Abstract
Nitric oxide (NO) is an important signaling molecule in animals and plants. In mammals, NO is produced from Arg by the enzyme NO synthase. In plants, NO synthesis from Arg using an NO synthase–type enzyme and from nitrite using nitrate reductase has been demonstrated previously. The data presented in this report strongly support the hypothesis that plant tissues also synthesize NO via the nonenzymatic reduction of apoplastic nitrite. As measured by mass spectrometry or an NO-reactive fluorescent probe, Hordeum vulgare (barley) aleurone layers produce NO rapidly when nitrite is added to the medium in which they are incubated. NO production requires an acid apoplast and is accompanied by a loss of nitrite from the medium. Phenolic compounds in the medium can increase the rate of NO production. The possible significance of apoplastic NO production for germinating grain and for plant roots is discussed.
INTRODUCTION
Nitric oxide (NO) is a gaseous free radical that diffuses readily through biomembranes. The half-life of NO in biological tissues is estimated to be <6 s (Thomas et al., 2001). This short half-life reflects the highly reactive nature of NO. NO reacts directly with metal complexes and other radicals and indirectly as a reactive nitrogen oxide species with DNA, proteins, and lipids (Wink and Mitchell, 1998). In animals, NO is a signal transduction element that functions in many tissues and interacts with multiple target compounds. The roles of NO in plants may be equally diverse. The participation of NO in plant disease resistance pathways has been reported on several occasions (Delledonne et al., 1998; Durner et al., 1998; Clarke et al., 2000; Foissner et al., 2000), and a role for NO in the abscisic acid (ABA) signal transduction pathway leading to stomatal closure also has been demonstrated (Neill et al., 2002). Other data, based largely on experiments in which plants or plant tissues were treated with NO donors, suggest roles for NO in oxidative stress, growth and development, mitochondrial activity, and programmed cell death (Beligni and Lamattina, 2001; Yamasaki et al., 2001; Beligni et al., 2002).
Because NO is a potent effecter of biological processes, renewed attention has been given to the mechanism of NO synthesis in plants. In animal systems, data from numerous sources have shown that NO is synthesized predominantly by the enzyme NO synthase (NOS). NOS converts l-Arg into l-citrulline in a NADPH-dependent reaction that releases one molecule of NO for each molecule of l-Arg. Assays for Arg-to-citrulline conversion and compounds that inhibit mammalian NOS have been used on several occasions to demonstrate that NO synthesis by a NOS-type enzyme also occurs in plants (Durner et al., 1998; Foissner et al., 2000). Recent evidence suggests that the NOS involved in plant defense responses is a variant P protein of the Gly decarboxylase complex (Chandok et al., 2003). However, it is clear that plants also synthesize NO from nitrite. Nitrite-dependent NO production has been observed for Glycine max (soybean) (Delledonne et al., 1998) and Helianthus annuus (sunflower) (Rockel et al., 2002), the algae Chlamydomonas reinhardtii (Sakihama et al., 2002) and Scenedesmus obliquus, and the cyanobacterium Anabaena doliolum (Mallick et al., 1999). In some if not all of these cases, NO is likely to be produced by nitrate reductase (NR), which reduces nitrate to nitrite and can further reduce nitrite to NO.
A nonenzymatic mechanism for the synthesis of NO from 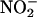 under acidic conditions is described by the reaction scheme shown in Equation 1 (Yamasaki, 2000):
under acidic conditions is described by the reaction scheme shown in Equation 1 (Yamasaki, 2000):
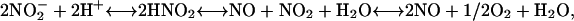 |
where nitrite is protonated to form nitrous acid (HNO2) in a freely reversible reaction that is favored at low pH. Through a series of reactions, two molecules of HNO2 interact and give rise to NO and nitrogen dioxide (NO2), and NO2 can be converted to NO plus 1/2O2. Reducing agents such as ascorbic acid (Yamasaki, 2000) and, as we show below, some phenolics can accelerate the rate of NO formation. Because the pKa for HNO2 is ∼3.2, this mechanism for NO synthesis is unlikely to occur in the cytosol of plant cells because the pH of the cytoplasm effectively prevents the formation of HNO2 (Yamasaki, 2000). It seemed possible to us, however, that NO could be produced in the apoplast by this mechanism given that (1) the apoplast is acidic (Drozdowicz and Jones, 1995; Yu et al., 2000; Fasano et al., 2001); (2) nitrite is present in the apoplast of some plant tissues, and nitrite-permeable transporters are found on the plasma membrane of plant cells (Aslam et al., 1994); and (3) the redox status of the apoplast is regulated, and antioxidants in their reduced form are found in the apoplast (Horemans et al., 2000).
We tested the hypothesis that NO is synthesized nonenzymatically by the chemical reduction of 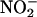 in the apoplast of Hordeum vulgare cv Himalaya (barley) aleurone layers. Aleurone layers are well suited for these experiments because they acidify the cell wall solution or incubation medium to pH 3 to 4 (Macnicol and Jacobsen, 1992; Drozdowicz and Jones, 1995), and the adjacent testa contains proanthocyanidins (Aastrup et al., 1984) that could act as antioxidants. Furthermore, H. vulgare aleurone layers contain nitrate-inducible NR, and
in the apoplast of Hordeum vulgare cv Himalaya (barley) aleurone layers. Aleurone layers are well suited for these experiments because they acidify the cell wall solution or incubation medium to pH 3 to 4 (Macnicol and Jacobsen, 1992; Drozdowicz and Jones, 1995), and the adjacent testa contains proanthocyanidins (Aastrup et al., 1984) that could act as antioxidants. Furthermore, H. vulgare aleurone layers contain nitrate-inducible NR, and 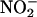 release from aleurone layers has been measured (Ferrari and Varner, 1970).
release from aleurone layers has been measured (Ferrari and Varner, 1970).
RESULTS
NO Delays Programmed Cell Death in H. vulgare Aleurone Layers
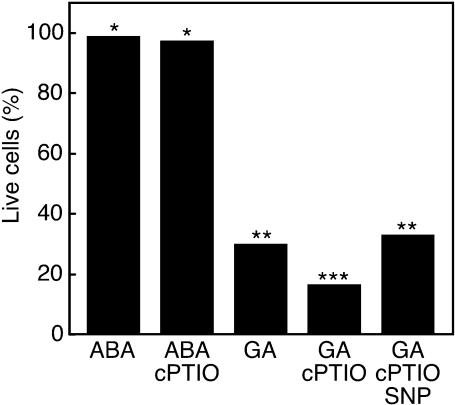
We showed previously that the NO donors sodium nitroprusside (SNP) and S-nitroso-N-acetylpenicillamine delayed gibberellin (GA)-induced programmed cell death (PCD) in H. vulgare aleurone layers (Beligni et al., 2002). In these cells, death is promoted by reactive oxygen species (Bethke and Jones, 2001; Fath et al., 2001), and NO delays death by acting as an antioxidant (Beligni et al., 2002). The data in Figure 1 extend these observations. When aleurone layers were treated with GA, 70% of cells were dead 48 h after hormone treatment, but virtually no cells were dead when layers were treated with ABA. Addition of the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3 oxide (cPTIO) shortly before the onset of PCD accelerates the rate of PCD in H. vulgare aleurone cells. As seen in Figure 1, when 200 μM cPTIO was added to GA-treated H. vulgare aleurone layers 18 h after hormone treatment, <17% of the cells were alive 48 h after hormone treatment. This is a significant difference with P = 0.02 in a Student's t test. This effect of cPTIO on PCD is likely to be specific for NO because adding the NO donor SNP (100 μM) along with cPTIO does not delay PCD in GA-treated layers, and cPTIO does not promote death of ABA-treated cells that are not undergoing PCD (Figure 1). These data further support our previous finding that NO is an effective modulator of the cell death program in H. vulgare aleurone cells. They also strongly suggest that aleurone layers produce NO and that endogenous NO plays a role in GA-induced PCD.
