Abstract
In animals, major classes of Rho guanine nucleotide exchange factors (GEFs) possess a Dbl (diffuse B-cell lymphoma)- homology (DH) domain that functions as a GEF-catalytic domain. However, no GEFs with the DH domain had been identified in plants. Recently, we found that the rice homolog of human SWAP70, Oryza sative (Os) SWAP70, containing the DH domain, exhibited GEF activity toward the rice Rho GTPase OsRac1, and regulates chitin-induced production of reactive oxygen species and defense gene expression in rice.1 Arabidopsis contains a single SWAP70 gene. A T-DNA insertion mutant of Arabidopsis SWAP70 was morphologically wild type. Measurement of in planta growth of Pseudomonas syringae DC3000 hrcC, a mutant incapable of type III effector delivery, revealed enhanced growth of the pathogen in the atswap70 mutant, indicating that AtSWAP70 is required for PAMP-triggered immunity. In addition, the atswap70 mutation reduced the RPM1-mediated hypersensitive response. These results suggested that AtSWAP70 plays a role in both PAMP- and effector-triggered immunity in Arabidopsis.
Keywords: GEF, Rop, GTPase, immunity, DH, defense, HR, PTI, ETI
Plants have developed multiple immune systems to protect them from pathogens.2 The first layer of defense is triggered by the recognition of pathogen-associated molecular patterns (PAMPs) by plant cell surface receptors, and is called PAMP-triggered immunity (PTI).3 To interfere with PTI, some pathogens have developed a type III secretion system, which deliver Type III effector proteins that suppress a variety of host immune responses. The second layer of defense depends on the ability of disease resistant (R) proteins to recognize effector proteins and induce robust immune responses including hypersensitive response (HR), and is called effector-triggered immunity (ETI).3
Rho family small GTPases function as key molecular signaling switches by cycling between GDP-bound inactive and GTP-bound active forms, which regulate many important cellular processes in animals and plants.4 The switch between GTP-bound and GDP-bound forms is controlled by three regulatory proteins: guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors (GDIs). GEFs promote the activation of small GTPases by catalyzing the exchange of GDP for GTP.4 Most Rho GEFs in animals contain a DH domain located at the N-terminus of the pleckstrin homology (PH) domain. Although these DH-PH-type Rho GEFs have large gene families and are widespread in animals, none have been found in plants.5 Instead, plants contain the Rac/Rop GEFs with a PRONE domain, which constitute a large family in the plant kingdom.6,7 There are 14 and 11 PRONE-type Rho GEFs in Arabidopsis and rice, respectively.8 Recently, we have identified OsSWAP70A as a novel Rac/Rop GEF for plants.1 SWAP70 contains both DH and PH domains, but their arrangement is the reverse of that in typical DH-PH-type Rho GEFs, wherein the DH domain is flanked by a C-terminal PH domain.9 OsSWAP70A showed GEF activity toward OsRac1,1 one of seven of rice Rac/Rop GTPases which regulate a series of PTI and ETI responses including cell death, the production of reactive oxygen species (ROS), the activation of pathogenesis-related genes, lignification, and the production of phytoalexin.10-12 In addition, OsSWAP70A regulates chitin-induced ROS production and defense gene expression possibly though OsRac1 in rice.1
Recently, we have reported that Arabidopsis contains a single SWAP70 gene (At2g30880).1 AtSWAP70 possessed a DH domain at the C-terminus of the PH domain (Fig. 1A), a characteristic of the human SWAP70 Rho GEF.9 Although similarity between the amino acid sequences of AtSWAP70 and human SWAP70 was limited in the PH and DH domains, AtSWAP70 was highly homologous (64% identity; 90% similarity) to OsSWAP70A overall. The PH and DH domains of AtSWAP70 had 85.1% and 64.5% identity to those of OsSWAP70A, respectively (Fig. 1B and C). The PH domain contained four basic amino acids conserved in the PH domains of animal SWAP70 proteins, which are critical for binding to phosphatidylinositol 3,4,5-triphosphate (PtdIns(3,4,5)P3).13 Predictions of the secondary structure of the AtSWAP70 DH domain using Jpred3 (www.compbio.dundee.ac.uk/www-jpred/) indicated entirely α-helices (data not shown), which coincided with the conserved three-dimensional structure of typical DH domains, suggesting that AtSWAP70 belongs to the DH family of Rho GEFs.
