Abstract
Comment on: Tran KV, et al. Cell Metab 2012; 15:222-9.
Keywords: adipogenesis, adipose tissue explants, brown adipose tissue, electron microscopy, endothelial cells, in vivo lineage-tracing, preadipocytes, white adipose tissue
Adipose tissue can be considered a multi-depot organ with extremely dynamic properties. Nutritional and environmental cues can strongly affect the cellular composition of this tissue and, as a result, promote the remodeling of the whole organ. A positive energy balance and cold exposure induce the growth and transformation of existing adipocytes and the formation of new cells. Both conditions induce the concomitant development of the vascular network to nourish the newly formed adipocytes.1 Recently, the attention of many researchers has been focused on the mechanisms underlying the emergence of brown adipocytes within classical white adipose tissue (WAT) depots as a possible strategy to curb obesity and accompanying disorders such as type 2 diabetes and cardiovascular diseases.2 In this area of study, the origin of preadipocytes (the cells committed to becoming adipocytes) in both WAT and brown adipose tissue (BAT) is an important question and has been addressed by different approaches. Several studies have identified specific markers for the precursor cells that give rise to committed preadipocytes and early adipocytes3, 4 and have suggested, in agreement with prior morphological studies,5 that the vascular wall of adipose tissue capillaries may serve as an adipogenic niche, where a still unknown local tissue microenvironment interacts with and regulates stem cell proliferation and differentiation.
At the beginning of the year, our group, in collaboration with Silvia Corvera’s team at the University of Massachusetts, published a paper in which it is concluded that endothelial cells (ECs) in adipose tissue capillaries give rise to white and brown adipocytes.6 The analysis of the ultrastructure of murine epididymal-WAT (eWAT, classic WAT) at postnatal days 6–8 (P6–8), when adipogenesis explodes into specific vasculo-adipocytic areas, allowed the discovery of characteristic endothelial-pericytic elements that led us to hypothesize that ECs may give rise to adipocytes. To provide evidence in support of this hypothesis, we performed lineage-tracing experiments using mice in which the activation of the promoter for the endothelial marker vascular endothelial (VE)-cadherin was detected using X-gal staining. In addition to ECs, at P6–8, adipocytes in the eWAT and subcutaneous WAT were positive for X-gal. Interestingly, X-gal staining was also observed in the interscapular BAT, and the reporter gene consistently co-localized with uncoupling protein 1 (UCP1), the brown adipocyte marker. To exclude the possibility that the adipocyte precursors are cells of hematopoietic origin (some of which also express VE-cadherin), we performed lineage-tracing studies using mice in which cells derived from hematopoietic precursors were not labeled. Once again, X-gal staining was observed in the capillaries as well as in the eWAT, inguinal and BAT depots. Finally, we asked whether human adipocytes also originate from ECs or EC-like cells. To this end, we cultured pieces of human adipose tissue, which, as previously shown, gives rise to capillary sprouts exposed to pro-angiogenic medium.7 Following treatment with an agonist of peroxisome proliferator-activated receptor-γ (PPARγ), which is the master regulator of adipogenesis, cells within capillary sprouts produced lipid droplets and exhibited molecular and morphological features consistent with white adipocytes. In particular, these cells showed decreased expression of mRNAs encoding EC proteins and increased expression of mRNAs encoding adipocyte proteins, including the transcription factor zinc-finger protein 423 (Zfp423), a marker of adipogenic cells.8
Notably, these observations were confirmed by another line of investigation performed in the laboratories of Bruce Spiegelman at Harvard Medical School. The authors, primarily through lineage-tracing experiments using Zfp423-driven GFP, identified a small subset of capillary ECs within the BAT and WAT that also express this marker, suggesting a contribution of specialized ECs to the adipose lineage.9
In conclusion, using morphological, genetic and functional approaches, we have obtained evidence that the ECs of the capillaries in developing WAT and BAT can differentiate into mature adipose cells. Based on these results, we suggest that capillary growth and adipocyte differentiation are highly coordinated events during embryogenesis and adulthood that satisfy the nutritional and/or thermogenic requirements of mammals. Dissecting the molecular machineries of this process may be of great interest in the physiopathology of obesity, where consistent experimental evidence suggests that the hypoxic damage of WAT in obese subjects is a primary defect leading to metabolic syndrome.10 In addition, our data suggest that white and brown adipocytes share the same precursor cell, i.e., the ECs (Fig. 1), and this existence of a shared precursor is consistent with the data supporting the physiological reversible transdifferentiating properties of the adipocytes in the adipose organ. Indeed, the ECs have a characteristic mature phenotype, allowing their specific function in capillaries. Thus, development of pericytes into adipocytes from ECs seems to follow the pathway of transdifferentiation rather than that of differentiation from a poorly differentiated stem cell, adding further support to the dynamic properties characteristic of this organ.
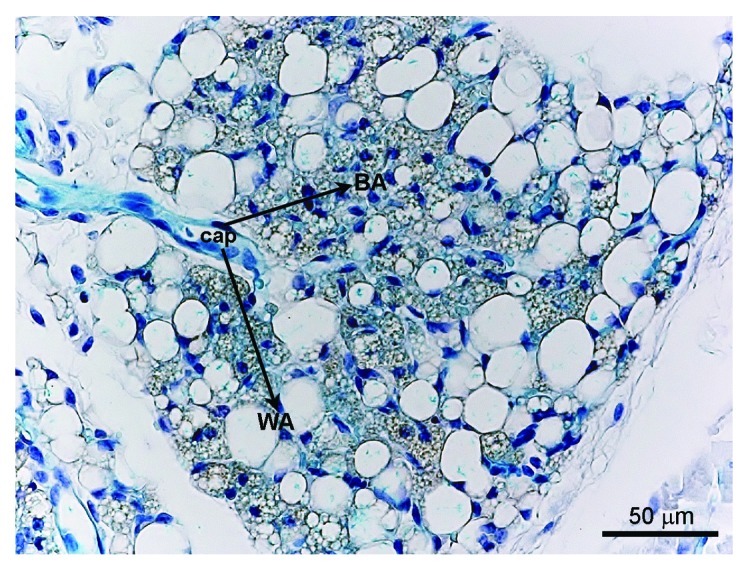
Figure 1. X-gal staining in a VE-cadherin-Cre-R26R mouse. In the inguinal adipose depot (i.e., mixed WAT/BAT depot), the blue precipitate indicating the presence of the reporter gene β-galactosidase is detectable in the endothelial cells of the capillaries (Cap), as expected, but also in the brown (BA) and white adipocytes (WA), suggesting their common origin from endothelial cells.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21255
References
- 1.Frontini A, et al. Cell Metab. 2010;11:253–6. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, et al. J Clin Invest. 2012;122:486–9. doi: 10.1172/JCI60941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodeheffer MS, et al. Cell. 2008;135:240–9. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Tang W, et al. Science. 2008;322:583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinti S, et al. J Submicrosc Cytol. 1984;16:243–51. [PubMed] [Google Scholar]
- 6.Tran KV, et al. Cell Metab. 2012;15:222–9. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gealekman O, et al. Circulation. 2011;123:186–94. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RK, et al. Nature. 2010;464:619–23. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta RK, et al. Cell Metab. 2012;15:230–9. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K, et al. J Clin Invest. 2011;121:2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]


