Abstract
We review the cellular and physiological mechanisms responsible for the regulation of blood flow in the retina and choroid in health and disease. Due to the intrinsic light sensitivity of the retina and the direct visual accessibility of fundus blood vessels, the eye offers unique opportunities for the non-invasive investigation of mechanisms of blood flow regulation. The ability of the retinal vasculature to regulate its blood flow is contrasted with the far more restricted ability of the choroidal circulation to regulate its blood flow by virtue of the absence of glial cells, the markedly reduced pericyte ensheathment of the choroidal vasculature, and the lack of intermediate filaments in choroidal pericytes. We review the cellular and molecular components of the neurovascular unit in the retina and choroid, techniques for monitoring retinal and choroidal blood flow, responses of the retinal and choroidal circulation to light stimulation, the role of capillaries, astrocytes and pericytes in regulating blood flow, putative signaling mechanisms mediating neurovascular coupling in the retina, and changes that occur in the retinal and choroidal circulation during diabetic retinopathy, age-related macular degeneration, glaucoma, and Alzheimer's disease. We close by discussing issues that remain to be explored.
Keywords: Blood flow, Retina, Choroid, Regulation, Functional hyperemia, Autoregulation, Pathology, Diabetic retinopathy, Microvasculature, Review
1. Introduction
1.1. Metabolic demands of the retina
The retina has the highest metabolic demands of any tissue in the body (Saari, 1987; Buttery et al., 1991). Studies utilizing oxygen microelectrodes (Alder et al., 1990) and immunohistochemical visualization of the activity of the enzyme cytochrome oxidase (Buttery et al., 1991) have shown that the outer segments of the photoreceptors are the most metabolically active layer of the retina. Because of the high metabolic activity of the retina, the ability to regulate blood flow is an essential feature of the mammalian retina. The conflicting requirements of sufficient blood supply and minimal interference with the light path to the photoreceptors have been met by the evolution of two vascular supplies – inherent intra-retinal vessels supply the inner two-thirds of the retina, while the choroidal circulation supplies the photoreceptors in the outer one-third of the retina. Further, in the retinas of primates, an avascular region at the fovea further facilitates high acuity vision.
1.2. Vascular supply of the retina
The human retinal vasculature is comprised of the central retinal artery, which enters the optic disc through the lamina cribrosa, where it branches into four principal intra-retinal arteries (Fig. 1A). Whilst termed retinal arteries, even the central retinal artery is only of a caliber of an arteriole and if accurate terminology is used, only retinal arterioles exist, not arteries. The arterioles bifurcate to form smaller arteriole branches and terminal arterioles, which feed into a capillary bed as they extend toward the peripheral retina. Retinal arterioles, due to the higher oxygen content of the blood they carry, are typically surrounded by a capillary-free zone, approximately 30–50 μm in diameter in monkeys (Okada and Ohta, 1994). The venous system of the retina has a similar arrangement with the central retinal venule leaving the eye through the optic disc to drain venous blood into the cavernous sinus. The terminal branches of the vessels, pre-capillary arterioles and post-capillary venules, are linked through anastomotic capillaries. Retinal capillaries are organized in an interconnecting two-layer network. A superficial layer is located in the nerve fiber and ganglion cell layers and a second lies deeper, in the inner nuclear and outer plexiform layers.
Fig. 1.
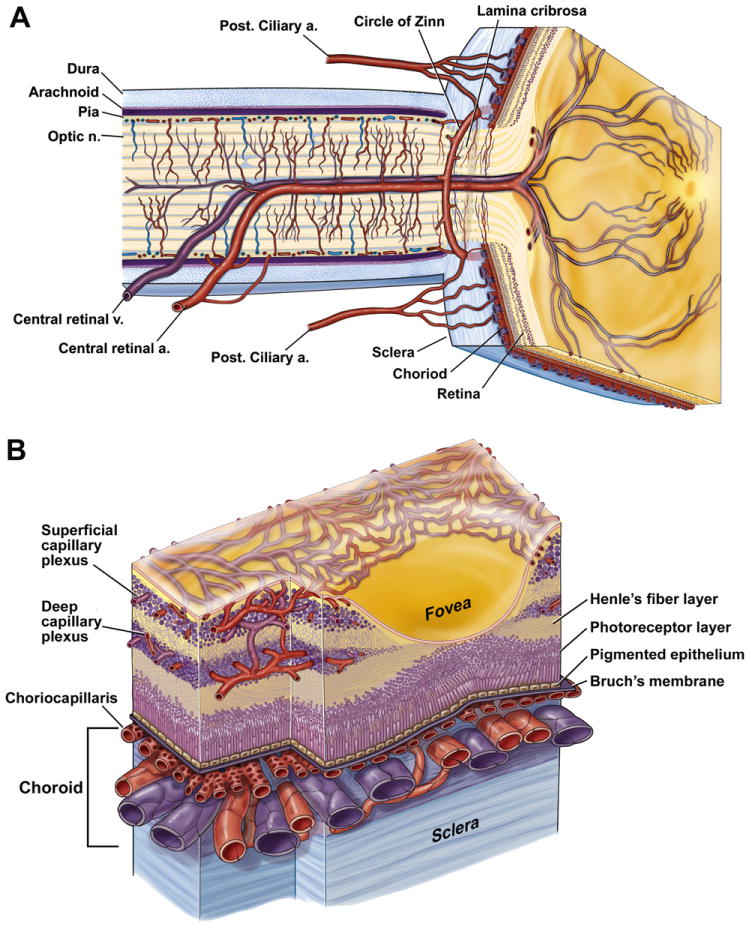
Anatomy of ocular circulation (a-artery, b-vein, n-nerve). A, Cut away drawing along the superior–inferior axis of the human eye through the optic nerve, showing the vascular supply to the retina and choroid. B, Drawing showing vasculature of the retina and choroid. Drawings by Dave Schumick from Anand-Apte and Hollyfield (2009).
In the mammalian retina, the vasculature in the nerve fiber and ganglion cell layers is known as the inner (or superficial) plexus, while the inner nuclear and outer plexiform layers receive blood from the deeper plexus located at the junction between them (Figs. 1B and 2A). The superficial plexus, contains arterioles, venules, and capillaries, while the deep vascular bed consists predominantly of capillary-sized vessels. Both the superficial and deep retinal plexus reach almost to the edge of the human (Fig. 2C) (Hughes et al., 2000; Chan-Ling et al., 2004a), cat (Chan-Ling et al., 1990), rat (Fig. 2D) (Stone et al., 1995) and mouse retina (Dorrell et al., 2002), except for a small avascular rim. The fovea, found only in primates, is also avascular; the thinness of the retina in this region permits adequate retinal oxygenation via the choroidal circulation (Engerman, 1976). The superior and inferior temporal vessels deviate in their paths to bypass the fovea and minimize their density in the temporal raphe region (Fig. 1A).
Fig. 2.
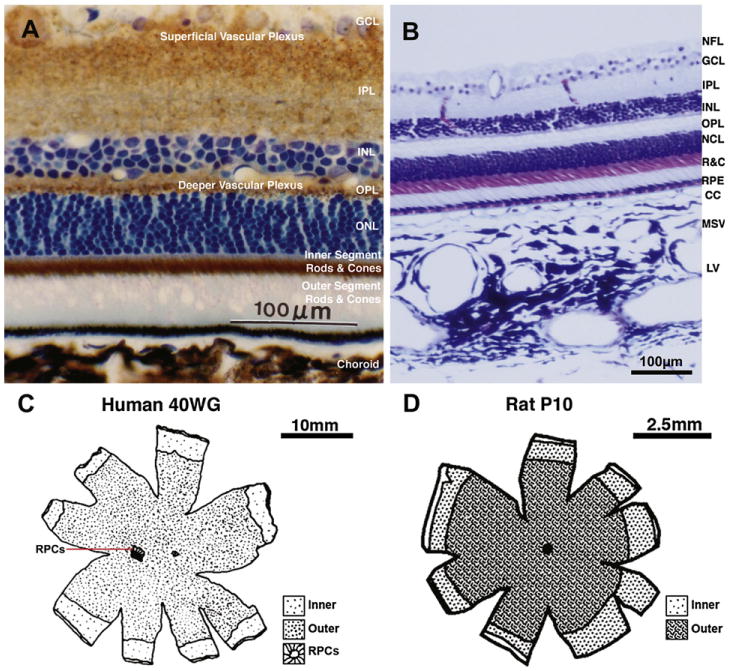
A, Transverse section of the rat retina, showing the relative expression of the metabolic enzyme cytochrome oxidase (COX) (reaction product in brown) throughout the retinal layers. COX histochemistry is used for mapping regional brain/retinal metabolism in animals, since there is a direct relation between enzyme activity and neuronal activity. The section has been counterstained with toluidine blue to demonstrate the location of cell nuclei layers. Note the highest level of cytochrome oxidase activity is in the inner segments of the rods and cones. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; inner and outer segments of the photoreceptors; from Buttery, PhD Thesis, 1990. B, Transverse section of the primate retina and choroid stained with Toluidine Blue and Nissl, showing retinal layers and the three vascular layers of the choroid. NFL, nerve fiber layer; R&C, rods and cones; RPE, retinal pigment epithelium; CC, choriocapillaris; MSV, medium and small choroidal vessels; LV, large choroidal vessels Chan-Ling, unpublished data. C, Map showing the outer limits of the inner (superficial) and outer (deep) vascular plexus as well as that of the radial peripapillary capillaries (RPCs) at 40 weeks gestation in the human retina (from Hughes et al., 2000). D, Map showing the outer limits of the inner (superficial) and outer (deep) vascular plexus at a postnatal day (P) 10 in the rat retina. No RPCs have been described in the rat retina to date; from Stone et al. (1995).
A third intra-retinal plexus, reported in the cat and human retina and known as the radial peripapillary capillaries (RPCs), is located in the nerve fiber layer in a small rim surrounding the optic nerve head (Chan-Ling et al., 1990; Hughes et al., 2000). These RPCs are located superficially around the optic nerve head, where the nerve fiber bundles are thickest, prior to exiting the retina (Henkind, 1967). Fig. 2C shows the extent of the RPC's surrounding the optic nerve head in the human. For detailed reviews of the retinal vasculature, see Chan-Ling (2008), Pournaras et al. (2008), Riva et al. (2011).
1.3. Fine structure of retinal vessels
In the human, the walls of the largest arterioles, near the optic disc, are comprised of five to seven layers of smooth muscle cells (tunica media). Smooth muscle actin filaments extend circumferentially around the retinal arterioles (Fig. 3A). After several branchings of the vascular network, the number of layers diminishes to just one or two in the retinal periphery. In retinal arterioles, the smooth muscle cells are orientated both circularly and longitudinally, each being surrounded by a basal lamina that contains an increasing amount of collagen toward the acellular adventitia (the tunica externa); reviewed in Pournaras et al. (2008). Endothelial cells (part of the tunica interna) are orientated longitudinally along the axis of the vessel and share their basement membrane with adjacent smooth muscle cells and pericytes. This basement membrane is composed of collagen IV, fibronectin, laminin, matrix metalloproteinases (MMPs-2, MMPs-9) and serine proteinase urokinase (UPA) and acts as an important regulatory matrix for the passage and sequestration of vasoactive agents and pro-survival growth factors; reviewed in Archer et al. (2007). In the smallest pre-capillary arterioles, the distribution of smooth muscle cells is frequently sporadic. Contrary to other vascular networks, the human retina lacks pre-capillary sphincters (Henkind and De Oliveira, 1968) and therefore the retinal capillaries are continuously perfused.
Fig. 3.
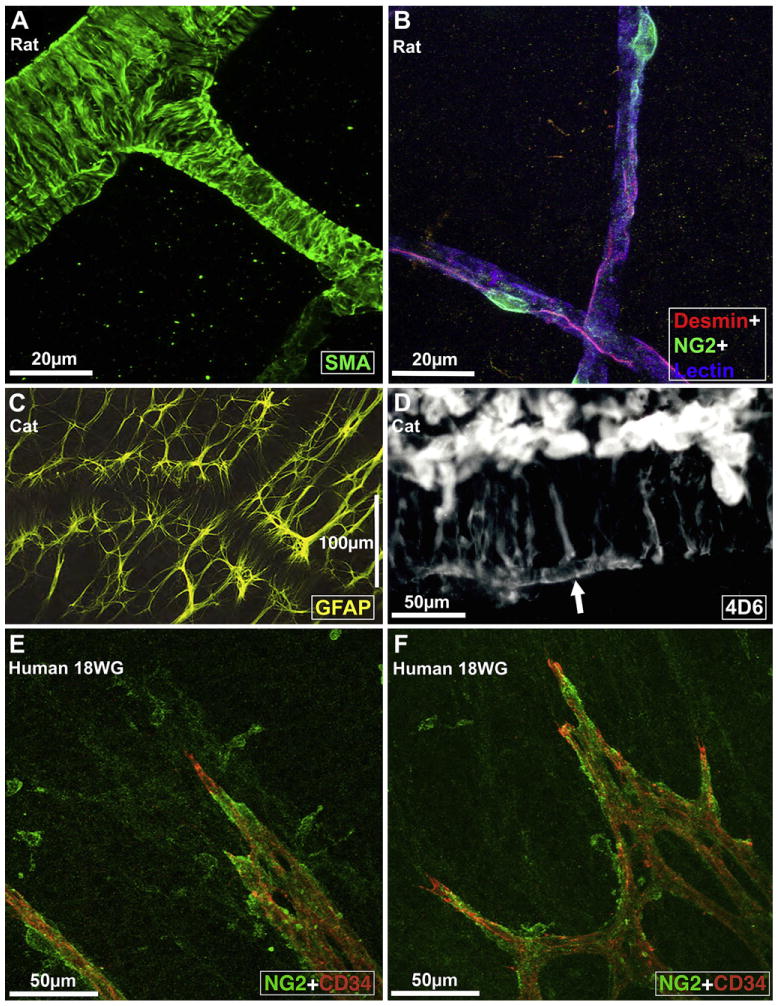
Fine structure of retinal vessels and glia ensheathment. A, Rat retinal arterioles (postnatal day 21) labeled for smooth muscle actin (SMA), a smooth muscle cell differentiation marker (from Hughes and Chan-Ling, 2004). B, Capillaries in the adult rat retina triple labeled with Griffonia Simplicifolia isolectin B4 (GS) lectin (blue), anti-desmin (red), and anti-NG2 (green); from Hughes and Chan-Ling (2004). C, Morphology of astrocytes immunolabeled with GFAP (at the level of the superficial vascular plexus) in the cat retina wholemount. Note close association between astrocytes, the vessel wall, and the nerve fiber bundles that run diagonally across the field of view; from (Chan-Ling, unpublished). D, The broken edge of a retinal wholemount, labeled with 4D6, a monoclonal antibody specific to Müller cells; from Dreher et al. (1988). The inner endfeet of Müller cells (top) are brightly fluorescent. At the outer margin of the inner nuclear layer Müller cell processes outline a capillary (arrow); from Dreher et al., 1988. E and F: Micrographs showing the intimate relationship between NG2+ pericytes and the CD34+ angiogenic tip cells of the developing retinal blood vessels in human retinal wholemounts at 18 weeks gestation. The NG2+ pericytes are located just ahead of the leading edge of patent CD34+ vessels; from Chan-Ling et al. (2011b).
The capillary unit consists of a continuous endothelium and intramural pericytes, which extend longitudinally along the capillary (Hughes and Chan-Ling, 2004) (Fig. 3B). Both cell types are in direct communication via gap junctional complexes (Oku et al., 2001) and share a common basement membrane. Regarding pericyte-to-endothelial cell ratios, a recent study of the human retina, utilizing ultrastructural criteria, showed a 94.5% frequency of pericyte coverage on human retinal capillaries (Chan-Ling et al., 2011b). Therefore, the retinal microvasculature is characterized by a uniquely high density of pericytes, substantially greater than that of human choriocapillaris, with an 11% relative frequency of pericyte coverage (Tilton et al., 1985; Chan-Ling et al., 2011b) or cerebral capillaries (Frank et al., 1987). In addition to numerous morphological characteristics, venules can be distinguished from arterioles by the size of the capillary-free zone around them; the zone is narrower around venules (Hogan and Feeney, 1963). The fine structure of venular muscle cells is similar to that of pericytes. Collagen fibrils are also seen in the outer layers of the basement membrane of these venules, and tend to increase in amount in the larger vessels (Ishikawa, 1963). Retinal microvessels are not-fenestrated and possess tight junctional complexes between the endothelial cells on their luminal aspect. The tight junctions represent the structural component of the inner blood-retinal barrier; for review see Chan-Ling (2006). Retinal arterioles, venules, and capillaries are closely ensheathed by macroglia. The superficial retinal vasculature is ensheathed by both astrocytes (Fig. 3C) and Müller cells, whilst the deep vascular plexus is ensheathed solely by Müller glia (Fig. 3D) (Holländer et al., 1991).
1.4. Vascular supply of the choroid
The choroidal circulation is derived primarily from the long and short ciliary arteries with some contribution from the anterior ciliary arteries. Histologically, the choroid is divided into five layers. Starting from the retinal side, these include Bruch's membrane, three vascular layers (the choroicapillaries, Sattler's layer and Haller's layer) and the suprachoroidea (Figs. 1B and 2B). Haller's layer includes large arteries and veins, while Sattler's layer is composed of medium and small arterioles that feed the capillary network of the choriocapillaris and venules. The choroidal arteries arise from the long and short posterior ciliary arteries and branches of Circle of Zinn (around the optic disc). The choriocapillaris is a highly anastomosed network of capillaries (with little or no basement membrane material), forming adense capillary network opposed to Bruch's membrane. Drainage of blood from the choroid is thought to occur exclusively through the vortex veins that ultimately merge with the ophthalmic vein (Ruskell, 1997). In contrast to the retina (Fig. 4G), choroidal microvessels are fenestrated (Bill et al., 1980), although the fenestrae are not as frequent in choroidal capillaries as in capillaries of other tissues (Chan-Ling et al., 2011a) (see Fig. 4H inset b). Unlike retinal vessels, the choroidal circulation is under neurogenic control. Sympathetic innervation includes noradrenergic and neuropeptide fibers (Bruun et al., 1984), whereas the parasympathetic nerves are primarily cholinergic (Bill and Sperber, 1990). For a comprehensive review on structure and function of the choroid see Nickla and Wallman (2010).
Fig. 4.
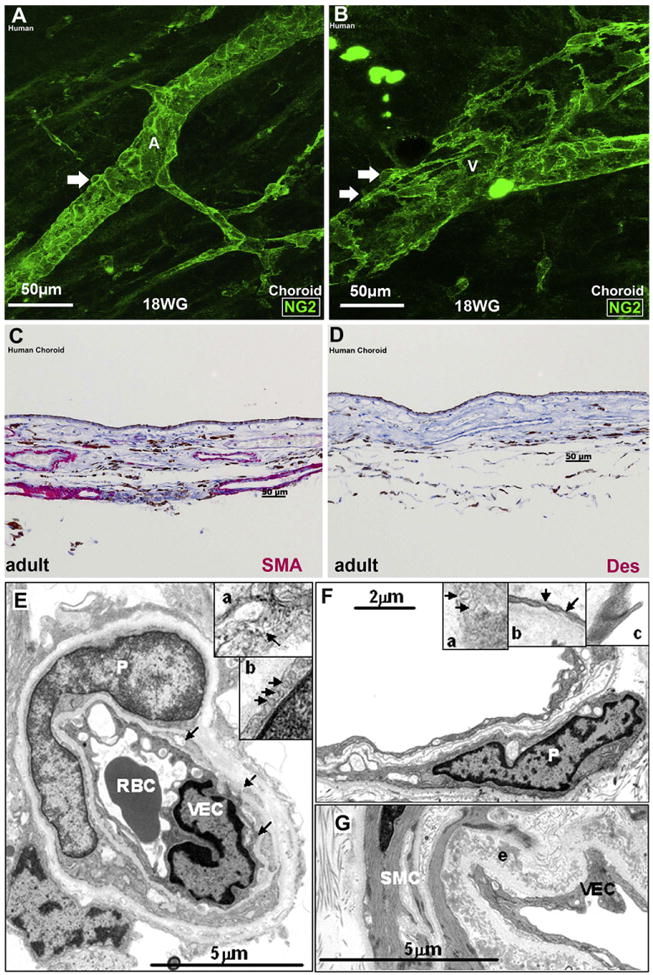
Pericyte and smooth muscle cell ensheathment of the retinal and choroidal vasculature. A and B: Human choroidal wholemounts at 18 weeks' gestation showing NG2+ somas (arrows) on the abluminal surface of an artery (A) and vein (V), respectively. Note that NG2+ pericyte ensheathment is more continuous in the artery than in the vein. C-D, Transverse sections of adult human choroid stained with SMA and desmin, respectively. (C) Most of the choroidal vessels are stained with SMA, (D) Desmin does not stain the choroidal vessels. The brown pigment in C and D is melanin. E, A transmission electron microscopy (TEM) of an adult human retinal capillary showing pericyte (P) ensheathment, red blood cell (RBC) and a vascular endothelial cell (VEC); arrows indicate fragments of pericyte cytoplasm. (Inset a) High magnification of a portion of the pericyte cytoplasm showing intermediate filaments (arrow). (Inset b) Portion of a pericyte showing pinocytotic vesicles (arrows). F, TEM of an adult human choroidal capillary with an associated pericyte (P). (Inset a) High magnification of the choroidal pericyte cytoplasm. Note the lack of filaments. Arrows: pinocytotic vesicles. (Inset b) High magnification of a vascular endothelial cell (VEC) showing fenestrae (arrows). (Inset c) High magnification of the junctions present in the VEC. G, TEM of an adult human choroidal arteriole. Well-developed smooth muscle cells (SMCs) with numerous organized thin filaments with focal densities are present. Pinocytotic vesicles and complete basal lamina are visible. An elastin (e) layer is also present in this vessel; from Chan-Ling et al. (2011b).
Although we designate vessels as arteries, arterioles, capillaries, venules and veins, the truth of the matter is that each segment of a vessel represents a continuum of vascular phenotype where the physiological characteristics as well as the proteins expressed and the cellular associations vary continuously along the vessel (Hughes and Chan-Ling, 2004). Thus, there are vessel segments that have characteristics of both arterioles and capillaries in certain parts of the retinal and choroidal vascular bed.
2. Development of retinal and choroidal circulation
Concomitant with the maturation of retinal neurons, the retina's vasculature develops to form an elaborate vascular tree that is well matched to the metabolic needs of the tissue (Chan-Ling et al., 1990). The formation of the intra-retinal vessels takes place via two distinct cellular processes under different molecular cues. Formation of the primordial superficial vessels of the central one-third of the human retina takes place via the process of vasculogenesis, the de novo formation of primitive vessels by differentiation from vascular precursor cells and formation of solid vascular cords followed by vessel patency. Formation of the remaining retinal vessels takes place via angiogenesis, the process of new vessel formation by budding or intussusceptive growth from existing blood vessels (Hughes et al., 2000). Thus, the outer two-thirds of the retina, the entire deep vascular plexus, and the increasing capillary density in the central one-third of the human retina is formed by the angiogenic process. In contrast, the human choroidal network appears to be established predominantly by hematopoietic differentiation and vasculogenesis with angiogenesis only adding to vascular density (Chan-Ling et al., 2011a).
In terms of creating the blood vessel unit, endothelial cell development concomitant with pericyte differentiation is the primary process see Fig. 3E and F and (Hughes and Chan-Ling, 2004). Subsequent morphogenic events consist of vessel guidance, branching, and recruitment of vascular-associated cells, including astrocytes, Müller cells, and macrophages. These events are critical for establishing functional circulation of the eye during development as well as during progression of neovascular disease. Only selected aspects of retinal vasculature development are discussed here, and the reader is referred to reviews by Provis (2001), Dorrellet al. (2007), Gariano(2003), Chan-Ling (2008), Anand-Apte and Hollyfield (2009) and Chan-Ling (2009) for a more complete description.
