was measured in the prone position with the hip in neutral position and knee bent to 90°, and hip external rotation was measured in the seated position with hips and knees bent to approximately 90°. Canvas straps stabilized the test leg and dynamometer during testing to isolate the uniplanar movement of interest (Figure 2). Dynamometer placement was based on the work of previous authors,21 who reported good reliability for handheld dynamometry in assessing lower extremity isometric strength. Ink markings were placed on the skin to ensure exact placement of the handheld dynamometer during posttesting.
Figure 2.
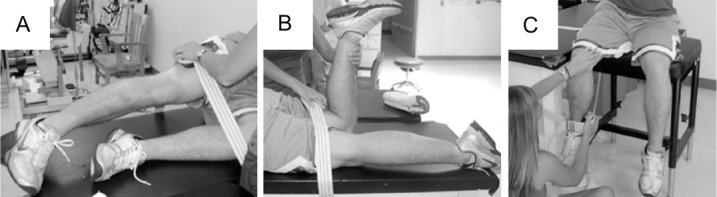
Strength testing positions. A, Hip abduction. B, Hip extension. C, Hip external rotation. The handheld dynamometer was placed at the distal end of the femur superior to the lateral epicondyle, just superior to the popliteal space on the posterior thigh, and proximal to the medial malleolus along the distal tibia for the 3 testing positions, respectively. This image is not of an actual patient.
After baseline testing, participants performed a 20-minute aerobic exercise treadmill protocol (modified Balke protocol15,16) in which they walked continuously at a speed of 3.5 mph (5.6 kph). This speed was based on preliminary work and previously used graded treadmill exercise protocols and was intended to allow volunteers to walk at a consistent but challenging pace that would avoid knee pain due to high-impact forces during inclined jogging. During the first 15 minutes, the treadmill incline was increased 1.0% per minute. For the final 5 minutes, the participants were allowed to modify the tread-mill incline (increase or decrease) to maintain an RPE of 15 to 17 for the remainder of the exercise protocol. After the aerobic exercise treadmill protocol, dynamic balance, surface EMG, muscle strength, and VJH were retested in the pretest order. To minimize the potential influence of recovery on the dependent measures, all posttesting was completed within 3 minutes of the end of the treadmill protocol, and the order of the tests was counterbalanced for each participant.
Data Processing
SEBT Data.
The distance from the starting point to maximal reach was measured in centimeters and normalized to the length of the participant's stance leg from the anterosuperior iliac spine to the medial malleolus. The average of 3 trials was used for analysis.
EMG Data.
A time epoch of 200 milliseconds (100 milliseconds before and 100 milliseconds after the instant of maximal reach distance during SEBT testing) was band-pass filtered (10–500 Hz) and processed using a 10-millisecond moving average root mean square algorithm. The mean root mean square value of the 200-millisecond window from each SEBT trial was used for analysis.
Vertical Jump Height. Time off the ground during the trials was identified using the digital signal captured from the pressure mat. Maximum VJH (in meters) was calculated using the following formula: VJH = (9.81 × T2)/8.
Force Data.
The average of 3 trials of maximal force obtained from the handheld dynamometer was normalized to body mass for analysis.
Statistical Analysis
The sample size was based on previously published21 estimates of variability in hip muscle strength assessment using handheld dynamometry. Anticipating that a large effect size (ie, 0.95) would result after the treadmill exercise protocol, we determined that 17 volunteers per group would be sufficient to detect statistically significant differences with a type I error rate of 5% and power exceeding 80%.