Figure 1.
The NO Scavenger cPTIO Promotes PCD in H. vulgare Aleurone Layers.
Freshly prepared aleurone layers were treated with ABA or GA. cPTIO (200 μM) alone or with the NO donor SNP (100 μM) was added to some layers 18 h later. Data represent the mean of ∼300 cells in each of 12 aleurone layers. The data are pooled from two independent experiments, both of which showed similar results. Means that are significantly different at the level of P = 0.05 in a Student's t test are indicated by different numbers of asterisks.
Aleurone Layers Produce NO from Nitrite
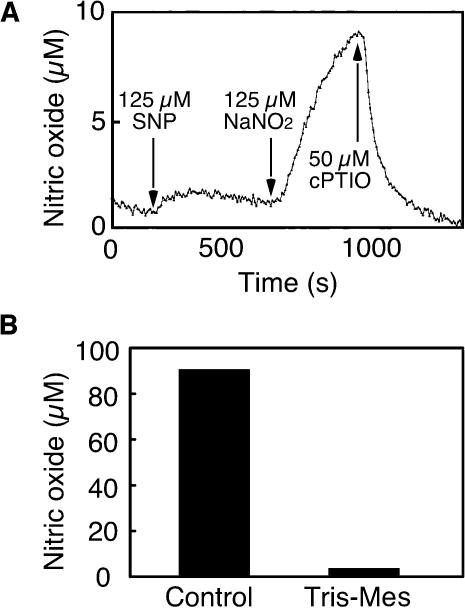
Because NO donors delayed PCD (Beligni et al., 2002) and the NO scavenger cPTIO accelerated PCD in H. vulgare aleurone layers (Figure 1), we examined further the potential for aleurone layers to synthesize NO. For the experiments described in Figures 2 and 3, NO production was measured using mass spectrometry. In these experiments, the sample cuvette contained a gas-permeable diaphragm, and the gases that diffused through this diaphragm were analyzed. When isolated aleurone layers that had not been incubated in solution or treated with a hormone were placed into a sample cuvette containing 20 mM CaCl2, a steady baseline for the signal corresponding to the mass of NO was established within a few minutes. To demonstrate that the mass spectrometer was suitable for measuring NO, the NO donor SNP (125 μM) was added to the sample cuvette. As seen in Figure 2A, there was a nearly instantaneous increase in the NO signal. To show that aleurone layers can produce NO from 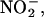 125 μM NaNO2 was added to the cuvette at the time indicated by the arrow in Figure 2A. NO synthesis was detected ∼15 s after addition of
125 μM NaNO2 was added to the cuvette at the time indicated by the arrow in Figure 2A. NO synthesis was detected ∼15 s after addition of 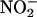 (Figure 2A), and peak production occurred within 8 min (data not shown). Addition of the NO scavenger cPTIO (50 μM) to the sample cuvette abolished the NO signal resulting from both SNP and
(Figure 2A), and peak production occurred within 8 min (data not shown). Addition of the NO scavenger cPTIO (50 μM) to the sample cuvette abolished the NO signal resulting from both SNP and  (Figure 2A). These data demonstrate that H. vulgare aleurone layers can make NO from
(Figure 2A). These data demonstrate that H. vulgare aleurone layers can make NO from 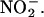 Similar experiments were done with whole H. vulgare grain, grain with the embryo removed, and whole grain split longitudinally. In each case, NO production was observed ∼15 s after adding
Similar experiments were done with whole H. vulgare grain, grain with the embryo removed, and whole grain split longitudinally. In each case, NO production was observed ∼15 s after adding 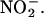 This lag was the same as that observed for our calibration procedure and reflects a delay inherent in the experimental setup. Significantly, this production of NO from
This lag was the same as that observed for our calibration procedure and reflects a delay inherent in the experimental setup. Significantly, this production of NO from 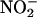 was pH dependent. Adding 2 mM Tris-Mes, pH 5.8, to the cuvette solution before adding 800 μM NaNO2 prevented NO synthesis (Figure 2B).
was pH dependent. Adding 2 mM Tris-Mes, pH 5.8, to the cuvette solution before adding 800 μM NaNO2 prevented NO synthesis (Figure 2B).
Figure 2.
Synthesis of NO by H. vulgare Aleurone Layers as Measured by Mass Spectrometry.
(A) Ten aleurone layers were placed in 2 mL of 20 mM CaCl2. At the times indicated, 125 μM SNP, 125 μM NaNO2, and 50 μM cPTIO were added. Note the increase in NO signal after SNP and NaNO2 additions and the quenching of both signals by cPTIO.
(B) Peak NO production after addition of 800 μM NaNO2 to 10 aleurone layers in 2 mL of 20 mM CaCl2 (control) or 2 mL of 2 mM Tris-Mes, pH 5.8.
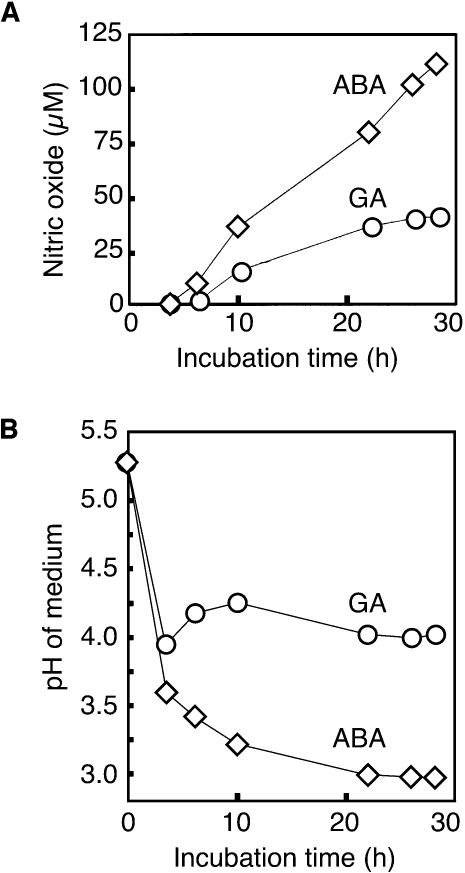
Figure 3.
NO Production by Aleurone Layer Medium.
(A) Incubation medium from aleurone layers treated with GA or ABA for the indicated times was diluted 1:10 and placed in the sample cuvette of a mass spectrometer. NaNO2 (800 μM) was added, and the peak NO signal was determined.
(B) pH of the medium in (A).
