Abstract
Ethylene plays a key role in promoting fruit ripening, so altering its biosynthesis/signaling could be an important means to delay this process. Nitric oxide (NO)-generated signals are now being shown to regulate ethylene pathways. NO signals have been shown to transcriptionally repress the expression of genes involved in ethylene biosynthesis enzymes and post-translationally modify methionine adenosyl transferase (MAT) activity through S-nitrosylation to reduce the availably of methyl groups required to produce ethylene. Additionally, NO cross-talks with plant hormones and other signal molecules and act to orchestrate the suppression of ethylene effects by modulating enzymes/proteins that are generally triggered by ethylene signaling at post-climacteric stage. Thus, medication of endogenous NO production is suggested as a strategy to postpone the climacteric stage of many tropical fruits.
Keywords: nitric oxide, ethylene, fruit ripening, reactive oxygen species
Nitric Oxide and Ethylene
Fruit ripening is a complex developmental phenomenon of genetically programmed biochemical and physiological processes culminating in desirable changes in the fruit's texture and sensorial attributes. Ethylene, a gaseous plant hormone is the key signal compound involved directly in the regulation of the ripening process in fruits at all its stages.1 Ethylene, both internal and external to the fruit, acts with environmental cues, coordinate the modulation of biochemical events in mature fruits culminating in ripening,2 the latter being an essential process of ecological and evolutionary significance. The ethylene biosynthesis pathway involves the participation of various proteins such as trans-membrane receptors, protein kinases, a membrane transporter-like regulator, and nuclear transcription factors (Fig. 1).3 Yang’s discovery of the components of the ethylene cycle in plants was a significant landmark in our understanding of plant growth regulation, senescence mechanisms and ripening. The immediate precursor of ethylene, 1aminocyclopropane carboxylic acid (ACC), is derived from S-adenosyl methionine through the action of the enzyme 1aminocyclopropane carboxylic acid synthase (ACS) and ACC is oxidized to liberate ethylene by 1-aminocyclopropane carboxylic acid oxidase (ACO).4 Ethylene biosynthesis in plants is regulated in two phases: the first phase operates during normal vegetative growth of plants while the second phase operates by a positive feedback mechanism, which is generally responsible for the rapid stimulation of ethylene production during ripening of climacteric fruits.5,6
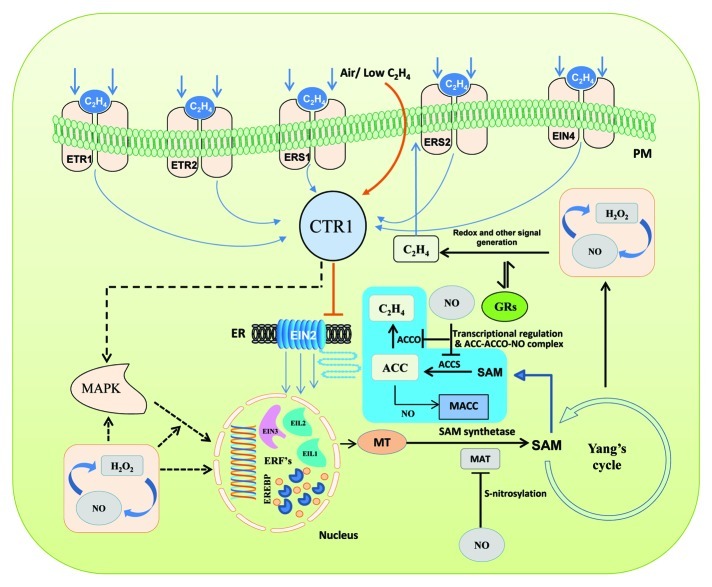
Figure 1. A schematic model of ethylene biosynthesis showing a few of its components as affected by NO during fruit ripening. (Straight arrows indicate established phenomena; dotted arrows indicate phenomena of unidentified mechanisms). Ethylene is perceived by a family of five membrane bound receptors (ETR1, ETR2, ERS1, ERS2 and EIN4) which are characterized having a sensor and a response regulator domain. In the absence of ethylene, the receptors activate the kinase activity of CTR1 (constitutive triple response 1) a negative regulator that suppresses downstream progression of signaling. CTR1 then actively suppresses the downstream responses, such that EIN2 and the EIN3/EIL family of transcription factors remain inactive. Upon perception of ethylene, the receptors no longer activate CTR1, thus activating the EIN3/EIL family of transcription factors. Ethylene insensitive 3 (EIN3) target is thought to be the ethylene response factor 1 (ERF1) gene. ERF1 encodes an ethylene response element binding protein (EREBP) that binds the GCC-box, a cis-element of many ethylene response genes, thus activating the ethylene-signaling pathway. In the ethylene cascade, autocatalytic activity of ethylene is reported to form SAM (S-adenosyl methionine) from methionine (MT) which is catalyzed by SAM synthetase, whereas SAM is catalyzed into Aminocyclopropane carboxylic acid (ACC) by ACC synthase and further oxidized into ethylene by ACC oxidase. Ethylene biosynthesis in this route was found affected by NO mainly through inhibition of SAM turnover via S-nitrosylation of transcriptionally produced methionine adenosyl transferase (MAT). Further, genes coding for ACCS and ACCO were downregulated (┴) by NO accounting for the reduction of ethylene. Stoichiometric reduction of ACC to 1-melonyl aminocyclopropane 1-carboxylic acid (MACC) and formation of a stable ternary ACC-ACCO-NO complex can also antagonize ethylene formation. Alternatively, reciprocal interaction of NO and hydrogen peroxide (H2O2) were also presumed affecting MAP kinase-mediated downstream components of ethylene biosynthesis. Growth regulators (GRs) and NO may also influence redox status and other signal generation. As a consequence, NO affects the yield of ethylene, thus delaying expressions of enzymes responsible for cell wall degradation, lignification and pigmentation of fruits conferring shelf life extension.
Genomics and proteomics studies have been central to revealing the components of ethylene signaling. Examples of such studies are elucidation of the ethylene receptors,7 constitutive triple response-kinases (ctr genes),8,9 transcriptional factors,10 ethylene response factors11 and the components of ethylene downstream cascade (Fig. 1).