We performed individual 2 × 2 mixed-model multivariate analyses of variances (ANOVAs) for each of the following preexercise-postexercise comparisons: (1) dynamic balance (normalized reach distance in the anterior, posteromedial, and posterolateral directions during the SEBT); (2) average root mean square of the gluteus medius EMG signal while performing the SEBT in the anterior, posteromedial, and posterolateral directions; and (3) maximum isometric hip muscle force output. In the case of a main effect, we proceeded with individual ANOVAs for each dependent variable. We performed an ANOVA for maximum, single-legged VJH. In the event of any significant interaction term, we used independent- and dependent-samples t test post hoc specific comparisons where appropriate. As an exploratory aim, we performed a subgroup analysis to compare graft types before and after aerobic exercise using individual 2 × 2 mixed-model ANOVAs. Test findings were considered statistically significant if P ≤ .05; we did not use a Bonferroni correction.22,23
RESULTS
Dynamic Balance
We observed a multivariate effect for time when we considered a linear combination of SEBT reach distance dependent variables (F3,30 = 4.2, P = .01). On average, posterolateral reach distance was reduced after aerobic exercise (ie, univariate main effect for time: F1,32 = 10.2, P = .003, η2 = 0.24) (Table 1). No differences were evident in anterior or posteromedial reach distances after exercise (P > .05). The ACLRs displayed shorter reach distances (ie, main effect for group) in the posterolateral (F1,32 = 6.7, P = .02, η2 = 0.17) and posteromedial (F1,32 = 4.4, P = .04, η2 = 0.12) directions but not in the anterior (P > .05) reach direction when compared with matched controls.
Table 1.
Star Excursion Balance Test Maximum Normalized Reach Distance (cm/cm) in Patients With Anterior Cruciate Ligament–Reconstructed Knees (ACLR), Control Group, and Total Sample (Mean ± SD)
| Direction | Group |
Total Sample |
P Valuea |
||||||
| ACLR |
Control |
Baseline | After Aerobic Exercise | Time | Group | Group × Time | |||
| Baseline | After Aerobic Exercise | Baseline | After Aerobic Exercise | ||||||
| Anterior | 0.76 ± 0.07 | 0.78 ± 0.08 | 0.76 ± 0.07 | 0.76 ± 0.09 | 0.76 ± 0.07 | 0.77 ± 0.09 | .40 | .66 | .58 |
| Posteromedial | 0.86 ± 0.12 | 0.85 ± 0.12 | 0.95 ± 0.12 | 0.93 ± 0.13 | 0.91 ± 0.13 | 0.89 ± 0.13 | .12 | .02b | .96 |
| Posterolateral | 0.83 ± 0.12 | 0.78 ± 0.11 | 0.91 ± 0.11 | 0.89 ± 0.12 | 0.87 ± 0.12 | 0.84 ± 0.13 | .003c | .04b | .17 |
a Findings from univariate analysis of variance.
b Less in ACLR patients than in the control group.
c Reduced from pretest to posttest in all participants.
Gluteus Medius Muscle Activation During Dynamic Balance
No multivariate main effect for gluteus medius muscle activation over time was demonstrated. On average (main effect for group), ACLRs exhibited lower gluteus medius EMG activity in the anterior direction than the control group (F1,33 = 7.1, P = .01, η2 = 0.20) (Table 2). We found no other differences in gluteus medius EMG activation between groups or after aerobic exercise.
Table 2.
Root Mean Square Gluteus Medius Muscle Activation During Maximum Normalized Star Excursion Balance Test Reach Distance Measurements in Patients With Anterior Cruciate Ligament–Reconstructed Knees (ACLR), Control Group, and Total Sample (Mean ± SD)
| Direction | Group |
Total Sample |
P Valuea |
||||||
| ACLR |
Control |
Baseline | After Aerobic Exercise | Time | Group | Group × Time | |||
| Baseline | After Aerobic Exercise | Baseline | After Aerobic Exercise | ||||||
| Anterior | 0.88 ± 0.27 | 0.84 ± 0.21 | 0.98 ± 0.20 | 1.07 ± 0.24 | 0.93 ± 0.24 | 0.96 ± 0.25 | .968 | .01b | .28 |
| Posteromedial | 1.45 ± 1.20 | 1.11 ± 0.61 | 1.30 ± 0.70 | 1.20 ± 0.77 | 1.37 ± 0.95 | 1.16 ± 0.70 | .15 | .91 | .42 |
| Posterolateral | 1.19 ± 0.87 | 1.10 ± 0.42 | 1.13 ± 0.49 | 1.08 ± 0.88 | 1.16 ± 0.68 | 1.09 ± 0.69 | .60 | .86 | .90 |
a Findings from univariate analysis of variance.
b Less in ACLR patients than in the control group.
Vertical Jump Height
No differences were noted in VJH between groups or after aerobic exercise (Table 3).
Table 3.