The NO production reported in Figure 2A could result from synthesis in the apoplast, the symplast, or both. To determine if apoplastic synthesis of NO could be demonstrated, we added 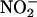 to media in which aleurone layers had been incubated for various lengths of time. For these experiments with isolated aleurone layers, the cell wall solution is in approximate equilibrium with the incubation medium. The incubation medium, therefore, can be regarded as an extended apoplastic space. Aleurone layers were incubated with either 5 μM ABA or 5 μM GA, and media were removed at intervals up to 28 h of incubation. When 800 μM NaNO2 was added to 2 mL of incubation media that had been diluted 1:10 with 20 mM CaCl2, a large increase in the NO signal was observed ∼15 s later. The peak NO signal for each treatment at each time is plotted against incubation time in Figure 3A. For both hormone treatments, NO was produced from the incubation medium when
to media in which aleurone layers had been incubated for various lengths of time. For these experiments with isolated aleurone layers, the cell wall solution is in approximate equilibrium with the incubation medium. The incubation medium, therefore, can be regarded as an extended apoplastic space. Aleurone layers were incubated with either 5 μM ABA or 5 μM GA, and media were removed at intervals up to 28 h of incubation. When 800 μM NaNO2 was added to 2 mL of incubation media that had been diluted 1:10 with 20 mM CaCl2, a large increase in the NO signal was observed ∼15 s later. The peak NO signal for each treatment at each time is plotted against incubation time in Figure 3A. For both hormone treatments, NO was produced from the incubation medium when 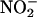 was added. Medium from ABA-treated layers gave a larger signal than that from GA-treated layers, and this most likely reflects the difference in pH between the media. As shown in Figure 3B, ABA-treated layers rapidly acidified their incubation medium to as low as pH 3.0, whereas GA-treated layers acidified their incubation medium to pH ∼4.0.
was added. Medium from ABA-treated layers gave a larger signal than that from GA-treated layers, and this most likely reflects the difference in pH between the media. As shown in Figure 3B, ABA-treated layers rapidly acidified their incubation medium to as low as pH 3.0, whereas GA-treated layers acidified their incubation medium to pH ∼4.0.
NO also was produced from 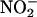 when
when 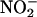 was added to media that had been passed through a 10,000 D size exclusion filter, suggesting that enzymes in the media were unlikely to be the source of NO (data not shown). NO production was not observed when
was added to media that had been passed through a 10,000 D size exclusion filter, suggesting that enzymes in the media were unlikely to be the source of NO (data not shown). NO production was not observed when 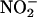 was added to medium that had not been used to incubate layers (data not shown). These data suggest that H. vulgare aleurone layers add components to the incubation medium that are required for NO synthesis from
was added to medium that had not been used to incubate layers (data not shown). These data suggest that H. vulgare aleurone layers add components to the incubation medium that are required for NO synthesis from 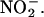 One of these is likely to be protons because buffering incubation media with Tris-Mes, pH 5.8, greatly reduced the NO signal.
One of these is likely to be protons because buffering incubation media with Tris-Mes, pH 5.8, greatly reduced the NO signal.
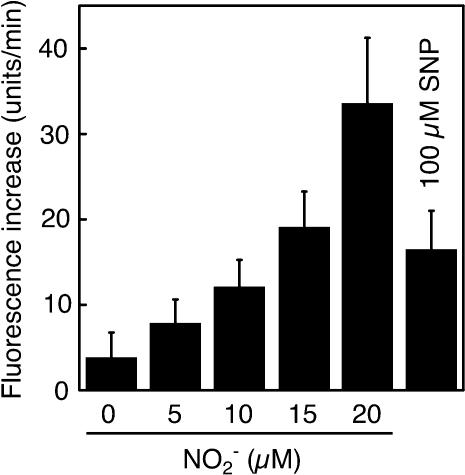
As an independent measurement technique, we used the NO-reactive fluorescent probe 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) (Kojima et al., 1999) to measure NO production from aleurone layer medium. The reaction of DAF-FM with NO but not 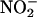 results in a large increase in fluorescence from the probe. As seen in Figure 4, low concentrations of nitrite (5 to 20 μM) resulted in a concentration-dependent increase in fluorescence from DAF-FM when added to aleurone layer medium. Addition of SNP (100 μM) in the absence of
results in a large increase in fluorescence from the probe. As seen in Figure 4, low concentrations of nitrite (5 to 20 μM) resulted in a concentration-dependent increase in fluorescence from DAF-FM when added to aleurone layer medium. Addition of SNP (100 μM) in the absence of 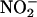 resulted in a similar increase in fluorescence. These latter data confirm that DAF-FM reacts with NO when in aleurone layer media, and they suggest that low concentrations of
resulted in a similar increase in fluorescence. These latter data confirm that DAF-FM reacts with NO when in aleurone layer media, and they suggest that low concentrations of 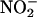 are sufficient to produce biologically significant amounts of NO.
are sufficient to produce biologically significant amounts of NO.
Figure 4.
Nitrite-Dependent NO Production from Aleurone Layer Media.
Nitrite at 0 to 20 μM was added to medium in which GA-treated H. vulgare aleurone layers were incubated for 18 h. SNP (100 μM) was added to the same medium without added nitrite. The rate of NO production was determined using the NO-reactive fluorescent probe DAF-FM. Data are means +sd of media from six flasks of aleurone layers and are representative of two independent replicates.
Phenolics Accumulate in the Apoplast of H. vulgare Aleurone Cells
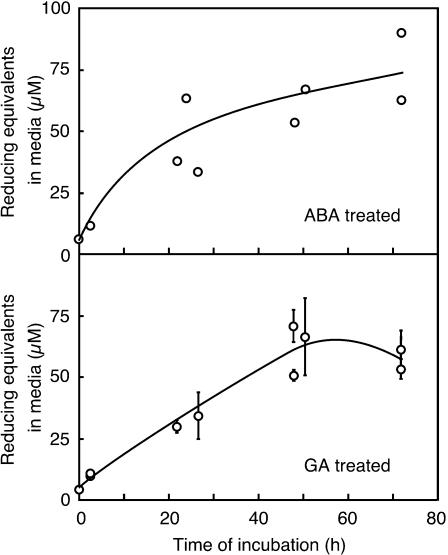
The data in Figures 3 and 4 show that media in which H. vulgare aleurone layers have been incubated have the capacity to produce large amounts of NO from 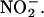 Because compounds that reduce HNO2 promote the synthesis of NO at low pH, we investigated the possibility that low molecular weight reductants or antioxidants were present in the incubation medium. The assay described by Omaye et al. (1979) measures the ability of a sample to reduce an iron-dipyridyl complex. This assay has been used to measure reduced ascorbate in plant extracts but is not specific for ascorbate. As shown in Figure 5, incubation media from ABA- or GA-treated aleurone layers accumulate compounds capable of reducing the iron-dipyridyl complex. We used purified ascorbic acid as a reference to determine the number of reducing equivalents present in the media at various times after hormone addition. For this comparison, 1 μM reducing equivalents reduce the iron-dipyridyl complex to the same extent as 1 μM ascorbic acid. Reducing equivalents accumulate in the media from both GA- and ABA-treated layers between zero time and 48 h. Medium from ABA-treated layers contains 30 to 60 μM reducing equivalents 24 h after hormone treatment, and this increases to 50 to 60 μM 48 h after hormone addition. The medium around GA-treated layers contains a comparable amount of reducing equivalents, with ∼35 μM at 24 h and 50 to 70 μM at 48 h. The volume of the apoplastic space in 10 H. vulgare grains is ∼5 to 10% of the incubation medium volume for 10 aleurone layers. Because of this, the concentration of reducing equivalents in the apoplast of whole grain is likely to be substantially higher than that in the incubation media.