There are many signals that regulate ethylene production and its perception in different organs of plants. Among the various signaling molecules, the participation of NO signal is of particular interest as this is now being shown to interfere with ethylene effects to directly and significantly influence fruit ripening.12 NO is a bio-active molecule which can regulate ethylene production via at least two mechanisms; through direct stoichiometric inhibition or suppressing the ethylene biosynthetic enzymes (see below). Several decades ago it was shown in various chemical reactions that NO inhibits the hydrogenation process during conversion of ethane (C2H2) to ethylene (C2H4) under a particular set of kinetic parameters.13 In a landmark study, Leshem et al.,14 delayed plant maturation and senescence with NO were related to stoichiometric reduction of ethylene.
Plants generate NO by various pathways. These are divided into oxidative and reductive categories.15 The most intensively studied enzyme is the cytosolic nitrate reductase (cNR) which uses nitrate as substrate and produce nitrite, which is further reduced to NO. In Arabidopsis, cNR is encoded by two genes which are NIA1 and NIA2. Antisense expression of nitrate reductase 2 (NIA2) leads to accumulation of nitrite and excess NO production in tobacco.16 In plants mitochondrial electron transport also produces NO at low oxygen conditions and during interaction with pathogens.17-19 Apart from these two pathways, the plasma membranes of roots produce NO via Nitrite-NO reductase activity. The second category of NO producing enzymes is operative via oxidative reaction. Most well-studied is nitric oxide synthase-like enzyme (NOS-like) which uses l-arginine as the substrate and produces NO. However, the existence of NOS-like enzyme in higher plants is still uncertain. The only evidence for NOS was based on an increase in NO production in the presence of larginine under specific physiological and developmental conditions and inhibition of NOS activity by arginine analogs.
Other pathways are based on the oxidation of polyamines (PA) or hydroxylamines20 and ROS induced NO production has been shown to act via hydroxylamine.21 Equally, plants can modify NO production through specific NO scavenging pathways. For instance, plant non symbiotic hemoglobins (Class 1) scavenge NO, Snitrosoglutathione reductase (GSNOR), and mitochondria actively scavenge NO.22
NO Effects on Post/harvest Quality
In many tropical fruits, climacteric upsurge of ethylene induces senescence affecting their post-climacteric storage. This drastically reduces quality attributes such as color, texture, nutritional composition and flavor. Senescence also predisposes fruits to invasion by saprophytic microbes. As explained above, knowledge of the direct relationship between NO and ethylene cycle has only recently come to light and so has relatively rarely been assessed within the context of fruit ripening. However, the loss of peach firmness was significantly retarded by NO treatment,23 which was attributed to the maintenance of cell membrane integrity and a reduced electrolyte leakage through delaying initiation of the senescence.24 NO also reduced the levels of diacylglycerol and triacylglycerol.25 NO inhibited the browning in apples26 and delayed the pericarp browning of Longan fruit (Dimocarpus longa) by minimizing pulp degradation and enhancing the total of soluble solids and ascorbic acid.27 NO treatment also improved the shelf life and desirable attributes of banana,28 tomato29 and Kiwifruit30,31 and in some climacteric and non-climacteric fruits (Table 1).
Table 1. Effect of nitric oxide on quality parameters of fruits.
| Fruit | Quality Parameters | Reference |
|---|---|---|
| Strawberry |
Extended post-harvest life |
14 |
| Peaches |
Enhanced firmness; higher integrity of cell membrane; prevention of early softening and rotting; decreased contents of diacylglycerol and triacylglycerol. Increased ascorbic acid and di-hydro ascorbic acid; delay in the initiation of the senescence of fruits |
23–25,42 |
| Longan |
Delayed pericarp browning and pulp breakdown and higher total soluble solids and ascorbic acid |
27 |
| Kiwifruit |
Extended post- harvest life, lower malondialdehyde and delay in loss of firmness and respiration, lower contents of soluble solids and malondialdehyde; higher contents of vitamin C and E. |
14,31,70,88 |
| Strawberry |
Extended postharvest life by reducing ethylene production and respiration rate |
101 |
| Banana |
Inhibited de-greening of peel and delayed softening of the pulp |
28 |
| Apple |
Delayed ethylene production and color development, inhibited early browning of fruits |
26,99 |
| Jujube | Increased red index; higher total phenol content; delayed the increase of soluble solids and decrease of vitamin C. | 95 |
The mechanisms of NO and ethylene cross talk
NO has now emerged as a novel signal molecule due to its distinct functions in the growth and development of the plants,32 flowering,33 fruit ripening and senescence,14,30 biotic stresses with a particular relevance to disease resistance34,35 and balancing cellular redox status.36,37 Of most relevance to this review, exogenous NO treatment affects fruit ripening and senescence14,30 and negatively impacts on ethylene emission from intact and fresh cut tomato fruits.29 NO is required in order to define the silhouette of fruits by competitively inhibiting ethylene-responsive components to delay senescence and extending shelf life.31,38 These observation clearly offer the prospect of managing post-harvest handling and storage of behaviors of horticultural produce by applying exogenous NO.14,39,40
As mentioned earlier, NO negates the autocatalytic biosynthesis of ethylene by binding to ACC oxidase, resulting in the formation of ACC oxidase-NO complex, which then forms a ternary stable complex, ACC-ACC oxidase-NO which biochemically reduces the ethylene production. Exponential reduction in ethylene formation in vivo was achieved by linear generation of NO through donors and infusion of gas in apple fruits where the level of reduction correlated with stoichiometric reduction.41 In addition, the produced 1-malonyl aminocyclopropane-1-carboxylic acid (MACC) is also reported to cause inhibition of the turnover of ethylene.23 NO and ROS reaction within the cell produces peroxynitrites, which affect the co-factors required for catalysis of ACC by ACS and ACO. That apart, the simultaneous treatment with NO and ethylene generating compounds competitively reduced in vivo ethylene levels in peach fruits,42 suggesting that NO could decrease ethylene output through inhibiting ACC synthase activity which concomitantly reduce ACC content.43 Differential expressions of homologs of ACS and ACO, in response to external and internal stimuli are controlled by NO both at transcriptional and post-transcriptional levels. NO delayed the expression of homologs of ACO but not ACS in tomatos44 and bananas.28 Transcript accumulation of the ethylene biosynthesis gene ACS2 in tobacco could be related to the concentration of applied NO45 suggesting that NO effect is mainly on ACO rather than ACS. Another important aspect of NO’s mode of ethylene regulation is through the regulation of the effect of hydrogen peroxide, the latter being an effective inducer of ethylene biosynthetic gene transcription.46 NO is a highly reactive molecule that can directly trigger ROS-linked redox changes by targeting transition metals (e.g., Fe, Cu and Zn) of signaling proteins, receptors, enzymes, transcription factors, DNA and proteins containing thiol groups via various modifications such as tyrosine nitration, Snitrosylation and metal nitrosylation. Given the importance of H2O2 in many physiological contexts which have economic relevance, the modulation of ethylene via H2O2 as well as NO needs further elucidation.