Maximum Single-Legged Vertical Jump Height (m) in Patients With Anterior Cruciate Ligament– Reconstructed Knees (ACLR), Control Group, and Total Sample (Mean ± SD)
| Time | ACLR | Control | Total Sample |
| Baseline | 0.16 ± 0.11 | 0.17 ± 0.08 | 0.17 ± 0.09 |
| After aerobic exercise | 0.17 ± 0.16 | 0.17 ± 0.09 | 0.17 ± 0.13 |
Isometric Hip Muscle Strength
A multivariate effect for time was seen for the linear combination of isometric muscle strength dependent variables (F3,30 = 5.0, P = .01) was considered. Specifically, after the aerobic exercise protocol, all participants experienced a reduction in hip extension (F1,30 = 7.6, P = .01, η2 = 0.19) and hip abduction strength (F1,30 = 4.4, P = .04, η2 = 0.12) but not in external rotation strength (F1,30 = 2.9, P = .09, η2 = 0.08) (Table 4). We observed an interaction suggesting a difference in hip extensor strength after aerobic exercise (F1,32 = 4.6, P = .04, η2 = 0.13): ACLRs displayed reduced hip extensor strength after exercise (t16 = 3.0, P = .01), but the control group did not (P > .05).
Table 4.
Hip Muscle Strength, Normalized to Body Weight (N/kg), in Patients With Anterior Cruciate Ligament– Reconstructed Knees (ACLR), Control Group, and Total Sample (Mean ± SD)
| Hip Muscle Strength | Group |
Total Sample |
P Valuea |
||||||
| ACLR |
Control |
Baseline | After Aerobic Exercise | Times | Group | Group × Time | |||
| Baseline | After Aerobic Exercise | Baseline | After Aerobic Exercise | ||||||
| Abduction | 0.82 ± 0.23 | 0.78 ± 0.25 | 0.85 ± 0.22 | 0.80 ± 0.28 | 0.83 ± 0.22 | 0.79 ± 0.26b | .04b | .77 | .86 |
| Extension | 0.53 ± 0.25 | 0.41 ± 0.18b | 0.43 ± 0.14 | 0.42 ± 0.16 | 0.47 ± 0.21 | 0.42 ± 0.17 | .01 b | .46 | .04c |
| External rotation | 0.31 ± 0.09 | 0.28 ± 0.10 | 0.37 ± 0.18 | 0.34 ± 0.14 | 0.34 ± 0.14 | 0.31 ± 0.12 | .10 | .18 | .99 |
a Findings from univariate analysis of variance.
b Reduced from pretest to posttest in all participants.
c Significant difference.
Subgroup Analysis: Graft Type
Patients whose reconstructions were performed with bone-tendon-bone grafts had greater external rotation strength (main effect for group: F1,14 = 5.6, P = .03) than those with hamstring grafts.
DISCUSSION
After aerobic exercise, we observed deteriorated dynamic balance and hip muscle strength. Contrary to our hypothesis, the changes in dynamic balance and VJH were not different between ACLRs and healthy, matched controls. However, ACLRs experienced a greater reduction in hip extensor strength after aerobic exercise than did the control group. This finding was consistent with our expectations of a greater reduction in hip extensor strength after a bout of aerobic exercise in recreationally active people with unilateral, primary ACL reconstructions. These changes were not observed for isometric hip external rotation or abduction strength. The findings from this study may help clinicians identify exercise-related compensations at the hip during clinical and sport-specific evaluations, thereby guiding therapeutic exercise prescriptions or other forms of proprioceptive or neuromuscular training. Although patients with a history of severe knee injury appear to be at higher risk for joint degeneration, we cannot say with any confidence whether differences in hip muscle strength after exercise precipitate the progression toward early-onset knee joint degeneration.
Hip extension force output is reduced in ACLRs at 3 months postoperatively and is restored by 1 year.11 Hamstring strength deficits have also been reported in ACLRs.24 However, data on hamstring strength deficits after ACL reconstruction conflict. Some authors25,26 reported greater hamstring strength deficits in patients with hamstring autografts, whereas others25,27 noted no differences in hamstring strength between those reconstructed with hamstring versus bone–patellar tendon–bone autografts. In the current study, patients who underwent reconstruction with hamstring grafts exhibited weaker hip external rotation strength and less gluteus medius muscle activation. Previous investigators have debated the existence of deteriorated quadriceps and hamstring muscle strength28,29 and gait30 among patients with reconstructions of different graft types. Generally speaking, it is intuitive that people who lose native hamstring tissue will be at risk for hamstring muscle strength problems, and those who lose a portion of the patellar tendon will be at risk for quadriceps strength problems. However, whether the observed changes in hip extensor weakness among ACLRs were due to donor site morbidity and whether this difference is clinically important during exercise is unknown.
