Abstract
The antagonism between abscisic acid (ABA) and gibberellin (GA) plays a key role in controlling seed germination,1,2 but the mechanism of antagonism during this process is not known. In the associated study,3 we investigated the relationship among ABA, reactive oxygen species (ROS), ascorbic acid (ASC) and GA during rice seed germination. ROS production is reduced by ABA, which hence results in decreasing ASC accumulation during imbibition. GA accumulation was also suppressed by a reduced ROS and ASC level, whereas application of exogenous ASC can partially rescue seed germination from ABA treatment. Further results show that production of ASC, which acts as a substrate in GA biosynthesis, was significantly inhibited by lycorine which thus suppressed the accumulation of GA. Consequently, expression of GA biosynthesis genes was suppressed by the low levels of ROS and ASC in ABA-treated seeds. These studies reveal a new role for ASC in mediating the antagonism between ABA and GA during seed germination in rice.
Keywords: ABA, GA, reactive oxygen species, ascorbic acid, seed germination, rice (Oryzasativa)
Seed germination is a complicated process, which is controlled by many plant hormones.4 Among them, the antagonism between ABA and GA plays a key role in controlling seed germination.5,6 Little is known about the mechanism of this antagonism in rice seed during germination. Previous works have shown that ROS plays a key role in seed biology.7-9 Different studies have proved ROS is a critical component in ABA signaling pathway in leaves and interacts with GA during seed germination,10,11 indicating that ROS could be good candidates in mediating the ABA and GA antagonism.
ASC was discovered in the late 1920s. Although it is well recognized as a powerful antioxidant,12 the question ‘what is the function of ASC?’ is still far from being answered.13,14 In orthodox seeds, neither ASC norASC peroxidase activity exist at the quiescent stage, but they re-start after a few hours from the onset of imbibition, which is suggested to be crucial to ensure seed germinability.15-17 Recently, ASC was found to act as a substrate for many 2-oxoacid-dependent dioxygenases (2-ODDs), which are involved in the synthesis of the plant hormones ethylene, GA, and ABA.18,19 Both of these two properties indicate a possible function during seed germination except the antioxidant role.