Another level of ethylene regulation is through post-translational modification via Snitrosylation. A good example of this is the inhibition of Adenosyl transferase-1 through Snitrosylation process. This results in reduced turnover of Sadenosyl methionine (SAM), thus regulating the chief precursor molecule for ACC production.47 The fruit-specific (localized) mitogen-activated protein kinase in tomatos inhibited ethylene levels acting as negative regulator of ethylene48; the action is being similar to CTR response of downregulation of ethylene in Arabidopsis thaliana.49
Interplay of NO with Phytohormones
In addition to the events discussed above which center on direct impacts of NO on ethylene production or signaling, indirect mechanisms exist where NO influences other phytohormones and signaling molecules which are intricately connected to ethylene biosynthesis (Fig. 2).50 NO interacts with various stress responsive signal networks, chiefly salicylic acid (SA), jasmonic acid (JA), ethylene and the coordination of secondary signal molecules such as cADP ribose, cGMP and Ca2+.51-55
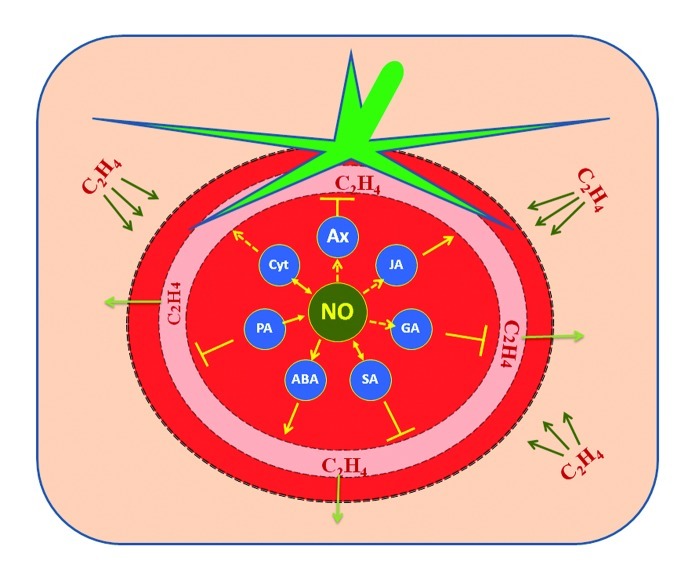
Figure 2. The overlapping interactions of polyamines (PA), jasmonic acid (JA), salicylic acid (SA), auxin (Aux), cytokinins (Cyt), abscisic acid (ABA) and gibberellic acid (GA) that are responsive to ethylene stimuli and their plausible relations with NO during ripening. Although NO's relation with phytohormones (Aux and GA) and signal molecule (JA) are yet to be established, the possible link between the ethylene biosynthesis inhibition (┴) by former and enhancement (→) by the latter can be hypothesized. The reciprocal interactions (↔) of NO with Cyt and SA affect the ethylene turnover during fruit ripening. NO's direct interaction with PA and ABA is known to negatively modulate ethylene biosynthesis. (Straight arrows indicate established phenomena; dotted arrows indicate phenomena of unidentified mechanisms).
SA, a phenolic molecule was found involved in ethylene biosynthesis, and hence fruit ripening.58 Both SA and NO are induced in plant cells during alleviation of various stresses,51,59 as well as their mutual interactions triggering effects. This interaction has been linked to ripening process, particularly when the transcription of ripening-specific genes are involved,56 and since ripening is also a process of senescence, one can expect reversal of senescence by NO signals functioning through alterations in phytohormones. Anthocyanins, the major secondary metabolites involved in fruit ripening imparting disease resistance and ecological role was found to be regulated by NO through cGMP activated chalcone synthase and ferredoxin NADP+-oxidoreductase. There is also evidence that SA, acting with NO, influenced a variety of patho-physiological responses involving calcium signals and casein kinase2 (CK2).60 Treatment with SA delayed the ripening in kiwifruit61 and suppressed the ethylene in banana62 by modulating the ethylene biosynthesis catalytic enzymes ACS in tomato63 and apple by ACO.64 The role of NO was also associated with SA action, and the latter disrupts the transcription of ethylene biosynthesis genes. The reciprocal control of NO and SA over each other was found to affect the ethylene metabolism in turn, which influenced the pattern of defense responses in tobacco, revealing the reciprocal antagonistic interplay of NO and ethylene.65 In addition, NO and ROS redox signaling networks were affected by SA during biotic stress.66 SA accumulation triggered by NO was found to suppress superoxide free radical and other ROS production67 and thereby aiding in the maintenance of cell membrane integrity and tissue senescence.
Jasmonic acid is another important stress signaling molecule, which is derived following lipoxygenase (LOX) mediated phospholipid metabolism and is also influenced by NO. Inhibition of wound induced H2O2 production and synthesis of proteinase inhibitor in tomato leaves were found to be mediated by both NO and JA.68 NO influences the JA-regulated induction of hypericin production in cell cultures of Hypericum perforatum following the addition of a fungal elicitor69 and JA caused a burst of NO during wound healing in Arabidopsis.59 Thus, both NO and JA were found to act synergistically in cellular stress responses as well as wound healing. Interestingly, NO-induced downregulation of LOX activity during post-climacteric period of fruit ripening70 suggesting an anti-ripening role for JA, as also suggested from the effects of exogenous application of JA on peach fruit.71 Application of strobulirin inhibited JA synthesis and with a concomitant decrease in ethylene production which in turn was linked with reduced lipid peroxidation. Crucially, co-application of NO and SA potentiated this effect.72 Since NO-JA-SA -ethylene interplay is clearly important, further studies are required to define which genes are up- or downregulated following co-treatment. This would allow the better application of these biochemical modulations in the efficient control of fruit ripening.