Because compounds that reduce HNO2 promote the synthesis of NO at low pH, we investigated the possibility that low molecular weight reductants or antioxidants were present in the incubation medium. The assay described by Omaye et al. (1979) measures the ability of a sample to reduce an iron-dipyridyl complex. This assay has been used to measure reduced ascorbate in plant extracts but is not specific for ascorbate. As shown in Figure 5, incubation media from ABA- or GA-treated aleurone layers accumulate compounds capable of reducing the iron-dipyridyl complex. We used purified ascorbic acid as a reference to determine the number of reducing equivalents present in the media at various times after hormone addition. For this comparison, 1 μM reducing equivalents reduce the iron-dipyridyl complex to the same extent as 1 μM ascorbic acid. Reducing equivalents accumulate in the media from both GA- and ABA-treated layers between zero time and 48 h. Medium from ABA-treated layers contains 30 to 60 μM reducing equivalents 24 h after hormone treatment, and this increases to 50 to 60 μM 48 h after hormone addition. The medium around GA-treated layers contains a comparable amount of reducing equivalents, with ∼35 μM at 24 h and 50 to 70 μM at 48 h. The volume of the apoplastic space in 10 H. vulgare grains is ∼5 to 10% of the incubation medium volume for 10 aleurone layers. Because of this, the concentration of reducing equivalents in the apoplast of whole grain is likely to be substantially higher than that in the incubation media.
Figure 5.
Reducing Equivalents Accumulate in the Incubation Medium around ABA- and GA-Treated H. vulgare Aleurone Layers.
Media were assayed for their ability to reduce an iron-dipyridyl complex. Reduced ascorbate was used as a standard. Data points are means ±sd for four flasks of aleurone layers, and the data are pooled from three independent experiments.
Ascorbate and glutathione are important reductants in plants, and ascorbate is present in the apoplast of plant leaves (Horemans et al., 2000). We assayed incubation media for the presence of ascorbate and glutathione using both enzyme-coupled assays and an HPLC system equipped with an electrochemical detector. These data are presented in Table 1. We were unable to detect ascorbate in the media using either method, and only trace amounts of glutathione were present. This makes it unlikely that either ascorbate or glutathione participate in the reduction of nitrous acid to NO in the apoplast of aleurone layers.
Table 1.
Concentration of Ascorbate and Glutathione in the Incubation Medium of H. vulgare Aleurone Layers as Determined by Enzyme-Coupled Assays and HPLC
| Concentration (μM)
|
Method
|
||||
|---|---|---|---|---|---|
| Time | ABA-Treated | GA-Treated | Enzyme Assay | ECD | |
| Total ascorbate | 21 h | 0.0 ± 0.0 | 0.0 ± 0.0 | * | |
| 48 h | 0.0 ± 0.0 | 0.0 ± 0.0 | * | ||
| Reduced ascorbate | 24 h | 0.0 ± 0.0 | 0.0 ± 0.0 | * | |
| Total glutathione | 24 h | 0.8 ± 0.0 | 0.8 ± 0.1 | * | |
| 48 h | 0.1 ± 0.1 | 0.5 ± 0.2 | * | ||
| GSH | 24 h | 0.0 ± 0.0 | 0.5 ± 0.2 | * | |
| GSSH | 24 h | 0.5 ± 0.6 | 0.7 ± 0.5 | * | |
Layers were incubated with either ABA or GA for the times indicated. The amounts of total ascorbate (reduced plus oxidized) and total glutathione were determined using enzyme-coupled assays. The amounts of reduced ascorbate, reduced glutathione (GSH), and fully oxidized glutathione (GSSH) were determined using an HPLC system equipped with an electrochemical detector (ECD). Each value is the mean ±sd of four flasks of aleurone layers.
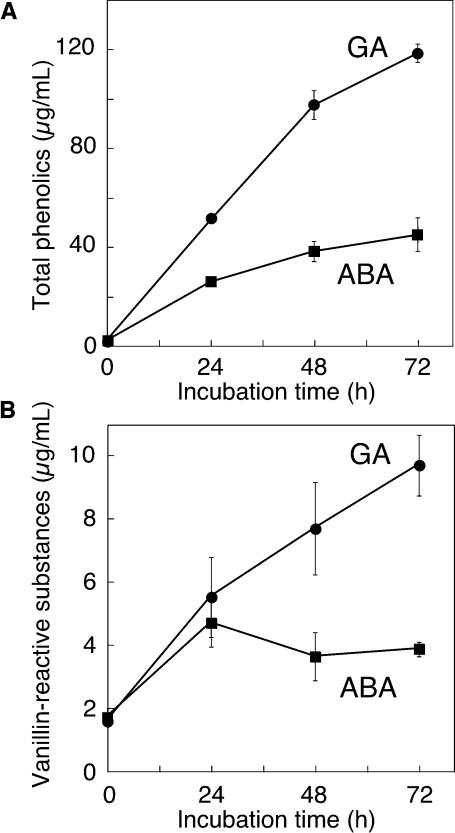
Phenolics also can function as reductants or antioxidants (Rice-Evans et al., 1996), and phenolic compounds are present in the apoplast of plant tissues and are especially abundant in the bran (embryo, aleurone, and testa/pericarp) and malt of H. vulgare (Jende-Strid and Moller, 1981; Aastrup et al., 1984; Goupy et al., 1999). As shown in Figure 6A, total phenolics accumulate in the media of ABA- and GA-treated aleurone layers to relatively high amounts. When layers were treated with either ABA or GA, total phenolics in the incubation media increased for at least 3 d. The rate of increase was greater in medium around GA-treated layers than around ABA-treated layers. Two days after hormone treatment, total phenolics were present at 100 μg/mL in medium from GA-treated layers but were present at <40 μg/mL in medium from ABA-treated layers. Much of this difference results from the accumulation of ferulic acid and ferulic acid conjugates that are abundant in the aleurone cell wall (Gubler and Ashford, 1985).
Figure 6.
Phenolic Compounds Accumulate in the Incubation Medium around ABA- and GA-treated H. vulgare Aleurone Layers.
Incubation media were assayed for the presence of total phenolics using Folin and Ciocalteu's phenol reagent (A) and for vanillin-reactive flavanols using vanillin (B). Data points in (A) are means ±sd for four (0 h) or eight flasks of aleurone layers. Data points in (B) are means ±sd of 4 (0 h), 5 (72 h), or 10 (24 and 48 h) flasks of aleurone layers.
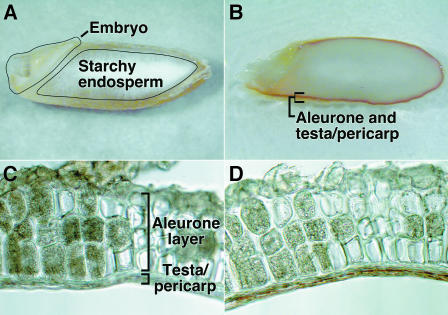
A specific class of phenolics reacts with vanillin and these include (+)-catechin, (−)-epicatechin, and procyanidins B1, B2, and C1, but not ferulic acid. These vanillin-reactive molecules are a subset of the phenolics with moderate-to-high antioxidant capabilities. Proanthocyanidins and catechin are present in H. vulgare grain (Jende-Strid and Moller, 1981; Aastrup et al., 1984), and vanillin has been used as a histological stain for proanthocyanidins in grain from several varieties of H. vulgare (Aastrup et al., 1984). Figure 7 shows that vanillin-reactive materials, which stain deep red, are present in the testa of H. vulgare cv Himalaya grain. Vanillin staining was restricted to a thin band of testa immediately outside the aleurone layer. This is seen clearly in Figures 7C and 7D, in which the same section is shown before (Figure 7C) and after (Figure 7D) vanillin staining. Vanillin-reactive materials were not apparent in the scutellum and embryo axis (Figure 7B).
Figure 7.
Proanthocyanidins in H. vulgare Grain Are Concentrated in the Testa.