We observed no differences in single-legged VJH between groups or after exercise. This was an unexpected finding, which may reflect the fact that our aerobic exercise protocol did not target the portions of the lower extremity motor-neuron pools more likely to contribute to explosive, powerful maneuvers such as the vertical jump. In a previous study,31 ACLRs experienced reduced single-legged hop distance after a resisted isotonic fatiguing protocol isolated to the knee extensors. Yet Gustavsson et al32 reported that vertical jump performance was restored 11 months after reconstruction. Our participants were 2 or more years postreconstruction, but it is clear from the SDs that vertical jump ability was highly variable, both at baseline and after the exercise protocol. This result indicates that our volunteers were heterogeneous in their ability to generate muscle power during an explosive maneuver.
Decreased or altered mechanoreceptor information about joint position8 in the injured knee may lead to modified neuromuscular control while the person attempts to maintain balance. In some ACLRs, static balance was not different from that in healthy controls8,33; however, in ACLRs, already impaired postural control may further deteriorate during static, unilateral balance34 as a response to a postural perturbation.8,35 In the current study, we observed worse dynamic balance, defined as normalized reach distance during the SEBT, in ACLRs compared with controls. These deficits were noted only in the rotational components of the SEBT, indicating that ACLRs may have difficulty or may be avoiding certain knee positions of rotary instability during a dynamic balance task. Excessive tibial rotation may result in abnormal loading of knee cartilage, potentially leading to joint degeneration.36
The ACLRs exhibited shorter reach distances when performing dynamic balance tasks that required greater lower extremity rotation. For example, their reach distance in the anterior direction of the SEBT was not different from that in the control group, but their reach distance in the posterolateral and posteromedial directions was different. Attempting to achieve the greatest possible reach distance is often accompanied by lower extremity rotational movements. However, no rotation is typically involved in the anterior reach direction of the SEBT (Figure 1). The findings of altered reach distances in the postero- medial and posterolateral directions are consistent with pre- vious descriptions of altered transverse-plane tibiofemoral kinematics after ACL reconstruction,37 especially during functional tasks.38 One group39 reported decreased internal tibial rotation in the ACL-reconstructed knee under simulated muscle loads, whereas another group40 noted decreased internal tibial rotation or increased external tibial rotation during running. Therefore, ACLRs may be more likely to exhibit reduced dynamic balance when the activities involve rotational movements at the knee joint, such as those experienced during the posterolateral and posteromedial reach directions of the SEBT.
We did not observe changes in gluteus medius activity after our aerobic exercise protocol. This may be because insufficient demand was placed on this muscle with a forward-walking exercise protocol, which may not have adequately challenged the frontal-plane hip stabilizers to reveal a difference between groups after exercise. However, ACLRs demonstrated less gluteus medius EMG activation during the anterior reach of the SEBT than did controls, even though no group differences were evident in the reach distances. The fact that ACLRs achieved anterior reach distances similar to those of controls indicates that the former group was using less gluteus medius activity while balancing. A potential explanation is that ACLRs were activating muscles other than the gluteus medius or exploring alternative knee, hip, and trunk positions while performing the anterior reach task. Anecdotally, the lack of activation may also result in a Trendelenburg posture during the anterior reach. A dropped hip on the reach side would further indicate that ACLRs had insufficient gluteus medius activation of the stance leg to maintain a level pelvis. However, this is only a possible rationale for our findings, and we did not make any attempt to standardize pelvic inclination or lateral tilting during SEBT procedures.
Previously, women were found to have reduced gluteus medius EMG activity while landing from a jump,41 which has been implicated as a potential source of noncontact injury risk. In the current study, reduced gluteus medius muscle activity represents a neuromuscular adaptation in ACLRs that may influence lower extremity stability during activity.