Fruit ripening is also modulated by growth regulators such as cytokinins, abscisic acid (ABA), indole-3-acetic acid (IAA) and gibberellins. Some literature has indicated that the action of these growth regulators could be influenced by NO and so would have wider developmental effects. Supporting this view, there are a few pharmacological studies in cell culture systems indicating NO influences involved in developmental function as well as actions during biotic and abiotic stress linked to these growth regulators,73 albeit via poorly elucidated mechanisms. In Japanese plums, auxin is an important signal which initiates and determines the date and rate of ripening in concert with ethylene, affecting ethylene-responsive transcriptional factors (ERF’S). Given the influence of NO on ethylene, it can be assumed that auxin effects were also modulated by NO.74
Although cytokinins are involved in senescence programming, their relationship with NO via the ethylene signal transduction pathway in ripening research has not been documented. However, NO and cytokinin were found synergistically involved in betalaine accumulation75 while cytokinins induced NO synthesis in cell cultures of parsley, tobacco and Arabidopsis.76 Involvement of NO also has also been recorded in apoptosis (programmed cell death), induced by cytokinins during biotic stress77,73 (Fig. 2). Gibberellic acid (GA), a plant hormone has been widely used for delaying the ripening in several fruits; but any possible interaction with NO has yet to be recorded. This last point notwithstanding, preliminary indications from the literature would suggested that a comprehensive characterization of the cross talk of NO with hormones such as auxins, cytokinin, gibberellins and ABA linked to regulation of the ethylene level, is urgently required.
PAs are important plant secondary metabolites produced that readily interact with nucleic acids, protein and phospholipids due to their ionic nature.78,79 PAs have been widely considered as anti-senescence metabolites as the addition can delay leaf senescence and the aging progress of plants.80 As fruit ripening is also a programmed senescence process, this role of PA is of relevance to fruit ripening. PAs were found to inhibit the transcript accumulation of wound inducible ACS and thus ethylene63 and enhanced the shelf life of pomegranate with improved quality attributes.81 This was perhaps to be expected as both ethylene and PA biosynthetic pathways share a common precursor molecule SAM, so that biochemical feedback mechanisms are to be expected. Putrescine application reduced the ethylene biosynthesis and delayed the softening of plum fruit.82 Similarly, spermidine and spermine reduced the ethylene synthesis by downregulating ACC synthase.83 Transgenics of tomatos engineered for higher levels of spermine and spermidine were observed to express changed ethylene production, and the biosynthesis of amino acids, isoprenoids and flavonoids as well as the accumulation of chaperones and other, stress proteins.84 Although one may expect NO involvement in these cases, it has not been demonstrated. One study has demonstrated that PA treatment induces a NO burst in Arabidopsis plants,85 which could also influence ethylene biosynthesis and, in other species, fruit ripening. Apart from a reduction of SAM, downstream responses to PA might also be mediated by NO, as suggested during stress phenomena86 possibly modifying the transcriptional regulation of ethylene biosynthetic genes rather than the feedback inhibition of enzymes of ethylene biosynthesis.
Role of NO in Post-Climacteric Biochemical Events
A series of changes in texture and color occur at post-climacteric phase of ripening that are directly linked to ethylene biosynthesis in fruits of both climacteric and non-climacteric types. Softening was significantly slowed down in post-climacteric period after the application of specific levels of NO in peaches,23 Japanese plums87 and bananas28 which can be correlated with the suppression of ethylene formation.43 Mechanistically, NO was shown to reduce the activities of cell wall softening enzymes-pectin methylesterase (PME) and β14endoglucanse in kiwifruit.88 However, more studies are required particularly to test any link with ripening-associated color and flavor development which are known to play ecological roles and offer protection against pathogen attack. It is well-known that phenolic compounds produced by the phenylpropanoid pathway and carotenoids contribute toward pigmentation are also associated with offering resistance to pathogens and scavenge free radicals within the fruit and also to the consumers. Equally, the excessive oxidative burst causes stress in plants mainly because of altered redox homeostasis, abnormal cell signaling resulting in massive disturbances of otherwise well-orchestrated cellular functions.89 Thus it is important to pursue research in the area of post-climacteric biochemical events regulated by ROS and modulated by NO, since this interaction has direct effects on cellular ethylene levels and other antioxidant actions during ripening. ROS also act as the senescence triggering factors, causing loss of cell-membrane integrity and functionality and NO has been shown to reduce ROS toxicity. NO prevented ROS mediated browning of harvested fruits, and also delayed senescence of ornamentals.90,91 NO may do this through the suppression of ROS generating enzymes or linked signaling cascades92 as demonstrated during biotic93 and abiotic68 stresses. Equally, NO upregulates the expression of major enzymes involved in quenching ROS such as catalase (CAT), peroxidases (POD) and superoxide dismutase (SOD) in peaches24 and kiwifruit.70 In kiwifruit, the ROS effects were significantly reversed by NO via genetic upregulation of SOD and CAT, and suppression of LOX resulting in the maintenance of vitamins C and E.70 Further, NO’s role as an antioxidant has been ascribed for its property of preventing the Fenton’s reaction, making the meager formation of hydroxyl radical.97 Likewise, NO affects the functioning of plant POD involved in cell wall lignification since it easily forms an iron-nitrosyl complex with haem iron.98 Free radical scavenging ability of NO has been demonstrated to preferentially quench o-quinone radicals, causing interruption of normal browning reactions occurring at the cut surfaces of fruit.99 On the other hand, the protection offered by NO to organic acids, particularly of ascorbic acid and vice versa may complement each other, converging in the prevention of browning. Phenylalanine ammonia lyase (PAL), the first key enzyme in the biosynthetic pathway of phenolic compounds that are known to cause browning in fruit is also triggered by various stress conditions,100 and probably these events are also reversed by NO in preventing browning. Longan, Lychee (Litchi chinensis) and the Indian date “jujube” (Ziziphus zizyphus) fruit treated with NO inhibited the activities of PAL, polyphenol oxidase (PPO) and POD.27,94,95 However, in plums although NO caused a delay in total phenol formation, it failed to suppress activities of PPO, POD and PAL.96
Lignification, which offers protection during biotic and abiotic stresses in higher plants is the other mechanism associated with oxidative burst, and the role of NO for enhanced lignification has been studied in several horticultural commodities for enhanced shelf life. Similarly, inhibition of LOX activity by NO was demonstrated in kiwifruit by binding its active site and inactivating the catalytic activity of the enzyme. Since, LOX catalyzes the formation of jasmonic acid (JA), and the latter being a growth regulator/signal molecule physiologically implied with ethylene,23 indicating LOX involvement in ripening being of great relevance.
