Abstract
Comment on: Johnson BN, et al. Proc Natl Acad Sci USA 2012; 109:6283-8.
Keywords: Bax, Parkinson’s disease, apoptosis, autophagy, mitochondria
Parkinson disease (PD) is a common neurodegenerative disorder affecting about 1–2% of the population over the age of 65.1 While most PD cases are sporadic, the rare, familial forms can provide unique insight into disease processes. Mutations within the PARK2 locus, which encodes the ubiquitin E3 ligase parkin,2 are the most common cause of autosomal recessive PD. Since loss-of-function parkin mutations are associated with neurodegeneration in humans, perhaps it’s not surprising that the expression of parkin is routinely associated with increased cell survival. Indeed, there is a surprisingly wide variety of cell stressors that are rescued by parkin, spanning chemical inducers of apoptosis, pro-oxidants and disease-associated proteins. For parkin to possess such a potent and almost omni-protective function, one would imagine it acting at a downstream level within a cell death/cell survival pathway. The fact that the pro-survival effects of parkin have been observed in Drosophila, rodents and cell culture systems derived from a variety of species further instructs us that the pathway(s) influenced by parkin are evolutionally conserved.
We recently reported that parkin-dependent ubiquitination of Bax is required for the anti-apoptotic function of parkin.3 In multiple systems, parkin reduced the mitochondrial accumulation of Bax under basal conditions and also inhibited acute stress-induced Bax translocation to the mitochondria. The discovery that a pro-apoptotic member of the Bcl-2 protein family is inactivated by parkin not only provides the underlying mechanism for the parkin-dependent regulation of cytochrome c release we previously described,4 but also satisfies the framework established by the parkin literature predicting a direct downstream function within a highly conserved cell death pathway.
Soon after the cloning of the PARKIN gene, numerous studies shed light on the function of parkin. Parallel efforts from many groups also revealed that parkin is dramatically sensitive to stress-induced mis-folding, aggregation and inactivation, with some studies reporting alterations in parkin solubility in diseased human brain tissue.5 However, it is difficult to reconcile the potent and widely accepted protective effects of parkin with the fact that at the biochemical level parkin is rapidly inactivated or made insoluble during cell stress. In the rational design of a pro-survival protein, many considerations would be made. At the primary sequence level, for example, highly reactive and modification-prone cysteines would be limited or excluded altogether; parkin has 35 cysteine residues. In terms of secondary structure, a stress-tolerant conformation would be desirable but is not found in parkin. The paradox of parkin-induced protection against stress and stress-induced parkin dysfunction inspired us to ask how parkin could promote cell survival in spite of an acute, stress-induced loss of parkin function. Our most recent data provide insight. We propose that a parkin-dependent reduction in mitochondrial Bax and the reduced propensity for apoptosis perpetuate a pro-survival state, even in the absence of parkin itself. Akin to how receptor densities establish long-term changes in the sensitivity of neurons to depolarization, by reducing mitochondrial Bax, parkin may establish a “desensitized state” within the intrinsic apoptotic pathway that persists long after parkin has been inactivated by stress.
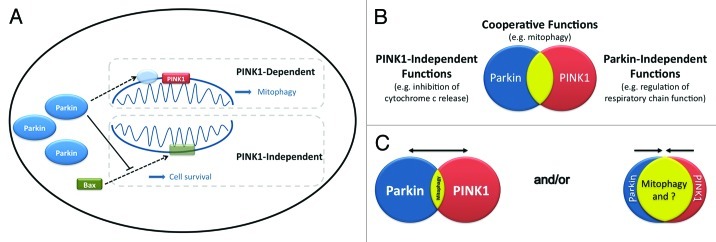
Parkin may not always act alone but rather functionally interact with another autosomal recessive PD gene product, PINK1. The existence of a discrete PINK1-Parkin pathway and the upstream placement of PINK1 was initially proposed by two studies demonstrating that parkin- and PINK1-null Drosophila displayed complementary phenotypes, and the ectopic expression of parkin rescued PINK1-null flies, but not vice versa.6,7 We now know that mitochondrial membrane depolarization induces the rapid recruitment of parkin to the mitochondria in a PINK1-dependent manner, where it facilitates mitochondrial degradation (mitophagy).8 PINK1 appears to serve as a powerful homing beacon for parkin, as PINK1 mis-localization within the cell can result in the aberrant co-localization of parkin,9 underscoring the commanding role of PINK1 in this function of parkin. However, the requirement of PINK1 for the earliest steps in parkin-induced mitophagy argues that this system cannot account for the parkin-dependent rescue of flies lacking PINK1. Perhaps more likely, the initial observation of parkin rescue involved a process distinct from parkin-induced mitophagy, a PINK1-independent parkin pathway, such as the parkin-dependent inactivation of a Bax-like protein3 and regulation of cytochrome c release.4 Therefore, available evidence would suggest the existence of at least two distinct pathways of parkin function (Fig. 1A), one requiring PINK1 (mitophagy) and the other being PINK1-independent (regulation of cytochrome c release). In our view, these two processes are not mutually exclusive, but rather likely coexist within the cell. Interestingly, multiple groups report a parkin-independent function of PINK1 in the regulation of electron transport chain activity, consistent with this perspective.10
Figure 1. The cooperative and independent functions of parkin and PINK1. (A) Parkin plays a role in two distinct mitochondrial pathways, a PINK1-dependent process initiating mitophagy, and a PINK1-independent pathway regulating the apoptotic release of cytochrome c. (B) Parkin and PINK1 each possess independent roles in mitochondrial biology, such as regulation of cytochrome c release and electron transport chain function, respectively, but also work together in mitophagy. (C) However, the relative importance of their individual and cooperative roles in the cell is not known and deserving of greater attention.
It is evident that parkin may promote cell survival through multiple avenues, while mitochondria remain at the epicenter of even this more expanded view. Therefore, we envisage a partial overlap model for parkin and PINK1 function (Fig. 1B). However, the relative importance of the cooperative PINK1-Parkin pathway and the individual roles of each of these disease-linked proteins may differ according to tissue and/or cell type (Fig. 1C). Certainly, their respective contributions to mitochondrial biology and cell health remain fertile ground for further debate and discovery.
Acknowledgments
Supported by NIH grant NS065013.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21261
References
- 1.Dauer W, et al. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Kitada T, et al. Nature. 1998;392:605–8. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 3.Johnson BN, et al. Proc Natl Acad Sci USA. 2012;109:6283–8. doi: 10.1073/pnas.1113248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AK, et al. Hum Mol Genet. 2009;18:4317–28. doi: 10.1093/hmg/ddp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosco DA, et al. Cold Spring Harb Perspect Biol. 2011;3:a007500. doi: 10.1101/cshperspect.a007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark IE, et al. Nature. 2006;441:1162–6. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 7.Park J, et al. Nature. 2006;441:1157–61. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 8.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125:795–9. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazarou M, et al. Dev Cell. 2012;22:320–33. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morais VA, et al. J Alzheimers Dis. 2010;20(Suppl 2):S255–63. doi: 10.3233/JAD-2010-100345. [DOI] [PubMed] [Google Scholar]