Abstract
Comment on: Adhikari D, et al. Hum Mol Gene 2012; 21:2476-84.
Keywords: Cdk1, meiosis, mitosis, oocyte meiosis resumption, protein phosphatase
Cell cycle progression in mammals involves multiple cyclin-dependent kinases (Cdks). Mice lacking Cdk2, Cdk3, Cdk4 or Cdk6 are viable, however, because Cdk1 can compensate for their loss by forming active complexes with A-, B-, E- and D-type cyclins in a stepwise manner. Thus, these Cdks are not essential for the mammalian cell cycle.1,2 In contrast, homozygous deletion of Cdk1 causes early embryonic death in mice, because Cdk1-null embryos cannot undergo mitosis.2,3 Cdk1 is, therefore, the only Cdk essential for driving the mitotic cell cycle in mammals.
In mammals, all oocytes are arrested at prophase of the first meiotic division when they are enclosed in individual ovarian follicles. Fully grown mouse oocytes resume meiosis as a result of a pre-ovulatory surge of luteinizing hormone (LH). This results in germinal vesicle (GV) breakdown (GVBD) followed by chromosome condensation, spindle formation and the completion of the first meiotic division.4 The resumption of meiosis in oocytes is often compared with the G2-M transition of mitosis, and the results from biochemical studies of mitosis are generalized to meiosis. Because Cdk1-null mice are not viable2,3 and all of the oocytes from Cdk2-null mice are eliminated very early during meiosis,5,6 it remained unknown whether it is Cdk1 or Cdk2 that is essential for the resumption of meiosis in oocytes.
Recent in vivo evidence has shown that Cdk1 is the only Cdk that is both essential and sufficient for driving the resumption of meiosis in mouse oocytes.7 By generating mouse models with oocyte-specific deletions of Cdk1 or Cdk2, this study showed that a lack of Cdk1 in oocytes results in a permanent arrest of the oocytes at the GV stage. On the other hand, mutant female mice lacking Cdk2 in their oocytes were fertile, and the oocytes resumed meiosis normally. Thus Cdk1 plays an essential role in driving both meiosis7 and mitosis.2,3
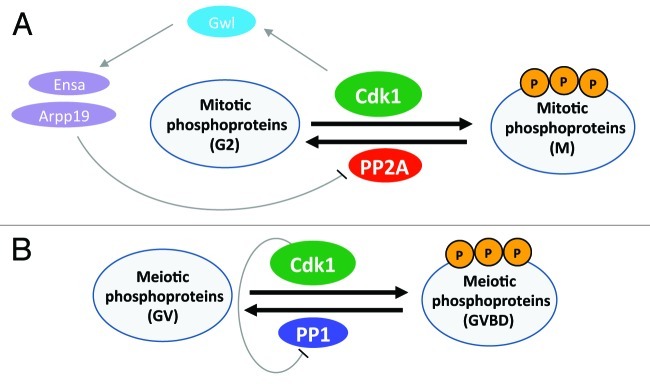
Although Cdk1 kinase activity is essential for the phosphorylation of mitotic phosphoproteins, inhibition of its counteracting protein phosphatase is also essential in order to maintain the phosphorylated status of the Cdk1 substrates that are required for entry into mitosis.8 Genetic and biochemical approaches have implicated protein phosphatase 2A (PP2A) in the dephosphorylation of mitotic phosphoproteins. Activation of Cdk1 leads to the phosphorylation and activation of Greatwall (Gwl) kinase, which, in turn, blocks PP2A. When PP2A is blocked, there is a net phosphorylation of mitotic phosphoproteins, and the cell enters into mitosis (Fig. 1A). In contrast, Adhikari et al.7 showed that permanent arrest of the Cdk1-null oocytes at the GV stage was caused by an inability to phosphorylate and suppress protein phosphatase 1 (PP1). Upon Cdk1 activation, PP1 is phosphorylated at Thr320 and becomes inactive.7 This allows the phosphorylation of meiotic phosphoproteins and the resumption of meiosis (Fig. 1B).
Figure 1. Schematic illustration of the roles of Cdk1 during the G2-M transition in mitosis and in the germinal vesicle breakdown that occurs during resumption of meiosis. (A) Cdk1 kinase is activated and promotes the phosphorylation of mitotic phosphoproteins at the G2-M transition in mitosis. In order to maintain the phosphorylation status of the Cdk1 substrates, PP2A is inactivated by activation of Gwl by Cdk1. Active Gwl inhibits PP2A through the activation of Arpp19 and Ensa. (B) During the resumption of meiosis in oocytes, Cdk1 is activated and promotes the phosphorylation of meiotic phosphoproteins. Maintenance of the phosphorylation status of the meiotic phosphoproteins is ensured by phosphorylation and inhibition of PP1 by Cdk1. P, phosphorylation; Arpp19, cAMP-regulated phosphoprotein 19; Ensa, α-endosulfine.
Although Gwl was initially identified as a regulator of mitotic entry in Drosophila melanogaster,9 its depletion from Xenopus laevis egg extracts also prevents mitotic entry.10 Moreover, silencing of MASTL (the human ortholog of Gwl) from human cell lines also leads to full activation of PP2A. This completely dephosphorylates the CDK1 substrates and prevents cells from entering mitosis.11 If PP1 is the Cdk1 substrate during oocyte meiotic resumption instead of PP2A, the question becomes whether or not Mastl kinase is still required for the resumption of meiosis in oocytes. Similarly, PP2A inhibition by Gwl kinase leads to Cdc25 activation during mitosis. This, in turn, activates Cdk1 by removing the inhibitory phosphorylations on Cdk1 that were the result of Wee1/Myt1 kinase activity.8 If Cdk1 phosphorylates and inactivates PP1 during the resumption of meiosis, a second question that arises is whether Mastl still affects the activity of Cdk1 through the regulation of Cdc25 activity. Thus, further research is needed to clarify the mechanism by which Cdk1 and its substrates are regulated during the meiotic maturation of oocytes. A better understanding of how Cdk1 is regulated and how it drives the resumption of meiosis in oocytes will help us to treat female infertility that results from oocytes that fail to resume meiotic maturation.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21254
References
- 1.Satyanarayana A, et al. Oncogene. 2009;28:2925–39. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 2.Santamaría D, et al. Nature. 2007;448:811–5. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- 3.Diril MK, et al. Proc Natl Acad Sci USA. 2012;109:3826–31. doi: 10.1073/pnas.1115201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehlmann LM. Reproduction. 2005;130:791–9. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 5.Ortega S, et al. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 6.Berthet C, et al. Curr Biol. 2003;13:1775–85. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Adhikari D, et al. Hum Mol Genet. 2012;21:2476–84. doi: 10.1093/hmg/dds061. [DOI] [PubMed] [Google Scholar]
- 8.Virshup DM, et al. Science. 2010;330:1638–9. doi: 10.1126/science.1199898. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, et al. J Cell Biol. 2004;164:487–92. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, et al. Mol Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Burgess A, et al. Proc Natl Acad Sci USA. 2010;107:12564–9. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]