Given these observations, the attractiveness of NO application to improve quality attributes of fruits could appear overwhelming. However, given the nature of NO and ROS toxicity at certain concentrations, precise monitoring of both NO and ROS thresholds is essential for maintaining the levels needed for desirable modulation of fruit ripening.
Conclusions and Perspectives
Ethylene-signaling in fruits is a tightly coordinated activity under the influence of several signals and phytohormones. Emerging information indicates that NO alters endogenous ethylene levels at various levels by modifying many pathways causing post-climacteric biochemical changes which are linked to fruit quality. Although NO controls ethylene stoichiometrically, its specific effects on different receptors and downstream signaling cascade is a subject for further verification. Understanding exactly how NO influences ethylene signaling will provide novel and economically important information which could allow the improvement of quality attributes of fruits for more extended periods. It may be that the initiation of NO production or supplementation of fruit packages with NO would be the relatively novel approaches to postpone the climacteric ethylene burst and thereby extend shelf-life.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19523
References
- 1.Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16(Suppl):S170–80. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Payasi A, Sanwal GG. Ripening of climacteric fruits and their control. J Food Biochem. 2010;34:679–710. doi: 10.1111/j.1745-4514.2009.00307.x. [DOI] [Google Scholar]
- 3.Chen YF, Gao Z, Kerris RJ, 3rd, Wang W, Binder BM, Schaller GE. Ethylene receptors function as components of high-molecular-mass protein complexes in Arabidopsis. PLoS One. 2010;5:e8640. doi: 10.1371/journal.pone.0008640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–89. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 5.Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, et al. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–86. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Malley RC, Rodriguez FI, Esch JJ, Binder BM, O’Donnell P, Klee HJ, et al. Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J. 2005;41:651–9. doi: 10.1111/j.1365-313X.2004.02331.x. [DOI] [PubMed] [Google Scholar]
- 8.Adams-Phillips L, Barry C, Kannan P, Leclercq J, Bouzayen M, Giovannoni J. Evidence that CTR1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Mol Biol. 2004;54:387–404. doi: 10.1023/B:PLAN.0000036371.30528.26. [DOI] [PubMed] [Google Scholar]
- 9.Qu X, Hall BP, Gao Z, Schaller GE. A strong constitutive ethylene-response phenotype conferred on Arabidopsis plants containing null mutations in the ethylene receptors ETR1 and ERS1. BMC Plant Biol. 2007;7:1–15. doi: 10.1186/1471-2229-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, et al. The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene signaling. Plant Cell. 2007;19:509–23. doi: 10.1105/tpc.106.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo O, Piqueras R, Śnchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–78. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manjunatha G, Lokesh V, Neelwarne B. Nitric oxide in fruit ripening: trends and opportunities. Biotechnol Adv. 2010;28:489–99. doi: 10.1016/j.biotechadv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Burnham HD, Pease RN. Inhibition of the hydrogenation of ethylene by nitric oxide. J Am Chem Soc. 1940;62:453–453. [Google Scholar]
- 14.Leshem YY, Wills RBH, Ku VVV. Evidence for the function of the free radical gas–nitric oxide (NO-) –as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem. 1998;36:825–33. doi: 10.1016/S0981-9428(99)80020-5. [DOI] [Google Scholar]
- 15.Gupta KJ. Protein S-nitrosylation in plants: photorespiratory metabolism and NO signaling. Sci Signal. 2011;4:jc1. doi: 10.1126/scisignal.2001404. [DOI] [PubMed] [Google Scholar]
- 16.Morot-Gaudry-Talarmain Y, Rockel P, Moureaux T, Quilleré I, Leydecker MT, Kaiser WM, et al. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta. 2002;215:708–15. doi: 10.1007/s00425-002-0816-3. [DOI] [PubMed] [Google Scholar]
- 17.Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot. 2005;56:2601–9. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- 18.Gupta KJ, Igamberdiev AU, Kaiser WM. New insights into the mitochondrial nitric oxide production pathways. Plant Signal Behav. 2010;5:999–1001. doi: 10.4161/psb.5.8.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modolo LV, Augusto O, Almeida IMG, Magalhaes JR, Salgado I. Nitrite as the major source of nitric oxide production by Arabidopsis thaliana in response to Pseudomonas syringae. FEBS Lett. 2005;579:3814–20. doi: 10.1016/j.febslet.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 20.Rümer S, Kapuganti JG, Kaiser WM. Oxidation of hydroxylamines to NO by plant cells. Plant Signal Behav. 2009;4:853–5. doi: 10.4161/psb.4.9.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rümer S, Gupta KJ, Kaiser WM. Plant cells oxidize hydroxylamines to NO. J Exp Bot. 2009;60:2065–72. doi: 10.1093/jxb/erp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta KJ, Kaiser WM. Production and scavenging of nitric oxide by barley root mitochondria. Plant Cell Physiol. 2010;51:576–84. doi: 10.1093/pcp/pcq022. [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Liu M, Zhou J. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol Technol. 2006;42:41–8. doi: 10.1016/j.postharvbio.2006.05.004. [DOI] [Google Scholar]