2.1. Roles of macroglia and macrophage in development of retinal vasculature
The angiogenic process of retinal vasculature development is regulated by oxygen levels within the retina. In response to ‘physiological hypoxia’ caused by the onset of neuronal activity (increased metabolic activity in maturing retinal neurons and photoreceptors), astrocytes and Müller cells respond by secreting vascular endothelial growth factor (VEGF165), inducing formation of superficial and deep layers of retinal vessels, respectively (Chan-Ling et al., 1990; Chan-Ling 1994; Chan-Ling et al., 1995; Stone et al., 1995; Zhang et al., 1999) (Fig. 5). Pericytes have also been suggested to express VEGF165, inducing the formation of retinal blood vessels in normal development (Darland et al., 2003). The importance of neuroglia in the development and maintenance of a healthy retinal plexus is supported by the fact that only species with retinal astrocytes have vascularized retinas (Schnitzer, 1988). Further, large numbers of proliferating astrocytes were shown to accompany the developing vessels as they migrate across the primate retina from the optic nerve (Sandercoe et al., 1999; Chan-Ling et al., 2009). For details on the relationship between the astrocytic and vascular cells lineages see Chan-Ling et al. (2011b, 2004a) and Dorrell et al., 2002. Although not directly proven, the close correlation in topography and timing between VEGF expression by neuroglia and vessel growth (Stone et al., 1995) supports the contribution of glial cells to vessel formation and survival. Recent observations, however, suggest astrocytes may also play an important role in vessel stabilization and pathological neovascularization (Scott et al., 2010; Weidemann et al., 2010). Therefore, astrocytes in the retina might have highly divergent roles during developmental, physiological angiogenesis, and ischemia-driven, pathological neovascularization.
Fig. 5.
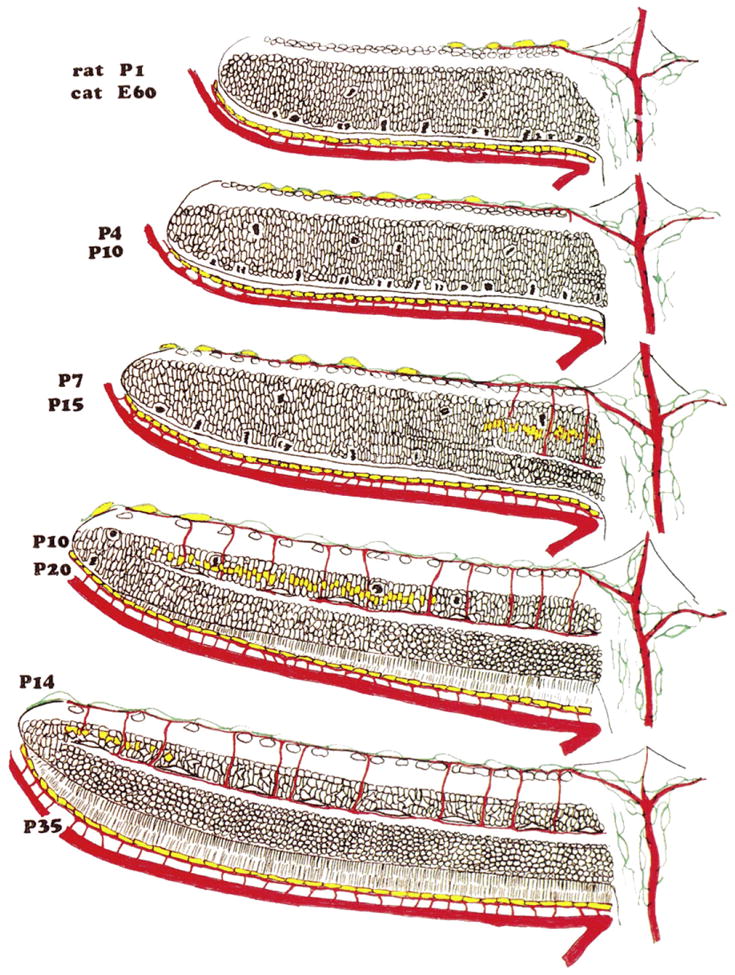
VEGF expression during formation of retinal blood vessels. Schematic representation of the retina from the optic disc (at right) to the periphery of the retina (at left) in rat and cat. Rat P1/Cat E60: The neural retina is comprised of two cellular layers. The outer (cytoblast) layer is still generating neurones, and mitotic figures are numerous at the outer surface of the neural retina, adjacent to the pigment epithelium. The choroid circulation is well formed, and the hyaloid artery extends through the optic disk to supply the vitreous body and the lens. Astrocytes (in green) have begun to migrate across the surface of the retina, and the superficial layer of vessels has begun to form. At this and subsequent ages, VEGF (in yellow) is expressed more strongly in the RPE than in astrocytes. Rat P4/Cat P10: The spread of astrocytes and superficial vessels has continued, and the first “descending” vessels have begun to bud from the superficial layer. VEGF expression is strong in the most peripheral astrocytes, but has faded in astrocytes near the optic disk. Rat P7/Cat P15: The separation of the cytoblast into the inner and outer nuclear layers, by the formation of the outer plexiform layer, has begun near the optic disk; where this separation has taken place, cell division has halted. Astrocytes have reached the edge of the retina, the most peripheral of them expressing VEGF and the superficial layer of vessels has spread correspondingly. Expression of VEGF is now apparent in the INL and, correspondingly, some descending vessels have reached the level of the deep vascular plexus, near the optic disk. Rat P10/Cat P20: The division of the retina into its layers is almost complete. VEGF expression in astrocytes has subsided, and expression in the INL is restricted to the peripheral margin of the retina. Rat P14/Cat P35: The formation of the neural retina and its vasculature is essentially complete. VEGF expression by astrocytes has subsided and expression in the INL is restricted to the peripheral margin of the retina; with modification from Stone et al. (1995).
Tissue resident and recruited macrophage populations have been implicated in developmental and pathologic neovascularisation (Polverini et al., 1977). However, recent studies have modified this earlier concept of macrophage association (Penfold et al., 1990; Checchin et al., 2006) to the concept that tissue macrophages act as cellular chaperones for vascular anastomosis (Fantin et al., 2010). Blood vessel networks expand in a two step process that begins with VEGF-A mediated angiogenic sprouting. This sprouting is induced by chemotactic gradients of VEGF-165 which stimulate proliferation of specialized vascular endothelial cells, termed tip cells (Gerhardt et al., 2003). The angiogenic process of vascular sprouting is following by vessel anastomosis. Utilizing both mouse mutants defective in macrophage development or VEGF signaling and live cell imaging in zebrafish, Fantin et al. (2010) found that macrophages that accumulate at sites of vessel fusion, are bridging tip cells from different vessel segments. Thus, macrophages play a major role in mediating the process of vascular anastomosis, a process central to increasing vascular density and establishing functional blood flow through a developing vascular plexus.
Cell adhesion and extracellular matrix molecules play an instrumental role in regulating the relationship between proliferating endothelial cells and their environment. Although the precise nature of the molecular interaction is not clear, evidence suggests that astrocytes guide endothelial cell growth and migration through R- cadherin (traditionally viewed as a neuronal cue) (Dorrell et al., 2002) and the selective expression of VEGF isoforms (Stalmans et al., 2002). Further, some isoforms of VEGF are soluble while others are bound; reviewed in Neufeld et al. (1999), Robinson and Stringer (2001). In this regard, it is of interest that cell sensitivity to soluble angiogenic mitogens may be modulated by physical interactions between cells and the extracellular matrix that alter cell shape and cytoskeletal structure. The mechanisms by which mechanical signals integrate with micro environmental cues to regulate neovascularization involve Rho kinase that modulates the balance between two antagonistic transcription factors, which in turn govern expression of the VEGF receptor VEGFR2 (Mammoto et al., 2009). Further, changes in pulsatile flow were shown to modulate expression of endothelium-derived vasoactive substances that control retinal pericyte apoptosis and proliferation (Walshe et al., 2011).
2.2. Comparative physiology of retinal and choroidal vascular development
While less is known about the cellular mechanisms and molecular cues that regulate the growth of blood vessels in the choroid than in the retina, recent studies have added to the understanding of the formation of the human choroidal vasculature (Hasegawa et al., 2007; Chan-Ling et al., 2011b, 2011a). It is evident, that choroidal vessels form earlier than those in the retina. In humans, the first retinal vessels form at the optic disc at approximately 14 weeks gestation (Ashton, 1970; Hughes et al., 2000), whereas primitive endothelium-lined elements – giving rise to choroidal vessels, are present as early as 29 days gestation (Ozanics et al., 1978) and an extensive patent choriocapillaris is evident an 8 week gestation (Chan-Ling et al., 2011a). These studies have shown that the first process in the formation of the human choroidal vasculature is the recruitment of circulating stem cells. This is followed by the process of vasculogenesis which takes place over the entire human choroid and is responsible for the primary formation of the choroidal vasculature, while angiogenic budding adds further to choroidal vascular density. Since vasculogenesis takes place independently of VEGF165; reviewed in Hughes et al. (2000), future studies need to determine the growth factors that regulate this process if a treatment for wet age-related macular degeneration is to be developed. Further differences between retinal and choroidal vascular beds are evidenced by the fact that endothelial cell proliferation in the choriocapillaris is not promoted by increased metabolic activity in the central retinal neurons (Allende et al., 2006). Further, during choroidal development, but not retinal development, nerve growth factor (NGF) was shown to regulate endothelial cell migration and proliferation (Steinle and Granger, 2003). In the developing mouse, disruption of the RPE-derived VEGF caused dramatic defects, including the absence of the choriocapillaris or a decrease in choroidal vascular density (Marneros et al., 2005; Le et al., 2010). These findings suggest that mechanisms regulating development of the choroidal and retinal vasculature are profoundly different.
2.3. Mural cell lineage
The same mural precursor cells (MPCs) of mesenchymal origin, likely give rise to both smooth muscle cells and pericytes, and the pattern of protein expression in these cells has been used to determine the point at which differentiation takes place (Hughes and Chan-Ling, 2004; Chan-Ling et al., 2011a). In the embryonic rat retinal vascular plexus, multiprocess MPCs express both NG2+, a transmembrane chondroitin sulfate proteoglycan, and smooth muscle actin (SMA). Around the time of birth, these cells begin to ensheath the maturing retinal vasculature, stop expressing SMA, and start expressing desmin, an intermediate filament protein typically expressed by pericytes. A continuum of phenotypes between the desmin+/−/NG2+/SMA+ MPCs and fully differentiated desmin+/−/NG2+/SMA+ smooth muscle cells and desmin+/NG2+/SMA+/− pericytes accompanies the development of the retinal vasculature, suggesting that the ensheathing MPCs are pluripotent cells capable of differentiating into both SMCs and pericytes as modulated by the vascular microenvironment. NG2+ pericytes are located just ahead of the leading edge of patent CD34+ vessel formation in the embryonic human retina (Fig. 3E and F). In the developing human choroid, cells with ultrastructural characteristics consistent with single, isolated MPCs are also seen scattered throughout the stroma (Chan-Ling et al., 2011a). NG2+ mural cells were seen closely ensheathing the vessels of the human choroid from an early age (Fig. 4A and B).
2.4. Pericyte intermediate filaments
In stark contrast to rat retinal pericytes (Hughes and Chan-Ling, 2004), recent ultrastructural and immunohistochemical studies of the human choroid demonstrate that choroidal pericytes do not express desmin, smooth muscle actin or intermediate filaments. Larger choroidal vessels, however, are SMA positive Fig. 4C; (Chan-Ling et al., 2011b). Immunohistochemistry staining for desmin, an accepted pericyte marker, confirmed this negative result in human choroidal pericytes (Fig. 4D). Desmin has been shown to be required in vascular smooth muscle cells in small resistance arteries for efficient control of agonist-induced vascular tone (Loufrani et al., 2002b) and microvascular remodeling (Loufrani et al., 2002a). A lack of desmin has also been noted in models of arteriogenesis (Cai et al., 2004) and may be important in the damage caused during desmin-related myopathies (Olive et al., 2004). In vimentin knockouts, mice were normal but their cells lacked structural stability. Functionally, this meant reduced pericyte motility and directional migration as well as diminished wound repair (Eckes et al., 2000). While further studies are required to fully understand the differences in retinal and choroidal pericytes, the lack of intermediate filaments in choroidal pericytes and the relative infrequency of pericyte ensheathment observed in the choroidal vasculature compared to retinal capillaries (Fig. 4E–G) (Chan-Ling et al., 2011b) suggests that choroidal pericytes have little contractile capability. Thus, choroidal pericytes, unlike retinal pericytes, may have a limited ability to control vascular tone (Chan-Ling et al., 2011b).
2.5. Expression of calponin and caldesmon – calcium regulating proteins
Calponin and caldesmon are associated with the thin filaments of smooth muscle cells and are thought to regulate tone in these cells; reviewed in (Akata, 2007). The timing and topography of calponin and caldesmon expression during development of the rat retinal vasculature suggest that smooth muscle cells' maturation indicated by expression of these two regulatory proteins, improves capacity to fine-tune autoregulatory responses (i.e. to changes in tissue oxygen levels) and may playan important role in the development of vessel stability (Hughes and Chan-Ling, 2004) (Fig. 6A). The extent of SMA expression by smooth muscle cells on retinal arterioles and venules increases markedly with smooth muscle cell maturation, and is followed first by calponin expression and then by caldesmon expression. In the choroid, calponin is only expressed on the larger vessels and caldesmon is not present (Chan-Ling et al., 2011b). The fact that SMA ensheathment in choroidal vessels is rather incomplete (Fig. 6B and C), caldesmon is not expressed at all and calponin is only expressed in a small proportion of vessels (Fig. 6D and F), provides further structural evidence for the limited blood flow regulatory ability of the choroid.
Fig. 6.
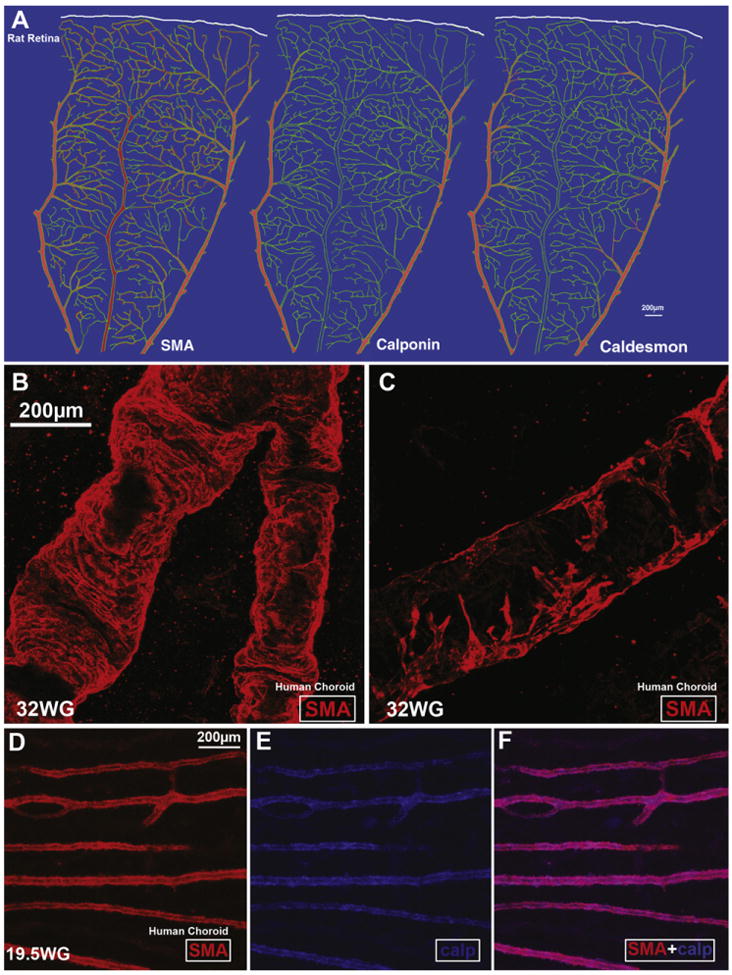
Expression of smooth muscle actin (SMA), calponin and caldesmon in the retinal and choroidal vasculature. A, Schematic representation of the extent of SMA, calponin, and caldesmon expression (red) in the vasculature (green) of an adult rat retina. Note that SMA expression extends the furthest, reaching the secondary arterioles with weaker expression on capillary and post-capillary venules. Caldesmon expression extends partially past the first branch point from the primary arteriole. In contrast, calponin expression is no longer detectable past the first branch point from the primary arterioles; from Hughes and Chan-Ling (2004). B–F, SMA staining in arterioles (B) and venules (C) in the human choroid at 32 weeks' gestation. Note the incomplete coverage of the venule with SMA filaments. (D–F) Human choroid at 19.5 weeks gestation labeled with calponin and SMA. Calponin and SMA are present only on the larger vessels (from Chan-Ling et al., 2011b).
3. Techniques for monitoring retinal and choroidal blood flow
3.1. Retinal blood flow
A variety of techniques have been developed to monitor retinal and choroidal blood flow. The Doppler effect has been employed in measuring the velocity, volume, and flux of blood through the capillaries and larger vessels of the optic nerve head and superficial retina. The reader is referred to reviews by Feke (2006), Riva and Falsini (2008). In the laser Doppler technique, the frequency of reflected laser light changes when scattered by red blood cells moving through vessels. The magnitude of the frequency shift is used to calculate the relative red blood cell velocity and density. The product of the velocity and density yields a measure of the blood flux. If the scattered laser light is observed in two directions, an absolute measure of the red blood cell velocity can be determined by a method known as bi-diretional laser Doppler velocimetry (Riva et al., 1981a; Garhofer et al., 2004a).
Laser Doppler flowmetry yields a measure of blood flow at a single region on the optic disc or retina. A two-dimensional picture of blood flow can be obtained by combining laser Doppler flowmetry and scanning laser ophthalmoscopy. In this technique, termed scanning laser Doppler flowmetry, the laser beam is rapidly scanned in a raster pattern across the surface of the retina, yielding laser Doppler measurements at multiple points (Michelson et al., 1996b).
Two-dimensional images of retinal blood flow can also be obtained using a related technique, laser speckle flowmetry (Cheng and Duong, 2007; Srienc et al., 2010; Nagahara et al., 2011). The retina or choroid is illuminated with a diffuse, coherent light and is imaged with a digital camera. Under coherent illumination, stationary regions of the retina appear speckled while moving regions (red blood cells moving through vessels) are less speckled. Quantitative evaluation of speckle contrast yields a two-dimensional image of blood flow with high spatial and temporal resolution.
Vascular responses to light stimulation can also be monitored by measuring the diameter of retinal arterioles. Real-time measurements of variations in the diameter of retinal vessels can be made with the retinal vessel analyzer (RVA) (Blum et al., 1999). However, the RVA can only be used for studying large vessels. For details on the reproducibility and sensitivity of the RVA see Polak et al. (2000). Vessel diameter can be also determined with high precision with confocal microscopy by using the line scan technique to repeatedly image a vessel along a line perpendicular to the vessel lumen (Seeliger et al., 2005; Srienc et al., 2010).
The velocity and density of white blood cells flowing through the macular vasculature can be determined using blue field stimulation, i.e. entoptic phenomenon, a technique in which a subject looks at a diffuse 430 nm blue light (Riva et al., 1981b). The blue light is absorbed by red blood cells, but not by white blood cells. Thus, as the occasional white blood cell interrupts the flow of red cells through the capillaries, the subject sees a field of “flying corpuscles.” For quantification, the subject is asked to match the perceived speed and density of the corpuscles they see to a set of simulated animations. Responses obtained with blue field stimulation are associated with blood flow in the temporal, but not nasal vessels, because the perifoveal capillaries are exclusively supplied by the temporal branches of the central retinal artery (Kiss et al., 2002).
Retinal blood flow can also be measured by video fluorescein angiography, which relies on rapid injection of a small bolus of fluorescein and the recording of retinal images detailing the passage of the dye with a scanning laser ophthalmoscope. The mean circulation transit time is determined using this technique (Bursell et al., 1992).
Global volumetric retinal blood flow, that reflects the overall retinal response to stimuli such as flicker, can be evaluated using Doppler (Fourier-domain) coherence tomography (Singh et al., 2010; Wang et al., 2011a), a functional extension of the standard optical coherence tomography (OCT) method originally used to obtain high-resolution, cross-sectional images of the retina (Huang et al., 1991) or to measure retinal functional changes with light stimulus (Bizheva et al., 2006; Srinivasan et al., 2006). In principle, Doppler OCT measures velocity profiles of fluid flow by detection of Doppler shifts of back-scattered light. A variety of different OCT probing schemes for assessing retinal and/or choroidal perfusion dynamics have been developed recently. A comprehensive review on these techniques is given in Geitzenauer et al. (2011).
Although blood flow measurement with magnetic resonance imaging (MRI) has been very successful in brain studies, its applicability to retinal blood flow was demonstrated only recently. The approach utilizes contrast-free imaging with the arterial spin labeling technique, where the endogenous water in the inflowing blood is magnetically labeled non-invasively. By investigating retinal responses to hyperoxic/hypercapnic challenges and effects of isoflurane and ketamine/xylazine anesthesia in rats and mice, the authors demonstrated that blood-flow magnetic resonance imaging has the potential to compliment optically based imaging techniques (Li et al., 2008; Muir and Duong, 2011). The feasibility of imaging and quantification of retinal blood flow with MRI in humans have been also investigated. Although, a clear blood flow signal was visualized, it did not have sufficient spatial resolution to distinguish the retina and choroid (Maleki et al., 2011). This challenge was overcomein the study of blood volume changes in the rat retina. By utilizing a blood-pool contrast agent, functional responses of retinal and choroidal vessels to visual stimulation were simultaneously resolved (Shih et al., 2011). More traditional magnetic resonance imaging techniques have been applied to investigate changes in tissue oxygenation using blood-oxygen level-dependent (BOLD) functional signals, which reflects changes in blood flow associated with visual stimulation (Duong et al., 2002; Cheng et al., 2006).
3.2. Choroidal blood flow
Measuring blood flow in the choroidal circulation is particularly challenging because the choroidal vessels are hidden from view by the retinal pigment epithelium. This is why, traditionally, choroidal blood flow has been studied indirectly. One method used to estimate choroidal blood flow is based on laserferometry. This fundus camera-based system illuminates the eye with a single beam laser diode along the optical axis. The light reflected from the surface of the cornea and retina produce interference fringes from which the distance between these two structures can be calculated. During the cardiac cycle, this distance changes and is associated with the fundus pulsation amplitude (FPA), an index of pulsatile choroidal perfusion. Alternatively, choroidal blood flow can be measured (also indirectly) with a computerized pneumotonometer, which records the cyclic variation in the intraocular pressure with each heartbeat (Silver and Farrell, 1994). Based on this measurement and the pressure–volume relation, the volume of blood entering the globe can be calculated (Silver and Geyer, 2000). For a comparison between laser interferometric measurement of fundus pulsation and pneumotonometric measurement of pulsatile ocular blood flow see Schmetterer et al. (2000a, 2000b). Response of the choroidal vasculature to stimulation can also be quantified with near-infrared Doppler flowmetry (Petrig and Riva, 1991). Illumination of the fundus with infrared light (above 800 nm) is advantageous since light at this wavelength can penetrate the retinal pigment epithelium (Geeraets et al., 1962). Application of laser Doppler flowmetry to the measurement of relative choroidal blood flow is discussed elsewhere (Riva, 2006; Preitner et al., 2004; Polska et al., 2004).
The presence of the overlaying retinal circulation represents a common difficulty in evaluating choroidal blood flow. The analysis can be facilitated by using indocyanine green, which, unlike fluorescein, is strongly bound to plasma proteins limiting diffusion of the indicator through fenestrated choroidal capillaries (Prunte and Niesel, 1988; Ciulla et al., 2002). A second key property of indocyanine green is the peak absorption and emission wavelengths in the near-infrared range, ensuring penetration deeper into the tissue and a stronger choroidal signal. Two-dimensional maps of choroidal circulation, with resolution comparable to indocyanine green angiography, can be constructed using laser speckle flowmetry. Since laser speckle flowmetry targets only the erythrocytes moving intravascularly, it generates a high contrast image of the choroidal vessels (Watanabe et al., 2008; Srienc et al., 2010).
