Abstract
The presence of apoplastic proteins without predicted signal peptide in the gene sequence suggests the existence of protein secretion independent of the ER/Golgi classical route. In animals, one of the pathways proposed for alternative protein secretion involves the release of exosomes to the extracellular space. Although this pathway has not been dissected in plants some indirect evidence is emerging. We have reported that apoplastic fractions of sunflower seeds contain exosome-like vesicles. Besides, these vesicles are enriched in the lectin Helja, which is immunolocalized in the extracellular space even if it the protein has no predicted signal peptide. Here we show that Helja is not glycosylated and its secretion is insensitive to brefeldin A, two of the major characteristics to discard ER/Golgi-mediated protein transport. Moreover, the levels of Helja in sunflower extracellular vesicles are not affected by brefeldin A treatment. Our results suggest that Helja could be exported through an exosome-mediated pathway and point out that this mechanism may be responsible for the secretion of at least part of the leaderless proteins detected in the extracellular compartment of plants.
Keywords: apoplast, protein secretion, signal sequence, non-classical secretion
The apoplast or extracellular environment of plant cells houses a complex and dynamic proteome which carries out multiple functions in plant metabolism and signaling. In the last decade interesting news have arisen concerning the mechanisms involved in eukaryotic protein secretion to the extracellular compartment. The classical view of protein secretion involves the recognition of a signal peptide in the N-terminus of extracellular proteins that directs their sorting to the endoplasmic reticulum and thereafter the transportation through the secretory pathway. Even if this mechanism seems to be responsible for most protein export, non-conventional protein transport has also been described in eukaryotic cells and is actively investigated in mammals.1,2 In other words, some extracellular proteins have been discovered that do not make use of the classical signal peptide-dependent secretory pathway. In plants, non-classical secretion has been poorly explored, although Cheng et al.3 have provided evidence on the salycilic acid-induced secretion of mannitol dehydrogenase, a normally symplastic enzyme, using a non-Golgi dependent pathway. In fact, plant non-classical protein secretion is recognized taking into account the large number of leaderless proteins detected in extracellular compartments, and has recently been discussed based on the concepts developed in other eukaryotic systems.4
The components and mechanisms required in unconventional protein secretion are being elucidated in mammalian cells and envisage four different mechanisms.5 One of them involves the release to the extracellular milieu of exosomes, which are vesicles secreted upon fusion of multivesicular endosomes with the plasma membrane. The purpose of this report is to integrate some experimental evidence that is arising in the field of plant protein export. Besides, we present novel data supporting the existence of exosome-mediated protein secretion in plants.
Apoplastic Vesicles and Proteins without Predicted Signal Peptide
While analyzing the extracellular proteome of sunflower seedlings we have found that around 60% of the proteins identified have no predicted signal peptide. Among them we have detected a jacalin-related lectin (Helja), whose extracellular localization was confirmed by immunolocalization experiments.6 Extracellular proteins lacking a predicted signal peptide, known as leaderless or non classically secreted, have also been systematically observed in apoplastic proteomes from Brassica,7 Arabidopsis8-10 and rice,11 among others. Apart from the increasing repertoire of leaderless proteins in extracellular plant fractions, the question is if there is any evidence on exosome-dependent protein secretion. Some experimental observations suggest that such a mechanism occurs in plant systems. In fact, we have already detected the presence of exosome-like structures in apoplastic fluids of sunflower.12 These particles, of 20–200 nm, have been shown to contain a Rab11A, typical proteins implicated in the production of vesicles from donor membranes and their fusion with acceptor ones. More important, we found that these vesicles were enriched in Helja, the extracellular leaderless lectin from apoplastic fluids recently characterized, suggesting that this protein may be secreted via exosome-like vesicles.