- 24.Flores FB, Śnchez-Bel P, Valdenegro M, Romojaro F, Martínez-Madrid MC, Egea MI. Effects of a pretreatment with nitric oxide on peach (Prunus persica L.) storage at room temperature. Eur Food Res Technol. 2008;227:1599–611. doi: 10.1007/s00217-008-0884-0. [DOI] [Google Scholar]
- 25.Zhu S, Zhou J. Effects of nitric oxide on fatty acid composition in peach fruits during storage. J Agric Food Chem. 2006;54:9447–52. doi: 10.1021/jf062451u. [DOI] [PubMed] [Google Scholar]
- 26.Pristijono P, Wills R, Golding J. Inhibition of browning on the surface of apple slices by short term exposure to nitric oxide (NO) gas. Postharvest Biol Technol. 2006;42:256–9. doi: 10.1016/j.postharvbio.2006.07.006. [DOI] [Google Scholar]
- 27.Duan X, Su X, You Y, Qu H, Li Y, Jiang Y. Effect of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chem. 2007;104:571–6. doi: 10.1016/j.foodchem.2006.12.007. [DOI] [Google Scholar]
- 28.Cheng G, Yang E, Lu W, Jia Y, Jiang Y, Duan X. Effect of nitric oxide on ethylene synthesis and softening of banana fruit slice during ripening. J Agric Food Chem. 2009;57:5799–804. doi: 10.1021/jf901173n. [DOI] [PubMed] [Google Scholar]
- 29.Aboul-Soud MAM. Exogenous nitric oxide negatively impacts on ethylene emissions from intact and fresh-cut tomato fruit. Res J Biol Sci. 2010;5:209–14. doi: 10.3923/rjbsci.2010.209.214. [DOI] [Google Scholar]
- 30.Leshem YY. Nitric oxide in plants: occurrence, function and use 2000; Springer Netherlands. [Google Scholar]
- 31.Zhu S, Sun L, Zhou J. Effects of different nitric oxide application on quality of kiwifruit during 20° C storage. Int J Food Sci Technol. 2010;45:245–51. doi: 10.1111/j.1365-2621.2009.02127.x. [DOI] [Google Scholar]
- 32.Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–21. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- 33.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, et al. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;305:1968–71. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 34.Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–8. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 35.Manjunatha G, Niranjan-Raj S, Prashanth GN, Deepak S, Amruthesh KN, Shetty HS. Nitric oxide is involved in chitosan-induced systemic resistance in pearl millet against downy mildew disease. Pest Manag Sci. 2009;65:737–43. doi: 10.1002/ps.1710. [DOI] [PubMed] [Google Scholar]
- 36.Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL. Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 2002;129:1642–50. doi: 10.1104/pp.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murgia I, de Pinto MC, Delledonne M, Soave C, De Gara L. Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J Plant Physiol. 2004;161:777–83. doi: 10.1016/j.jplph.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Leshem YY, Pinchasov Y. Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripening of strawberries Fragaria anannasa (Duch.) and avocados Persea americana (Mill.) J Exp Bot. 2000;51:1471–3. doi: 10.1093/jexbot/51.349.1471. [DOI] [PubMed] [Google Scholar]
- 39.Leshem Y, Wills R. Harnessing senescence delaying gases nitric oxide and nitrous oxide: a novel approach to postharvest control of fresh horticultural produce. Biol Plant. 1998;4:1–10. doi: 10.1023/A:1001779227767. [DOI] [Google Scholar]
- 40.Wills RBH, Ku VVV, Leshem YY. Fumigation with nitric oxide to extend the postharvest life of strawberries. Postharvest Biol Technol. 2000;18:75–9. doi: 10.1016/S0925-5214(99)00061-7. [DOI] [Google Scholar]
- 41.Rudell DR, Mattheis JP. Nitric oxide and nitrite treatments reduce ethylene evolution from apple fruit disks. HortScience. 2006;41:1462–5. [Google Scholar]
- 42.Liu MC, Song WH, Zhu SH, Zhou J. Effects of nitric oxide and exogenous ethylene treatments on ethylene biosynthesis in Feicheng Peach. Agric Sci China. 2007;6:290–5. doi: 10.1016/S1671-2927(07)60047-9. [DOI] [Google Scholar]
- 43.Zhu S, Zhou J. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chem. 2007;100:1517–22. doi: 10.1016/j.foodchem.2005.12.022. [DOI] [Google Scholar]
- 44.Eum HL, Kim HB, Choi SB, Lee SK. Regulation of ethylene biosynthesis by nitric oxide in tomato (Solanum lycopersicum L.) fruit harvested at different ripening stages. Eur Food Res Technol. 2009;228:331–8. doi: 10.1007/s00217-008-0938-3. [DOI] [Google Scholar]
- 45.Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, et al. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants. Plant Physiol. 2006;142:595–608. doi: 10.1104/pp.106.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jakubowicz M, Gałgańska H, Nowak W, Sadowski J. Exogenously induced expression of ethylene biosynthesis, ethylene perception, phospholipase D, and Rboh-oxidase genes in broccoli seedlings. J Exp Bot. 2010;61:3475–91. doi: 10.1093/jxb/erq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindermayr C, Saalbach G, Bahnweg G, Durner J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J Biol Chem. 2006;281:4285–91. doi: 10.1074/jbc.M511635200. [DOI] [PubMed] [Google Scholar]
- 48.Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci U S A. 2000;97:5663–8. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klee H, Tieman D. The tomato ethylene receptor gene family: Form and function. Physiol Plant. 2002;115:336–41. doi: 10.1034/j.1399-3054.2002.1150302.x. [DOI] [PubMed] [Google Scholar]