Imbibed H. vulgare grain were sectioned longitudinally ([A] and [B]), and isolated aleurone layers were sectioned transversely ([C] and [D]). The deep-red peripheral band of vanillin staining in (B) is lacking in the unstained grain (A). The same section through the aleurone layers and attached testa is shown before (C) and after (D) staining. Note that only the region immediately outside of the aleurone layer is stained with vanillin.
Although initially concentrated in the testa, vanillin-reactive substances accumulate in aleurone layer incubation media. As shown in Figure 6B, vanillin-reactive substances were present in the media around both ABA- and GA-treated layers and constitute ∼10% of total phenolics. Although vanillin-reactive substances accumulated for 3 d in the medium around GA-treated layers and reached a concentration of 10 μg/mL of medium, they increased for only 1 d in ABA media and then remained relatively constant at 4 μg/mL.
Phenolics Can Increase NO Production from Nitrite
We used an artificial medium and the NO-reactive probe DAF-FM to understand more clearly the potential for NO production from 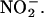 The rate at which the fluorescence from a DAF-FM–containing solution increases depends both on the rate at which NO is produced and on the rate at which NO reacts with molecules in the solution other than DAF-FM. Phenolics could participate in either of these processes. Catechin was used for these experiments as a model phenolic that is present in H. vulgare. The data in Figures 5 and 6 are likely to be underestimates for the concentrations of reducing equivalents (∼50 μM) and phenolics that could act as reductants (∼10 μg/mL) that are found in H. vulgare grain. Physiological concentrations of phenolics are likely to be at least 10 times higher because the volume of the incubation medium is at least 10 times that of the equivalent apoplastic space. Furthermore, a concentration gradient is likely to exist within the grain, with the highest concentration near the testa and aleurone. Phenolics are most abundant in these tissues in dry and freshly imbibed grain (Figure 7; Gubler and Ashford, 1985). Because of this, we used catechin at two concentrations: 34 μM (10 μg/mL) and 340 μM (100 μg/mL). The latter is likely to approximate more closely the in vivo condition.
The rate at which the fluorescence from a DAF-FM–containing solution increases depends both on the rate at which NO is produced and on the rate at which NO reacts with molecules in the solution other than DAF-FM. Phenolics could participate in either of these processes. Catechin was used for these experiments as a model phenolic that is present in H. vulgare. The data in Figures 5 and 6 are likely to be underestimates for the concentrations of reducing equivalents (∼50 μM) and phenolics that could act as reductants (∼10 μg/mL) that are found in H. vulgare grain. Physiological concentrations of phenolics are likely to be at least 10 times higher because the volume of the incubation medium is at least 10 times that of the equivalent apoplastic space. Furthermore, a concentration gradient is likely to exist within the grain, with the highest concentration near the testa and aleurone. Phenolics are most abundant in these tissues in dry and freshly imbibed grain (Figure 7; Gubler and Ashford, 1985). Because of this, we used catechin at two concentrations: 34 μM (10 μg/mL) and 340 μM (100 μg/mL). The latter is likely to approximate more closely the in vivo condition.
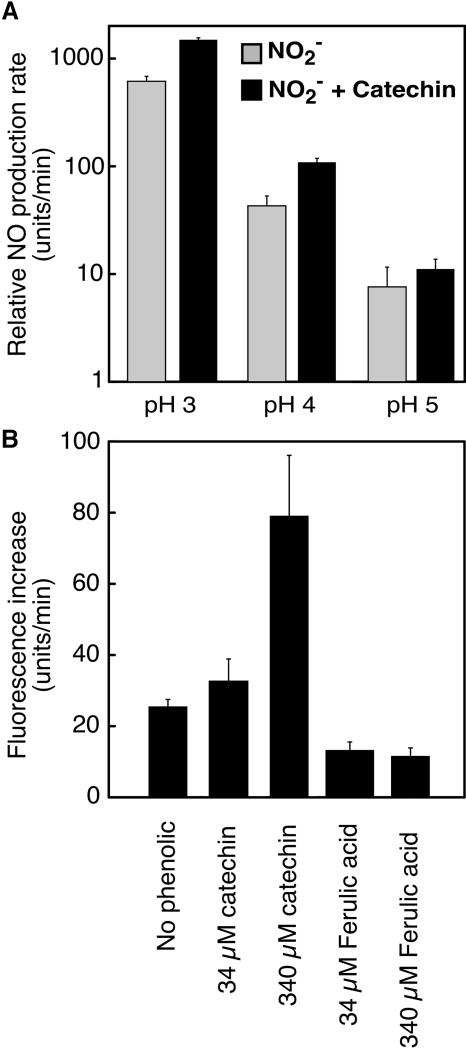
As expected, the production of NO from 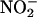 was very sensitive to the pH of the medium. As shown in Figure 8A on a log scale, an increase in pH of one unit decreased net NO production rates by approximately one order of magnitude. Catechin stimulated the production of NO from
was very sensitive to the pH of the medium. As shown in Figure 8A on a log scale, an increase in pH of one unit decreased net NO production rates by approximately one order of magnitude. Catechin stimulated the production of NO from 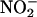 at pH 3.0 to 5, with the largest percentage increases occurring at pH 3.0 and 4. Addition of 340 μM (100 μg/mL) catechin at pH 3.0 or 4 increased NO production to ∼250% of controls (Figure 8A).
at pH 3.0 to 5, with the largest percentage increases occurring at pH 3.0 and 4. Addition of 340 μM (100 μg/mL) catechin at pH 3.0 or 4 increased NO production to ∼250% of controls (Figure 8A).
Figure 8.
The Rate of Nonenzymatic NO Production from Nitrite Depends on the pH and Phenolic Content of the Medium.
(A) Relative NO production rates (log scale) at pH 3, 4, and 5 in the presence or absence of 340 μM catechin. Data are means ±sd of three or four determinations and are representative of two replicates.
(B) The effect of catechin and ferulic acid on NO production. Data are means ±sd of four determinations and are representative of two replicates.
The effect of phenolic compounds on net NO production as measured by DAF-FM varied with the compound added. Addition of catechin at 34 or 340 μM (10 or 100 μg/mL) increased net NO production, and data from one typical experiment are shown in Figure 8B. At pH 3.5, 34 μM catechin increased the rate of NO production to ∼30% higher than the spontaneous rate. At 340 μM, net NO production was 300% of the spontaneous rate. Ferulic acid at 34 and 340 μM, however, decreased the rate of net NO production to less than that observed in the absence of added phenolics (Figure 8B).