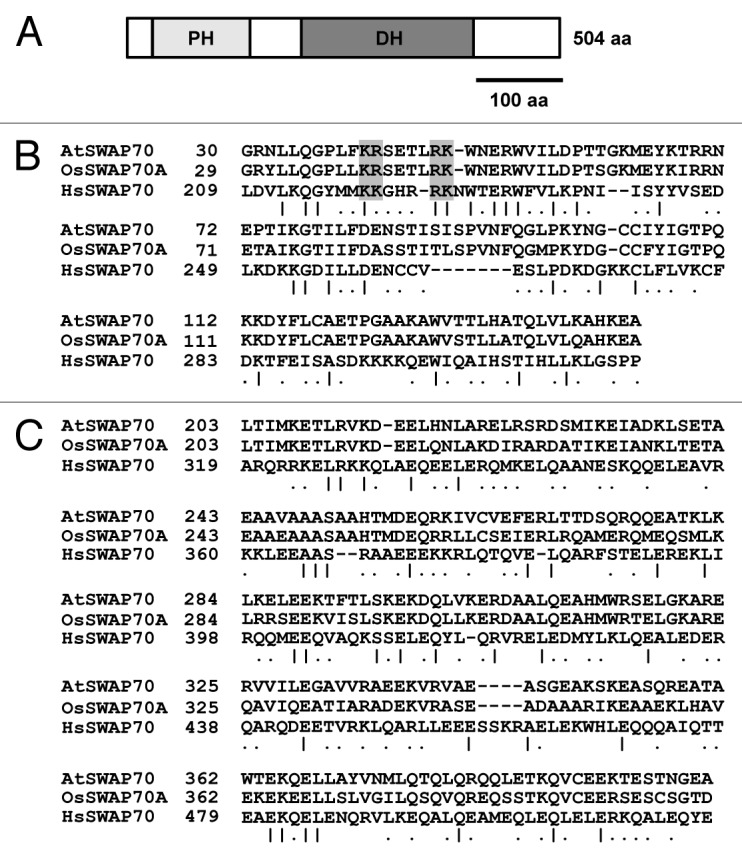
Figure 1. Identification of Arabidopsis SWAP70. (A) Schematic representation of AtSWAP70, showing the Pleckstrin homology (PH) and Dbl homology (DH) domains. (B) Comparison of amino acid sequence between the PH domains of AtSWAP70, OsSWAP70A and human SWAP70. Identical and similar amino acid residues are indicated by vertical bars and dots, respectively. Shaded boxes indicate PtdIns(3,4,5)P3-binding sites. (C) Alignment of the amino acid sequences of the DH domains of AtSWAP70, OsSWAP70A and human SWAP70. Identical and similar amino acid residues are indicated by vertical bars and dots, respectively.
To evaluate the role of AtSWAP70, we analyzed a mutant (GABI_096E03) of AtSWAP70, in which T-DNA was inserted at the 6th intron (Fig. 2A). No transcript of AtSWAP70 was detected in the mutant by RT-PCR using AtSWAP70-specific primers (Primer A and Primer B) (Fig. 2B). The atswap70 mutant was morphologically wild type, suggesting that AtSWAP70 was dispensable for normal plant development under our growth conditions. To examine the possible function of AtSWAP70 in PTI responses, we measured the in planta growth of Pseudomonas syringae DC3000 and P. syringae DC3000 hrcC, a type III secretion system-deficient mutant unable to deliver type III effectors into host cells. The growth of P. syringae DC3000 hrcC was suppressed as compared with P. syringae DC3000 (Fig. 2C), which is explained by the fact that P. syringae DC3000 hrcC is unable to inhibit PTI because of a loss of the type III effector delivery. The growth of P. syringae DC3000 hrcC in atswap70 was 2-fold that in the wild-type Col-0 (Fig. 2C), although no significant difference between the wild type and atswap70 in the growth of P. syringae DC3000 was found, suggesting that PTI was partially compromised in the atswap70 mutant.