- 50.Wilhelmová N, Fuksová H, Srbová M, Miková D, Mýtinová Z, Procházková D, et al. The effect of plant cytokinin hormones on the production of ethylene, nitric oxide, and protein nitrotyrosine in ageing tobacco leaves. Biofactors. 2006;27:203–11. doi: 10.1002/biof.5520270118. [DOI] [PubMed] [Google Scholar]
- 51.Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci U S A. 1998;95:10328–33. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wendehenne D, Pugin A, Klessig DF, Durner J. Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001;6:177–83. doi: 10.1016/S1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- 53.Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–8. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebrun-Garcia A, Durner J, et al. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol. 2004;135:516–29. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. Nitric oxide signalling functions in plant-pathogen interactions. Cell Microbiol. 2004;6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 56.Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman DW, et al. Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J. 2004;2:359–66. doi: 10.1111/j.1467-7652.2004.00085.x. [DOI] [PubMed] [Google Scholar]
- 57.Bowler C, Neuhaus G, Yamagata H, Chua NH. Cyclic GMP and calcium mediate phytochrome phototransduction. Cell. 1994;77:73–81. doi: 10.1016/0092-8674(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 58.Rhoads DM, McIntosh L. Salicylic acid regulation of respiration in higher plants: alternative oxidase expression. Plant Cell. 1992;4:1131–9. doi: 10.1105/tpc.4.9.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta. 2004;218:938–46. doi: 10.1007/s00425-003-1178-1. [DOI] [PubMed] [Google Scholar]
- 60.Zottini M, Costa A, De Michele R, Ruzzene M, Carimi F, Lo Schiavo F. Salicylic acid activates nitric oxide synthesis in Arabidopsis. J Exp Bot. 2007;58:1397–405. doi: 10.1093/jxb/erm001. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Chen KS, Chen QJ, Zhang SL, Ren YP. Effects of Acetylsalicylic acid (ASA) and ethylene treatments on ripening and softening of postharvest kiwifruit. Acta Bot Sin. 2003;45:1447–52. [Google Scholar]
- 62.Srivastava MK, Dwivedi UN. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000;158:87–96. doi: 10.1016/S0168-9452(00)00304-6. [DOI] [PubMed] [Google Scholar]
- 63.Li N, Parsons BL, Liu DR, Mattoo AK. Accumulation of wound-inducible ACC synthase transcript in tomato fruit is inhibited by salicylic acid and polyamines. Plant Mol Biol. 1992;18:477–87. doi: 10.1007/BF00040664. [DOI] [PubMed] [Google Scholar]
- 64.Fan X, Mattheis J, Fellman J. Inhibition of apple fruit 1-aminocyclopropane-1-carboxylic acid oxidase activity and respiration by acetylsalicyclic acid. J Plant Physiol. 1996;149:469–71. doi: 10.1016/S0176-1617(96)80151-9. [DOI] [Google Scholar]
- 65.Mur LAJ, Laarhoven LJJ, Harren FJM, Hall MA, Smith AR. Nitric oxide interacts with salicylate to regulate biphasic ethylene production during the hypersensitive response. Plant Physiol. 2008;148:1537–46. doi: 10.1104/pp.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, et al. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci U S A. 2000;97:8849–55. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Chen K, Zhang S, Ferguson I. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Biol Technol. 2003;28:67–74. doi: 10.1016/S0925-5214(02)00172-2. [DOI] [Google Scholar]
- 68.Orozco-Ćrdenas ML, Ryan CA. Nitric oxide negatively modulates wound signaling in tomato plants. Plant Physiol. 2002;130:487–93. doi: 10.1104/pp.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu MJ, Dong JF, Zhu MY. Nitric oxide mediates the fungal elicitor-induced hypericin production of Hypericum perforatum cell suspension cultures through a jasmonic-acid-dependent signal pathway. Plant Physiol. 2005;139:991–8. doi: 10.1104/pp.105.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu S, Sun L, Liu M, Zhou J. Effect of nitric oxide on reactive oxygen species and antioxidant enzymes in kiwifruit during storage. J Sci Food Agric. 2008;88:2324–31. doi: 10.1002/jsfa.3353. [DOI] [Google Scholar]
- 71.Ziosi V, Bonghi C, Bregoli AM, Trainotti L, Biondi S, Sutthiwal S, et al. Jasmonate-induced transcriptional changes suggest a negative interference with the ripening syndrome in peach fruit. J Exp Bot. 2008;59:563–73. doi: 10.1093/jxb/erm331. [DOI] [PubMed] [Google Scholar]
- 72.Venancio WS, Rodrigues MAT, Begliomini E, de Souza NL. Physiological effects of strobilurin fungicides on plants. Ponta Grossa. 2003;9:59–68. [Google Scholar]
- 73.Carimi F, Zottini M, Costa A, Cattelan I, De Michele R, Terzi M, et al. NO signalling in cytokinin-induced programmed cell death. Plant Cell Environ. 2005;28:1171–8. doi: 10.1111/j.1365-3040.2005.01355.x. [DOI] [Google Scholar]
- 74.El-Sharkawy I, Kim WS, Jayasankar S, Svircev AM, Brown DC. Differential regulation of four members of the ACC synthase gene family in plum. J Exp Bot. 2008;59:2009–27. doi: 10.1093/jxb/ern056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scherer GFE, Holk A. NO donors mimic and NO inhibitors inhibit cytokinin action in betalaine accumulation in Amaranthus caudatus. Plant Growth Regul. 2000;32:345–50. doi: 10.1023/A:1010750111550. [DOI] [Google Scholar]
- 76.Tun NN, Holk A, Scherer GFE. Rapid increase of NO release in plant cell cultures induced by cytokinin. FEBS Lett. 2001;509:174–6. doi: 10.1016/S0014-5793(01)03164-7. [DOI] [PubMed] [Google Scholar]
- 77.Carimi F, Zottini M, Formentin E, Terzi M, Lo Schiavo F. Cytokinins: new apoptotic inducers in plants. Planta. 2003;216:413–21. doi: 10.1007/s00425-002-0862-x. [DOI] [PubMed] [Google Scholar]