The data in Figures 3 and 4 demonstrate that aleurone layer incubation media can produce NO from 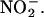 If phenolics accelerate NO production as indicated by Equation 1, then it is expected that addition of a phenolic, such as catechin, to aleurone layer medium will accelerate the conversion of
If phenolics accelerate NO production as indicated by Equation 1, then it is expected that addition of a phenolic, such as catechin, to aleurone layer medium will accelerate the conversion of 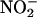 to NO. To test this, we added NaNO2 (100 μM) to incubation medium from flasks of ABA- or GA-treated H. vulgare aleurone layers and monitored the
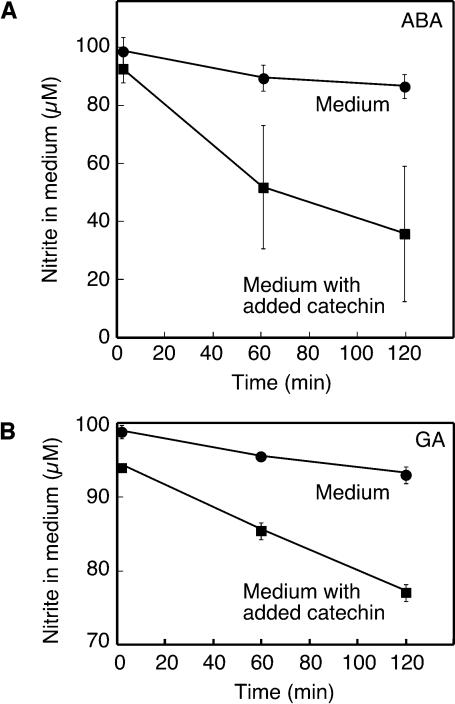
to NO. To test this, we added NaNO2 (100 μM) to incubation medium from flasks of ABA- or GA-treated H. vulgare aleurone layers and monitored the 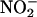 concentration of the medium with time using the Griess reaction. As shown in Figure 9, in the absence of added catechin, the nitrite concentration decreased to ∼90 μM in 2 h. When 100 μg/mL of catechin was added to replicate samples of media, the rate of
concentration of the medium with time using the Griess reaction. As shown in Figure 9, in the absence of added catechin, the nitrite concentration decreased to ∼90 μM in 2 h. When 100 μg/mL of catechin was added to replicate samples of media, the rate of 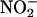 loss increased such that after 2 h, it was <80 μM in GA medium and 40 μM in ABA medium (Figure 9).
loss increased such that after 2 h, it was <80 μM in GA medium and 40 μM in ABA medium (Figure 9).
Figure 9.
Catechin Accelerates the Rate of Nitrite Loss from Aleurone Layer Medium.
Nitrite (100 μM) was added to medium in which ABA-treated (A) or GA-treated (B) aleurone layers had been incubated for 24 h or to medium containing added catechin (340 μM). Medium nitrite concentration was determined at the times indicated. Data points are means ±sd of medium from two or three flasks of aleurone layers, and the data in (A) and (B) are representative of at least two independent replicates.
DISCUSSION
The data presented in Figures 2, 3, and 4 demonstrate that H. vulgare aleurone layers and aleurone layer incubation media can synthesize NO from 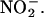 Additional data strongly support the conclusion that the mechanism for NO synthesis is that indicated by Equation 1 and occurs in the apoplast (Figures 2, 4, and 9). In particular, we have shown that NO synthesis requires an acidic pH in the apoplast (Figures 2B and 8A), that NO synthesis occurs when
Additional data strongly support the conclusion that the mechanism for NO synthesis is that indicated by Equation 1 and occurs in the apoplast (Figures 2, 4, and 9). In particular, we have shown that NO synthesis requires an acidic pH in the apoplast (Figures 2B and 8A), that NO synthesis occurs when 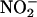 is added to the incubation medium alone (Figures 3A and 4), and that coincident with NO synthesis is a loss of
is added to the incubation medium alone (Figures 3A and 4), and that coincident with NO synthesis is a loss of 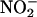 from the apoplast (Figure 9). We conclude that aleurone layers establish conditions that make it inevitable that apoplastic
from the apoplast (Figure 9). We conclude that aleurone layers establish conditions that make it inevitable that apoplastic 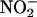 will be converted to NO. NO synthesis after
will be converted to NO. NO synthesis after 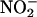 addition to plant tissues has been reported previously, but this is evidence for the nonenzymatic synthesis of NO in the apoplast.
addition to plant tissues has been reported previously, but this is evidence for the nonenzymatic synthesis of NO in the apoplast.
The nonenzymatic production of NO from 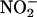 is accelerated by reducing agents, and we demonstrate that reducing equivalents accumulate to 50 to 70 μM in the incubation medium of isolated aleurone layers (Figure 5). This concentration may substantially underestimate the concentration that would accumulate within the apoplast of whole grain because the volume of the incubation medium used for 10 aleurone layers is 10 to 20 times as large as the apoplast of 10 H. vulgare grain. Neither ascorbate nor glutathione accumulate in the incubation medium of H. vulgare aleurone layers (Table 1); rather, it is phenolics that are found at relatively high concentrations (Figure 6). Vanillin-reactive substances are present in the apoplast and these can support the rapid synthesis of NO from nitrite (Figure 8). We speculate that most of the phenolics, other than ferulic acid and ferulic acid conjugates, originate from the testa (Figure 7). The loss of proanthocyanidins from the seed coats during malting of H. vulgare grain is well established (Yadav et al., 2001).
is accelerated by reducing agents, and we demonstrate that reducing equivalents accumulate to 50 to 70 μM in the incubation medium of isolated aleurone layers (Figure 5). This concentration may substantially underestimate the concentration that would accumulate within the apoplast of whole grain because the volume of the incubation medium used for 10 aleurone layers is 10 to 20 times as large as the apoplast of 10 H. vulgare grain. Neither ascorbate nor glutathione accumulate in the incubation medium of H. vulgare aleurone layers (Table 1); rather, it is phenolics that are found at relatively high concentrations (Figure 6). Vanillin-reactive substances are present in the apoplast and these can support the rapid synthesis of NO from nitrite (Figure 8). We speculate that most of the phenolics, other than ferulic acid and ferulic acid conjugates, originate from the testa (Figure 7). The loss of proanthocyanidins from the seed coats during malting of H. vulgare grain is well established (Yadav et al., 2001).
Our data show that the apoplast of both GA- and ABA-treated H. vulgare aleurone layers can produce NO from 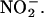 The pH of both is sufficiently acidic (Figure 3B), and both contain reducing agents (Figures 5 and 6). It is expected, however, that
The pH of both is sufficiently acidic (Figure 3B), and both contain reducing agents (Figures 5 and 6). It is expected, however, that 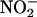 availability will be greater in the endosperm of germinating grain (where aleurone layers are in the GA-treated state) than it is in nongerminating grain (where aleurone layers are in the ABA-treated state). Germination, usually defined as the rupture of the seed coats by the radicle and/or coleoptile, will increase the rate of
availability will be greater in the endosperm of germinating grain (where aleurone layers are in the GA-treated state) than it is in nongerminating grain (where aleurone layers are in the ABA-treated state). Germination, usually defined as the rupture of the seed coats by the radicle and/or coleoptile, will increase the rate of 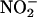 entry from the soil solution, and this source of
entry from the soil solution, and this source of 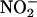 may be augmented by
may be augmented by 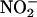 produced enzymatically by nitrate-inducible NR in the aleurone layer (Ferrari and Varner, 1970).
produced enzymatically by nitrate-inducible NR in the aleurone layer (Ferrari and Varner, 1970).
NO is a fast-acting signaling molecule that is effective at low concentrations over tissue level distances. The physiological significance of NO production in cereal grains may depend on the source of 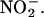 We speculate that NO may play a role in coordinating the activities of the aleurone layer with those of the embryo axis or scutellum. Data presented in Figure 3B and previously published data (Macnicol and Jacobsen, 1992; Drozdowicz and Jones, 1995) have shown that the pH of the endosperm cavity is low enough to promote the formation of HNO2.