The existence of apoplastic vesicles in plants has also been reported in other experimental systems but no function has still been assigned to these structures. Hence, extracellular lipoprotein particles have been detected in tomato apoplastic fluids.13 Interestingly, they contain several defense-related proteins (including a leaderless aspartyl protease) and also the phospholipid phosphatidylinositol 4-phosphate, previously shown to trigger the activation of defense responses.14 This evidence obtained in our laboratory joins additional observations of An et al.,15,16 who reported the release of exosome-like vesicles in barley leaves attacked by a pathogenic fungus. Recently, Wang et al.17 have reported the presence of EXPO, exosome-like structures released by Arabidopsis and tobacco cells, probably associated to unconventional secretion. Overall, accumulated evidence suggest that exosome-like structures carrying specific proteins may be delivered to the extracellular compartment of plant cell to accomplish still undiscovered functions.
An Extracellular Lectin Detected in Exosome-like Vesicles Fulfills the Requirements for Non-Classical Secretion
Three basic observations have led to the proposal of alternative pathways of eukaryotic protein secretion:1,2 (a) the lack of a classical signal peptide in the secreted protein, (b) the exclusion of these proteins from the secretory organelles and consequently the lack of post-translational modifications such as N-gycosylation occurring therein, and (c) resistance of these transport processes to brefeldin A, an inhibitor of ER/Golgi-dependent protein secretion. We have previously deduced that Helja was a leaderless protein,6 which was confirmed by the analysis of its genomic sequence. In fact, the complete gene sequence from Helianthus annuus Helja available at the Sunflower Genome Project18 (scaffold 12-HO_S039609, gene HA412r201104S039609.1) revealed the absence of a signal peptide in the predicted protein. So, we have further investigated whether this extracellular lectin fulfills the rest of the requirements to be considered non-classically secreted. In order to test Helja glycosylation we have purified this protein from seed apoplastic fluids using a mannose affinity chromatography followed by fractionation on SDS-PAGE and detection of putative glycosydic moieties. Figure 1 shows that a glycoprotein specific stain was unable to detect Helja (Fig. 1A) even in the presence of high levels of the protein (Fig. 1B). Given that adequate controls were performed it can be concluded that Helja is not glycosylated. Additionally, we have analyzed if brefeldin A was able to avoid Helja secretion, since treatments with this inhibitor result in the inability of ER derived vesicles to fuse with Golgi cisternae.19 To that aim we have incubated sunflower seeds in the presence of this inhibitor and apoplastic fluids were then obtained. As expected for an inhibitor of classical secretion, brefeldin A drastically affected the protein pattern of apoplastic fluids, as reveled by Coomassie blue staining (Fig. 2A). However, brefeldin A treated seeds do not show reduced levels of Helja. Moreover, the levels of Helja contained in the vesicular exosome-like fraction prepared by centrifugation of apoplastic fluids also remained unchanged upon brefeldin A treatment (Fig. 2B).
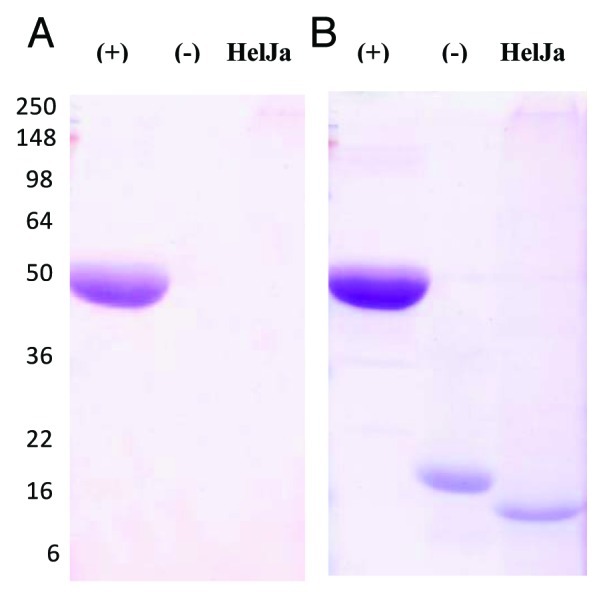
Figure 1. Helja is not glycosylated. A purified fraction of Helja was obtained from sunflower extracellular fluids (EF) collected from 10 g of seeds as previously described20 and subsequent purification on a D-mannose-agarose chromatography.6 Twenty μg of the mannose-eluted fraction (Helja) was analyzed in a 12% SDS-PAGE and sequentially stained with GelCode Glycoprotein dye (Pierce) (A) and Coomassie brilliant blue (B). Radish peroxidase was used as positive glycosylation control (+) and soybean trypsin inhibitor as a negative control (-). The molecular weight markers are indicated on the left.