4. Regulation of retinal blood flow
The retina tends to maintain a constant blood flow in the face of variations in perfusion pressure, blood gasses and intraocular pressure. This is an intrinsic autoregulatory response since the potential influence of autonomic innervation can be excluded (Laties, 1967; Ye et al., 1990) and the contribution of circulating hormones and neurotransmitters on retinal vascular resistance is generally assumed to be negligible due to the blood-retinal barrier (Delaey and Van De Voorde, 2000b). Experimentally, autoregulation of the retinal microcirculation is assessed by provocation methods, including (i) a change in systemic blood pressure due to muscular exertion, postural change, or drug-induced effects (Blum et al., 1999; Tachibana et al., 1982; Polak et al., 2000), (ii) oxygen, carbogen, or CO2 inhalation, (iii) elevation of intraocular pressure with a suction cup (Ernest et al., 1972; Chen et al., 1993).
4.1. Pressure autoregulation and myogenic tone
It is well documented that autoregulation in the retina and optic nerve head is effective within a wide range of perfusion pressures (Fig. 7). In normal subjects, an experimental elevation of intraocular pressure up to 29 mmHg (resulting in a 36% decrease in perfusion pressure), is adequately compensated by retinal autoregulation (Riva et al., 1981b; Grunwald et al., 1984b). For greater increases in intraocular pressure, the retinal vasculature was shown to behave almost passively (Grunwald et al., 1988). The autoregulatory response was also demonstrated during periods of static exercise, where a 34% rise in perfusion pressure resulted in a rise in flow of only 4–8% (Dumskyj et al., 1996). Similar results were reported for experiments investigating retinal and optic nerve head blood flow during dynamic and static exercise (Harris et al., 1996a; Movaffaghy et al., 1998).
Fig. 7.
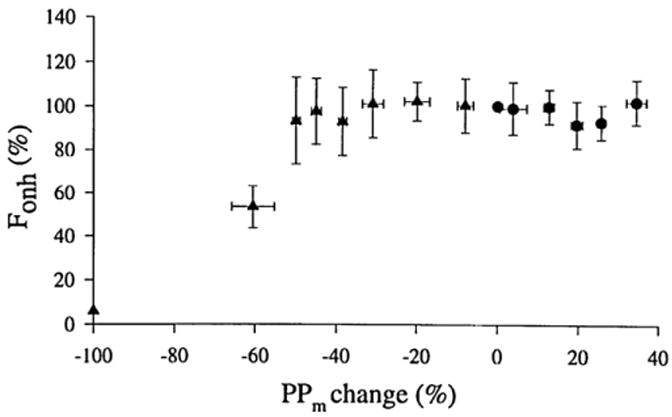
Autoregulation of blood flow in the retina. Blood flow in the human optic nerve head (Fonh) remains stable over a range of perfusion pressures (PPm). Perfusion pressure changes were induced either by increases in blood pressure (circles) or by elevation of intraocular pressure (triangles). Fonh values were normalized to 100% at baseline. The change in PPm is expressed as percentage of baseline pressure; from Movaffaghy et al. (1998).
The normal retinal hemodynamic response to increases in perfusion pressure is an increase in vascular resistance (Robinson et al., 1986; Dumskyj et al., 1996; Jeppesen et al., 2007). This behavior, termed the myogenic response, is intrinsic to smooth muscle cells, and independent of metabolic and hormonal influences. Active contractile responses to counteract increased transmural pressure were first described over a century ago (Bayliss, 1902) and several mechanisms have been advanced to explain this phenomenon. The prevailing view is that myogenic constriction is initiated by depolarization of vascular smooth muscle cells, leading to Ca2+ entry via voltage-gated Ca2+ channels (Knot and Nelson, 1998; Kotecha and Hill, 2005). However, depolarization-independent mechanisms also appear to be involved, as pressure-induced contraction is still observed after depolarization by potassium (Delaey and Van De Voorde, 2000a). One mechanism involved in the development of myogenic tone in isolated human retinal arterioles is the activation of the Rho kinase signaling pathway, which modulates contractile myofilament sensitivity to Ca2+ (Hein et al., 2010). The mechanistic basis of the myogenic response is reviewed in detail by Davis and Hill (1999).
4.2. Regulation by blood gases and pH
Evidence for regulation of retinal blood flow by blood gases comes from reports that breathing pure oxygen decreases retinal blood flow by ∼30% (Fallon et al., 1985). Retinal blood vessels are also sensitive to variations in the partial pressure of carbon dioxide (pCO2). Hypercapnic conditions (without hyperoxia) elicit a significant increase in retinal arteriolar and capillary diameter and blood flow (Alm and Bill, 1972b; Venkataraman et al., 2008). Acidosis-induced relaxation of tone in preconstricted isolated porcine retinal arterioles is associated with a hyperpolarization and a decrease in [Ca2+]i in the smooth muscle cells. The same study demonstrated that the vascular response to normocapnic and hypercapnic acidosis was similar, hence independent of CO2, and not mediated by NO (Hessellund et al., 2006). In isolated rat retinae, pharmacological inhibition of carbonic anhydrase, resulting in extracellular acidification, is associated with an increase in capillary diameter (Reber et al., 2003).
4.3. Responses to visual stimulation
In their classical paper published more than 100 years ago, Roy and Sherrington hypothesized that the brain possess an intrinsic mechanism by which its vascular supply can be varied locally in response to variations of functional activity (Roy and Sherrington, 1890). A substantial body of evidence demonstrates that, as in the brain, blood flow in the optic nerve head (Riva et al., 1991; Falsini et al., 2002) and retina is regulated by neuronal activity (Formaz et al., 1997; Garhofer et al., 2004a; Bek et al., 2008).
4.3.1. Light/dark transition
In humans, transition from light to dark conditions results in an increase in retinal blood flow of 40–70% (Feke et al., 1983; Riva et al., 1983) (Fig. 8A). This active hemodynamic response was attributed to an increase in blood velocity rather than to a change in the diameter of the larger retinal vessels, presumably due to a decrease in the hemodynamic resistance of downstream capillaries. The observation that systolic and diastolic blood flow velocities in the central retinal artery were markedly increased in darkness supports this view (Havelius et al., 1999). It is believed that an increase in retinal perfusion compensates for the elevated oxygen consumption in the dark by the photoreceptors (Linsenmeier, 1986).
Fig. 8.
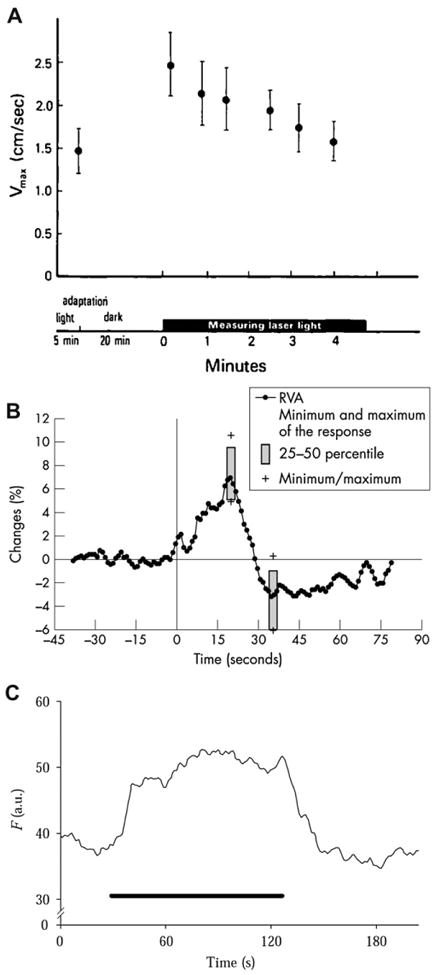
Change in retinal vascular diameter and retinal blood flow to light stimulation. A, Maximum red blood cell velocity (Vmax) measured in a vein using laser Doppler velocimetry after 5 min of fundus illumination and after 20 min of darkness in humans; from Riva et al. (1983). B, Mean arterial diameter response to flicker measured using the retinal vessel analyzer (RVA) in humans; from Nagel and Vilser (2004). C, Blood velocity response to flicker stimulation measured from the rim of the optic disc in cats with laser Doppler flowmetry; from Riva et al. (2005).
4.3.2. Flicker stimulation
Flicker stimulation evokes increases in retinal vessel diameter and retinal blood flow (Fig. 8B and C). A blood flow increase in the primate retina, evoked by flicker stimulation and measured using labeled microspheres, was reported by Bill and Sperber (1990). Since then, blood flow responses to luminance and chromatic modulation have been characterized (Polak et al., 2002; Riva et al., 2001; Falsini et al., 2002; Kotliar et al., 2004; Wang et al., 2011a) and employed for studying the relation between neuronal activity and vascular responses. For instance, a correlation between red-green heterochromatic flicker-induced changes in human optic nerve head blood flow combined with changes in harmonic components of the ERG response, suggests a contribution from both the inner and outer retina to neurovascular coupling (Falsini et al., 2002). Additional experiments demonstrate that blood flow responses are driven by both parvo and magnocellular pathways (Riva et al., 1991, 2005).
In humans, the temporal dynamics of arterial diameter changes to diffuse flicker reveal that neurovascular coupling occurs rapidly, within 1 s (reviewed by Riva et al., 2005). Upon cessation of a stimulus, arterial diameter and red blood cell velocity decrease to below baseline values, whereas vein diameter returns to baseline levels without an undershoot (Garhofer et al., 2004a; Nagel and Vilser, 2004). Flicker-induced dilatation of arteries can range from 1 to 7% (Garhofer et al., 2004a; Polak et al., 2002; Nagel et al., 2004). While some studies report no significant difference between arterial and venous responses to flicker (Lecleire-Collet et al., 2011), others find that dilation in veins and venules is less pronounced, ranging from 1 to 6% (Polak et al., 2002).
Several studies have demonstrated a correlation between flicker-induced increases in blood flow and changes in blood-oxygen levels. The partial pressure of O2 (pO2), measured with a phosphorescence sensor, showed that rat retinal arterial and capillary pO2 levels and arteriovenous pO2 differences increase in response to flickering light (Shakoor et al., 2006). In the same study, no change in choroidal pO2 was seen. Similar results were found in humans, where flicker stimulation resulted in an increase in venous oxygen saturation (sO2) (Hammer et al., 2011).
4.3.3. Retinal vascular responses to local light stimulation
Less data is available on local regulation of blood flow within the retina and is limited to animal studies. A functional magnetic resonance imaging study in cat demonstrated that a flickering stimulus, presented to one half of the retina, resulted in increased blood flow that was largely confined to that half of the retina (Fig. 9) (Duong et al., 2002). A laser speckle flowmetry study in rat showed that when the retina is stimulated with a small flickering spot, blood flow increases are largest in the stimulated region (Fig. 10) (Srienc et al., 2010). These studies demonstrate that the retina possesses mechanisms for regulating retinal blood flow at a local level.
Fig. 9.
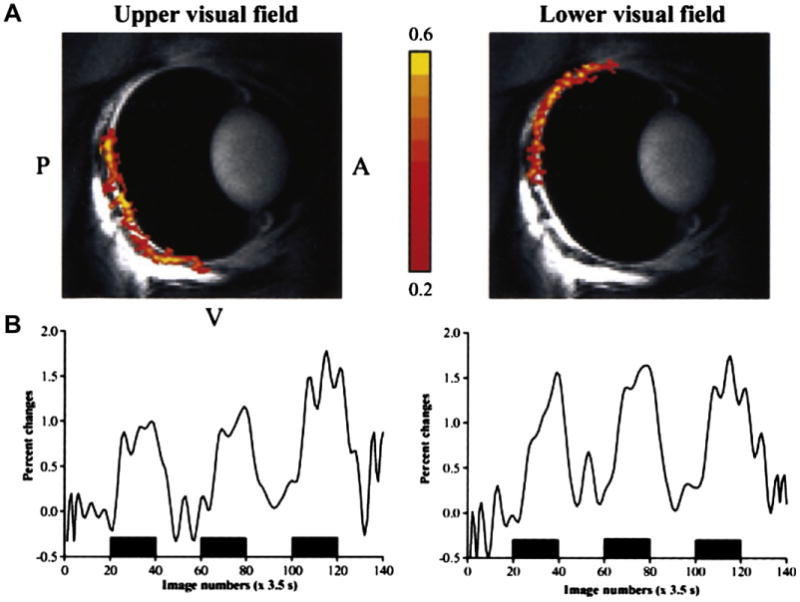
Local changes in blood flow evoked by focal stimulation. (A) Functional magnetic resonance imaging (fMRI) images of the cat eye stimulated with a drifting grating presented to the upper visual field (left) and the lower visual field (right). Red and yellow regions indicate increased blood flow. (B) Percent change in fMRI signals evoked by visual stimulation (black bars); from Duong et al. (2002).
Fig. 10.
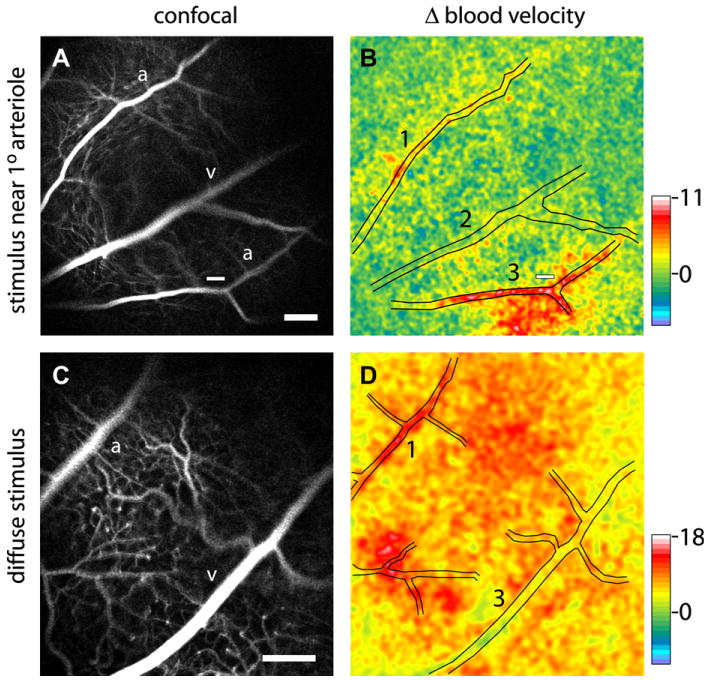
Blood velocity increases evoked by focal (top) and diffuse (bottom) flickering light stimulation in the rat retina. The left panels show confocal images of the retina and right panels show blood velocity changes measured with laser speckle flowmetry. Focal stimulation (small white bars in A and B) evokes a local increase in blood flow (red region in B) while diffuse stimulation evokes a blood flow increase over the entire retina (red and yellow regions in D). Pseudocolor scale bars indicate percent change in blood velocity; from Srienc et al. (2010).
4.4. Control of retinal arteriolar tone and smooth muscle contractility
The tone of retinal arterioles is dependent on changes in smooth muscle contractility influenced by free intracellular Ca2+. Calcium levels are regulated by the balance between Ca2+ influx/release into the cytoplasm and Ca2+-removal from the cell. Plasmalemmal ion channels play an important role in the regulation of intracellular Ca2+, both by providing pathways for Ca2+ entry and by regulating cell membrane potentials. In rat retinal vessels, activation of L-type Ca2+ channels in vessels >18 μm diameter produces a transient increase in intracellular Ca2+ (Scholfield and Curtis, 2000). K+ currents are important physiological regulators of resting membrane potential of vascular smooth muscle cells. Activation of voltage-dependent KV, BK (McGahon et al., 2007b, 2007a) and KATP channels (Hein et al., 2006; Ishizaki et al., 2009) hyperpolarize vascular smooth muscle cells, opposing constriction.
Calcium ions can indirectly control smooth muscle contractility by modulating BK channels activity. In rat cerebral arteries, the synchronized opening of BK channels by Ca2+ sparks (localized Ca2+ release events), was shown to evoke spontaneous transient outward current and vasodilatation (Nelson et al., 1995; Knot et al., 1998). More recently, spontaneous Ca2+ events in small retinal arterioles (diameter <40 μm) were shown to play a contractile role. Ca2+ events originating from ryanodine receptors in the sarcoplasmic reticulum (SR) shown to act as building blocks for more prolonged, global Ca2+ signals (Tumelty et al., 2007). Whether the contractile effect of local Ca2+ signals observed in isolated retinal arterioles is tissue specific or represents a feature of arterioles throughout the body remains to be addressed.
Retinal arteriolar myocytes also have a Ca2+-induced Cl− current, which may be activated by Ca2+ entry through L-type Ca2+ channels or Ca2+ release from intracellular stores. This current appears to contribute to agonist-induced retinal vasoconstriction (McGahon et al., 2009). In retinal vascular smooth muscle cells, Ca2+-removal via the Na+/Ca2+ exchanger, the SERCA pump and the plasma membrane Ca2+-ATPase have been shown to play an important role in regulating cytosolic Ca2+ levels (Scholfield et al., 2007).
4.5. Contribution of arterioles and capillaries to activity-induced responses
Investigations of functional hyperemia in the brain suggest that arterioles, rather than capillaries, are the primary site of blood flow regulation. Arteriolar flow changes following sensory or electrical stimulation occur at the same time (Matsuura et al., 1999; Hillman et al., 2007), or just before (Vanzetta et al., 2005) changes in capillary perfusion, suggesting that the predominant hemodynamic changes originate upstream of capillaries.
On the other hand, the fine spatial resolution of changes in cerebral blood flow following sensory stimulation suggests regulation of flow at the capillary level. Evidence for active regulation of cerebral capillary tone in vivo is more limited. A recent study demonstrated that although capillaries can actively change their diameter, active regulation of capillary diameter does not occur during sensory stimulation (Fernandez-Klett et al., 2010). Although the limited evidence available points to the initiation of blood flow increases at the arteriolar level, it remains to be determined whether arterioles are the primary site of blood flow regulation.
During neurovascular coupling, the local dilation of arterioles in an area of activation will not result in a substantial increase in blood flow unless upstream vessels also dilate. How vasodilator and vasoconstrictor responses are conveyed to upstream locations is unclear. Coordinated vasoactive responses may relyon coupling and communication between cells within the vessel wall. In central nervous system, vascular endothelial cells and smooth muscle cells are electrically coupled (Little et al., 1995). Hyperpolarization of an individual SMC can spread through endothelial cells and to smooth muscle cells via myoendothelial coupling and evoke a coordinated dilating response along the length of an arteriole (Zhang et al., 2011). Of note, the patterns of gap junctional staining within the rat retina shows regional specificity, suggesting a homogenous distribution of gap junction proteins Cxs37 and 40 expressed in large radiating arterioles but absent in smaller vessels (Kuo et al., 2008).
4.6. Pericyte regulation of retinal capillary diameter
Several lines of evidence suggest that pericytes may contribute to active regulation of blood flow in the retina. For reviews, see Chakravarthy and Gardiner, 1999; Puro, 2007; Hamilton et al., 2010. However, definitive experiments have not, as yet, been conducted (see also Section 4.5).
It is well established that pericytes possess the complement of muscle contractile proteins (Herman and D'Amore, 1985) and are capable of initiating vasomotor signals that can be propagated along the length of capillaries to other pericytes (Peppiatt et al., 2006; Puro, 2007). They constrict in response to angiotensin II (Matsugi et al., 1997b), norepinephrine (Markhotina et al., 2007), GABA antagonists (Peppiatt et al., 2006) and ATP (Kawamura et al., 2003) (Fig. 11). In turn, vasoactive molecules such as vasoactive intestinal peptide (VIP), pituitary adenylate cyclase activating peptide (PACAP; Markhotina et al., 2007) and NO donors (Haefliger and Anderson, 1997; Haefliger et al., 1997) relax retinal pericytes.
Fig. 11.
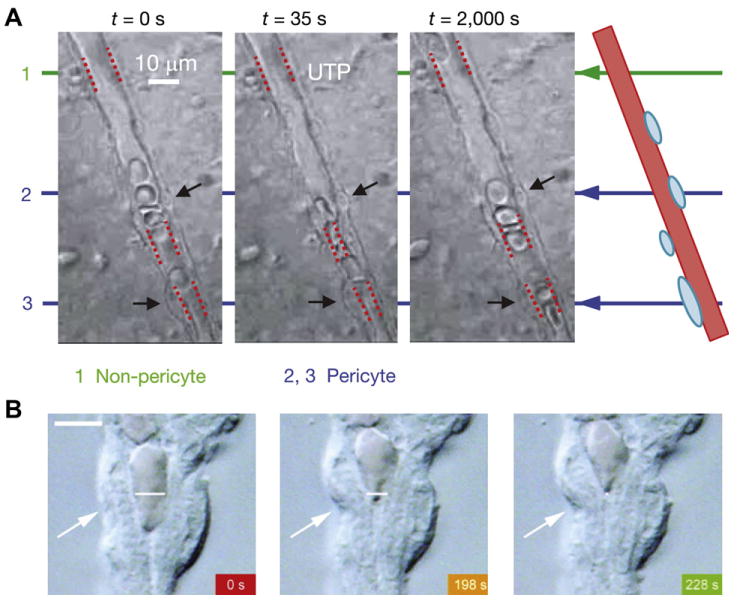
Contractile responses of retinal pericytes. A, UTP-induced constriction of a capillary lumen adjacent to two pericytes (indicated by black arrows) in the rat retina; from Peppiatt et al. (2006). B, Cholinergic agonist-induced constriction of a pericyte-containing capillary from a freshly isolated adult rat retina. The arrows point to a pericyte that constricts during exposure to oxotremorine-M. Scale bar, 5 μm; from Wu et al. (2003a).
The mechanisms responsible for regulation of pericyte contractile tone are similar to those of vascular smooth muscle cells. Pericytes express a variety of ion channels and transporters (i.e. Na+/Ca2+ exchangers; Yamanishi et al., 2006); reviewed by Puro (2007), that are likely to regulate pericyte function and mediate changes in capillary diameter. K+ channels regulate the resting membrane potential and excitability of retinal pericytes (Quignard et al., 2003; Ishizaki et al., 2009; Matsushita and Puro, 2006). Activation of these currents represents a common signaling pathway for mediating a decrease in pericyte contractile tone in response to prostacyclin (PGI2; (Burnette and White, 2006)), sodium nitroprusside (Haefliger et al., 1994), and β-adrenoceptor agonists (Quignard et al., 2003).
As in vascular smooth muscle cells, the change in pericyte contractile tone correlates with intracellular Ca2+ concentration (Sakagami et al., 1999, 2001; Wu et al., 2003a). Thus, ATP constricts retinal capillaries by raising pericyte intracellular Ca2+ through P2X7 and P2Y receptors (Kawamura et al., 2003). Depolarizing chloride current that is dependent, in part, upon the Ca2+ influx through voltage-dependent (i.e. VGCC) and voltage-independent (i.e. NSCs) channels also contributes to pericyte constriction (Sakagami et al., 2001, 1999). The repetitive opening of chloride channels is thought to enhance net Ca2+ influx during prolonged exposure of pericytes to serum derived molecules, i.e. insulin-like growth factor 1 (IGF-1; Sakagami et al., 1999).
The tone of pericyte-containing microvessels is thought to be governed not only by a balance between Ca2+-mediated contractility and NO-mediated relaxation, but also by a phenomenon termed Ca2+-sensitization. Emerging data from morphometric analysis of pericyte shape and contractile phenotype suggests a contribution of Rho signaling to modulation of actomyosin-based contractility in these mural cells (Kutcher et al., 2007).
5. Mechanisms of functional hyperaemia
The mechanisms that mediate functional hyperemia remain controversial. For many years, metabolic feedback mechanisms were thought to mediate the regulation of blood flow in response to changes in neuronal activity. As originally proposed by Roy and Sherrington, metabolic feedback would work as follows: Increases in metabolism which accompany neuronal activity would lower O2 and glucose levels and produce vasoactive metabolites (Roy and Sherrington, 1890). These metabolites would elicit vasodilation and increased blood flow, which, in turn, would restore O2 and glucose levels. In the brain, these metabolites include adenosine, lactate, CO2, protons (acidification) and a drop in O2.