Here in the associate study, we also showed that ROS production is reduced especially in the embryo region by a relative high level of ABA during germination in rice seed. As a result, the ASC production is reduced by the perturbed redox state in the imbibing seeds. The suppression is not only found in the treatments of ABA, but also in the treatments of DPI, a ROS scavenger, and lycorine which is of inhibitor of ASC biosynthesis (Fig. 1A), further suggesting that ABA suppresses ASC production is mediated by a reduced ROS production during imbibition in rice seed. Interestingly, when ASC was applied to imbibing seeds treated with ABA, the inhibition of seed germination by ABA is partially rescued by 1mM ASC application, indicating that ASC is involved in the process that ABA inhibits seed germination.
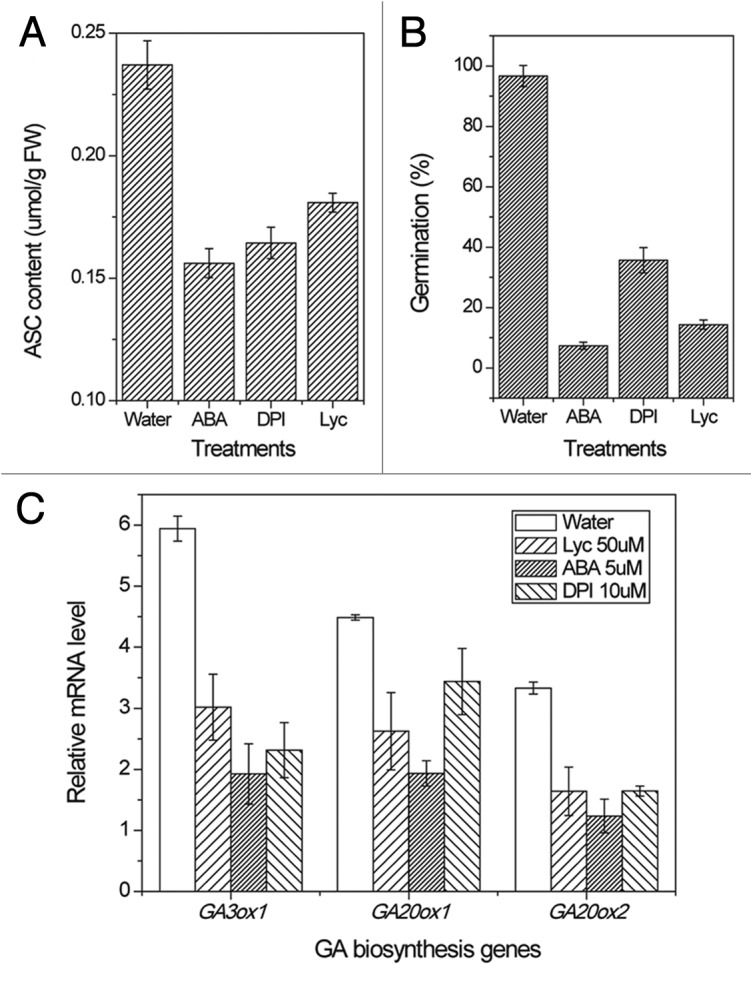
Figure 1. Inhibition of ASC production (A) and the consequent effect on seed germination (B) and GA accumulation (C). Rice seeds were imbibed at 28 °C in the presence of water, ABA, diniconazole, Tiron, DPI and lycorine solutions for 36h. Seed samples were collected and stored at –80 °C for ASC determination and QRT-PCR. Values are means ± SD (n = 5).
It is suggested that suppressing the ASC content during seed germination will lead to other consequences, because ASC is much more than just an antioxidant.13,20 To answer this question, an inhibitor, lycorine, was used since this alkaloid has beenproved to be a strong inhibitor of (l-galactono-c-lactone) GaL dehydrogenase (EC 1.3.2.3), the last enzyme of the ASC biosynthetic pathway, both in vivo21-23 and in vitro,24 was applied to the imbibing seed in rice. Consequently, seed germination was inhibited by lycorine, which is similar to the inhibition effect of ABA and DPI (Fig. 1B). This result further proves that ASC plays a role in ABA signaling pathway during seed germination. In agreement with our result, a recent work has revealed that low ASC triggers ABA- and jasmonate-dependent signaling pathways that together regulate growth through ABI4.25
Since the GA accumulation which is indicated by the expression of GA biosynthesis genes and amylase activities, similarly to ASC production, is reducedby ABA and DPI treatments during seed germination. It is quite possible that ASC plays a key role in mediating the antagonism between ABA and GA during seed germination. Indeed, exogenous application of lycorine reduced the expression of key genes in the GA biosynthesis pathway in seeds treated with lycorine, which is similar to treatment with ABA and DPI (Fig. 1C). The similar effect of ABA and lycorine on GA accumulation has revealed that antagonism between abscisic acid and gibberellins is partially mediated by ascorbic acid during seed germination in rice.