Our exercise protocol was intended to exert our participants and induce a subexhaustive level of muscular fatigue. The time between the end of the treadmill protocol and completion of the postexercise measurements may confound any study designed to examine the immediate influence of fatigue on neuromuscular function. Previous authors42 reported balance deficits that persisted 5 minutes after a 20-minute fatiguing exercise protocol but resolved completely after an additional 15 minutes of rest. In our study, RPE values were similar to those reported previously,42 and all testing was completed within 5 minutes after exercise. We performed extensive pilot and practice sessions to enable expedient and accurate data collection in the minutes after our exercise protocol. We also counterbalanced the order of testing. Therefore, although we cannot confirm the state of fatigue in the minutes after exercise, we offer the following assurances. An investigation of recovery of neuromuscular function42 included absolute rest during the postexercise period. This was not the case for our participants, who continued to work at high intensity while performing maximal jump tasks, isometric contractions, and dynamic balance. In addition, if participants experienced recovery immediately after the exercise tasks, we would expect that the values of our dependent measures would vary greatly. As shown in the tables, it is clear that variability, as reported with SDs, did not change much from pretest to posttest. However, we cannot state with certainty that the level of neuromuscular fatigue was consistent during all postexercise measures; this is a potential confounding factor that may explain some of our nonsignificant findings.
This study may be limited by the aerobic exercise protocol used to induce fatigue. Our concern was to be able to stringently control the level of exertion experienced by each participant for appropriate comparisons. Yet the fitness levels of “recreationally active” people may vary. The exercise protocol we used was intended to fatigue the participants. Previous researchers,43 using similar graded treadmill protocols, showed that young, healthy people walking at a constant speed of 3.5 mph (5.6 kph) for 20 minutes on a gradually increasing incline experienced average heart rates exceeding 180 beats per minute. More contemporary models of fitness assessment in young adults use treadmill tests that incorporate concurrent increases in treadmill incline and speed to maximally exert participants, but this was not our intention. We wanted volunteers to experience a consistent level of fatigue with minimal risk for becoming exhausted or experiencing pain during the test, both of which could potentially confound the outcome measures. The use of RPE has also been reported as a valid and reliable method for regulating exercise intensity44: an RPE value of 15 corresponds with an average exercise intensity exceeding 70% V02max.45 Furthermore, the 6–20 RPE scale was developed to predict heart rate during exercise (ranging from 60–200 beats per minute, respectively). Therefore, according to the common method for estimating maximal heart rate (220 – age), at an RPE between 15 and 17, participants were exercising at more than 80% of their recommended maximal heart rate. Thus, our participants completed a period of exercise that progressed to a moderate level of intensity. The changes experienced during this bout of exercise may be magnified during longer-duration and higher-intensity sport-specific exercise; therefore, clinicians and researchers can use our findings to recognize exercise-related adaptations that may represent coping strategies for injured people who are fatigued. Neuromuscular differences between ACLRs and the healthy control group after the low-demand exercise protocol we used may be amplified during more strenuous, exhausting, or sport-specific exercise protocols. We cannot comment on whether these changes existed before the initial injury.
In conclusion, neuromuscular changes after aerobic exercise occurred in both ACLRs and controls. The ACLRs may experience greater deficits in hip extensor strength after aerobic exercise. Reduced reach distances in ACLRs may represent a protective mechanism against excessive tibiofemoral rotation during dynamic balance. These findings have potential implications for identifying patients who may be at risk for reinjury or long-term joint degeneration. Clinicians should characterize weaknesses in both the resting state and after aerobic exercise in recreationally active patients with a history of ACL reconstruction.