We speculate that NO may play a role in coordinating the activities of the aleurone layer with those of the embryo axis or scutellum. Data presented in Figure 3B and previously published data (Macnicol and Jacobsen, 1992; Drozdowicz and Jones, 1995) have shown that the pH of the endosperm cavity is low enough to promote the formation of HNO2. 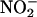 entering the grain from soil solution or released by the embryo axis, the scutellum, or the aleurone layer to the apoplast/endosperm cavity would result in NO production that could be sensed by the other tissues. In other experiments, we have shown that the NO donor SNP breaks dormancy of H. vulgare grain and Arabidopsis thaliana seeds and that the NO scavenger cPTIO strengthens dormancy (R. Jones, J. Jacobsen, and F. Gubler, unpublished data). In this context, NO would be an ideal signal for coordinating the activities of the embryo axis, scutellum, and aleurone layer in real time. Because of its relatively short half-life, NO would be especially advantageous as a short-term signal, whereas plant hormones such as GA or ABA are more suitable for establishing relatively sustained signals.
entering the grain from soil solution or released by the embryo axis, the scutellum, or the aleurone layer to the apoplast/endosperm cavity would result in NO production that could be sensed by the other tissues. In other experiments, we have shown that the NO donor SNP breaks dormancy of H. vulgare grain and Arabidopsis thaliana seeds and that the NO scavenger cPTIO strengthens dormancy (R. Jones, J. Jacobsen, and F. Gubler, unpublished data). In this context, NO would be an ideal signal for coordinating the activities of the embryo axis, scutellum, and aleurone layer in real time. Because of its relatively short half-life, NO would be especially advantageous as a short-term signal, whereas plant hormones such as GA or ABA are more suitable for establishing relatively sustained signals.
Another hypothesis for the role of NO in cereal grains is that 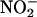 entering germinated grain from the soil solution is converted to NO that would act as an antimicrobial agent. NO produced in this way could minimize pathogen growth and maximize the availability of stored nutrient reserves to the embryo. Microbial attack is likely to be a problem in germinating grains and seeds. The modified starch in germinating cereal grains, for example, is a rich medium for microbial growth that is located outside of the embryo. Once the seed coats are ruptured by the growing embryo axis, the embryo must compete with microorganisms for these nutrients. Soils contain
entering germinated grain from the soil solution is converted to NO that would act as an antimicrobial agent. NO produced in this way could minimize pathogen growth and maximize the availability of stored nutrient reserves to the embryo. Microbial attack is likely to be a problem in germinating grains and seeds. The modified starch in germinating cereal grains, for example, is a rich medium for microbial growth that is located outside of the embryo. Once the seed coats are ruptured by the growing embryo axis, the embryo must compete with microorganisms for these nutrients. Soils contain 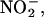 with the concentration in the soil solution depending on the extent and frequency of nitrogen addition (e.g., fertilization) and the activity of nitrifying and denitrifying bacteria. Nitrite concentrations can be greater than several hundred micromolar (Court et al., 1962; Stevens et al., 1998), though values of 10 to 50 μM are more common in agricultural soils (Binnerup and Sorensen, 1992; Stevens et al., 1998).
with the concentration in the soil solution depending on the extent and frequency of nitrogen addition (e.g., fertilization) and the activity of nitrifying and denitrifying bacteria. Nitrite concentrations can be greater than several hundred micromolar (Court et al., 1962; Stevens et al., 1998), though values of 10 to 50 μM are more common in agricultural soils (Binnerup and Sorensen, 1992; Stevens et al., 1998).
The nonenzymatic synthesis of NO from 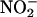 requires a low pH, and this may be the factor that restricts this mechanism of NO synthesis to discrete locations in the plant body. Accurately measuring apoplastic pH is difficult, but the apoplast of leaves often is reported to be pH 6 to 6.5 (Yu et al., 2000). In light of this, it is unlikely that apoplastic synthesis of NO is significant in leaves and perhaps not in photosynthetic tissues in general. In addition to seeds, roots are another location in plants where apoplastic synthesis of NO may be significant (Stöhr and Ullrich, 2002). Roots are regularly exposed to
requires a low pH, and this may be the factor that restricts this mechanism of NO synthesis to discrete locations in the plant body. Accurately measuring apoplastic pH is difficult, but the apoplast of leaves often is reported to be pH 6 to 6.5 (Yu et al., 2000). In light of this, it is unlikely that apoplastic synthesis of NO is significant in leaves and perhaps not in photosynthetic tissues in general. In addition to seeds, roots are another location in plants where apoplastic synthesis of NO may be significant (Stöhr and Ullrich, 2002). Roots are regularly exposed to 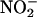 in the soil solution,
in the soil solution, 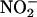 near the surface of roots can be much higher than
near the surface of roots can be much higher than 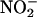 in the bulk soil (Binnerup and Sorensen, 1992), and the plasma membrane of root cells contains NR (Stöhr and Ullrich, 2002). Furthermore, transient acidification of the apoplast often is associated with altered nutrient supply or with stimuli that trigger signal transduction events in roots. For example, adventitious roots are initiated by auxins, which have been shown to trigger cell wall acidification. A recent report suggests that NO is a signal transduction intermediate leading to adventitious root formation and that auxin treatment of roots results in a transient increase in NO production (Pagnussat et al., 2002). These two observations may be linked, in that cell wall acidification may increase NO production by increasing the availability of HNO2. Another instance of root acidification occurs during the gravitropic response of A. thaliana roots. In this case, the pH of the root cap decreased from pH 5.5 to ∼4.5 within 2 min (Fasano et al., 2001). It remains to be seen if there is a commensurate increase in NO production at this location, but this is an intriguing possibility. Plant roots also contain an enzyme that catalyzes the formation of NO from nitrite (Stöhr and Ullrich, 2002). It will be important to determine the relative amounts of NO produced by this enzyme relative to nonenzymatic NO production and other sources of NO.
in the bulk soil (Binnerup and Sorensen, 1992), and the plasma membrane of root cells contains NR (Stöhr and Ullrich, 2002). Furthermore, transient acidification of the apoplast often is associated with altered nutrient supply or with stimuli that trigger signal transduction events in roots. For example, adventitious roots are initiated by auxins, which have been shown to trigger cell wall acidification. A recent report suggests that NO is a signal transduction intermediate leading to adventitious root formation and that auxin treatment of roots results in a transient increase in NO production (Pagnussat et al., 2002). These two observations may be linked, in that cell wall acidification may increase NO production by increasing the availability of HNO2. Another instance of root acidification occurs during the gravitropic response of A. thaliana roots. In this case, the pH of the root cap decreased from pH 5.5 to ∼4.5 within 2 min (Fasano et al., 2001). It remains to be seen if there is a commensurate increase in NO production at this location, but this is an intriguing possibility. Plant roots also contain an enzyme that catalyzes the formation of NO from nitrite (Stöhr and Ullrich, 2002). It will be important to determine the relative amounts of NO produced by this enzyme relative to nonenzymatic NO production and other sources of NO.
In Homo sapiens (human), nonenzymatic production of NO from nitrite occurs in the stomach, the oral cavity, on the surface of the skin, and in urine (Weitzberg and Lundberg, 1998). In these cases, the nitrite needed to make NO is thought to be produced by bacteria, and NO serves as both a signaling molecule and as part of host defenses against pathogens (Weitzberg and Lundberg, 1998). Parallels between these sites of nonenzymatic NO production and those proposed here for plants can be drawn easily. The endosperm cavity of germinating cereal grains, for example, is analogous in many ways to the H. sapiens stomach. The challenge for future research on plants, as well as mammals, is to define more clearly the biological significance of the nonenzymatic synthesis of NO.