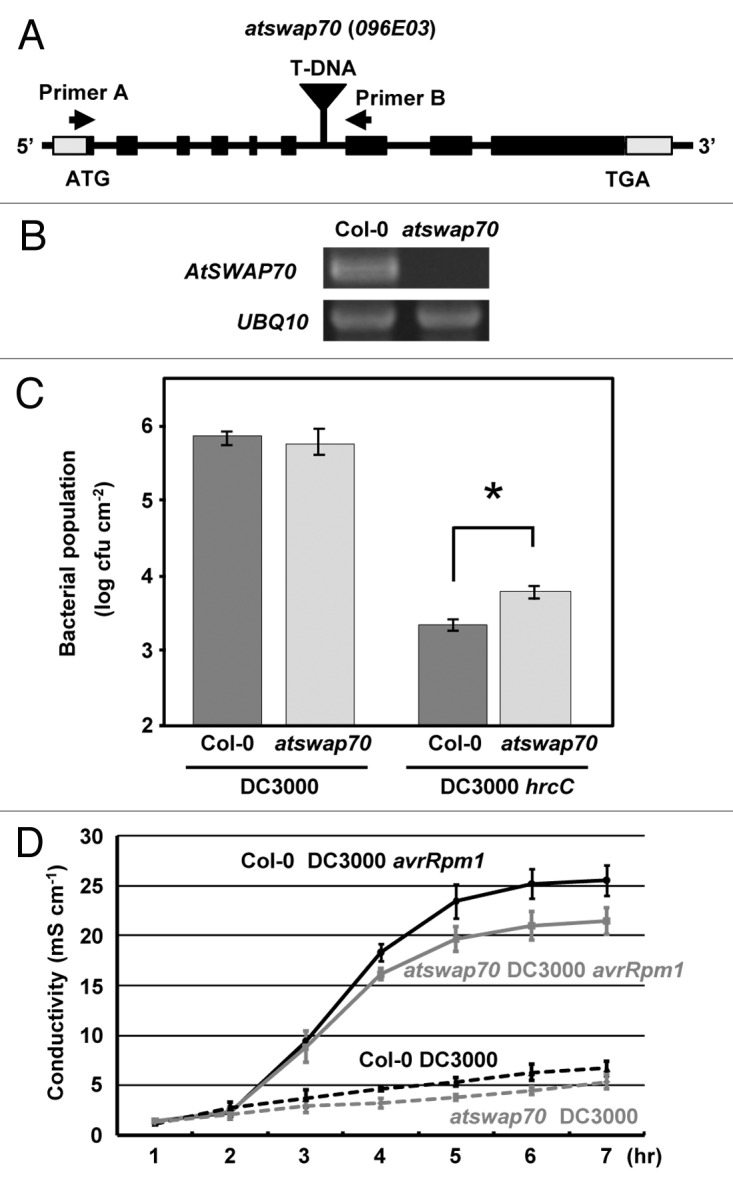
Figure 2. The AtSWAP70 T-DNA insertion mutation partially compromised PTI and ETI. (A) Schematic map of the T-DNA insertion mutation in AtSWAP70. The black and gray boxes indicate the translated and untranslated regions, respectively. T-DNA is located at the 6th intron. The arrows indicate primers (primer A and primer B) used for RT-PCR. (B) The RT-PCR analysis of AtSWAP70 mRNA in the mutant. Arabidopsis Ubiquitin (UBQ)10 was used as a control. (C) Bacterial growth with Pst DC3000 and Pst DC3000 hrcC in atswap70 and Col-0 at 3 d post-inoculation. An asterisk indicates a significant difference with the t-test at p < 0.01. (D) Conductivity of a solution containing four leaf disks from either Col-0 or atswap70 inoculated with Pst DC3000 (avrRpm1) or Pst DC3000 at 107 cfu/ml.
