Abstract
Comment on: Feng CN, et al. Cell Cycle 2012; 11:2486-94.
Approximately 20% of human breast cancers overexpress the HER2 gene, a state that is associated with an aggressive clinical course with high metastatic propensity. The development of HER2 targeting agents such as trastuzumab have greatly improved the outcome for these patients, representing one of the most successful molecularly targeted therapies. Although HER2 protein overexpression usually results from gene amplification, this is not always the case, and it is unclear what accounts for increased HER2 protein expression in these cases. Reporting in Cell Cycle, Feng et al. demonstrated a significant association of Lin28 expression with the expression of HER2, both of which were associated with poor clinical outcome.1 Lin28 was originally described as a gene regulating developmental timing in worms.2 Lin28 directly modulates the expression of a number of genes post-transcriptionally.3 Feng et al. demonstrated that Lin28 binds to target sites present in HER2 mRNA, leading to enhanced HER2 protein expression. This confirmed a prior study which reported that Lin28 was overexpressed in HER2-positive breast cancers.4
Lin28 is known to be an important stem cell regulatory gene. In fact, it is one of four factors sufficient to reprogram human somatic cells into induced pluripotent stem cells.5 More recently, Lin28 has been found to have profound effects on normal and malignant stem cells through the posttranscriptional downregulation of the microRNA Let-7. The inverse regulatory relationship between Lin28 and Let-7 microRNA has been well documented during normal development, cell metabolism and tumorigenicity. On the one hand, Let-7 negatively regulates stemness by repressing self-renewal and promoting differentiation in normal development and cancer, while Lin28 is expressed at higher levels in undifferentiated cells and decreases during cellular differentiation. Lin28 has been reported to function as an oncogene that promotes cellular transformation, an effect that was abrogated by expression of Let-7.6 In breast cancer cells, endogenous levels of Let-7 mRNAs were found to be markedly reduced in mammospheres and in cancer stem cells that displayed a CD44+/CD24- phenotype, while levels were significantly increased in more differentiated cells forming the tumor bulk.7 Expression of Let-7 in breast cancer stem cells inhibited their capacity for self-renewal and induced differentiation, while downregulation of Let-7 in differentiated cells promoted their de-differentiation and acquisition of CSC properties. Lin28 and Let-7 have also been shown to be involved in sustaining an inflammatory feedback loop involving interleukin-6 and NFκB, a loop that drives the breast cancer stem cell population (see Fig. 1).
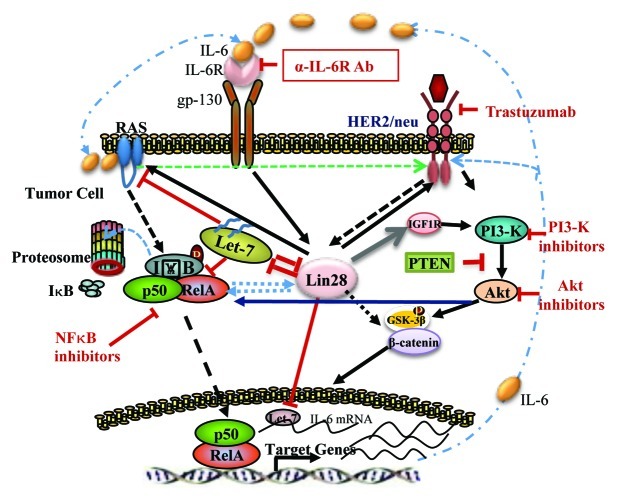
Figure 1.
Interaction of Lin28 and HER2 signaling pathways. Lin28 and HER2 interact to regulate cancer stem cells. Potential inhibitors of these pathways are shown.
Our laboratory and others have also implicated the HER2 gene in the regulation of breast cancer stem cells. This occurs through activation of the Wnt pathway through GSK3B and β-catenin phosphorylation.8 Overexpression of HER2 in normal mammary epithelial cells and mammary carcinomas increases the population of CSCs, a state associated with increased CSC self-renewal and tumorigenicity. Loss of PTEN function in these cells generates a trastuzumab-resistant CSC population through activation of an IL-6 inflammatory loop involving NFκB. This is associated with generation of an epithelial-mesenchymal transition (EMT) invasive phenotype. NFκB plays an essential role in HER2 induced oncogenesis by providing signals that maintain mammary tumor-initiating cells.9 The observation that HER2 can regulate NFκB,10 and that NFκB can regulate Lin28,11 coupled with the report of Feng et al. that Lin28, in turn, regulates HER2 suggests that these two important stem cell regulatory genes may interact in a positive feedback loop (see Fig. 1).
Lin28 is one of the vital factors in the networks of different regulatory elements that may be responsible for the elevated HER2 expression in a subpopulation of cells in HER2 non-amplified tumors. These networks also involve multiple CSC regulators such as HER2, Akt, NFκB and IL-6. As illustrated in Figure 1, targeting these pathways may reduce the CSC population improving patient outcomes.
Disclosures
Max S. Wicha is a scientific advisor and holds equity in Oncomed Pharmaceuticals and is a scientific advisor for Veristem.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/21395
References
- 1.Feng CN, et al. Cell Cycle. 2012;11:2486–94. doi: 10.4161/cc.20893. [DOI] [PubMed] [Google Scholar]
- 2.Moss EG, et al. Cell. 1997;88:637–46. doi: 10.1016/S0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 3.Lei XX, et al. Nucleic Acids Res. 2012;40:3574–84. doi: 10.1093/nar/gkr1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piskounova E, et al. Cell. 2011;147:1066–79. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, et al. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan SR, et al. Nat Genet. 2009;41:843–8. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu F, et al. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Korkaya H, et al. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, et al. Proc Natl Acad Sci USA. 2007;104:15852–7. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merkhofer EC, et al. Oncogene. 2010;29:1238–48. doi: 10.1038/onc.2009.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iliopoulos D, et al. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]