Adenosine levels rise with neuronal activity and could mediate functional hyperemia as adenosine dilates arterioles following activation of vascular smooth muscle A2A receptors and subsequent opening of KATP channels (Gidday et al., 1996; Hein et al., 2005). Adenosine also relaxes cultured retinal pericytes by activation of the A2 receptor-adenylate cyclase-cAMP system (Matsugi et al., 1997a) and enhances capillary blood flow in the optic nerve head by activation of ATP-sensitive potassium channels through the A1 and A2a receptor pathway (Hirao et al., 2004). Lactate is released by both neurons and glial cells following neuronal activity and can mediate vascular dilation. In the retina, flickering light elicits increases in extracellular lactic acid (Ames et al., 1992; Wang et al., 1997). Further, lactate infusion decreases retinal arterial flicker response in humans (Garhofer et al., 2003).
Although products of neuronal metabolism do modulate vascular tone, it is not clear whether they play a primary role in mediating functional hyperemia in the brain or in the retina; reviewed in Attwell et al. (2010). Neuronal activity results in the release of many other factors that are also vasoactive. Active neurons release the vasodilatory agent NO and in both the brain and retina. In the cerebellum blocking NO production reduces activity-dependent vasodilation (Yang and Iadecola, 1997; Iadecola et al., 1995). Light-evoked hyperemia in the optic nerve head and in the retina is reduced by lowering NO with nitric oxide synthase (NOS) blockers (Buerk et al., 1996; Kondo et al., 1997; Dorner et al., 2003). In addition, recent work in both the brain and the retina indicate that arachidonic acid (AA) metabolites, produced by glial cells or as a consequence of glial cell activity, can also elicit vasodilation and increased blood flow; for review see Attwell et al. (2010). When production of these metabolites is blocked, functional hyperemia is substantially reduced, indicating that these factors play an important role in mediating functional hyperemia. Undoubtedly, multiple mechanisms contribute to the functional hyperemia response, both in the retina and in the brain. Although the question is by no means resolved, it is likely that feed forward mechanisms, in which products of neuronal activity, including NO, K+ and AA metabolites, elicit vasodilation and contribute substantially to functional hyperemia. It remains to be determined whether metabolic feedback mechanisms also contribute significantly to functional hyperemia. Specific mechanisms that could contribute to functional hyperemia in the retina are reviewed in the following sections.
5.1. Potassium signaling
Potassium is a vasoactive agent at low concentrations. When K+ levels surrounding arteries and arterioles are increased modestly (up to ∼10 mM), the vessels dilate. Potassium acts by two mechanisms to dilate vessels. It increases the open probability of inwardly rectifying K+ (Kir) channels in smooth muscle cells, hyperpolarizing the cells and lowering cytoplasmic Ca2+ levels (Knot et al., 1996). It also raises the activity of the smooth muscle cell Na+/K+ ATPase, which also results in cell hyperpolarization (Horiuchi et al., 2002). (At concentrations above 15 mM, K+ constricts vessels by depolarizing smooth muscle cells.)
Extracellular K+ levels, [K+]O, within the inner retina rise significantly with light stimulation (Karwoski et al., 1985). The increase in [K+]O in the cat retina in response to a flickering light coincides with the increase in blood flow (Buerk et al., 1995), suggesting that the excess K+ resulting from neuronal activity contributes to functional hyperemia. A K+-driven vasodilation could be mediated by an efflux of K+ from glial cells, a mechanism termed K+ siphoning (Paulson and Newman, 1987; Kofuji and Newman, 2004; Filosa et al., 2006). This K+ siphoning mechanism would work as follows: Potassium that is released from active neurons into the extracellular space flows into glial cells, depolarizing the cells and generating an efflux of K+ from other cell regions. There is a high density of K+ channels on glial cell endfeet which ensheath blood vessels (Newman, 1986). Thus, the K+ released from neurons is directed through glial cells onto vascular smooth muscle cells, where it elicits vasodilation. In the retina, both astrocyte and Müller cell endfeet have high K+ conductance and ensheath blood vessels and both types of glial cells could mediate, in theory, functional hyperemia. KIR 4.1 is the principal K+ channel in retinal Müller cells (Kofuji et al., 2000, 2002). This channel mediates the generation of field potentials and the regulation of extracellular K+ in the retina (Kofuji et al., 2000).
The K+ siphoning hypothesis of functional hyperemia has been tested in the retina (Metea et al., 2007). Individual astrocytes and Müller cells were depolarized by current pulses applied through a patch pipette. Although adjacent vessels displayed light-evoked vasodilation and dilated when exposed to elevated K+ in the superfusate, they did not dilate in response to glial cell depolarization. In addition, light-evoked vasodilations were not reduced in Kir4.1 KO animals where K+ efflux from glial cell endfeet should be absent. Both results demonstrate that, contrary to the K+ siphoning hypothesis, K+ efflux from glial cells does not contribute significantly to functional hyperemia, at least in the retina (Metea et al., 2007).
Nelson, Filosa and colleagues have suggested an alternate mechanism by which K+ could mediate functional hyperemia. They propose that neuronal activity results in the opening of BK channels on astrocyte endfeet, mediated by increases in Ca2+ and, perhaps, arachidonic acid metabolites (Gebremedhin et al., 2003; Filosa et al., 2006). The opening of BK channels would generate an efflux of K+ from astrocyte endfeet onto blood vessels, producing a localized elevation in [K+]o and leading to vasodilation. Blocking BK channels reduces vasodilation in the brain, suggesting that this K+-based mechanism contributes to functional hyperemia (Filosa et al., 2006). Although retinal Müller cells express BK channels (Newman, 1985; Bringmann et al., 1999), there is no direct evidence that K+ efflux from BK channels contributes to functional hyperemia in the retina.
5.2. Arachidonic acid metabolite-mediated signaling
Many arachidonic acid metabolites are vasoactive and are thought to contribute to the functional hyperemia response (Fig. 12). Prostaglandins and epoxyeicosatrienoic acids (EETs) dilate vessels while the AA metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) constricts vessels. The AA metabolite-signaling pathway is thought to work as follows: neuronal activity, through the release of neurotransmitters, stimulates astrocytes, leading to elevated Ca2+ levels in the glial cells. Elevated Ca2+ activates the Ca2+-dependent PLA2, leading to the production of AA from membrane phospholipids. Increased AA results in the production of AA metabolites, either within astrocytes themselves or in vascular smooth muscle cells; reviewed by Attwell et al. (2010).
Fig. 12.
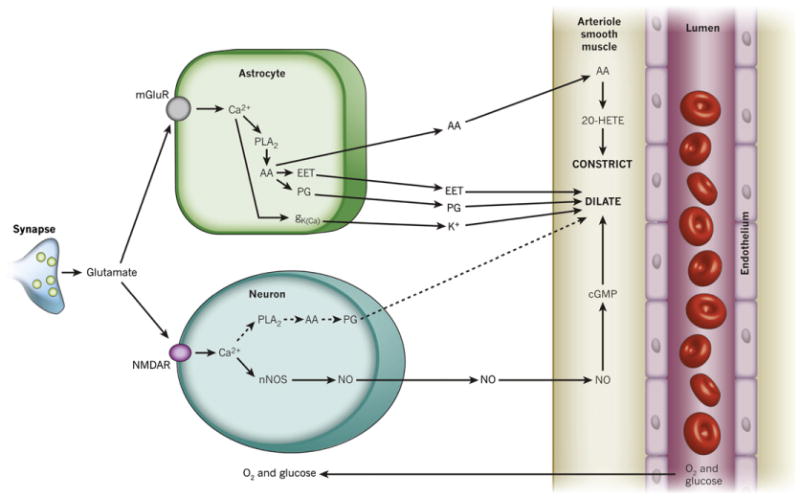
Summary of signaling pathways that mediate neurovascular coupling in the central nervous system. Synaptically released glutamate acts on NMDA receptors in neurons (NMDAR) to raise [Ca2+]i, causing neuronal nitric oxide synthase (nNOS) to release NO, which activates smooth muscle guanylate cyclase. Raised [Ca2+]i may also (dashed line) generate arachidonic acid (AA) from phospholipase A2 (PLA2), which is converted to prostaglandins (PG) that dilate vessels. Glutamate also raises [Ca2+]i in astrocytes by activating metabotropic glutamate receptors (mGluR), generating arachidonic acid and three types of AA metabolites: prostaglandins and EETs in astrocytes, which dilate vessels, and 20-HETE in smooth muscle, which constricts vessels. A rise of [Ca2+]i in astrocyte endfeet may also activate Ca2+-gated K+ channels (gK(Ca); alternative abbreviation: BK), releasing K+, which also dilates vessels. In the retina, Müller glial cells are activated by ATP rather than glutamate released from neurons. Calcium increases in Müller cells result in the release PG and EETs onto smooth muscle cells, which dilate vessels, and 20-HETE production, which constricts vessels; from Attwell et al. (2010).
The AA metabolite hypothesis has received strong support in recent years. Stimulation of astrocytes in brain slices (Takano et al., 2006) and in the cortex in vivo (Niwa et al., 2000) results in a prostaglandin-dependent vasodilation which is blocked by inhibition of cyclooxygenase, a prostaglandin-synthesizing enzyme. Interrupting the signaling from neurons to astrocytes by blocking metabotropic glutamate receptors (mGluRs), which mediate astrocyte stimulation in the brain, substantially reduces the vasodilation (Zonta et al., 2003; Petzold et al., 2008), indicating that this mechanism contributes significantly to the regulation of blood flow. Glial cells have more complex effects on blood vessels, however. In brain slice experiments, astrocyte stimulation can also result in vasoconstriction mediated by production of 20-HETE (Mulligan and MacVicar, 2004), suggesting that glial cells can regulate blood flow, not only by dilating vessels, but also by constricting them.
A similar AA metabolite mechanism operates in the retina. Stimulation of retinal astrocytes or Müller cells by photolysis of caged Ca2+ or caged IP3, results in increased cytoplasmic Ca2+ levels and in vasomotor responses in adjacent vessels (Metea and Newman, 2006) (Fig. 13). Glial stimulation can result in both vasodilatation and vasoconstriction, depending on experimental conditions. As in the brain, glial-evoked vasoconstrictions are mediated by production of 20-HETE (Metea and Newman, 2006), while glial-evoked vasodilations are mediated by the production of EETs as well as prostaglandins (Mishra et al., 2011). In the retina, signaling from neurons to astrocytes is mediated by ATP and glial purinergic P2Y receptors (Newman, 2005). Interruption of signaling from neurons to glial cells by addition of purinergic antagonists nearly abolishes light-evoked vasodilations and constrictions. These results demonstrate that glial cells and AA metabolite production contribute significantly to functional hyperemia in the retina.
Fig. 13.
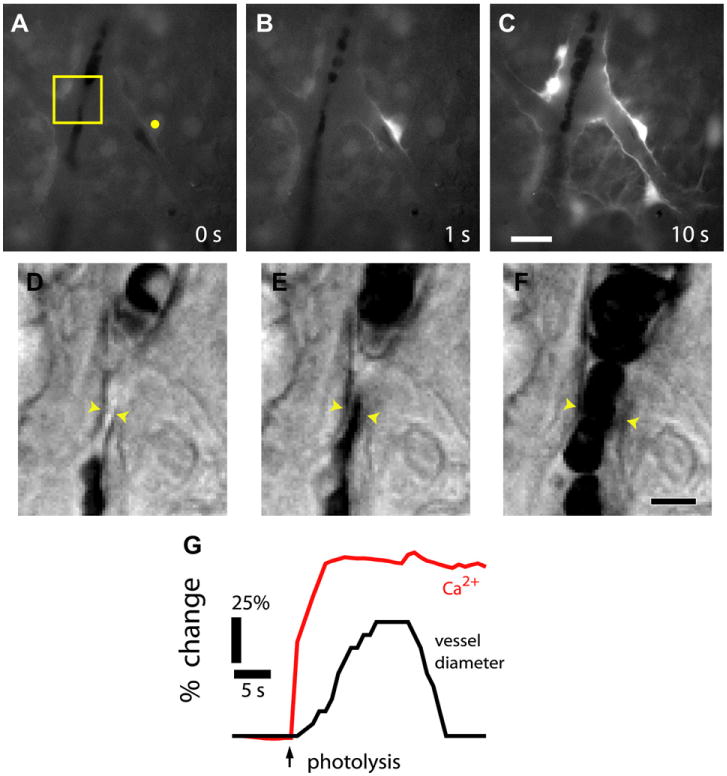
Glial cell stimulation evokes vasodilation of retinal arterioles. A–C, Fluorescence images showing a Ca2+ increase that propagates through several glial cells following stimulation by photolysis of caged-Ca2+ (yellow dot in A). D-F, IR-DIC images of the boxed region in A, each acquired 0.5 s after the corresponding image above. Glial stimulation evokes a dilation of the arteriole, indicated by the yellow arrowheads. G, Time course of glial Ca2+ change and vessel dilation; from Metea and Newman (2006).
5.3. Nitric oxide and oxygen modulation of neurovascular coupling
Nitric oxide dilates blood vessels by reducing K+ conductance in vascular smooth muscle cells (Feletou and Vanhoutte, 2006). NO also contributes to the regulation of blood flow in the retina by modulating glia-to-vessel signaling. When NO levels are raised experimentally by addition of NO donors, light- evoked and glial-evoked vasodilations are reduced, sometime revealing a vaso constricting component of the response (Metea and Newman, 2006). This modulatory effect of NO could be mediated by NO inhibition of the synthesis of vasodilators, including EETs (Udosen et al., 2003).
Oxygen has a similar modulatory effect on functional hyperemia in the brain and retina. In brain slices, increasing O2 results in the attenuation of glial-evoked vasodilation and the appearance of vasoconstrictions (Gordon et al., 2008). A similar modulatory effect is observed in the isolated retina, where increasing O2 in the superfusate from 21% to 100% results in a decrease in light-evoked and glial-evoked vasodilation (Mishra et al., 2011). However, raising pO2 in the retina in vivo does not alter light-evoked vasodilatory responses (Mishra et al., 2011). This is because pO2 does not increase substantially in the retina in vivo, even when an animal breaths 100% O2 (Yu et al., 1999).
6. Blood flow regulation in the choroid
In contrast to the retina, the choroidal circulation is controlled by extrinsic autonomic innervation. Decreases in choroidal blood flow are mediated by activation of sympathetic efferent nerves that release noradrenaline, activating alpha 1-adrenoceptors on vascular smooth muscle cells (Alm, 1977; Kawarai and Koss, 1998). In turn, increases in choroidal blood flow are mediated by parasympathetic efferent nerves which act via NO signaling (Nilsson, 1996). The choroid also receives rich innervation from trigeminal sensory fibers which contain calcitonin gene-related peptide (de Hoz et al., 2008) that are thought to mediate light-evoked control of the choroidal circulation (Okamoto et al., 2010). The following paragraphs give an overview of regulatory responses to alterations in perfusion pressure, blood gases levels and light conditions in the choroid.
6.1. Pressure regulation
It was long assumed that the choroidal circulation shows little regulation in response to changes in perfusion pressure. A linear or close to linear pressure-flow relationship was reported in early animal studies using a variety of techniques, indicating that the choroidal circulation behaves like a passive vascular bed (Bill, 1962; Friedman, 1970; Armaly and Araki, 1975; Gherezghiher et al., 1991). More recent studies have reported, in contrast, that moderate decreases in perfusion pressure, experimentally adjusted by manipulating intraocular pressure or mean arterial pressure, result in significant compensation in choroidal blood flow (Riva et al., 1997b; Bogner et al., 2011; Polska et al., 2007) (Fig. 14).
Fig. 14.
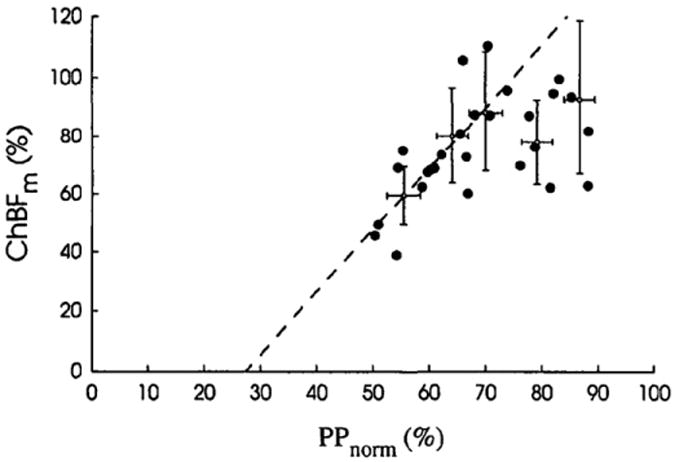
Autoregulation of choroidal blood flow in humans. Change in mean choroidal blood flow (ChBFm) is plotted versus mean ocular perfusion pressure (PPnorm), where PP was decreased by slowly increasing the intraocular pressure. Both ChBFm and PP are normalized. Note that relationship is not linear, indicating an autoregulatory mechanism. Error bars represent the 95% confidence interval; from Riva et al. (1997b).
Further evidence of autoregulation comes from studies that investigated the response of choroidal blood flow to increases in the ocular perfusion pressure, which in human studies is usually induced by static and dynamic exercise (Riva et al., 1997a; Lovasik et al., 2003; Polska et al., 2007). A large increase in the ocular perfusion pressure induced by stationary biking was shown to coincide with only a moderate increase in choroidal blood flow (Lovasik et al., 2003). Similarly, Riva and colleagues reported an increase in the choroidal blood flow of only 12% even though isometric exercise raised the ocular perfusion pressure by as much as 60% (Riva et al., 1997a). Although definitive experimental data are lacking, some results indicate either a myogenic and/or a neuronal contribution to choroidal blood flow regulation in face of changes in perfusion pressure (Polska et al., 2007; Kiel, 1999).
6.2. Regulation by blood gases
Animal studies suggest that choroidal flow is strongly dependent on pCO2, but shows little reactivity to changes in pO2 (Alm and Bill, 1972a; Friedman and Chandra, 1972; Riva et al., 1994b; Flower et al., 1995). These observations are in keeping with studies on the effect of arterial blood gases on choroidal blood and ocular fundus pulsation amplitude in humans, as evaluated by laser Doppler flowmetry and laser interferometry respectively (Schmetterer et al., 1995; Geiser et al., 2000). Choroidal sensitivity to CO2 varies with species. Raising pCO2 increases choroidal blood flow in cats (Alm and Bill, 1972a; Friedman and Chandra, 1972) and humans (Riva et al., 1994a; Geiser et al., 2000), but not in rats (Wang et al., 2008).
6.3. Choroidal vascular responses to light stimulation
There is general agreement that blood flow in the retinal vasculature is regulated by light and varies with changes with background luminance and flicker. In contrast, blood flow regulation by light in the choroidal circulation is still a matter of controversy.
6.3.1. Light/dark transition
Recent studies have shown that the light to dark transition in humans is associated with a decrease in choroidal blood flow. Further, when dark-adapted eyes were exposed to room light, choroidal blood flow was increased (Fuchsjager-Mayrl et al., 2001; Longo et al., 2000) (Fig. 15). A decrease in choroidal blood flow during dark adaptation could be due to a change in oxygen consumption in the outer retina. This is unlikely, however, as the choroidal circulation is insensitive to pO2 changes (Schmetterer et al., 1995; Geiser et al., 2000). Moreover, the elevated oxygen consumption in the dark by photoreceptors would lead to an increase in choroidal perfusion, rather than a decrease. In addition, changes in choroidal blood flow were reported not only in the stimulated eye but also in the contralateral eye (Fuchsjager-Mayrl et al., 2001; Longo et al., 2000), indicating that the choroidal response is under neural control.
Fig. 15.
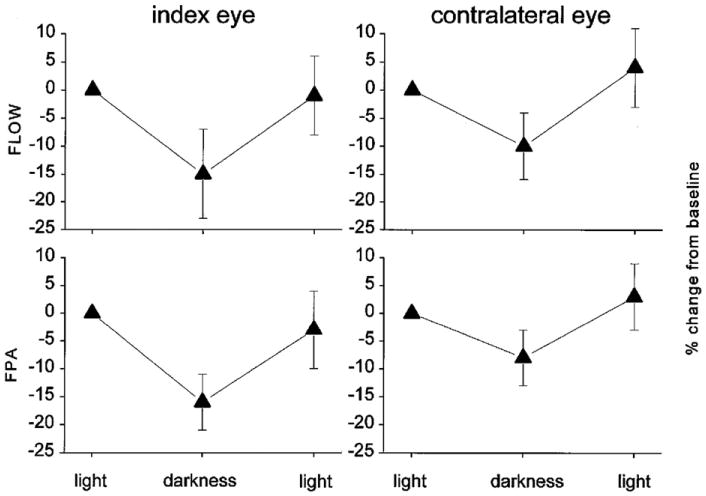
Light responses of the choroidal vasculature. Change in choroidal blood flow (FLOW), measured with laser Doppler flowmetry, and the fundus pulsation amplitude (FPA), measured with laser interferometry, during darke–light transitions. Note that transition from light to dark results in a decrease in choroidal blood flow, not only in the stimulated (index) eye, but also in the contralateral eye; from Fuchsjager-Mayrl et al. (2001).
It has been suggested that the choroidal circulation is necessary for the maintenance of a stable temperature in the outer layers of the retina (Parver et al., 1982). Changes in choroidal hemodynamics that occur during light and dark adaptation could contribute to passive dissipation of the heat induced by light. This notion is supported by the observation that when intraocular pressure was increased, the retinal-choroidal temperature in the macula of anesthetized monkeys decreased in room light but increased after exposure to a bright light source (Parver et al., 1980).
6.3.2. Flicker stimulation
In humans tested with laser Doppler flowmetry and laser interferometry, diffuse flickering light has no effect on choroidal blood flow (Garhofer et al., 2002). However, a blue-flickering stimulus evokes a decrease in the subfoveal choroidal blood flow. Based on this result and a subsequent analysis of the ERG b-wave, it was concluded that an increase in rod activity results in a decrease in choroidal flow in the foveal-macular zone and an increase in the retinal periphery (Lovasik et al., 2005). In rats, choroidal vessels do not respond to changing luminance, frequency, and wavelength as demonstrated by layer-specific functional MRI (Shih et al., 2011). In chicks, a flickering light evokes an increase in choroidal blood flow that is due to an increase of blood volume but not velocity, an effect that has been attributed to local vasodilation (Shih et al., 1997). It should be noted that the chick eye, in contrast to the mammalian eye, lacks intrinsic retinal vessels.
6.4. Comparison of retinal and choroidal regulatory mechanisms
The metabolic needs of the eye are supplied by two separate vascular systems, the retinal and the choroidal vasculature, having very different hemodynamic properties. The retinal circulation has a substantially lower blood flow and a higher level of oxygen extraction than does the choroidal circulation. There are significant differences in the regulatory mechanisms operating in the retinal and choroidal vasculature as well. The regulatory role of extrinsic factors is relatively minor in the retina, since it lacks autonomic innervation, whereas choroidal vessels have strong autonomic input. Retinal blood flow exhibits autoregulation over a wide range of perfusion pressures. Although initially controversial, autoregulation in the choroidal vasculature also occurs. While there is agreement that blood gases (pO2 and pCO2) control retinal blood flow, the choroidal circulation is largely insensitive to changes in pO2, and, in some species, to pCO2 as well. In general, light stimulation has been found to have little effect in the choroidal circulation. This is in stark contrast to the retina, where the vascular responses to light–dark transitions and to flicker are well characterized.
7. Regulation of ocular blood flow in disease
Normal retinal and choroidal blood flow is altered in a number of disorders that affect the eye, including diabetic retinopathy, glaucoma, age-related macular degeneration, and Alzheimer's disease. Changes in responses to flickering light, as well as altered rates of basal blood flow, are observed under pathological conditions. The following sections detail changes in ocular blood flow that are observed under a number of conditions.