Acknowledgments
This work was supported by Hong Kong Research Grants Council (HKBU 262809) and Hong Kong Baptist University Strategic Development Fund (SDF 090910P03).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19919
References
- 1.Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Curr Opin Plant Biol. 2002;5:33–6. doi: 10.1016/S1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- 2.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–23. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 3.Ye N, Zhu G, Liu Y, Zhang A, Li Y, Liu R, et al. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by ABA in rice seeds. J Exp Bot. 2012 doi: 10.1093/jxb/err336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewley JD. Seed Germination and Dormancy. Plant Cell. 1997;9:1055–66. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281–307. doi: 10.1079/SSR2005218. [DOI] [Google Scholar]
- 6.Seo M, Nambara E, Choi G, Yamaguchi S. Interaction of light and hormone signals in germinating seeds. Plant Mol Biol. 2009;69:463–72. doi: 10.1007/s11103-008-9429-y. [DOI] [PubMed] [Google Scholar]
- 7.Oracz K, El-Maarouf Bouteau H, Farrant JM, Cooper K, Belghazi M, Job C, et al. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007;50:452–65. doi: 10.1111/j.1365-313X.2007.03063.x. [DOI] [PubMed] [Google Scholar]
- 8.El-Maarouf-Bouteau H, Bailly C. Oxidative signaling in seed germination and dormancy. Plant Signal Behav. 2008;3:175–82. doi: 10.4161/psb.3.3.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller K, Carstens AC, Linkies A, Torres MA, Leubner-Metzger G. The NADPH-oxidase AtrbohB plays a role in Arabidopsis seed after-ripening. New Phytol. 2009;184:885–97. doi: 10.1111/j.1469-8137.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 10.Ye N, Zhu G, Liu Y, Li Y, Zhang J. ABA controls H₂O₂ accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011;52:689–98. doi: 10.1093/pcp/pcr028. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Ye N, Liu R, Chen M, Zhang J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot. 2010;61:2979–90. doi: 10.1093/jxb/erq125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–79. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 13.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta. 2002;1569:1–9. doi: 10.1016/S0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 14.Herschbach C, Scheerer U, Rennenberg H. Redox states of glutathione and ascorbate in root tips of poplar (Populus tremula X P. alba) depend on phloem transport from the shoot to the roots. J Exp Bot. 2010;61:1065–74. doi: 10.1093/jxb/erp371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tommasi F, Paciolla C, Arrigoni O. The ascorbate system in recalcitrant and orthodox seeds. Physiol Plant. 1999;105:193–8. doi: 10.1034/j.1399-3054.1999.105202.x. [DOI] [Google Scholar]
- 16.De Gara L, Paciolla C, De Tullio MC, Motto M, Arrigoni O. Ascorbate-dependent hydrogen peroxide detoxification and ascorbate regeneration during germination of a highly productive maize hybrid: evidence of an improved detoxification mechanism against reactive oxygen species. Physiol Plant. 2000;109:7–13. doi: 10.1034/j.1399-3054.2000.100102.x. [DOI] [Google Scholar]
- 17.De Tullio C, Arrigoni O. The ascorbic acid system in seeds: to protect and to serve. Seed Sci Res. 2003;13:249–60. doi: 10.1079/SSR2003143. [DOI] [Google Scholar]
- 18.Prescott AG, John P. Dioxygenases: molecular structure and role in plant metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:245–71. doi: 10.1146/annurev.arplant.47.1.245. [DOI] [PubMed] [Google Scholar]
- 19.Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, et al. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003;15:939–51. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innocenti AM, Mazzuca S, Bitonti MB, De Gara L, Liso R, Arrigoni O. Endogenous rhythm of ascorbic acid in seedlingroots of broad bean. Plant Physiol Biochem. 1994;32:521–5. [Google Scholar]
- 21.Arrigoni O, Arrigoni-Liso R, Calabrese G. Lycorine as an inhibitor of ascorbic acid biosynthesis. Nature. 1975;256:513–4. doi: 10.1038/256513a0. [DOI] [Google Scholar]
- 22.De Tullio C, De Gara L, Paciolla C, Arrigoui O. Dehydroascorbate-reducing proteins in maize are induced by the ascorbate biosynthesis inhibitor lycorine. Plant Physiol Biochem. 1998;36:433–40. doi: 10.1016/S0981-9428(98)80207-6. [DOI] [Google Scholar]
- 23.Onofri S, Barreca D, Garuccio I. Effects of lycorine on growth and effects of L-galactonic acid-gamma-lactone on ascorbic acid biosynthesis in strains of Cryptococcus laurentii isolated from Narcissus pseudonarcissus roots and bulbs. Antonie Van Leeuwenhoek. 2003;83:57–61. doi: 10.1023/A:1022903504795. [DOI] [PubMed] [Google Scholar]
- 24.Arrigoni O, De Gara L, Paciolla C, Evidente A, De Pinto MC, Liso R. Lycorine: a powerful inhibitor of L-galactono-c-lactonedehydrogenase activity. J Plant Physiol. 1997;150:362–4. doi: 10.1016/S0176-1617(97)80134-4. [DOI] [Google Scholar]
- 25.Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, et al. The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell. 2011;23:3319–34. doi: 10.1105/tpc.111.090100. [DOI] [PMC free article] [PubMed] [Google Scholar]