REFERENCE
- 1.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 2.Ingram JG, Fields SK, Yard EE, Comstock RD. Epidemiology of knee injuries among boys and girls in US high school athletics. Am J Sports Med. 2008;36(6):1116–1122. doi: 10.1177/0363546508314400. [DOI] [PubMed] [Google Scholar]
- 3.Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321–2328. doi: 10.2106/JBJS.H.00539. [DOI] [PubMed] [Google Scholar]
- 4.Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 5.Cebesoy O. What are the risk factors in the development of osteoarthritis following ACL reconstruction? Int Orthop. 2006;30(5):431–432. doi: 10.1007/s00264-006-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 7.Drechsler WI, Cramp MC, Scott OM. Changes in muscle strength and EMG median frequency after anterior cruciate ligament reconstruction. Eur J Appl Physiol. 2006;98(6):613–623. doi: 10.1007/s00421-006-0311-9. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman M, Schrader J, Koceja D. An investigation of postural control in postoperative anterior cruciate ligament reconstruction patients. J Athl Train. 1999;34(2):130–136. [PMC free article] [PubMed] [Google Scholar]
- 9.Lysholm M, Ledin T, Odkvist LM, Good L. Postural control: a comparison between patients with chronic anterior cruciate ligament insufficiency and healthy individuals. Scand J Med Sci Sports. 1998;8(6):432–438. doi: 10.1111/j.1600-0838.1998.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 10.Hiemstra LA, Gofton WT, Kriellaars DJ. Hip strength following hamstring tendon anterior cruciate ligament reconstruction. Clin J Sport Med. 2005;15(3):180–182. doi: 10.1097/01.jsm.0000157795.93004.ea. [DOI] [PubMed] [Google Scholar]
- 11.Geoghegan JM, Geutjens GG, Downing ND, Colclough K, King RJ. Hip extension strength following hamstring tendon harvest for ACL reconstruction. Knee. 2007;14(5):352–356. doi: 10.1016/j.knee.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Yanagawa T, Shelburne K, Serpas F, Pandy M. Effect of hamstrings muscle action on stability of the ACL-deficient knee in isokinetic extension exercise. Clin Biomech (Bristol, Avon) 2002;17(9–10):705–712. doi: 10.1016/s0268-0033(02)00104-3. [DOI] [PubMed] [Google Scholar]
- 13.Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture. 2003;17(2):159–169. doi: 10.1016/s0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- 14.Hewett TE, Myer GD, Ford KR. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. et al. [DOI] [PubMed] [Google Scholar]
- 15.Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise Testing: European Respiratory Society. Eur Respir J. 1997;10(11):2662–2689. doi: 10.1183/09031936.97.10112662. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society, American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 17.Plisky PJ, Rauh MJ, Kaminski TW, Underwood FB. Star Excursion Balance Test as a predictor of lower extremity injury in high school basketball players. J Orthop Sports Phys Ther. 2006;36(12):911–919. doi: 10.2519/jospt.2006.2244. [DOI] [PubMed] [Google Scholar]
- 18.Olmsted LC, Carcia CR, Hertel J, Shultz SJ. Efficacy of the Star Excursion Balance Tests in detecting reach deficits in subjects with chronic ankle instability. J Athl Train. 2002;37(4):501–506. [PMC free article] [PubMed] [Google Scholar]
- 19.Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008;27(3):353–370. vii. doi: 10.1016/j.csm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Kleissen RF. Effects of electromyographic processing methods on computer-averaged surface electromyographic profiles for the gluteus medius muscle. Phys Ther. 1990;70(11):716–722. doi: 10.1093/ptj/70.11.716. [DOI] [PubMed] [Google Scholar]
- 21.Kelln BM, McKeon PO, Gontkof LM, Hertel J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil. 2008;17(2):160–170. doi: 10.1123/jsr.17.2.160. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):313. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 23.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiemstra LA, Webber S, MacDonald PB, Kriellaars DJ. Contralateral limb strength deficits after anterior cruciate ligament reconstruction using a hamstring tendon graft. Clin Biomech (Bristol, Avon) 2007;22(5):543–550. doi: 10.1016/j.clinbiomech.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Ardern CL, Webster KE, Taylor NF, Feller JA. Hamstring strength recovery after hamstring tendon harvest for anterior cruciate ligament reconstruction: a comparison between graft types. Arthroscopy. 2010;26(4):462–469. doi: 10.1016/j.arthro.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Ageberg E, Roos HP, Silbernagel KG, Thomee R, Roos EM. Knee extension and flexion muscle power after anterior cruciate ligament reconstruction with patellar tendon graft or hamstring tendons graft: a cross-sectional comparison 3 years post surgery. Knee Surg Sports Traumatol Arthrosc. 2009;17(2):162–169. doi: 10.1007/s00167-008-0645-4. [DOI] [PubMed] [Google Scholar]