7.1. Diabetic retinopathy
Diabetic retinopathy is a microvascular complication of diabetes and a leading cause of visual impairment and blindness worldwide (Klein et al., 1984). Alterations in retinal capillaries are the hallmark of diabetic retinopathy and include multiple occlusions due to increased leukocyte entrapment (Ogura, 2000), increased permeability of the vessel wall with focal leakage occurring primarily at the location between the deep retinal capillary net and the ascending venules (Ben-Nun et al., 2004) and, in the proliferative form, growth of newly-formed vessels (neovascularization).
Retinal circulatory changes precede overt clinical diabetic retinopathy and include a reduction in the functional hyperemia response, the response to hyperoxic challenge, and changes in basal blood flow. Basal blood flow changes are characterized by retinal hypoperfusion in early diabetes with a shift to retinal hyper-perfusion as diabetic retinopathy progresses. However, there are quantitative and qualitative inconsistencies in the data; for reviews see Pemp and Schmetterer (2008), Curtis et al. (2009), which can be explained by several factors, including the stage of diabetes, the techniques used, the site of measurement in the retina (e.g. nasal versus temporal; Grunwald et al., 1992), and finally, demographic parameters. Similarly, conflicting results in diabetic animals is likely due to differences in species and strains and type of disease model used. Since glucose plasma levels are a determinant of retinal blood flow (Findl et al., 2000a), acute and/or chronic hyperglycemia may also contribute to contradictory findings concerning retinal blood flow during different stages of diabetes and diabetic retinopathy (Grunwald et al., 1987).
7.1.1. Hemodynamic dysfunction in diabetic retinopathy
Several studies have documented a reduction in light-evoked vasodilation in diabetic patients (Garhofer et al., 2004b; Nguyen et al., 2009; Pemp et al., 2009) (Fig. 16A). This reduction in the functional hyperemia response has recently been reproduced in an animal model of diabetic retinopathy (Mishra and Newman, 2010, 2012). Importantly, the reduction in the vasodilatory response in patients and in an animal model is observed before the appearance of overt clinical retinopathy (Mandecka et al., 2007; Mishra and Newman, 2010). Therefore, vascular responses to flicker stimulation can be a useful screening tool for early detection of diabetic retinopathy (Nguyen et al., 2008; Mishra and Newman, 2010), see also Section 7.5. The reduction in flicker-induced vasodilation in an animal model of diabetes has been reversed by inhibiting the enzyme inducible nitric oxide synthase (iNOS) (Mishra and Newman, 2010, 2012) (Fig. 16B and C). iNOS inhibition is believed to be efficacious because it lowers nitric oxide levels, which interfere with neurovascular coupling mechanisms (Metea and Newman, 2006).
Fig. 16.
Reduced functional hyperemia response in the diabetic retina. A, Flicker-induced dilation of retinal arteries and veins is reduced in patients with type 1 diabetes; from Pemp et al. (2009). B and C, Flicker-induced vasodilation is reduced in the diabetic rat retina. The iNOS inhibitor aminoguanidine, given intravenously (diabetic AG-IV), or in drinking water (diabetic AG-H2O), reverses the loss of vasodilation; from Mishra and Newman (2012).
Other studies on hemodynamic abnormalities in retinal circulation focus on blunted autoregulatory responses to hyperoxic/hypoxic challenge. Fallcon et al. speculated that the smaller hyperemic response to hypoxia seen in the capillary macular region in proliferative retinopathy is due to the presence of large areas of non-perfusion in the diabetic retina (Fallon et al., 1987). In large retinal vessels, a smaller than normal reduction in blood flow velocity during pure oxygen breathing has been reported before the onset of overt retinopathy (Grunwald et al., 1984a, 1984b). In another study, when hyperoxia was imposed on patients with early diabetic retinopathy, there were no hemodynamic effects (although diabetic subjects had basal vascular abnormalities). However, hyperoxia did reverse the visual dysfunction present in the patients, i.e. a reduction in contrast sensitivity (Harris et al., 1996b). Further, the fact that diabetic retinopathy is frequently a focal disease with microaneurysms and capillary segments with reduced perfusion/blood flow, leads us to suggest that detection of hemodynamic dysfunction could be more sensitive if undertaken near sites of microaneurysms and capillary dysfunction.
7.1.2. Neuronal and glial dysfunction in diabetes
Although clinically observed microvascular changes are the hallmark used to classify diabetic retinopathy, there are also significant neuronal and glial changes that occur early during the development of the disease.
Changes in the electroretinogram (ERG), a measure of neuronal function in the retina, occur in early stages of diabetic retinopathy. There is a reduction in the amplitude of the ERG a- and b-waves (Barber et al., 1998; Phipps et al., 2004) as well as changes in the oscillatory potentials (Hancock and Kraft, 2004; Lecleire-Collet et al., 2011), which are generated by inner retinal neurons. Morphological studies have confirmed that diabetes results in a loss of retinal neurons (Martin et al., 2004); for review see Barber et al. (2011). Neurodegenerative changes in early diabetic retinopathy also occur in photoreceptors (Park et al., 2003), as well as within the inner-nuclear layer (van Dijk et al., 2009). The neuronal cell loss was shown to induce microglial reactivity (Zeng et al., 2000).
In animal and human studies of diabetic retinopathy, a number of changes are observed in Müller glial cells. These include increased expression of glial fibrillary acidic protein (GAFP) (Barber et al., 2000; Mizutani et al., 1998), a sign of reactivity, down-regulation of glutamine synthetase, upregulation of iNOS (Mishra and Newman, 2010), a loss of osmotic balance associated with a decrease in K+ currents, and a disruption of voltage-dependent cell functions (i.e. uptake of neurotransmitters) (Pannicke et al., 2006; Bringmann et al., 2002). Disruption of glutamate uptake could contribute to excitotoxicity of retinal neurons due to accumulation of glutamate (Puro, 2002). As reviewed above, disruption of glial-mediated neurovascular coupling is believed to be responsible for reduced light-induced vasodilation in the diabetic retina.
7.1.3. Pericyte dysfunction in diabetes and other disorders
Although changes in pericytes have been observed in pathology in many vascular beds, the changes that occur in retinal capillaries are by far the best documented; reviewed in Motiejunaite and Kazlauskas (2008), Hamilton et al. (2010), Armulik et al. (2011). The loss of pericytes in retinal microvessels is one of the earliest morphological changes that takes place in diabetic retinopathy (Cogan and Kuwabara, 1967) (Fig. 17). Pericyte loss is associated with development of microaneurysms and shunt vessel formation in the diabetic retina (Speiser et al., 1968; De Oliveira, 1966). Increased blood glucose concentrations could contribute to pericyte loss (Kern and Engerman, 1995; Kern et al., 2000). Cell culture experiments have demonstrated that high glucose increases apoptosis in retinal pericytes (Podesta et al., 2000; Pomero et al., 2003). As pericytes from adult retina show little, if any, replicative capability (Engerman et al., 1967), accelerated apoptosis can readily account for pericyte dropout and the formation of pericyte ghosts – the empty pockets in the basement membranes at the sites from which pericytes have disappeared. Growing evidence suggests that pericytes can be actively depleted under hyper-glycaemic conditions by non- apoptotic mechanisms as well. For instance, reduced pericyte coverage of retinal capillaries occurs as a result of pericyte migration from underlying vessels into the perivascular parenchym and involves angiopoetin-2 and its tyrosine kinase receptor, Tie-2 (Pfister et al., 2008, 2010).
Fig. 17.
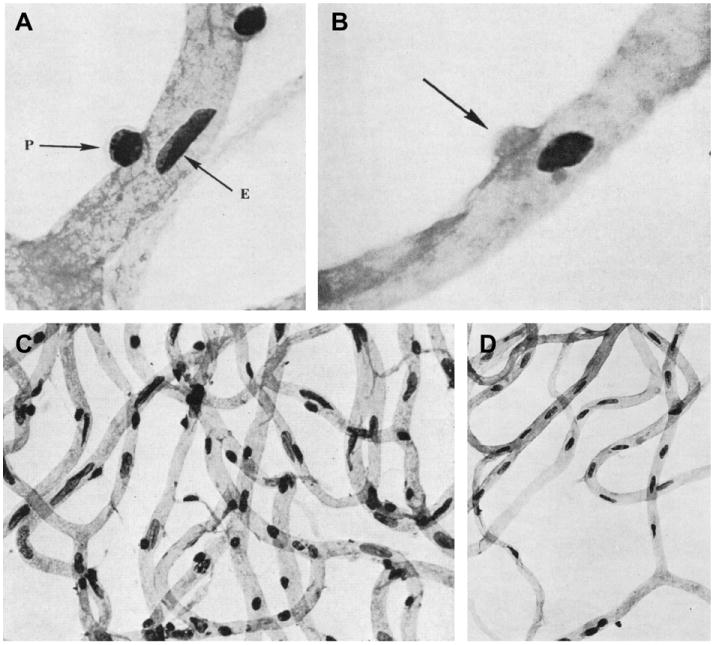
Loss of pericytes in diabetic retinopathy. A, Normal human retinal capillaries showing intramural pericytes (P) and endothelial cells (E). B, Mural ghost pericyte (arrow) in a human diabetic retina. C, Capillary meshwork in a healthy retina, showing a normal ratio of endothelial cells to pericytes. D, Capillary meshwork in a diabetic retina. Note marked loss of pericytes (round nuclei) with preservation of endothelial cells (elongated nuclei). Trypsin digestion, periodic acid-Schiff (PAS) and hematoxylin stain; from Speiser et al. (1968).
A common pathophysiologic mechanism linking chronic hyperglycemia to pericyte pathology is mitochondrial over production of reactive oxygen species (Li et al., 1998) and increased formation of advanced glycation end products (AGEs; Stitt et al., 2004). Activation of protein kinase C (PKC), aldose reductase (Robison et al., 1990) and active nuclear factor (NF-κB; Romeo et al., 2002) also contribute; see reviews by Obrosova and Kador (2011), Zong et al. (2011). The link between glycation and oxidative stress was directly demonstrated in an in vivo model of diabetes (Brouwers et al., 2011). Pericyte apoptosis in hyperglycemia has also been attributed to the loss of the survival actions of platelet-derived growth factor (PDGF) (Haribalaganesh et al., 2009; Geraldes et al., 2009).
The molecular basis for hyperglycemia-induced changes in pericyte function include closure of gap junction pathways in retinal microvessels and inhibition of Cx43 expression and subsequent vascular cell apoptosis and development of acellular capillaries (Oku et al., 2001; Bobbie et al., 2010). In addition, there are several lines of evidence demonstrating compromised contractile activity of retinal pericytes, as a consequence of the diabetes-induced alteration in ion channel function and Ca2+ homeostasis; reviewed by Puro (2007). Impairment of voltage-driven vasomotor responses through inhibition of voltage-dependent calcium channels by spermine (Matsushita et al., 2010) or inhibition of endothelin-1 (Et-1) – evoked pericyte contraction via disruption of ETA receptor signaling (Hughes et al., 2004) have been implicated.
Another early histopathological sign of diabetic retinopathy of relevance to pericyte function is the thickening of the basement membrane resulting from increased synthesis of collagen IV, fibronectin, and laminin and/or reduced degradation by catabolic enzymes; reviewed by Gardiner et al. (2007). Modification of vascular basement membrane may reduce bioavailability of pro-survival factors for retinal pericytes and inhibitors of angiogenesis in the eye, i.e. PDGF (Lindblom et al., 2003). In general, the physiological consequences of compromised two-way communication between endothelial cells and pericytes manifest themselves as a loss of functional integrity of the capillary unit; reviewed by Armulik et al. (2005). In the retina, mural cell loss and/or dedifferentiation, characterized by aberrant cell phenotype and reduced desmin ensheathment ratio (DER) – a quantitative measure of the pericyte-endothelial cell interaction and vessel stability (Chan-Ling et al., 2004b), were also implicated in formation of neovessels in the retinopathy of prematurity (Hughes et al., 2007).
Alterations in pericytes with age might also contribute to the development of age-related morphological and physiological abnormalities of the retinal vasculature. In two independent studies of the rat retina, aging caused the breakdown of normal vascular architecture, i.e. increase in number of acellular capillaries, loss of capillary patency, and reduced pericyte-endothelial cell contact (Hughes et al., 2006). These changes resembled the anatomical and histological lesions associated with impaired vascular regulation seen in diabetic retinopathy (Hughes et al., 2004; Roy et al., 2010).
Pericyte pathology is associated with a reduction in blood flow seen in models of retinal ischemia (Yemisci et al., 2009). In the brain, experimental ischemia caused by occlusion of the middle cerebral artery results in the constriction of pericytes and reduction of blood flow in capillaries. Constriction is caused by both oxidative and nitrative stress and persists even after the occlusion is removed. Reperfusion-induced oxidative and nitrative injury contributes to pericyte pathology and perhaps to the reduction in blood flow observed following stroke. Retinal pericytes show similar constriction following exposure to oxygen-glucose deprived solution, in an ex vivo model of ischemia (Yemisci et al., 2009).
7.2. Glaucoma
Glaucoma is one of the most common causes of blindness in industrialized nations (Quigley and Broman, 2006). It is a family of optic neuropathies characterized by the selective loss of retinal ganglion cells and their axons, resulting in progressive reduction of the visual field. Although elevated intraocular pressure has been identified as a primary risk factor for development of glaucoma, the disease progresses in a large portion of patients despite therapeutic intervention to lower intraocular pressure (Leske et al., 2007). Abnormal vascular regulation at the retinal, choroidal and peripapillary level are associated with pathogenesis of the disease (Kornzweig et al., 1968; Grunwald et al., 1984b; Ulrich et al., 1996). Recent observations demonstrate that retinal vascular reactivity to normoxic hypercapnia is reduced in patients with untreated and progressive primary open-angle glaucoma (Venkataraman et al., 2010). Compromised autoregulatory capacity, i.e. a larger increase of choroidal blood flow in open-angle glaucoma patients in response to exercise-induced blood pressure rise, is also observed (Fig. 18A) (Portmann et al., 2011).
Fig. 18.
Choroidal blood flow in glaucoma and age-related macular degeneration. A, Choroidal blood flow in glaucoma patients, measured with laser Doppler flowmetry (LDF) at baseline (dark gray bar), during a hand-grip exercise (black bar), and during recovery (light gray bars). Note the lowered blood flow in primary open-angle glaucoma (POAG) patients; from Portmann et al. (2011). B, Reduced choroidal blood flow in age-related macular degeneration (AMD). Relative choroidal blood flow (ChBFlow) in control subjects (Normal) and AMD patients, measured with laser Doppler flowmetry. Patients were divided into three groups according to their ophthalmoscopic AMD features associated with increased risk of choroidal neovascularization; from Grunwald et al. (2005).
Elevated intraocular pressure associated with glaucoma is expected to cause decreased ocular blood flow and micro-angiopathy. Indeed, several studies have shown decreased blood flow in the optic nerve head, retina and choroid in patients with glaucoma (Michelson et al., 1996a; Findl et al., 2000b; Grunwald et al., 1998; Portmann et al., 2011; Wang et al., 2011b) (Fig. 18A). A decrease in the number of capillaries in the optic nerve head and the atrophy of the peripapillary capillaries supplying the retinal nerve fiber layer has been reported (Kornzweig et al., 1968; Gottanka et al., 2005).
A relationship between glaucomatous optic neuropathy and the endothelin system has been demonstrated. Chronic local administration of endothelin-1 to the optic nerve head induces a reduction in optic nerve head blood flow and is associated with a loss of retinal ganglion cells (Chauhan et al., 2004; Cioffi et al., 1995). Moreover, plasma levels and aqueous humor concentration of biochemical markers of endothelial function are higher in glaucoma patients, supporting a role of endothelin in optic nerve ischemia (Kaiser et al., 1995; Sugiyama et al., 1995; Ghanem et al., 2011). Oral administration of dual ETA and ETB receptor antagonists significantly increased retinal, choroidal and optic nerve head blood flow in patients with glaucoma as well as healthy subjects (Resch et al., 2009). Vascular dysregulation related to an imbalance of endothelium-derived vasoactive agents in glaucoma patients – see also Flammer and Mozaffarieh, 2008, occurs at the level of small ocular vessels, as well as retrobulbar vessels (Galassi et al., 2011). In light of the observations by Grieshaber et al. (2007), dysregulation of the ocular circulation in combination with activation of retinal astrocytes and Müller cells may underlie the pathogenic events in the onset of open-angle glaucoma; reviewed in Prasanna et al. (2010).
7.3. Age-related macular degeneration
Age-related macular degeneration (AMD) is the major cause of irreversible blindness in elderly patients worldwide. The stages of age-related maculopathy are categorized as early, with presence of drusen and minor visual symptoms, and late, encompassing both dry (geographic atrophy) and wet (neovascular), in which severe loss of vision is common (Bird et al., 1995).
The hypothesis that clinical manifestations of AMD are attributable to impaired choroidal perfusion has gained support in recent studies; these are summarized in previous reviews: Harris et al., 1999; Lutty et al., 1999; Ciulla et al., 2002; Pemp and Schmetterer, 2008. In brief, reduced choroidal flow velocities in patients with AMD have been measured (Ciulla et al., 1999; Uretmen et al., 2003). Increased mean times for arterial, capillary, and venous filling, and reduced capillary density in patients with AMD, indicative of reduced choroidal blood flow, have been reported using angiographic techniques (Ciulla et al., 2002; Prunte and Niesel, 1988). Abnormal choroidal blood flow in AMD has also been suggested based on laser Doppler experiments (Grunwald et al., 2005; Xu et al., 2010), pneumotonometry (Chen et al., 2001; Mori et al., 2001) and interferometry (Schmetterer et al., 1998) (Fig. 18B).
The hemodynamic model of AMD pathogenesis postulates that increased scleral rigidity due to atherosclerotic plaque formation results in an increased resistance to blood flow and abnormal venous drainage in the choroid (Friedman et al., 1989). This, in turn, alters retinal pigment epithelium function, causing accumulation of lipoproteins and subsequent retinal damage (RPE atrophy, abnormal Bruch's membrane permeability) seen in early AMD; reviewed by Friedman (2008). Progression to the neovascular stage involves angiogenic factors such as VEGF, which are induced by hypoxia and ischemia (Kliffen et al., 1997; Frank, 1997). The subject of increased scleral rigidity in AMD patients with choroidal neovascularization has also been investigated (Pallikaris et al., 2006). An alternate pathological model of AMD postulates that drusen formation causes an aggressive inflammatory response in genetically predisposed individuals (Kubista et al., 2011; Williamson et al., 2011), and the inflammation then causes secondary damage to the retinal pigment epithelium; reviewed by Anderson et al. (2010).
Although changes in the RPE have classically been identified as the principal causative factor in AMD, it is now recognized that the inner retina and, more specifically macroglia, are also involved in the disease. Changes in the inner retina in AMD patients that resemble advanced retinal aging have been reported. These changes included hypertrophic reactive astrocytes at the inner surface of the retina, with filaments forming dense packs (Ramirez et al., 1996; Mansour et al., 2008) and Müller cells with GFAP immunoreactivity displaying loss of structural integrity (Wu et al., 2003b; Ramirez et al., 1996). More importantly, a reduction in the ratio of astroytes to neurons have been reported in the aged rat retina (Mansour et al., 2008), suggesting a compromised ability of astrocytes to maintain homeostasis during aging. Since AMD is a disease of aging, this change in astrocyte to neuronal ratio may partly underlie the pathogenesis of AMD.
7.4. Alzheimer's disease
Recent studies suggest that structural abnormalities of the brain vasculature and the dysregulation of blood flow contribute to the pathogenesis of Alzheimer's disease (AD) (Girouard and Iadecola, 2006; Grammas et al., 1999). Individuals with AD have visual abnormalities in contrast sensitivity, motion and depth perception, color discrimination, and blurred vision; for review see Guo et al. (2010). Classically, these defects were attributed to cortical disease (Justino et al., 2001; Kergoat et al., 2002). However, a growing number of reports document the involvement of the retina in the neurodegenerative changes present in AD. Recent studies investigating retinal changes in mouse models of AD have shown immunoreactivity against amyloid-β in the cells of the ganglion cell and the nerve fiber layers. Amyloid-β deposits were also detected in the retinal and choroidal microvasculature (Ning et al., 2008; Shimazawa et al., 2008). These changes were associated with increased microglial reactivity (Perez et al., 2009; Howlett et al., 2011); reviewed by Chiu et al. (2011).
Histological studies have revealed degeneration of optic nerve axons and a loss of retinal ganglion cells in AD individuals (Parisi, 2003; Iseri et al., 2006). Electrophysiological investigations reveal abnormalities in the pattern electroretinogram with delayed implicit times and reduced amplitude, indicating abnormal retinal ganglion cell function in subjects with AD (Parisi, 2003).
Recent studies support the hypothesis that abnormal cerebro-vascular function is involved in the progression of AD (Ruitenberg et al., 2005); reviewed by de la Torre (2009). This could be true for retinal blood flow as well. Hemodynamic data from patients with mild or moderate AD revealed a significant narrowing of retinal veins and reduced venous blood flow accompanying neuronal damage (Berisha et al., 2007). Based on the finding that AD patients have amyloid-β/collagen fibril deposition in the walls of cerebral vessels (Vinters et al., 1996; Kalaria and Pax, 1995), it is legitimate to speculate that retinal hypoperfusion also results from an increase in venous wall thickness, resulting in a narrowed lumen (Michelson et al., 2007). This hypothesis remains to be demonstrated experimentally. It will also be important to determine whether functional hyperemia and autoregulation are compromised in the retinas of AD patients.
7.5. Retinal microvascular changes as a screening tool
The transparency of the ocular media combined with the fact that the retina is developmentally an extension of the brain, makes the eye an ideal location for the detection of systemic diseases that affect the microcirculation. Screening and early diagnostics of cardiovascular pathologies are growing in importance because of increasing life expectancy in industrialized countries. Routine ophthalmoscopic examination has long been an element of prognostic assessment for diabetes and hypertension. Epidemiological studies focus on a spectrum of quantitative and qualitative retinal vascular manifestations including retinal arteriolar dilatation (Cheung et al., 2008), retinal arteriolar narrowing (Nguyen et al., 2008; Grauslund et al., 2009), and a smaller ratio of the retinal arteriolar to venular caliber – due to either narrower arterioles or wider venules (Wong et al., 2005). Alterations in microvascular geometry, with larger arteriolar branching angle and increased arteriolar tortuosity being markers of diabetic microvascular complications (Sasongko et al., 2010) and, venular caliber, being associated with systemic inflammation (Wong et al., 2006), have also been examined. Clinical studies can also benefit from new methodological approaches that focus on dynamic retinal vessel responses to stimuli such as light stimulation, hypercapnia and hyperoxia, and changes in perfusion pressure.
8. Future directions
While much is known about the development of the retinal and choroidal vasculature, the structure of the vascular unit and the contractile machinery of vascular smooth muscle cells, mechanisms mediating neurovascular coupling, and the responses of retinal and choroidal vessels to visual and autoregulatory stimuli, many questions remain to be answered. Several key issues that remain to be addressed are outlined in the following paragraphs.
Although there is a detailed understanding of the growth factors that regulate angiogenesis in the eye, little is known regarding the nature of the regulators of vasculogenesis. What are the growth factors that regulate formation of retinal and choroidal blood vessels via vasculogenesis? Since the development of the central one-third of the superficial human retinal vascular plexus and the majority of the human choroidal vascular plexus occurs via vasculogenesis, identification of vasculogenic growth factors could have a major influence on the treatment of retinal and choroidal neovascularisation that is not responsive to VEGF165 treatment.
New insights are needed regarding changes that occur with aging and pathology in the ability of the choroidal vascular plexus to regulate its blood flow. This vascular plexus supplies the retinal photoreceptors, and any change in its ability to regulate blood flow could have major implications for the health of the photoreceptors.
We know little about the functional significance of the absence of desmin intermediate filaments in choroidal pericytes and about the paucity of pericyte ensheathmenton the ability of the choroidal plexus to regulate blood flow. Do compensatory mechanisms exists that allows for the regulation of choroidal blood flow?