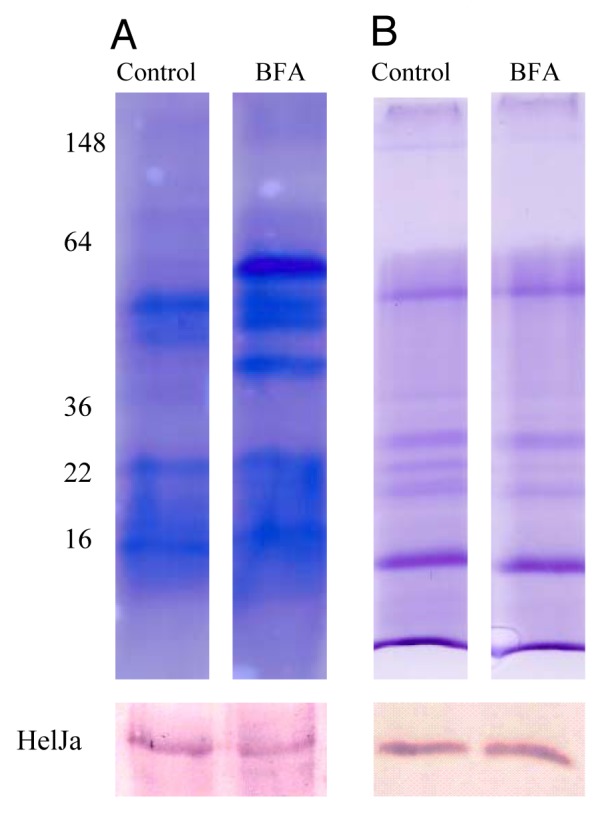
Figure 2. Helja secretion is not affected by brefeldin A treatment. Sunflower seeds were imbibed in water (Control) or 50 μg ml−1 brefeldin A (BFA) for 2 h and subjected to extracellular fluid (EF) extraction or extracellular vesicles preparation as previously described.12 The EF (5 g of seeds, A) or the vesicles (15 g of seeds, B) were fractionated by SDS-PAGE and the proteins were stained with Coomassie blue (upper panel) or transferred to nitrocellulose for immunodetection of Helja (lower panel). The molecular weight markers are indicated on the left.
In conclusion, we show that Helja secretion responds to the three premises accepted for non-classically secreted proteins:1,2 this leaderless protein is not glycosylated and its secretion is not affected by brefeldin A. Furthermore, the fact that Helja is the major protein detected in extracellular exosome-like vesicles and its content is not affected by brefeldin treatment suggests Helja export through an exosome-mediated pathway. Although the origin and role of these vesicles in plants is still unknown, this report points out that such a mechanism must be considered for the secretion of at least part of the proteins lacking a signal peptide detected in extracellular plant compartments.