- 78.Feuerstein BG, Williams LD, Basu HS, Marton LJ. Implications and concepts of polyamine-nucleic acid interactions. J Cell Biochem. 1991;46:37–47. doi: 10.1002/jcb.240460107. [DOI] [PubMed] [Google Scholar]
- 79.Corley E, Wolosiuk RA, Hertig CM. Regulation of the activation of chloroplast fructose-1,6-bisphosphatase: inhibition by spermidine and spermine. Biochem Biophys Res Commun. 1983;115:707–14. doi: 10.1016/S0006-291X(83)80202-2. [DOI] [PubMed] [Google Scholar]
- 80.Polyamines ST. Annu Rev Plant Physiol. 1985;36:117–43. doi: 10.1146/annurev.pp.36.060185.001001. [DOI] [Google Scholar]
- 81.Mirdehghan S, Rahemi M, Castillo S, Martínez-Romero D, Serrano M, Valero D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol Technol. 2007;44:26–33. doi: 10.1016/j.postharvbio.2006.11.010. [DOI] [Google Scholar]
- 82.Khan AS, Singh Z, Abbasi NA. Pre-storage putrescine application suppresses ethylene biosynthesis and retards fruit softening during low temperature storage in ‘Angelino’ plum. Postharvest Biol Technol. 2007;46:36–46. doi: 10.1016/j.postharvbio.2007.03.018. [DOI] [Google Scholar]
- 83.Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140:103–25. doi: 10.1016/S0168-9452(98)00218-0. [DOI] [Google Scholar]
- 84.Mattoo AK, Handa AK. Higher polyamines restore and enhance metabolic memory in ripening fruit. Plant Sci. 2008;174:386–93. doi: 10.1016/j.plantsci.2008.01.011. [DOI] [Google Scholar]
- 85.Tun NN, Santa-Catarina C, Begum T, Silveira V, Handro W, Floh EIS, et al. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006;47:346–54. doi: 10.1093/pcp/pci252. [DOI] [PubMed] [Google Scholar]
- 86.Arasimowicz-Jelonek M, Floryszak-Wieczorek J, Kubiś J. Interaction between polyamine and nitric oxide signaling in adaptive responses to drought in cucumber. J Plant Growth Regul. 2009;28:177–86. doi: 10.1007/s00344-009-9086-7. [DOI] [Google Scholar]
- 87.Singh S, Singh Z, Swinny E. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell) Postharvest Biol Technol. 2009;53:101–8. doi: 10.1016/j.postharvbio.2009.04.007. [DOI] [Google Scholar]
- 88.Zhang B, Chen K, Bowen J, Allan A, Espley R, Karunairetnam S, et al. Differential expression within the LOX gene family in ripening kiwifruit. J Exp Bot. 2006;57:3825–36. doi: 10.1093/jxb/erl151. [DOI] [PubMed] [Google Scholar]
- 89.Bailey-Serres J, Mittler R. The roles of reactive oxygen species in plant cells. Plant Physiol. 2006;141:311–6. doi: 10.1104/pp.104.900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farias-Eisner R, Chaudhuri G, Aeberhard E, Fukuto JM. The chemistry and tumoricidal activity of nitric oxide/hydrogen peroxide and the implications to cell resistance/susceptibility. J Biol Chem. 1996;271:6144–51. doi: 10.1074/jbc.271.11.6144. [DOI] [PubMed] [Google Scholar]
- 91.Durzan DJ, Pedroso MC. Nitric oxide and reactive nitrogen oxide species in plants. Biotechnol Genet Eng Rev. 2002;19:293–337. doi: 10.1080/02648725.2002.10648032. [DOI] [PubMed] [Google Scholar]
- 92.Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microbe Interact. 2000;13:1380–4. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- 93.2BBMałolepsza U, Rózalska S. Nitric oxide and hydrogen peroxide in tomato resistance. Nitric oxide modulates hydrogen peroxide level in o-hydroxyethylorutin-induced resistance to Botrytis cinerea in tomato. Plant Physiol Biochem. 2005;43:623–35. doi: 10.1016/j.plaphy.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Kramchote S, Wongs-Aree V, Srilaong A, Uthairatanakij S. Kanlayanarat. Influence of nitric oxide on pericap browning of litchi cv. ‘Chakrapad’, in Europe-Asia Symposium on Quality Management in Postharvest Systems-Eurasia 2007. 804, pp. 249–254. [Google Scholar]
- 95.Zhu S, Sun L, Zhou J. Effects of nitric oxide fumigation on phenolic metabolism of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao) in relation to fruit quality. LWT-Food Sci Technol. 2009;42:1009–14. doi: 10.1016/j.lwt.2008.12.012. [DOI] [Google Scholar]
- 96.Li J, Yi C, Yang E, Qu H, Jiang Y, Duan X, et al. Effect of nitric oxide on disorder development and quality maintenance of Plum fruit stored at low temperature, in Europe-Asia Symposium on Quality Management in Postharvest Systems-Eurasia 2007; 804, pp. 549–554. [Google Scholar]
- 97.Wink DA, Cook JA, Pacelli R, Liebmann J, Krishna MC, Mitchell JB. Nitric oxide (NO) protects against cellular damage by reactive oxygen species. Toxicol Lett. 1995;82-83:221–6. doi: 10.1016/0378-4274(95)03557-5. [DOI] [PubMed] [Google Scholar]
- 98.Ferrer M, Ros Barceló A. Differential effects of nitric oxide on peroxidase and H2O2 production by the xylem of Zinnia elegans. Plant Cell Environ. 1999;22:891–7. doi: 10.1046/j.1365-3040.1999.00459.x. [DOI] [Google Scholar]
- 99.Pristijono P, Wills R, Golding J. Use of the nitric oxide-donor compound, diethylenetriamine-nitric oxide (DETANO), as an inhibitor of browning in apple slices. J Hortic Sci Biotechnol. 2008;83:555–8. [Google Scholar]
- 100.Nguyen TBT, Ketsa S, van Doorn WG. Relationship between browning and the activities of polyphenoloxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol Technol. 2003;30:187–93. doi: 10.1016/S0925-5214(03)00103-0. [DOI] [Google Scholar]
- 101.Wills RB, Soegiarto L, Bowyer MC. Use of a solid mixture containing diethylenetriamine/nitric oxide (DETANO) to liberate nitric oxide gas in the presence of horticultural produce to extend postharvest life. Nitric Oxide. 2007;17:44–9. doi: 10.1016/j.niox.2007.05.003. [DOI] [PubMed] [Google Scholar]