The mechanisms mediating neurovascular coupling in the retina remain unclear. Recent evidence suggests that the release of arachidonic acid metabolites, including PGE2 and EETs, from retinal glial cells is largely responsible for mediating light-evoked vasodilation. But does the release of vasoactive agents from neurons directly onto vessels also contribute to functional hyperemia? Do NO, adenosine, lactate and K+ also function as neurovascular signaling agents in the retina, as they do in the brain?
Pericytes respond to neuronal activity and vasoactive agents by relaxing or constricting. Yet we do not know whether these cells actively regulate retinal blood flow in capillaries, either under normal or pathological conditions.
Reported responses of the choroidal vasculature to photoreceptor stimulation and autoregulatory stimuli vary depending on species and even between different laboratories investigating the response. Photoreceptors depend on the choroidal circulation for oxygen and glucose and new insights into how choroidal blood flow is regulated are needed. Further, we do not know whether all species represent suitable models for characterizing the regulation of retinal and choroidal circulation in humans.
The loss of functional hyperemia is one of the earliest changes to be detected in diabetic retinopathy. Yet, we do not understand the functional consequences of the loss of this vascular response. Does the loss of functional hyperemia lead to retinal hypoxia and to the death of neurons or vascular elements. A better understanding of this process may lead to new therapies for preventing diabetes-related blindness.
Little is known about how increases in intraocular pressure accompanying glaucoma affect retinal or choroidal circulation. Such knowledge could lead to new insights into the role of vascular-associated changes in the etiology of glaucoma-related blindness.
Acknowledgments
The authors thank Mr. Sam Adamson for assistance with digital imaging. Supported by Fondation Leducq of France, the National Institutes of Health of the United States (EY004077), the National Health and Medical Research Council of Australia (#571100, 1005730), the Rebecca L. Cooper Medical Research Foundation, the Brian M Kirby Foundation – Gift of Sight Initiative, and the NSW Optometrist Registration Board – Best Practice Grant.
References
- Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J Anesth. 2007;21:232–242. doi: 10.1007/s00540-006-0488-4. [DOI] [PubMed] [Google Scholar]
- Alder VA, Ben-Nun J, Cringle SJ. PO2 profiles and oxygen consumption in cat retina with an occluded retinal circulation. Invest Ophthalmol Vis Sci. 1990;31:1029–1034. [PubMed] [Google Scholar]
- Allende A, Madigan MC, Provis JM. Endothelial cell proliferation in the choriocapillaris during human retinal differentiation. Br J Ophthalmol. 2006;90:1046–1051. doi: 10.1136/bjo.2006.092080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm A, Bill A. The oxygen supply to the retina. I. Effects of changes in intraocular and arterial blood pressures, and in arterial PO2 and PCO2 on the oxygen tension in the vitreous body of the cat. Acta Physiol Scand. 1972a;84:261–274. doi: 10.1111/j.1748-1716.1972.tb05177.x. [DOI] [PubMed] [Google Scholar]
- Alm A, Bill A. The oxygen supply to the retina. II. Effects of high intraocular pressure and of increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. A study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Acta Physiol Scand. 1972b;84:306–319. doi: 10.1111/j.1748-1716.1972.tb05182.x. [DOI] [PubMed] [Google Scholar]
- Alm A. The effect of sympathetic stimulation on blood flow through the uvea, retina and optic nerve in monkeys (Macacca irus) Exp Eye Res. 1977;25:19–24. doi: 10.1016/0014-4835(77)90241-x. [DOI] [PubMed] [Google Scholar]
- Ames A, III, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Apte B, Hollyfield JG. Developmental anatomy of the retinal and choroidal vasculature. In: Besharse J, Bok D, editors. Encyclopedia of the Eye. Academic Press, Elsevier Books; London: 2009. pp. 9–15. [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Ret Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DB, Gardiner TA, Stitt AW. Retinal Vascular Disease. Springer; 2007. Functional anatomy, fine structure and basic pathology of the retinal vasculature; pp. 3–23. [Google Scholar]
- Armaly MF, Araki M. Effect of ocular pressure on choroidal circulation in the cat and Rhesus monkey. Invest Ophthalmol. 1975;14:584–591. [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Ashton N. Retinal angiogenesis in the human embryo. Br Med Bull. 1970;26:103–106. doi: 10.1093/oxfordjournals.bmb.a070758. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–791. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. Invest Ophthalmol Vis Sci. 2000;41:3561–3568. [PubMed] [Google Scholar]
- Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2008;246:763–769. doi: 10.1007/s00417-008-0766-y. [DOI] [PubMed] [Google Scholar]
- Ben-Nun J, Alder VA, Constable IJ. Retinal microvascular patency in the diabetic rat. Int Ophthalmol. 2004;25:187–192. doi: 10.1007/s10792-004-5815-x. [DOI] [PubMed] [Google Scholar]
- Berisha F, Feke GT, Trempe CL, McMeel JW, Schepens CL. Retinal abnormalities in early Alzheimer's disease. Invest Ophthalmol Vis Sci. 2007;48:2285–2289. doi: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- Bill A, Sperber GO. Control of retinal and choroidal blood flow. Eye. 1990;4:319–325. doi: 10.1038/eye.1990.43. [DOI] [PubMed] [Google Scholar]
- Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980;100:332–336. [PubMed] [Google Scholar]
- Bill A. Intraocular pressure and blood flow through the uvea. Arch Ophthalmol. 1962;67:336–348. doi: 10.1001/archopht.1962.00960020338010. [DOI] [PubMed] [Google Scholar]
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- Bizheva K, Pflug R, Hermann B, Povazay B, Sattmann H, Qiu P, Anger E, Reitsamer H, Popov S, Taylor JR, Unterhuber A, Ahnelt P, Drexler W. Optophysiology: depth-resolved probing of retinal physiology with functional ultrahigh-resolution optical coherence tomography. Proc Natl Acad Sci U S A. 2006;103:5066–5071. doi: 10.1073/pnas.0506997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J. Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefes Arch Clin Exp Ophthalmol. 1999;237:296–300. doi: 10.1007/s004170050236. [DOI] [PubMed] [Google Scholar]
- Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010;51:3758–3763. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner B, Tockner B, Runge C, Strohmaier C, Trost A, Branka M, Radner W, Kiel JW, Schroedl F, Reitsamer HA. The effect of vasopressin on choroidal blood flow, intraocular pressure, and orbital venous pressure in rabbits. Invest Ophthalmol Vis Sci. 2011;52:7134–7140. doi: 10.1167/iovs.11-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Biedermann B, Reichenbach A. Expression of potassium channels during postnatal differentiation of rabbit Müller glial cells. Eur J Neurosci. 1999;11:2883–2896. doi: 10.1046/j.1460-9568.1999.00706.x. [DOI] [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Uhlmann S, Kohen L, Wiedemann P, Reichenbach A. Membrane conductance of Müller glial cells in proliferative diabetic retinopathy. Can J Ophthalmol. 2002;37:221–227. doi: 10.1016/s0008-4182(02)80113-2. [DOI] [PubMed] [Google Scholar]
- Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, Schrauwen P, Brownlee M, Stehouwer CD, Schalkwijk CG. Over-expression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats. J Biol Chem. 2011;286:1374–1380. doi: 10.1074/jbc.M110.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun A, Ehinger B, Sundler F, Tornqvist K, Uddman R. Neuropeptide Y immunoreactive neurons in the guinea-pig uvea and retina. Invest Ophthalmol Vis Sci. 1984;25:1113–1123. [PubMed] [Google Scholar]
- Buerk DG, Riva CE, Cranstoun SD. Frequency and luminance-dependent blood-flow and K+ ion changes during flicker stimuli in cat optic nerve head. Invest Ophthalmol Vis Sci. 1995;36:2216–2227. [PubMed] [Google Scholar]
- Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc Res. 1996;52:13–26. doi: 10.1006/mvre.1996.0040. [DOI] [PubMed] [Google Scholar]
- Burnette JO, White RE. PGI2 opens potassium channels in retinal pericytes by cyclic AMP-stimulated, cross-activation of PKG. Exp Eye Res. 2006;83:1359–1365. doi: 10.1016/j.exer.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Bursell SE, Clermont AC, Shiba T, King GL. Evaluating retinal circulation using video fluorescein angiography in control and diabetic rats. Curr Eye Res. 1992;11:287–295. doi: 10.3109/02713689209001782. [DOI] [PubMed] [Google Scholar]
- Buttery RG, Hinrichsen CF, Weller WL, Haight JR. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vis Res. 1991;31:169–18. 7. doi: 10.1016/0042-6989(91)90110-q. [DOI] [PubMed] [Google Scholar]
- Cai WJ, Kocsis E, Scholz D, Luo X, Schaper W, Schaper J. Presence of Cx37 and lack of desmin in smooth muscle cells are early markers for arteriogenesis. Mol Cell Biochem. 2004;262:17–23. doi: 10.1023/b:mcbi.0000038201.43148.20. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Gardiner TA. Endothelium-derived agents in pericyte function/dysfunction. Prog Ret Eye Res. 1999;18:511–527. doi: 10.1016/s1350-9462(98)00034-2. [DOI] [PubMed] [Google Scholar]
- Chan-Ling TL, Halasz P, Stone J. Development of retinal vasculature in the cat: processes and mechanisms. Curr Eye Res. 1990;9:459–478. doi: 10.3109/02713689008999612. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division: Evidence that ‘Physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- Chan-Ling T. Glial, neuronal and vascular interactions in the mammalian retina. In: Osborne N, Chader G, editors. Progress in Retinal Research. Vol. 13. Pergamon Press; Oxford: 1994. pp. 357–389. Highest ranking journal in ophthalmology. [Google Scholar]
- Chan-Ling T, McLeod DS, Hughes S, Baxter L, Chu Y, Hasegawa T, Lutty GA. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004a;45:2020–2032. doi: 10.1167/iovs.03-1169. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Page MP, Gardiner T, Baxter L, Rosinova E, Hughes S. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am J Pathol. 2004b;165:1301–1313. doi: 10.1016/S0002-9440(10)63389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, Chu Y, Baxter L, Weible M, II, Hughes S. In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia. 2009;57:39–53. doi: 10.1002/glia.20733. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Dahlstrom JE, Koina ME, McColm JR, Sterling RA, Bean EG, Adamson S, Hughes S, Baxter LC. Evidence of hematopoietic differentiation, vasculogenesis and angiogenesis in the formation of human choroidal blood vessels. Exp Eye Res. 2011a;92:361–376. doi: 10.1016/j.exer.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Koina ME, McColm JR, Dahlstrom JE, Bean E, Adamson S, Yun S, Baxter L. Role of CD44+ stem cells in mural cell formation in the human choroid: evidence of vascular instability due to limited pericyte ensheathment. Invest Ophthalmol Vis Sci. 2011b;52:399–410. doi: 10.1167/iovs.10-5403. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T. Blood–Brain Barriers. Wiley-VCH Verlag GmbH & Co; KgaA: 2006. The blood retinal interface: similarities and contrasts with the blood-brain interface; pp. 701–724. [Google Scholar]
- Chan-Ling T. Vasculogenesis and angiogenesis in formation of the human retinal vasculature cell-cell interactions and molecular cues. In: Penn JS, editor. Retinal and Choroidal Angiogenesis. Springer; Netherlands: 2008. pp. 119–138. [Google Scholar]
- Chan-Ling T. Development of the Retinal Vasculature. In: Besharse J, Bok D, editors. Encyclopedia of the Eye. Vol. 2. Academic Press, Elsevier Books; London: pp. 22–33. Unpublished data. D-L, Chapter 4. [Google Scholar]
- Chauhan BC, LeVatte TL, Jollimore CA, Yu PK, Reitsamer HA, Kelly ME, Yu DY, Tremblay F, Archibald ML. Model of endothelin-1-induced chronic optic neuropathy in rat. Invest Ophthalmol Vis Sci. 2004;45:144–152. doi: 10.1167/iovs.03-0687. [DOI] [PubMed] [Google Scholar]
- Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006;47:3595–3602. doi: 10.1167/iovs.05-1522. [DOI] [PubMed] [Google Scholar]
- Chen C, Reed JF, III, Rice DC, Gee W, Updike DP, Salathe EP. Biomechanics of ocular pneumoplethysmography. J Biomech Eng. 1993;115:231–238. doi: 10.1115/1.2895480. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Cheng CY, Lee AF, Lee FL, Chou JC, Hsu WM, Liu JH. Pulsatile ocular blood flow in asymmetric exudative age related macular degeneration. Br J Ophthalmol. 2001;85:1411–1415. doi: 10.1136/bjo.85.12.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Duong TQ. Simplified laser-speckle-imaging analysis method and its application to retinal blood flow imaging. Opt Lett. 2007;32:2188–2190. doi: 10.1364/ol.32.002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Nair G, Walker TA, Kim MK, Pardue MT, Thule PM, Olson DE, Duong TQ. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A. 2006;103:17525–17530. doi: 10.1073/pnas.0605790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Rogers SL, Donaghue KC, Jenkins AJ, Tikellis G, Wong TY. Retinal arteriolar dilation predicts retinopathy in adolescents with type 1 diabetes. Diabetes Care. 2008;31:1842–1846. doi: 10.2337/dc08-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu K, Chan TF, Wu A, Leung IY, So KF, Chang RC. Neurodegeneration of the retina in mouse models of Alzheimer's disease: what can we learn from the retina? Age (Dordr) 2011 doi: 10.1007/s11357-011-9260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi GA, Orgul S, Onda E, Bacon DR, Van Buskirk EM. An in vivo model of chronic optic nerve ischemia: the dose-dependent effects of endothelin-1 on the optic nerve microvasculature. Curr Eye Res. 1995;14:1147–1153. doi: 10.3109/02713689508995821. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Harris A, Chung HS, Danis RP, Kagemann L, McNulty L, Pratt LM, Martin BJ. Color Doppler imaging discloses reduced ocular blood flow velocities in nonexudative age-related macular degeneration. Am J Ophthalmol. 1999;128:75–80. doi: 10.1016/s0002-9394(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Harris A, Kagemann L, Danis RP, Pratt LM, Chung HS, Weinberger D, Garzozi HJ. Choroidal perfusion perturbations in non-neovascular age related macular degeneration. Br J Ophthalmol. 2002;86:209–213. doi: 10.1136/bjo.86.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan DG, Kuwabara T. The mural cell in perspective. Arch Ophthalmol. 1967;78:133–139. doi: 10.1001/archopht.1967.00980030135005. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye. 2009;23:1496–1508. doi: 10.1038/eye.2009.108. [DOI] [PubMed] [Google Scholar]
- Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D'Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- de Hoz R, Ramirez AI, Salazar JJ, Rojas B, Ramirez JM, Trivino A. Substance P and calcitonin gene-related peptide intrinsic choroidal neurons in human choroidal whole-mounts. Histol Histopathol. 2008;23:1249–1258. doi: 10.14670/HH-23.1249. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Cerebrovascular and cardiovascular pathology in Alzheimer's disease. Int Rev Neurobiol. 2009;84:35–48. doi: 10.1016/S0074-7742(09)00403-6. [DOI] [PubMed] [Google Scholar]
- De Oliveira F. Pericytes in diabetic retinopathy. Br J Ophthalmol. 1966;50:134–143. doi: 10.1136/bjo.50.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaey C, Van De Voorde J. Pressure-induced myogenic responses in isolated bovine retinal arteries. Invest Ophthalmol Vis Sci. 2000a;41:1871–1875. [PubMed] [Google Scholar]
- Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000b;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285:H631–H636. doi: 10.1152/ajpheart.00111.2003. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated byendothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M, Smith LEH. Retinal vascular development. In: Joussen AM, Gardner TW, Kirchhof B, Ryan SJ, editors. Retinal Vascular Disease. Springer Berlin; Heidelberg: 2007. pp. 24–37. [Google Scholar]
- Dreher Z, Wegner M, Stone J. Muller cell endfeet at the inner surface of the retina: light microscopy. Vis Neurosci. 1988;1:169–180. doi: 10.1017/s0952523800001449. [DOI] [PubMed] [Google Scholar]
- Dumskyj MJ, Eriksen JE, Dore CJ, Kohner EM. Autoregulation in the human retinal circulation: assessment using isometric exercise, laser Doppler velocimetry, and computer-assisted image analysis. Microvasc Res. 1996;51:378–392. doi: 10.1006/mvre.1996.0034. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Ngan SC, Ugurbil K, Kim SG. Functional magnetic resonance imaging of the retina. Invest Ophthalmol Vis Sci. 2002;43:1176–1181. [PMC free article] [PubMed] [Google Scholar]
- Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113(Pt. 13):2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–743. [PubMed] [Google Scholar]
- Engerman RL. Development of the macular circulation. Invest Ophthalmol. 1976;15:835–840. [PubMed] [Google Scholar]
- Ernest JT, Archer D, Krill AE. Ocular hypertension induced by scleral suction cup. Invest Ophthalmol. 1972;11:29–34. [PubMed] [Google Scholar]
- Fallon TJ, Maxwell D, Kohner EM. Retinal vascular autoregulation in conditions of hyperoxia and hypoxia using the blue field entoptic phenomenon. Ophthalmology. 1985;92:701–705. doi: 10.1016/s0161-6420(85)33978-7. [DOI] [PubMed] [Google Scholar]
- Fallon TJ, Maxwell DL, Kohner EM. Autoregulation of retinal blood flow in diabetic retinopathy measured by the blue-light entoptic technique. Ophthalmology. 1987;94:1410–1415. doi: 10.1016/s0161-6420(87)33271-3. [DOI] [PubMed] [Google Scholar]
- Falsini B, Riva CE, Logean E. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. 2002;43:2309–2316. [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116:829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feke GT, Zuckerman R, Green GJ, Weiter JJ. Response of human retinal blood flow to light and dark. Invest Ophthalmol Vis Sci. 1983;24:136–141. [PubMed] [Google Scholar]
- Feke GT. Laser Doppler instrumentation for the measurement of retinal blood flow: theory and practice. Bull Soc Belge Ophtalmol. 2006:171–184. [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Findl O, Dallinger S, Rami B, Polak K, Schober E, Wedrich A, Ries E, Eichler HG, Wolzt M, Schmetterer L. Ocular haemodynamics and colour contrast sensitivity in patients with type 1 diabetes. Br J Ophthalmol. 2000a;84:493–498. doi: 10.1136/bjo.84.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findl O, Rainer G, Dallinger S, Dorner G, Polak K, Kiss B, Georgopoulos M, Vass C, Schmetterer L. Assessment of optic disk blood flow in patients with open-angle glaucoma. Am J Ophthalmol. 2000b;130:589–596. doi: 10.1016/s0002-9394(00)00636-x. [DOI] [PubMed] [Google Scholar]
- Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43:317–321. doi: 10.3129/i08-056. [DOI] [PubMed] [Google Scholar]
- Flower RW, Fryczkowski AW, McLeod DS. Variability in choriocapillaris blood flow distribution. Invest Ophthalmol Vis Sci. 1995;36:1247–1258. [PubMed] [Google Scholar]
- Formaz F, Riva CE, Geiser M. Diffuse luminance flicker increases retinal vessel diameter in humans. Curr Eye Res. 1997;16:1252–1257. doi: 10.1076/ceyr.16.12.1252.5021. [DOI] [PubMed] [Google Scholar]
- Frank RN, Dutta S, Mancini MA. Pericyte coverage is greater in the retinal than in the cerebral capillaries of the rat. Invest Ophthalmol Vis Sci. 1987;28:1086–1091. [PubMed] [Google Scholar]
- Frank RN. Growth factors in age-related macular degeneration: pathogenic and therapeutic implications. Ophthalmic Res. 1997;29:341–353. doi: 10.1159/000268032. [DOI] [PubMed] [Google Scholar]
- Friedman E, Chandra SR. Choroidal blood flow. III. Effects of oxygen and carbon dioxide. Arch Ophthalmol. 1972;87:70–71. doi: 10.1001/archopht.1972.01000020072015. [DOI] [PubMed] [Google Scholar]
- Friedman E, Ivry M, Ebert E, Glynn R, Gragoudas E, Seddon J. Increased scleral rigidity and age-related macular degeneration. Ophthalmology. 1989;96:104–108. doi: 10.1016/s0161-6420(89)32936-8. [DOI] [PubMed] [Google Scholar]
- Friedman E. Choroidal blood flow. Pressure-flow relationships. Arch Ophthalmol. 1970;83:95–99. doi: 10.1001/archopht.1970.00990030097018. [DOI] [PubMed] [Google Scholar]
- Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2008;146:348–349. doi: 10.1016/j.ajo.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Fuchsjager-Mayrl G, Polska E, Malec M, Schmetterer L. Unilateral light-dark transitions affect choroidal blood flow in both eyes. Vis Res. 2001;41:2919–2924. doi: 10.1016/s0042-6989(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Galassi F, Giambene B, Varriale R. Systemic vascular dysregulation and retrobulbar hemodynamics in normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2011;52:4467–4471. doi: 10.1167/iovs.10-6710. [DOI] [PubMed] [Google Scholar]
- Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation. 2007;14:25–38. doi: 10.1080/10739680601072123. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Huemer KH, Zawinka C, Schmetterer L, Dorner GT. Influence of diffuse luminance flicker on choroidal and optic nerve head blood flow. Curr Eye Res. 2002;24:109–113. doi: 10.1076/ceyr.24.2.109.8164. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Zawinka C, Huemer KH, Schmetterer L, Dorner GT. Flicker light-induced vasodilatation in the human retina: effect of lactate and changes in mean arterial pressure. Invest Ophthalmol Vis Sci. 2003;44:5309–5314. doi: 10.1167/iovs.03-0587. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Zawinka C, Resch H, Huemer KH, Dorner GT, Schmetterer L. Diffuse luminance flicker increases blood flow in major retinal arteries and veins. Vis Res. 2004a;44:833–838. doi: 10.1016/j.visres.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004b;88:887–891. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF. Cellular mechanisms in retinal vascular development. Prog Retin Eye Res. 2003;22:295–306. doi: 10.1016/s1350-9462(02)00062-9. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activites of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci. 2003;23:1678–1687. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraets WJ, Williams RC, Chang G, Ham WTJ, Guerry D, III, Schmidt FH. The relative absorption of thermal energy in retina and choroid. Invest Ophthalmol. 1962;1:340–347. [PubMed] [Google Scholar]
- Geiser MH, Riva CE, Dorner GT, Diermann U, Luksch A, Schmetterer L. Response of choroidal blood flow in the foveal region to hyperoxia and hyperoxia-hypercapnia. Curr Eye Res. 2000;21:669–676. [PubMed] [Google Scholar]
- Geitzenauer W, Hitzenberger CK, Schmidt-Erfurth UM. Retinal optical coherence tomography: past, present and future perspectives. Br J Ophthalmol. 2011;95:171–177. doi: 10.1136/bjo.2010.182170. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-d and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem AA, Elewa AM, Arafa LF. Endothelin-1 and nitric oxide levels in patients with glaucoma. Ophthalmic Res. 2011;46:98–102. doi: 10.1159/000323584. [DOI] [PubMed] [Google Scholar]
- Gherezghiher T, Okubo H, Koss MC. Choroidal and ciliary body blood flow analysis: application of laser Doppler flowmetry in experimental animals. Exp Eye Res. 1991;53:151–156. doi: 10.1016/0014-4835(91)90068-p. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Maceren RG, Shah AR, Meier JA, Zhu Y. KATP channels mediate adenosine-induced hyperemia in retina. Invest Ophthalmol Vis Sci. 1996;37:2624–2633. [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gordon GRJ, Choi HB, Rungta RL, Ellis-Davies GCR, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottanka J, Kuhlmann A, Scholz M, Johnson DH, Lutjen-Drecoll E. Pathophysiologic changes in the optic nerves of eyes with primary open angle and pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2005;46:4170–4181. doi: 10.1167/iovs.05-0289. [DOI] [PubMed] [Google Scholar]
- Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer's disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauslund J, Hodgson L, Kawasaki R, Green A, Sjolie AK, Wong TY. Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia. 2009;52:2213–2217. doi: 10.1007/s00125-009-1459-8. [DOI] [PubMed] [Google Scholar]
- Grieshaber MC, Orgul S, Schoetzau A, Flammer J. Relationship between retinal glial cell activation in glaucoma and vascular dysregulation. J Glaucoma. 2007;16:215–219. doi: 10.1097/IJG.0b013e31802d045a. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Brucker AJ, Sinclair SH, Petrig BL. Altered retinal vascular response to 100% oxygen breathing in diabetes mellitus. Ophthalmology. 1984a;91:1447–1452. doi: 10.1016/s0161-6420(84)34124-0. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Stone RA, Keates EU, Petrig BL. Retinal autor-egulation in open-angle glaucoma. Ophthalmology. 1984b;91:1690–1694. doi: 10.1016/s0161-6420(84)34091-x. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Martin DB, Quint AR, Epstein PA. Effect of an insulin-induced decrease in blood glucose on the human diabetic retinal circulation. Ophthalmology. 1987;94:1614–1620. doi: 10.1016/s0161-6420(87)33257-9. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Kozart DM. Retinal circulation during a spontaneous rise of intraocular pressure. Br J Ophthalmol. 1988;72:754–758. doi: 10.1136/bjo.72.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Riva CE, Baine J, Brucker AJ. Total retinal volumetric blood flow rate in diabetic patients with poor glycemic control. Invest Ophthalmol Vis Sci. 1992;33:356–363. [PubMed] [Google Scholar]
- Grunwald JE, Piltz J, Hariprasad SM, DuPont J. Optic nerve and choroidal circulation in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2329–2336. [PubMed] [Google Scholar]
- Grunwald JE, Metelitsina TI, DuPont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033–1038. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- Guo L, Duggan J, Cordeiro MF. Alzheimer's disease and retinal neuro-degeneration. Curr Alzheimer Res. 2010;7:3–14. doi: 10.2174/156720510790274491. [DOI] [PubMed] [Google Scholar]
- Haefliger IO, Anderson DR. Oxygen modulation of guanylate cyclase-mediated retinal pericyte relaxations with 3-morpholino-sydnonimine and atrial natriuretic peptide. Invest Ophthalmol Vis Sci. 1997;38:1563–1568. [PubMed] [Google Scholar]
- Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci. 1994;35:991–997. [PubMed] [Google Scholar]
- Haefliger IO, Chen Q, Anderson DR. Effect of oxygen on relaxation of retinal pericytes by sodium nitroprusside. Graefes Arch Clin Exp Ophthalmol. 1997;235:388–392. doi: 10.1007/BF00937289. [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:5. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Vilser W, Riemer T, Liemt F, Jentsch S, Dawczynski J, Schweitzer D. Retinal venous oxygen saturation increases by flicker light stimulation. Invest Ophthalmol Vis Sci. 2011;52:274–277. doi: 10.1167/iovs.10-5537. [DOI] [PubMed] [Google Scholar]
- Hancock HA, Kraft TW. Oscillatory potential analysis and ERGs of normal and diabetic rats. Invest Ophthalmol Vis Sci. 2004;45:1002–1008. doi: 10.1167/iovs.03-1080. [DOI] [PubMed] [Google Scholar]
- Haribalaganesh R, Sheikpranbabu S, Elayappan B, Venkataraman D, Gurunathan S. Pigment-epithelium-derived factor down regulates hyperglycemia-induced apoptosis via PI3K/Akt activation in goat retinal pericytes. Angiogenesis. 2009;12:381–389. doi: 10.1007/s10456-009-9159-z. [DOI] [PubMed] [Google Scholar]
- Harris A, Arend O, Bohnke K, Kroepfl E, Danis R, Martin B. Retinal blood flow during dynamic exercise. Graefes Arch Clin Exp Ophthalmol. 1996a;234:440–444. doi: 10.1007/BF02539410. [DOI] [PubMed] [Google Scholar]
- Harris A, Arend O, Danis RP, Evans D, Wolf S, Martin BJ. Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol. 1996b;80:209–213. doi: 10.1136/bjo.80.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Chung HS, Ciulla TA, Kagemann L. Progress in measurement of ocular blood flow and relevance to our understanding of glaucoma and age-related macular degeneration. Prog Retin Eye Res. 1999;18:669–687. doi: 10.1016/s1350-9462(98)00037-8. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, McLeod DS, Bhutto IA, Prow T, Merges CA, Grebe R, Lutty GA. The embryonic human choriocapillaris develops by hemo-vasculogenesis. Dev Dyn. 2007;236:2089–2100. doi: 10.1002/dvdy.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelius U, Hansen F, Hindfelt B, Krakau T. Human ocular vasodynamic changes in light and darkness. Invest Ophthalmol Vis Sci. 1999;40:1850–1855. [PubMed] [Google Scholar]
- Hein TW, Yuan Z, Rosa RH, Jr, Kuo L. Requisite roles of A2A receptors, nitric oxide, and KATP channels in retinal arteriolar dilation in response to adenosine. Invest Ophthalmol Vis Sci. 2005;46:2113–2119. doi: 10.1167/iovs.04-1438. [DOI] [PubMed] [Google Scholar]
- Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- Hein TW, Rosa RH, Jr, Yuan Z, Roberts E, Kuo L. Divergent roles of nitric oxide and rho kinase in vasomotor regulation of human retinal arterioles. Invest Ophthalmol Vis Sci. 2010;51:1583–1590. doi: 10.1167/iovs.09-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkind P, De Oliveira LF. Retinal arteriolar annuli. Invest Ophthalmol. 1968;7:584–591. [PubMed] [Google Scholar]
- Henkind P. Radial peripapillary capillaries of the retina. I. Anatomy: human and comparative. Br J Ophthalmol. 1967;51:115–123. doi: 10.1136/bjo.51.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman IM, D'Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985;101:43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessellund A, Aalkjaer C, Bek T. Effect of acidosis on isolated porcine retinal vessels. Curr Eye Res. 2006;31:427–434. doi: 10.1080/02713680600681236. [DOI] [PubMed] [Google Scholar]
- Hillman EM, Devor A, Bouchard MB, Dunn AK, Krauss GW, Skoch J, Bacskai BJ, Dale AM, Boas DA. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. Neuroimage. 2007;35:89–104. doi: 10.1016/j.neuroimage.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Oku H, Goto W, Sugiyama T, Kobayashi T, Ikeda T. Effects of adenosine on optic nerve head circulation in rabbits. Exp Eye Res. 2004;79:729–735. doi: 10.1016/j.exer.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Feeney L. The ultrastructure of the retinal vessels. II The small vessels. J Ultrastruct Res. 1963;9:29–46. doi: 10.1016/s0022-5320(63)80034-9. [DOI] [PubMed] [Google Scholar]
- Holländer H, Makarov F, Dreher Z, Van Driel D, Chan-Ling T, Stone J. Structure of the macroglia of the retina: the sharing and division of labour between astrocytes and Muller cells. J Comp Neurol. 1991;313:587–603. doi: 10.1002/cne.903130405. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- Howlett DR, Bate ST, Collier S, Lawman A, Chapman T, Ashmeade T, Marshall I, Anderson PJ, Philpott KL, Richardson JC, Hille CJ. Characterisation of amyloid-induced inflammatory responses in the rat retina. Exp Brain Res. 2011 doi: 10.1007/s00221-011-2819-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Chan-Ling T. Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci. 2004;45:2795–2806. doi: 10.1167/iovs.03-1312. [DOI] [PubMed] [Google Scholar]
- Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–1228. [PubMed] [Google Scholar]
- Hughes SJ, Wall N, Scholfield CN, McGeown JG, Gardiner TA, Stitt AW, Curtis TM. Advanced glycation endproduct modified basement membrane attenuates endothelin-1 induced [Ca2+]i signalling and contraction in retinal microvascular pericytes. Mol Vis. 2004;10:996–1004. [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27:1838–1847. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Baxter L, Chan-Ling T. Changes in pericytes and smooth muscle cells in the kitten model of retinopathy of prematurity: implications for plus disease. Invest Ophthalmol Vis Sci. 2007;48:1368–1379. doi: 10.1167/iovs.06-0850. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Li J, Ebner TJ, Xu X. Nitric oxide contributes to functional hyperemia in cerebellar cortex. Am J Physiol. 1995;268:R1153–R1162. doi: 10.1152/ajpregu.1995.268.5.R1153. [DOI] [PubMed] [Google Scholar]
- Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006;26:18–24. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- Ishikawa T. Fine structure of retinal vessels in man and the macaque monkey. Invest Ophthalmol. 1963;2:1–15. [PubMed] [Google Scholar]
- Ishizaki E, Fukumoto M, Puro DG. Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J Physiol. 2009;587:2233–2253. doi: 10.1113/jphysiol.2009.169003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P, Sanye-Hajari J, Bek T. Increased blood pressure induces a diameter response of retinal arterioles that increases with decreasing arteriolar diameter. Invest Ophthalmol Vis Sci. 2007;48:328–331. doi: 10.1167/iovs.06-0360. [DOI] [PubMed] [Google Scholar]
- Justino L, Kergoat M, Bergman H, Chertkow H, Robillard A, Kergoat H. Neuroretinal function is normal in early dementia of the Alzheimer type. Neurobiol Aging. 2001;22:691–695. doi: 10.1016/s0197-4580(01)00234-2. [DOI] [PubMed] [Google Scholar]
- Kaiser HJ, Flammer J, Wenk M, Luscher T. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to postural changes. Graefes Arch Clin Exp Ophthalmol. 1995;233:484–488. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Pax AB. Increased collagen content of cerebral microvessels in Alzheimer's disease. Brain Res. 1995;705:349–352. doi: 10.1016/0006-8993(95)01250-8. [DOI] [PubMed] [Google Scholar]
- Karwoski CJ, Newman EA, Shimazaki H, Proenza LM. Light-evoked increases in extracellular K+ in the plexiform layers of amphibian retinas. J Gen Physiol. 1985;86:189–213. doi: 10.1085/jgp.86.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, Puro DG. ATP: a vasoactive signal in the pericyte-containing micro-vasculature of the rat retina. J Physiol. 2003;551.3:787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarai M, Koss MC. Sympathetic vasoconstriction in the rat anterior choroid is mediated by a1-adrenoceptors. Eur J Pharmacol. 1998;363:35–40. doi: 10.1016/s0014-2999(98)00790-0. [DOI] [PubMed] [Google Scholar]
- Kergoat H, Kergoat MJ, Justino L, Chertkow H, Robillard A, Bergman H. Visual retinocortical function in dementia of the Alzheimer type. Gerontology. 2002;48:197–203. doi: 10.1159/000058350. [DOI] [PubMed] [Google Scholar]
- Kern TS, Engerman RL. Galactose-induced retinal microangiopathy in rats. Invest Ophthalmol Vis Sci. 1995;36:490–496. [PubMed] [Google Scholar]
- Kern TS, Tang J, Mizutani M, Kowluru RA, Nagaraj RH, Romeo G, Podesta F, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp Eye Res. 1999;69:413–429. doi: 10.1006/exer.1999.0717. [DOI] [PubMed] [Google Scholar]
- Kiss B, Polska E, Dorner G, Polak K, Findl O, Mayrl GF, Eichler HG, Wolzt M, Schmetterer L. Retinal blood flow during hyperoxia in humans revisited: concerted results using different measurement techniques. Microvasc Res. 2002;64:75–85. doi: 10.1006/mvre.2002.2402. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, DeMets DL, Kaufman I, Voss PS. Prevalence of diabetes mellitus in southern Wisconsin. Am J Epidemiol. 1984;119:54–61. doi: 10.1093/oxfordjournals.aje.a113725. [DOI] [PubMed] [Google Scholar]
- Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508.1:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Zimmererman PA, Nelson MT. Extracellular K+-induced hyper-polarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J Physiol. 1996;492.2:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508(Pt. 1):211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Ceelen PW, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, Thomzig A, Veh RW, Bringmann A, Reichenbach A. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia. 2002;39:292–303. doi: 10.1002/glia.10112. [DOI] [PubMed] [Google Scholar]
- Kondo M, Wang L, Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand. 1997;75:232–235. doi: 10.1111/j.1600-0420.1997.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Kornzweig AL, Eliasoph I, Feldstein M. Selective atrophy of the radial peripapillary capillaries in chronic glaucoma. Arch Ophthalmol. 1968;80:696–702. doi: 10.1001/archopht.1968.00980050698002. [DOI] [PubMed] [Google Scholar]
- Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1326–H1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- Kotliar KE, Vilser W, Nagel E, Lanzl IM. Retinal vessel reaction in response to chromatic flickering light. Graefes Arch Clin Exp Ophthalmol. 2004;242:377–392. doi: 10.1007/s00417-003-0847-x. [DOI] [PubMed] [Google Scholar]
- Kubista KE, Tosakulwong N, Wu Y, Ryu E, Roeder JL, Hecker LA, Baratz KH, Brown WL, Edwards AO. Copy number variation in the complement factor H-related genes and age-related macular degeneration. Mol Vis. 2011;17:2080–2092. [PMC free article] [PubMed] [Google Scholar]
- Kuo IY, Chan-Ling T, Wojcikiewicz RJ, Hill CE. Limited intravascular coupling in the rodent brainstem and retina supports a role for glia in regional blood flow. J Comp Neurol. 2008;511:773–787. doi: 10.1002/cne.21873. [DOI] [PubMed] [Google Scholar]
- Kutcher ME, Kolyada AY, Surks HK, Herman IM. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol. 2007;171:693–701. doi: 10.2353/ajpath.2007.070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties AM. Central retinal artery innervation. Absence of adrenergic innervation to the intraocular branches. Arch Ophthalmol. 1967;77:405–409. doi: 10.1001/archopht.1967.00980020407021. [DOI] [PubMed] [Google Scholar]
- Le YZ, Bai Y, Zhu M, Zheng L. Temporal requirement of RPE-derived VEGF in the development of choroidal vasculature. J Neurochem. 2010;112:1584–1592. doi: 10.1111/j.1471-4159.2010.06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecleire-Collet A, Audo I, Aout M, Girmens JF, Sofroni R, Erginay A, Le Gargasson JF, Mohand-Said S, Meas T, Guillausseau PJ, Vicaut E, Paques M, Massin P. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011;52:2861–2867. doi: 10.1167/iovs.10-5960. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Li W, Liu X, He Z, Yanoff M, Jian B, Ye X. Expression of apoptosis regulatory genes by retinal pericytes after rapid glucose reduction. Invest Ophthalmol Vis Sci. 1998;39:1535–1543. [PubMed] [Google Scholar]
- Li Y, Cheng H, Duong TQ. Blood-flow magnetic resonance imaging of the retina. Neuroimage. 2008;39:1744–1751. doi: 10.1016/j.neuroimage.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TL, Beyer EC, Duling BR. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am J Physiol. 1995;268:H729–H739. doi: 10.1152/ajpheart.1995.268.2.H729. [DOI] [PubMed] [Google Scholar]
- Longo A, Geiser M, Riva CE. Subfoveal choroidal blood flow in response to light-dark exposure. Invest Ophthalmol Vis Sci. 2000;41:2678–2683. [PubMed] [Google Scholar]
- Loufrani L, Li Z, Levy BI, Paulin D, Henrion D. Excessive microvascular adaptation to changes in blood flow in mice lacking gene encoding for desmin. Arterioscler Thromb Vasc Biol. 2002a;22:1579–1584. doi: 10.1161/01.atv.0000032652.24932.1a. [DOI] [PubMed] [Google Scholar]
- Loufrani L, Matrougui K, Li Z, Levy BI, Lacolley P, Paulin D, Henrion D. Selective microvascular dysfunction in mice lacking the gene encoding for desmin. FASEB J. 2002b;16:117–119. doi: 10.1096/fj.01-0505fje. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Riva CE, Petrig BL, Geiser M. Choroidal blood flow during exercise-induced changes in the ocular perfusion pressure. Invest Ophthalmol Vis Sci. 2003;44:2126–2132. doi: 10.1167/iovs.02-0825. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Kergoat H, Wajszilber MA. Blue flicker modifies the subfoveal choroidal blood flow in the human eye. Am J Physiol Heart Circ Physiol. 2005;289:H683–H691. doi: 10.1152/ajpheart.01187.2004. [DOI] [PubMed] [Google Scholar]
- Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in chorio- capillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35. [PubMed] [Google Scholar]
- Maleki N, Dai W, Alsop DC. Blood flow quantification of the human retina with MRI. NMR Biomed. 2011;24:104–111. doi: 10.1002/nbm.1564. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes Care. 2007;30:3048–3052. doi: 10.2337/dc07-0927. [DOI] [PubMed] [Google Scholar]
- Mansour H, Chamberlain CG, Weible MW, Hughes S, Chu Y, Chan-Ling T. Aging-related changes in astrocytes in the rat retina: imbalance between cell proliferation and cell death reduces astrocyte availability. Aging Cell. 2008;7:526–540. doi: 10.1111/j.1474-9726.2008.00402.x. [DOI] [PubMed] [Google Scholar]
- Markhotina N, Liu GJ, Martin DK. Contractility of retinal pericytes grown on silicone elastomer substrates is through a protein kinase A-mediated intracellular pathway in response to vasoactive peptides. IET Nanobiotechnol. 2007;1:44–51. doi: 10.1049/iet-nbt:20060019. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Fan J, Yokoyama Y, Gerber HP, Ferrara N, Crouch RK, Olsen BR. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167:1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004;45:3330–3336. doi: 10.1167/iovs.04-0247. [DOI] [PubMed] [Google Scholar]
- Matsugi T, Chen Q, Anderson DR. Adenosine-induced relaxation of cultured bovine retinal pericytes. Invest Ophthalmol Vis Sci. 1997a;38:2695–2701. [PubMed] [Google Scholar]
- Matsugi T, Chen Q, Anderson DR. Contractile responses of cultured bovine retinal pericytes to angiotensin II. Arch Ophthalmol. 1997b;115:1281–1285. doi: 10.1001/archopht.1997.01100160451011. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Puro DG. Topographical heterogeneity of Kir currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol. 2006;573:483–495. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Fukumoto M, Kobayashi T, Kobayashi M, Ishizaki E, Minami M, Katsumura K, Liao SD, Wu DM, Zhang T, Puro DG. Diabetes-induced inhibition of voltage-dependent calcium channels in the retinal microvasculature: role of spermine. Invest Ophthalmol Vis Sci. 2010;51:5979–5990. doi: 10.1167/iovs.10-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Fujita H, Seki C, Kashikura K, Kanno I. Hemodynamics evoked by microelectrical direct stimulation in rat somatosensory cortex. Comp Biochem Physiol A. 1999;124:47–52. doi: 10.1016/s1095-6433(99)00086-0. [DOI] [PubMed] [Google Scholar]
- McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, Scholfield CN, McGeown JG, Curtis TM. Diabetes downregulates large-conductance Ca2+-activated potassium β1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007a;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahon MK, Dawicki JM, Arora A, Simpson DA, Gardiner TA, Stitt AW, Scholfield CN, McGeown JG, Curtis TM. Kv1.5 is a major component underlying the A-type potassium current in retinal arteriolar smooth muscle. Am J Physiol Heart Circ Physiol. 2007b;292:H1001–H1008. doi: 10.1152/ajpheart.01003.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahon MK, Needham MA, Scholfield CN, McGeown JG, Curtis TM. Ca2+-activated Cl current in retinal arteriolar smooth muscle. Invest Ophthalmol Vis Sci. 2009;50:364–371. doi: 10.1167/iovs.08-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson G, Langhans MJ, Groh MJ. Perfusion of the juxtapapillary retina and the neuroretinal rim area in primary open angle glaucoma. J Glaucoma. 1996a;5:91–98. [PubMed] [Google Scholar]
- Michelson G, Schmauss B, Langhans MJ, Harazny J, Groh MJ. Principle, validity, and reliability of scanning laser Doppler flowmetry. J Glaucoma. 1996b;5:99–105. [PubMed] [Google Scholar]
- Michelson G, Warntges S, Baleanu D, Welzenbach J, Ohno-Jinno A, Pogorelov P, Harazny J. Morphometric age-related evaluation of small retinal vessels by scanning laser Doppler flowmetry: determination of a vessel wall index. Retina. 2007;27:490–498. doi: 10.1097/01.iae.0000243032.33738.f7. [DOI] [PubMed] [Google Scholar]
- Mishra A, Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia. 2010;58:1996–2004. doi: 10.1002/glia.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Newman EA. Aminoguanidine reverses the loss of functional hyperemia in a rat model of diabetic retinopathy. Front Neuroenergetics. 2012;3:10. doi: 10.3389/fnene.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Hamid A, Newman EA. Oxygen modulation of neurovascular coupling in the retina. Proc Natl Acad Sci U S A. 2011;108:17827–17831. doi: 10.1073/pnas.1110533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- Mori F, Konno S, Hikichi T, Yamaguchi Y, Ishiko S, Yoshida A. Pulsatile ocular blood flow study: decreases in exudative age related macular degeneration. Br J Ophthalmol. 2001;85:531–533. doi: 10.1136/bjo.85.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motiejunaite R, Kazlauskas A. Pericytes and ocular diseases. Exp Eye Res. 2008;86:171–177. doi: 10.1016/j.exer.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Movaffaghy A, Chamot SR, Petrig BL, Riva CE. Blood flow in the human optic nerve head during isometric exercise. Exp Eye Res. 1998;67:561–568. doi: 10.1006/exer.1998.0556. [DOI] [PubMed] [Google Scholar]
- Muir ER, Duong TQ. MRI of retinal and choroidal blood flow with laminar resolution. NMR Biomed. 2011;24:216–223. doi: 10.1002/nbm.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nagahara M, Tamaki Y, Tomidokoro A, Araie M. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci. 2011;52:87–92. doi: 10.1167/iovs.09-4422. [DOI] [PubMed] [Google Scholar]
- Nagel E, Vilser W. Flicker observation light induces diameter response in retinal arterioles: a clinical methodological study. Br J Ophthalmol. 2004;88:54–56. doi: 10.1136/bjo.88.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel E, Vilser W, Lanzl I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Invest Ophthalmol Vis Sci. 2004;45:1486–1492. doi: 10.1167/iovs.03-0667. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Newman EA. Voltage-dependent calcium and potassium channels in retinal glial cells. Nature. 1985;317:809–811. doi: 10.1038/317809a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Regional specialization of the membrane of retinal glial cells and its importance to K+ spatial buffering. Ann N Y Acad Sci. 1986;481:273–286. doi: 10.1111/j.1749-6632.1986.tb27158.x. [DOI] [PubMed] [Google Scholar]
- Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25:5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, Simpson R, Shaw J, Wong TY. Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes. 2008;57:536–539. doi: 10.2337/db07-1376. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W, Wong TY. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes Care. 2009;32:2075–2080. doi: 10.2337/dc09-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SF. Nitric oxide as a mediator of parasympathetic vasodilation in ocular and extraocular tissues in the rabbit. Invest Ophthalmol Vis Sci. 1996;37:2110–2119. [PubMed] [Google Scholar]
- Ning A, Cui J, To E, Ashe KH, Matsubara J. Amyloid-beta deposits lead to retinal degeneration in a mouse model of Alzheimer disease. Invest Ophthalmol Vis Sci. 2008;49:5136–5143. doi: 10.1167/iovs.08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Kador PF. Aldose reductase/polyol inhibitors for diabetic retinopathy. Curr Pharm Biotechnol. 2011;12:373–385. doi: 10.2174/138920111794480642. [DOI] [PubMed] [Google Scholar]
- Ogura Y. In vivo evaluation of leukocyte dynamics in the retinal and choroidal circulation. Jpn J Ophthalmol. 2000;44:322–323. doi: 10.1016/s0021-5155(00)00164-7. [DOI] [PubMed] [Google Scholar]
- Okada S, Ohta Y. Microvascular pattern of the retina in the Japanese monkey (Macaca fuscata fuscata) Scanning Microsc. 1994;8:415–427. [PubMed] [Google Scholar]
- Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149:235–242. doi: 10.1016/j.pain.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001;42:1915–1920. [PubMed] [Google Scholar]
- Olive M, Goldfarb L, Moreno D, Laforet E, Dagvadorj A, Sambuughin N, Mar-tinez-Matos JA, Martinez F, Alio J, Farrero E, Vicart P, Ferrer I. Desmin-related myopathy: clinical, electrophysiological, radiological, neuro-pathological and genetic studies. J Neurol Sci. 2004;219:125–137. doi: 10.1016/j.jns.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ozanics V, Rayborn ME, Sagun D. Observations on the ultrastructure of the developing primate choroid coat. Exp Eye Res. 1978;26:25–45. doi: 10.1016/0014-4835(78)90149-5. [DOI] [PubMed] [Google Scholar]
- Pallikaris IG, Kymionis GD, Ginis HS, Kounis GA, Christodoulakis E, Tsilimbaris MK. Ocular rigidity in patients with age-related macular degeneration. Am J Ophthalmol. 2006;141:611–615. doi: 10.1016/j.ajo.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes HP, Bringmann A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006;55:633–639. doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- Parisi V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer's disease. Semin Ophthalmol. 2003;18:50–57. doi: 10.1076/soph.18.2.50.15855. [DOI] [PubMed] [Google Scholar]
- Park SH, Park JW, Park SJ, Kim KY, Chung JW, Chun MH, Oh SJ. Apoptotic death of photoreceptors in the streptozotocin-induced diabetic rat retina. Diabetologia. 2003;46:1260–1268. doi: 10.1007/s00125-003-1177-6. [DOI] [PubMed] [Google Scholar]
- Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol. 1980;89:641–646. doi: 10.1016/0002-9394(80)90280-9. [DOI] [PubMed] [Google Scholar]
- Parver LM, Auker CR, Carpenter DO. The stabilizing effect of the choroidal circulation on the temperature environment of the macula. Retina. 1982;2:117–120. doi: 10.1097/00006982-198200220-00008. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemp B, Schmetterer L. Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol. 2008;43:295–301. doi: 10.3129/i08-049. [DOI] [PubMed] [Google Scholar]
- Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci. 2009;50:4029–4032. doi: 10.1167/iovs.08-3260. [DOI] [PubMed] [Google Scholar]
- Penfold PL, Provis JM, Madigan MC, van DD, Billson FA. Angiogenesis in normal human retinal development: the involvement of astrocytes and macrophages. Graefes Arch Clin Exp Ophthalmol. 1990;228:255–263. doi: 10.1007/BF00920031. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Lumayag S, Kovacs B, Mufson EJ, Xu S. β-amyloid deposition and functional impairment in the retina of the APPswe/PS1DE9 transgenic mouse model of Alzheimer's disease. Invest Ophthalmol Vis Sci. 2009;50:793–800. doi: 10.1167/iovs.08-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrig BL, Riva CE. Near-IR retinal laser Doppler velocimetry and flow-metry: new delivery and detection techniques. Appl Opt. 1991;30:2073–2078. doi: 10.1364/AO.30.002073. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister F, Feng Y, vom HF, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57:2495–2502. doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010;47:59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- Phipps JA, Fletcher EL, Vingrys AJ. Paired-flash identification of rod and cone dysfunction in the diabetic rat. Invest Ophthalmol Vis Sci. 2004;45:4592–4600. doi: 10.1167/iovs.04-0842. [DOI] [PubMed] [Google Scholar]
- Podesta F, Romeo G, Liu WH, Krajewski S, Reed JC, Gerhardinger C, Lorenzi M. Bax is increased in the retina of diabetic subjects and is associated with pericyte apoptosis in vivo and in vitro. Am J Pathol. 2000;156:1025–1032. doi: 10.1016/S0002-9440(10)64970-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak K, Dorner G, Kiss B, Polska E, Findl O, Rainer G, Eichler HG, Schmetterer L. Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol. 2000;84:1285–1290. doi: 10.1136/bjo.84.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak K, Schmetterer L, Riva CE. Influence of flicker frequency on flicker-induced changes of retinal vessel diameter. Invest Ophthalmol Vis Sci. 2002;43:2721–2726. [PubMed] [Google Scholar]
- Polska E, Polak K, Luksch A, Fuchsjager-Mayrl G, Petternel V, Findl O, Schmetterer L. Twelve hour reproducibility of choroidal blood flow parameters in healthy subjects. Br J Ophthalmol. 2004;88:533–537. doi: 10.1136/bjo.2003.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polska E, Simader C, Weigert G, Doelemeyer A, Kolodjaschna J, Scharmann O, Schmetterer L. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest Ophthalmol Vis Sci. 2007;48:3768–3774. doi: 10.1167/iovs.07-0307. [DOI] [PubMed] [Google Scholar]
- Polverini PJ, Cotran PS, Gimbrone MA, Jr, Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Pomero F, Allione A, Beltramo E, Buttiglieri S, D'Alu F, Ponte E, Lacaria A, Porta M. Effects of protein kinase C inhibition and activation on proliferation and apoptosis of bovine retinal pericytes. Diabetologia. 2003;46:416–419. doi: 10.1007/s00125-003-1044-5. [DOI] [PubMed] [Google Scholar]
- Portmann N, Gugleta K, Kochkorov A, Polunina A, Flammer J, Orgul S. Choroidal blood flow response to isometric exercise in glaucoma patients and patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2011;52:7068–7073. doi: 10.1167/iovs.11-7758. [DOI] [PubMed] [Google Scholar]
- Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Ret Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Krishnamoorthy R, Yorio T. Endothelin, astrocytes and glaucoma. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preitner A, Orgul S, Prunte C, Flammer J. Measurement procedures in confocal choroidal laser Doppler flowmetry. Curr Eye Res. 2004;28:233–240. doi: 10.1076/ceyr.28.4.233.27830. [DOI] [PubMed] [Google Scholar]
- Provis JM. Development of the primate retinal vasculature. Prog Retin Eye Res. 2001;20:799–821. doi: 10.1016/s1350-9462(01)00012-x. [DOI] [PubMed] [Google Scholar]
- Prunte C, Niesel P. Quantification of choroidal blood-flow parameters using indocyanine green video-fluorescence angiography and statistical picture analysis. Graefes Arch Clin Exp Ophthalmol. 1988;226:55–58. doi: 10.1007/BF02172719. [DOI] [PubMed] [Google Scholar]
- Puro DG. Diabetes-induced dysfunction of retinal Muller cells. Trans Am Ophthalmol Soc. 2002;100:339–352. [PMC free article] [PubMed] [Google Scholar]
- Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quignard JF, Harley EA, Duhault J, Vanhoutte PM, Feletou M. K+ channels in cultured bovine retinal pericytes: effects of beta-adrenergic stimulation. J Cardiovasc Pharmacol. 2003;42:379–388. doi: 10.1097/00005344-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Trivino A, Ramirez AI, Salazar JJ, Garcia-Sanchez J. Structural specializations of human retinal glial cells. Vis Res. 1996;36:2029–2036. doi: 10.1016/0042-6989(95)00322-3. [DOI] [PubMed] [Google Scholar]
- Reber F, Gersch U, Funk RW. Blockers of carbonic anhydrase can cause increase of retinal capillary diameter, decrease of extracellular and increase of intracellular pH in rat retinal organ culture. Graefes Arch Clin Exp Ophthalmol. 2003;241:140–148. doi: 10.1007/s00417-002-0560-1. [DOI] [PubMed] [Google Scholar]
- Resch H, Karl K, Weigert G, Wolzt M, Hommer A, Schmetterer L, Garhofer G. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest Ophthalmol Vis Sci. 2009;50:358–363. doi: 10.1167/iovs.08-2460. [DOI] [PubMed] [Google Scholar]
- Riva CE, Falsini B. Functional laser Doppler flowmetry of the optic nerve: physiological aspects and clinical applications. Prog Brain Res. 2008;173:149–163. doi: 10.1016/S0079-6123(08)01111-4. [DOI] [PubMed] [Google Scholar]
- Riva C, Grunwald J, Sinclair S, O'Keefe K. Fundus camera based retinal LDV. Appl Opt. 1981a;20:117–120. doi: 10.1364/AO.20.000117. [DOI] [PubMed] [Google Scholar]
- Riva CE, Sinclair SH, Grunwald JE. Autoregulation of retinal circulation in response to decrease of perfusion pressure. Invest Ophthalmol Vis Sci. 1981b;21:34–38. [PubMed] [Google Scholar]
- Riva CE, Grunwald JE, Petrig BL. Reactivity of the human retinal circulation to darkness: a laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 1983;24:737–740. [PubMed] [Google Scholar]
- Riva CE, Harino S, Shonat RD, Petrig BL. Flicker evoked increase in optic nerve head blood flow in anesthetized cats. Neurosci Lett. 1991;128:291–296. doi: 10.1016/0304-3940(91)90282-x. [DOI] [PubMed] [Google Scholar]
- Riva CE, Cranstoun SD, Grunwald JE, Petrig BL. Choroidal blood flow in the foveal region of the human ocular fundus. Invest Ophthalmol Vis Sci. 1994a;35:4273–4281. [PubMed] [Google Scholar]
- Riva CE, Cranstoun SD, Mann RM, Barnes GE. Local choroidal blood flow in the cat by laser Doppler flowmetry. Invest Ophthalmol Vis Sci. 1994b;35:608–618. [PubMed] [Google Scholar]
- Riva CE, Titze P, Hero M, Movaffaghy A, Petrig BL. Choroidal blood flow during isometric exercises. Invest Ophthalmol Vis Sci. 1997a;38:2338–2343. [PubMed] [Google Scholar]
- Riva CE, Titze P, Hero M, Petrig BL. Effect of acute decreases of perfusion pressure on choroidal blood flow in humans. Invest Ophthalmol Vis Sci. 1997b;38:1752–1760. [PubMed] [Google Scholar]
- Riva CE, Falsini B, Logean E. Flicker-evoked responses of human optic nerve head blood flow: luminance versus chromatic modulation. Invest Ophthalmol Vis Sci. 2001;42:756–762. [PubMed] [Google Scholar]
- Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Ret Eye Res. 2005;24:183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Riva CE, Alm A, Pouraras CJ. Ocular circulation. In: Kaufman PL, Alm A, Levin LA, Nilsson SFE, ver Hoeve J, WU SM, editors. Adler's Physiology of the Eye. Mosby: Elsevier; 2011. pp. 243–273. [Google Scholar]
- Riva CE. Sub-foveal choroidal blood flow by LDF: measurement and application to the physiology and pathology of the choroidal circulation. Bull Soc Belge Ophtalmol. 2006;302:185–194. [PubMed] [Google Scholar]
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986;27:722–726. [PubMed] [Google Scholar]
- Robison WG, Jr, Tillis TN, Laver N, Kinoshita JH. Diabetes-related histopathologies of the rat retina prevented with an aldose reductase inhibitor. Exp Eye Res. 1990;50:355–366. doi: 10.1016/0014-4835(90)90136-i. [DOI] [PubMed] [Google Scholar]
- Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-κB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–108. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Tonkiss J, Roy S. Aging increases retinal vascular lesions characteristic of early diabetic retinopathy. Biogerontology. 2010;11:447–455. doi: 10.1007/s10522-010-9263-x. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den HT, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Peripapillary venous drainage from the choroid: a variable feature in human eyes. Br J Ophthalmol. 1997;81:76–79. doi: 10.1136/bjo.81.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari JC. Metabolism and photochemistry in the retina. In: Moses RA, Hart WM, editors. Adler's Physiology of the Eye Clinical Application. Mosby; St Louis: 1987. pp. 356–373. [Google Scholar]
- Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J Physiol. 1999;521.3:637–650. doi: 10.1111/j.1469-7793.1999.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami K, Kawamura H, Wu DM, Puro DG. Nitric oxide/cGMP-induced inhibition of calcium and chloride currents in retinal pericytes. Microvasc Res. 2001;62:196–203. doi: 10.1006/mvre.2001.2343. [DOI] [PubMed] [Google Scholar]
- Sandercoe TM, Madigan MC, Billson FA, Penfold PL, Provis JM. Astrocyte proliferation during development of the human retinal vasculature. Exp Eye Res. 1999;69:511–523. doi: 10.1006/exer.1999.0730. [DOI] [PubMed] [Google Scholar]
- Sasongko MB, Wang JJ, Donaghue KC, Cheung N, Benitez-Aguirre P, Jenkins A, Hsu W, Lee ML, Wong TY. Alterations in retinal micro-vascular geometry in young type 1 diabetes. Diabetes Care. 2010;33:1331–1336. doi: 10.2337/dc10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmetterer L, Wolzt M, Lexer F, Alschinger C, Gouya G, Zanaschka G, Fassolt A, Eichler HG, Fercher AF. The effect of hyperoxia and hypercapnia on fundus pulsations in the macular and optic disc region in healthy young men. Exp Eye Res. 1995;61:685–690. doi: 10.1016/s0014-4835(05)80019-3. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Kruger A, Findl O, Breiteneder H, Eichler HG, Wolzt M. Topical fundus pulsation measurements in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1998;236:160–163. doi: 10.1007/s004170050058. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Dallinger S, Findl O, Eichler HG, Wolzt M. A comparison between laser interferometric measurement of fundus pulsation and pneu-motonometric measurement of pulsatile ocular blood flow. 1. Baseline considerations. Eye. 2000a;14:39–45. doi: 10.1038/eye.2000.9. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Dallinger S, Findl O, Graselli U, Eichler HG, Wolzt M. A comparison between laser interferometric measurement of fundus pulsation and pneumotonometric measurement of pulsatile ocular blood flow. 2. Effects of changes in pCO2 and pO2 and of isoproterenol. Eye. 2000b;14:46–52. doi: 10.1038/eye.2000.10. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Astrocytes in mammalian retina. Prog Ret Res. 1988;7:209–231. [Google Scholar]
- Scholfield CN, Curtis TM. Heterogeneity in cytosolic calcium regulation among different microvascular smooth muscle cells of the rat retina. Microvasc Res. 2000;59:233–242. doi: 10.1006/mvre.1999.2227. [DOI] [PubMed] [Google Scholar]
- Scholfield CN, McGeown JG, Curtis TM. Cellular physiology of retinal and choroidal arteriolar smooth muscle cells. Microcirculation. 2007;14:11–24. doi: 10.1080/10739680601072115. [DOI] [PubMed] [Google Scholar]
- Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One. 2010;5:e11863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger MW, Beck SC, Pereyra-Munoz N, Dangel S, Tsai JY, Luhmann UF, van de Pavert SA, Wijnholds J, Samardzija M, Wenzel A, Zrenner E, Narfstrom K, Fahl E, Tanimoto N, Acar N, Tonagel F. In vivo confocal imaging of the retina in animal models using scanning laser ophthalmoscopy. Vis Res. 2005;45:3512–3519. doi: 10.1016/j.visres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Shakoor A, Blair NP, Mori M, Shahidi M. Chorioretinal vascular oxygen tension changes in response to light flicker. Invest Ophthalmol Vis Sci. 2006;47:4962–4965. doi: 10.1167/iovs.06-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YF, Lin SY, Huang JK, Jian SW, Lin LL, Hung PT. The choroidal blood flow response after flicker stimulation in chicks. J Ocul Pharmacol Ther. 1997;13:213–218. doi: 10.1089/jop.1997.13.213. [DOI] [PubMed] [Google Scholar]
- Shih YY, De la Garza BH, Muir ER, Rogers WE, Harrison JM, Kiel JW, Duong TQ. Lamina-specific functional MRI of retinal and choroidal responses to visual stimuli. Invest Ophthalmol Vis Sci. 2011;52:5303–5310. doi: 10.1167/iovs.10-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazawa M, Inokuchi Y, Okuno T, Nakajima Y, Sakaguchi G, Kato A, Oku H, Sugiyama T, Kudo T, Ikeda T, Takeda M, Hara H. Reduced retinal function in amyloid precursor protein-over-expressing transgenic mice via attenuating glutamate-N-methyl-d-aspartate receptor signaling. J Neurochem. 2008;107:279–290. doi: 10.1111/j.1471-4159.2008.05606.x. [DOI] [PubMed] [Google Scholar]
- Silver DM, Farrell RA. Validity of pulsatile ocular blood flow measurements. Surv Ophthalmol. 1994;38(Suppl):S72–S80. doi: 10.1016/0039-6257(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Silver DM, Geyer O. Pressure–volume relation for the living human eye. Curr Eye Res. 2000;20:115–120. [PubMed] [Google Scholar]
- Singh AS, Kolbitsch C, Schmoll T, Leitgeb RA. Stable absolute flow estimation with Doppler OCT based on virtual circumpapillary scans. Biomed Opt Express. 2010;1:1047–1058. doi: 10.1364/BOE.1.001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser P, Gittelsohn AM, Patz A. Studies on diabetic retinopathy. 3. Influence of diabetes on intramural pericytes. Arch Ophthalmol. 1968;80:332–337. doi: 10.1001/archopht.1968.00980050334007. [DOI] [PubMed] [Google Scholar]
- Srienc AI, Kurth-Nelson ZL, Newman EA. Imaging retinal blood flow with laser speckle flowmetry. Front Neuroenergetics. 2010;2:128. doi: 10.3389/fnene.2010.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan VJ, Wojtkowski M, Fujimoto JG, Duker JS. In vivo measurement of retinal physiology with high-speed ultrahigh-resolution optical coherence tomography. Opt Lett. 2006;31:2308–2310. doi: 10.1364/ol.31.002308. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D'Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinle JJ, Granger HJ. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton Neurosci. 2003;108:57–62. doi: 10.1016/S1566-0702(03)00151-6. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Hughes SJ, Canning P, Lynch O, Cox O, Frizzell N, Thorpe SR, Cotter TG, Curtis TM, Gardiner TA. Substrates modified by advanced glycation end-products cause dysfunction and death in retinal pericytes by reducing survival signals mediated by platelet-derived growth factor. Diabetologia. 2004;47:1735–1746. doi: 10.1007/s00125-004-1523-3. [DOI] [PubMed] [Google Scholar]
- Stone J, Itin A, Lon T, Pe'er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Moriya S, Oku H, Azuma I. Association of endothelin-1 with normal tension glaucoma: clinical and fundamental studies. Surv Ophthalmol. 1995;39(Suppl. 1):S49–S56. doi: 10.1016/s0039-6257(05)80073-6. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Gotoh F, Ishikawa Y. Retinal vascular autoregulation in normal subjects. Stroke. 1982;13:149–155. doi: 10.1161/01.str.13.2.149. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Miller EJ, Kilo C, Williamson JR. Pericyte form and distribution in rat retinal and uveal capillaries. Invest Ophthalmol Vis Sci. 1985;26:68–73. [PubMed] [Google Scholar]
- Tumelty J, Scholfield N, Stewart M, Curtis T, McGeown G. Ca2+-sparks constitute elementary building blocks for global Ca2+-signals in myocytes of retinal arterioles. Cell Calcium. 2007;41:451–466. doi: 10.1016/j.ceca.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udosen IT, Jiang H, Hercule HC, Oyekan AO. Nitric oxide-epoxygenase interactions and arachidonate-induced dilation of rat renal microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H2054–H2063. doi: 10.1152/ajpheart.00075.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich A, Ulrich C, Barth T, Ulrich WD. Detection of disturbed autoregulation of the peripapillary choroid in primary open angle glaucoma. Ophthalmic Surg Lasers. 1996;27:746–757. [PubMed] [Google Scholar]
- Uretmen O, Akkin C, Erakgun T, Killi R. Color Doppler imaging of choroidal circulation in patients with asymmetric age-related macular degeneration. Ophthalmologica. 2003;217:137–142. doi: 10.1159/000068559. [DOI] [PubMed] [Google Scholar]
- van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, van Velthoven ME, Schlingemann RO, Verbraak FD, Abramoff MD. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzetta I, Hildesheim R, Grinvald A. Compartment-resolved imaging of activity-dependent dynamics of cortical blood volume and oximetry. J Neurosci. 2005;25:2233–2244. doi: 10.1523/JNEUROSCI.3032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman ST, Hudson C, Fisher JA, Rodrigues L, Mardimae A, Flanagan JG. Retinal arteriolar and capillary vascular reactivity in response to isoxic hypercapnia. Exp Eye Res. 2008;87:535–542. doi: 10.1016/j.exer.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Venkataraman ST, Hudson C, Rachmiel R, Buys YM, Markowitz SN, Fisher JA, Trope GE, Flanagan JG. Retinal arteriolar vascular reactivity in untreated and progressive primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2043–2050. doi: 10.1167/iovs.09-3630. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Wang ZZ, Secor DL. Brain parenchymal and microvascular amyloid in Alzheimer's disease. Brain Pathol. 1996;6:179–195. doi: 10.1111/j.1750-3639.1996.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Walshe TE, Connell P, Cryan L, Ferguson G, O'Brien C, Cahill PA. The role of pulsatile flow in controlling microvascular retinal endothelial and pericyte cell apoptosis and proliferation. Cardiovasc Res. 2011;89:661–670. doi: 10.1093/cvr/cvq341. [DOI] [PubMed] [Google Scholar]
- Wang L, Kondo M, Bill A. Glucose metabolism in cat outer retina. Effects of light and hyperoxia. Invest Ophthalmol Vis Sci. 1997;38:48–55. [PubMed] [Google Scholar]
- Wang L, Grant C, Fortune B, Cioffi GA. Retinal and choroidal vaso-reactivity to altered PaCO2 in rat measured with a modified microsphere technique. Exp Eye Res. 2008;86:908–913. doi: 10.1016/j.exer.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fawzi AA, Tan O, Zhang X, Huang D. Flicker-induced changes in retinal blood flow assessed by Doppler optical coherence tomography. Biomed Opt Express. 2011a;2:1852–1860. doi: 10.1364/BOE.001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fawzi AA, Varma R, Sadun AA, Zhang X, Tan O, Izatt JA, Huang D. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011b;52:840–845. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Fujii H, Kishi S. Imaging of choroidal hemodynamics in eyes with polypoidal choroidal vasculopathy using laser speckle phenomenon. Jpn J Ophthalmol. 2008;52:175–181. doi: 10.1007/s10384-007-0521-7. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JF, McLure CA, Guymer RH, Baird PN, Millman J, Cantsilieris S, Dawkins RL. Almost total protection from age-related macular degeneration by haplotypes of the regulators of complement activation. Genomics. 2011 doi: 10.1016/j.ygeno.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol. 2003a;284:H2083–H2090. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- Wu KH, Madigan MC, Billson FA, Penfold PL. Differential expression of GFAP in early v late AMD: a quantitative analysis. Br J Ophthalmol. 2003b;87:1159–1166. doi: 10.1136/bjo.87.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Grunwald JE, Metelitsina TI, DuPont JC, Ying GS, Martin ER, Dunaief JL, Brucker AJ. Association of risk factors for choroidal neo-vascularization in age-related macular degeneration with decreased foveolar choroidal circulation. Am J Ophthalmol. 2010;150:40–47. doi: 10.1016/j.ajo.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H925–H934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- Yang G, Iadecola C. Obligatory role of NO in glutamate-dependent hyperemia evoked from cerebellar parallel fibers. Am J Physiol. 1997;272:R1155–R1161. doi: 10.1152/ajpregu.1997.272.4.R1155. [DOI] [PubMed] [Google Scholar]
- Ye XD, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990;31:1731–1737. [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Yu DY, Cringle SJ, Alder V, Su EN. Intraretinal oxygen distribution in the rat with graded systemic hyperoxia and hypercapnia. Invest Ophthalmol Vis Sci. 1999;40:2082–2087. [PubMed] [Google Scholar]
- Zeng XX, Ng YK, Ling EA. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Vis Neurosci. 2000;17:463–471. doi: 10.1017/s0952523800173122. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Porat RM, Alon T, Keshet E, Stone J. Tissue oxygen levels control astrocyte movement and differentiation in developing retina. Dev Brain Res. 1999;118:135–145. doi: 10.1016/s0165-3806(99)00140-6. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wu DM, Xu GZ, Puro DG. The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J Physiol. 2011;589:2383–2399. doi: 10.1113/jphysiol.2010.202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Curr Diab Rep. 2011;11:244–252. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