- 27.Moisala AS, Jarvela T, Kannus P, Jarvinen M. Muscle strength evaluations after ACL reconstruction. Int J Sports Med. 2007;28(10):868–872. doi: 10.1055/s-2007-964912. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DC, DeBerardino TM, Nelson BJ. Patellar tendon versus hamstring tendon autografts for anterior cruciate ligament reconstruction: a randomized controlled trial using similar femoral and tibial fixation methods. Am J Sports Med. 2009;37(10):1946–1957. doi: 10.1177/0363546509339577. et al. [DOI] [PubMed] [Google Scholar]
- 29.Lautamies R, Harilainen A, Kettunen J, Sandelin J, Kujala UM. Isokinetic quadriceps and hamstring muscle strength and knee function 5 years after anterior cruciate ligament reconstruction: comparison between bone–patellar tendon– bone and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc. 2008;16(11):1009–1016. doi: 10.1007/s00167-008-0598-7. [DOI] [PubMed] [Google Scholar]
- 30.Webster KE, Wittwer JE, O'Brien J, Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med. 2005;33(2):247–254. doi: 10.1177/0363546504266483. [DOI] [PubMed] [Google Scholar]
- 31.Augustsson J, Thomee R, Karlsson J. Ability of a new hop test to determine functional deficits after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):350–356. doi: 10.1007/s00167-004-0518-4. [DOI] [PubMed] [Google Scholar]
- 32.Gustavsson A, Neeter C, Thomee P. A test battery for evaluating hop performance in patients with an ACL injury and patients who have undergone ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):778–788. doi: 10.1007/s00167-006-0045-6. et al. [DOI] [PubMed] [Google Scholar]
- 33.Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC., III. Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37(3):262–268. [PMC free article] [PubMed] [Google Scholar]
- 34.Zouita Ben Moussa A, Zouita S, Dziri C, Ben Salah FZ. Single-leg assessment of postural stability and knee functional outcome two years after anterior cruciate ligament reconstruction. Ann Phys Rehabil Med. 2009;52(6):475–484. doi: 10.1016/j.rehab.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Henriksson M, Ledin T, Good L. Postural control after anterior cruciate ligament reconstruction and functional rehabilitation. Am J Sports Med. 2001;29(3):359–366. doi: 10.1177/03635465010290031801. [DOI] [PubMed] [Google Scholar]
- 36.Stergiou N, Ristanis S, Moraiti C, Georgoulis AD. Tibial rotation in anterior cruciate ligament (ACL)-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med. 2007;37(7):601–613. doi: 10.2165/00007256-200737070-00004. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament– reconstructed knees. Arthroscopy. 2009;25(7):760–766. doi: 10.1016/j.arthro.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Chouliaras V, Ristanis S, Moraiti C, Stergiou N, Georgoulis AD. Effectiveness of reconstruction of the anterior cruciate ligament with quadrupled hamstrings and bone–patellar tendon– bone autografts: an in vivo study comparing tibial internal-external rotation. Am J Sports Med. 2007;35(2):189–196. doi: 10.1177/0363546506296040. [DOI] [PubMed] [Google Scholar]
- 39.Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33(2):240–246. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 40.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 41.Hart JM, Garrison JC, Kerrigan DC, Palmieri-Smith R, Ingersoll CD. Gender differences in gluteus medius muscle activity exist in soccer players performing a forward jump. Res Sports Med. 2007;15(2):147–155. doi: 10.1080/15438620701405289. [DOI] [PubMed] [Google Scholar]
- 42.Susco TM, Valovich McLeod TC, Gansneder BM, Shultz SJ. Balance recovers within 20 minutes after exertion as measured by the Balance Error Scoring System. J Athl Train. 2004;39(3):241–246. [PMC free article] [PubMed] [Google Scholar]
- 43.Wells JG, Balke B, Van Fossan DD. Lactic acid accumulation during work: a suggested standardization of work classification. J Appl Physiol. 1957;10(1):51–55. doi: 10.1152/jappl.1957.10.1.51. [DOI] [PubMed] [Google Scholar]
- 44.Dunbar CC, Robertson RJ, Baun R. The validity of regulating exercise intensity by ratings of perceived exertion. Med Sci Sports Exerc. 1992;24(1):94–99. et al. [PubMed] [Google Scholar]
- 45.Morris M, Lamb KL, Hayton J, Cotterrell D, Buckley J. The validity and reliability of predicting maximal oxygen uptake from a treadmill-based sub-maximal perceptually regulated exercise test. Eur J Appl Physiol. 2010;109(5):938–938. doi: 10.1007/s00421-010-1439-1. [DOI] [PubMed] [Google Scholar]