Acknowledgments
This work was supported by grants from the ANPCYT, CONICET and the University of Mar del Plata, Argentina. MR and LDLC are members of the research career of CONICET. We would like to thank Loren Rieseberg (University of British Columbia), leader of the Sunflower Genome Project (www.genomecanada.ca/medias/pdf/EN/Genomics-of-Sunflower.pdf), Patrick Vincourt and Jerome Gouzy (INRA, France) for providing the genomic sequence of Helja, the gene annotation and the predicted protein.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19675
References
- 1.Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–19. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 2.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–14. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheng F-Y, Zamski E, Guo W-W, Pharr DM, Williamson JD. Salicylic acid stimulates secretion of the normally symplastic enzyme mannitol dehydrogenase: a possible defense against mannitol-secreting fungal pathogens. Planta. 2009;230:1093–103. doi: 10.1007/s00425-009-1006-3. [DOI] [PubMed] [Google Scholar]
- 4.Cheng F-Y, Williamson JD. Is there leaderless protein secretion in plants? Plant Signal Behav. 2010;5:129–31. doi: 10.4161/psb.5.2.10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–55. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 6.Pinedo M, Regente M, Elizalde M, Quiroga IY, Pagnussat L, Jorrin-Novo J, et al. Extracellular sunflower proteins: Evidence on non-classical secretion of a jacalin related lectin. Protein Pept Lett. 2012 doi: 10.2174/092986612799363163. In press. [DOI] [PubMed] [Google Scholar]
- 7.Basu U, Francis JL, Whittal RM, Stephens JL, Wang Y, Zaiane OR, et al. Extracellular proteomes of Arabidopsis thaliana and Brassica napus roots: analysis and comparison by MudPIT and LC-MS/MS. Plant Soil. 2006;286:357–76. doi: 10.1007/s11104-006-9048-9. [DOI] [Google Scholar]
- 8.Slabas AR, Ndimba B, Simon WJ, Chivasa S. Proteomic analysis of the Arabidopsis cell wall reveals unexpected proteins with new cellular locations. Biochem Soc Trans. 2004;32:524–8. doi: 10.1042/BST0320524. [DOI] [PubMed] [Google Scholar]
- 9.Kwon HK, Yokoyama R, Nishitani K. A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol. 2005;46:843–57. doi: 10.1093/pcp/pci089. [DOI] [PubMed] [Google Scholar]
- 10.Oh IS, Park AR, Bae MS, Kwon SJ, Kim YS, Lee JE, et al. Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell. 2005;17:2832–47. doi: 10.1105/tpc.105.034819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung YH, Jeong SH, Kim SH, Singh R, Lee JE, Cho YS, et al. Systematic secretome analyses of rice leaf and seed callus suspension-cultured cells: workflow development and establishment of high-density two-dimensional gel reference maps. J Proteome Res. 2008;7:5187–210. doi: 10.1021/pr8005149. [DOI] [PubMed] [Google Scholar]
- 12.Regente M, Corti-Monzón G, Maldonado AM, Pinedo M, Jorrín J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583:3363–6. doi: 10.1016/j.febslet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Gonorazky G, Laxalt AM, Dekker HL, Rep M, Munnik T, Testerink C, et al. Phosphatidylinositol 4-phosphate is associated to extracellular lipoproteic fractions and is detected in tomato apoplastic fluids. Plant Biol (Stuttg) 2012;14:41–9. doi: 10.1111/j.1438-8677.2011.00488.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonorazky G, Laxalt AM, Testerink C, Munnik T, de la Canal L. Phosphatidylinositol 4-phosphate accumulates extracellularly upon xylanase treatment in tomato cell suspensions. Plant Cell Environ. 2008;31:1051–62. doi: 10.1111/j.1365-3040.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 15.An Q, Hückelhoven R, Kogel K-H, van Bel AJE. Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 2006;8:1009–19. doi: 10.1111/j.1462-5822.2006.00683.x. [DOI] [PubMed] [Google Scholar]
- 16.An Q, van Bel AJE, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2:4–7. doi: 10.4161/psb.2.1.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Ding Y, Wang J, Hillmer S, Miao Y, Lo SW, et al. EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell. 2010;22:4009–30. doi: 10.1105/tpc.110.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunflower Genome Project ( www.genomecanada.ca/medias/pdf/EN/Genomics-of-Sunflower.pdf)
- 19.Nebenführ A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–8. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regente M, Corti Monzón G, de la Canal L. Phospholipids are present in extracellular fluids of imbibing sunflower seeds and are modulated by hormonal treatments. J Exp Bot. 2008;59:553–62. doi: 10.1093/jxb/erm329. [DOI] [PubMed] [Google Scholar]