To elucidate whether AtSWAP70 participates in ETI induced by the recognition of AvrRpm1, a Pseudomonas Type III effector, with the Arabidopsis NB-LRR R-protein RPM1, we quantified RPM1-dependent HR by monitoring electrolyte leakage.14 Wild-type Col-0 leaves inoculated with P. syringae DC3000 (avrRpm1) displayed increased ion leakage at 3–6 h after inoculation, which was not detected in Col-0 inoculated with P. syringae DC3000. The atswap70 mutant showed significantly less ion leakage than Col-0 in repeated experiments (Fig. 2D). It is therefore likely that AtSWAP70 positively regulates RPM1-dependent HR. However, there was no difference in bacterial growth between Col-0 and atswap70 (data not shown).
The data shown here indicates that AtSWAP70 plays important roles in PTI and ETI, suggesting that AtSWAP70 controls host immune responses possibly through regulation of ROP/Rac activity in Arabidopsis. In fact, rice OsRac1 activated by OsSWAP70A has been demonstrated to function as a key regulator for PTI and ETI.15,16 Although there are 11 ROP GTPases in Arabidopsis,8 no involvement of these ROP GTPase in immune responses has been found. The identification of ROP GTPases regulated by AtSWAP70 would help us to understand the roles of ROPs in Arabidopsis immune responses.
Acknowledgments
This research was supported by Grants-in-aid from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN), KAKENHI (19380028 and 23380028), and Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy from Ministry of Education, Culture, Sports, Science and Technology, 2011–2015 (S1101035) to T.K.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19562
References
- 1.Yamaguchi K, Imai K, Akamatsu A, Mihashi M, Hayashi N, Shimamoto K, et al. SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J. 2012 doi: 10.1111/j.1365-313X.2011.04874.x. In press. [DOI] [PubMed] [Google Scholar]
- 2.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–14. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 5.Berken A. ROPs in the spotlight of plant signal transduction. Cell Mol Life Sci. 2006;63:2446–59. doi: 10.1007/s00018-006-6197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005;436:1176–80. doi: 10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18:366–81. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y, Kawasaki T, Shimamoto K, Yang Z. ROP/RAC GTPase. In: Yang Z, ed. In Annual plant reviews. 33. Wiley-Blackwell Publishing, 2008:64-99. [Google Scholar]
- 9.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–63. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 10.Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, et al. The small GTP-binding protein rac is a regulator of cell death in plants. Proc Natl Acad Sci U S A. 1999;96:10922–6. doi: 10.1073/pnas.96.19.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, et al. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci U S A. 2006;103:230–5. doi: 10.1073/pnas.0509875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci U S A. 2001;98:759–64. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka T, Ihara S, Fukui Y. Cooperation of DEF6 with activated Rac in regulating cell morphology. J Biol Chem. 2007;282:2011–8. doi: 10.1074/jbc.M605153200. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki T, Nam J, Boyes DC, Holt BF, 3rd, Hubert DA, Wiig A, et al. A duplicated pair of Arabidopsis RING-finger E3 ligases contribute to the RPM1- and RPS2-mediated hypersensitive response. Plant J. 2005;44:258–70. doi: 10.1111/j.1365-313X.2005.02525.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Hamada S, Fujiwara M, Zhu T, Thao NP, Wong HL, et al. The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe. 2010;7:185–96. doi: 10.1016/j.chom.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Kawano Y, Akamatsu A, Hayashi K, Housen Y, Okuda J, Yao A, et al. Activation of a Rac GTPase by the NLR family disease resistance protein Pit plays a critical role in rice innate immunity. Cell Host Microbe. 2010;7:362–75. doi: 10.1016/j.chom.2010.04.010. [DOI] [PubMed] [Google Scholar]