METHODS
Preparation of Aleurone Layers and Aleurone Layer Incubation Media
H. vulgare cv Himalaya grain (1991 and 1999 harvests) were obtained from the Department of Agronomy, Washington State University, Pullman, WA. Aleurone layers were prepared as described (Schuurink et al., 1996). For experiments in which NO production was measured by mass spectrometry, samples were prepared in one of two ways. For experiments using intact aleurone layers, isolated layers were kept in a Petri dish on Whatman 1 filter paper (Clifton, NJ) moistened with distilled water until needed (up to 4 h). For experiments using incubation media, 35 aleurone layers were incubated in 10 mL of 20 mM CaCl2 containing either 5 μM GA or 5 μM ABA, and incubation media was removed at the times indicated (Figure 3). For all other experiments, 10 aleurone layers were incubated in 3 mL of 20 mM CaCl2 containing either 5 μM ABA or 5 μM GA.
Cell Viability Assays
Determinations for live and dead cells treated with cPTIO or cPTIO plus SNP were made as described (Beligni et al., 2002).
Measurement of NO
NO production was measured using a mass spectrometer (IsoPrime; Micromass, Manchester, UK) run in fixed accelerating and magnet setting modes with masses M/e = 28 (N2), M/e = 30 (NO), and M/e = 32 (O2) focused on individual Faraday collectors. The signal from the three collectors was recorded continuously by a purpose-built data acquisition system, and mass intensity values were recorded every 5 s. Aleurone layers or incubation medium were placed in a gas-tight liquid sample cuvette (2 mL volume). Gases were sampled continuously and passed from the cuvette to the mass spectrometer through a polyethylene membrane that partitioned the cuvette chamber from the vacuum of the mass spectrometer. The NO donor SNP (Merck, Darmstadt, Germany) and the NO scavenger cPTIO (Molecular Probes, Eugene, OR) were used to authenticate the NO signal. NO for signal quantification was generated by quantitatively reducing KNO2 with KI (Berkels et al., 2001). For measurements of NO production from aleurone layers, 10 aleurone layers were placed in the sample cuvette with 2 mL of 20 mM CaCl2. SNP, NaNO2, or cPTIO were added as indicated. For experiments with Tris-Mes, 10 aleurone layers were added to 2 mL of 20 mM CaCl2 or 1.8 mL of 20 mM CaCl2 plus 0.2 mL of 20 mM Tris-Mes, pH 5.8, before adding 50 mM NaNO2 to a final concentration of 800 μM. For measurements of NO production from incubation media, 0.2 mL of media were diluted to 2 mL with 20 mM CaCl2 before addition of 50 mM NaNO2 to a final concentration of 800 μM.
NO production also was measured using the NO-reactive fluorescent probe DAF-FM (5.5 mM stock solution in DMSO; Molecular Probes). Fluorescence from DAF-FM–containing solutions was measured at 515 nm using a Hitachi F-4500 fluorescence spectrophotometer (Hitachi High Technologies America, San Jose, CA) with 495-nm excitation. For measurements of NO production from artificial media, reaction mixtures contained 20 μL of a 1:50 dilution of the DAF-FM stock; 1.76 mL of 10 mM succinate, pH 3, 4, or 5, as indicated; 20 or 200 μL of catechin or ferulic acid; and 20 μL of 1 mM NaNO2 in a final volume of 2 mL. Fluorescence from DAF-FM is pH sensitive and decreases as the pH decreases below pH 6. To compare NO production at different pH levels, a calibration curve was developed by adding 0.1 to 1 mL of reaction mixture (pH 3 to 5) to 2 mL of 50 mM Hepes, pH 7.75, and determining the fluorescence at pH >7 relative to that at a specific acidic pH. For measurements of NO from aleurone layer media, the reaction mixture contained 280 μL of filter sterilized media, 35 μL of a 1:100 dilution of the DAF-FM stock, and 35 μL of SNP (1 mM) or water. After the appropriate time, 0.1 mL of reaction mixture was added to 2 mL of 50 mM Hepes, pH 7.75, and the fluorescence from the mixture was determined.
Measurement of Reducing Equivalents
Reducing equivalents were measured using the dipyridyl reaction (Omaye et al., 1979). Briefly, medium was added 1:1 to 10% (w/w) metaphosphoric acid and centrifuged (12,000g) for 15 min at 4°C. The supernatant (100 μL) was added to 250 μL of 0.15 M potassium phosphate buffer, pH 7.4, containing 5 mM EDTA and 100 μL of distilled water. Samples were incubated at room temperature for 10 min before adding color development solution (0.62 mL) containing 10% trichloroacetic acid, 55% orthophosphoric acid, 4% dipyridyl, and 15% FeCl3 (1:1:1:0.11). Reactions were mixed and then incubated for 40 min at 40°C. Samples were centrifuged at room temperature (12,000g) for 3 min, and the OD525 of the supernatant was determined and compared with a standard curve made using purified ascorbate (Sigma, St. Louis, MO).
Measurement of Total Phenolics and Vanillin-Reactive Substances
For total phenolics, aleurone medium was added 1:1 to 10% (w/w) metaphosphoric acid, centrifuged at 12000g for 20 min at 4°C, and the clear supernatant used as the sample. Reaction mixtures contained 50 μL of sample, 500 μL of distilled water, and 50 μL of Folin and Ciocalteu's phenol reagent (Sigma). After a 5-min incubation, 300 μL of 1 M Na2CO2 was added, and 2 h later, the absorbance at 765 nm was determined. Vanillin-reactive substances were quantified using the method described by Sun et al. (1998). Samples were prepared by resuspending freeze-dried, filter sterilized media in methanol to one-fifth the original volume. Vanillin-reactive materials in H. vulgare grain were determined using the method described by Aastrup et al. (1984). Imbibed, whole grain was sectioned longitudinally by hand with a razor blade. Aleurone layers were sectioned transversely with a Lancer Vibratome (Technical Products International, St. Louis, MO) and stained for up to 30 min with 1% vanillin in 6 M HCl.
Measurement of Nitrite, Ascorbate, and Glutathione
Nitrite content of the incubation medium was measured using the Griess reaction (Granger et al., 1996). Enzymatic assays for ascorbate were performed using the procedure described by Foyer et al. (1983). Glutathinone was assayed as described by Griffith (1980). Ascorbate and glutathione measurements using HPLC were done as described (Müller-Moulé et al., 2003). For HPLC assays, aleurone medium was added 1:1 to 10% (w/w) metaphosphoric acid, centrifuged at 12000g for 20 min at 4°C, and the clear supernatant used as the sample. For enzymatic assays of glutathione, the clear supernatant was neutralized with 1.5 volumes of 0.5 M K-phosphate, pH 7.25.
Acknowledgments
This work was supported by grants from the National Science Foundation and the Torrey Mesa Research Institute, San Diego, CA. The assistance of Denise Schichnes and Steve Ruzin of the College of Natural Resources Biological Imaging Facility is gratefully acknowledged.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Paul C. Bethke (pcbethke@nature.berkeley.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.017822.
References
- Aastrup, S., Outtrup, H., and Erdal, K. (1984). Location of the proanthocyanidins in the barley grain. Carlsberg Res. Commun. 49, 105–109. [Google Scholar]
- Aslam, M., Travis, R.L., and Huffaker, R.C. (1994). Stimulation of Nitrate and Nitrite Efflux by Ammonium in Barley (Hordeum vulgare L.) Seedlings. Plant Physiol. 106, 1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beligni, M.V., Fath, A., Bethke, P.C., Lamattina, L., and Jones, R.L. (2002). Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol. 129, 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beligni, M.V., and Lamattina, L. (2001). Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 24, 267–278. [Google Scholar]
- Berkels, R., Purol-Schnabel, S., and Roesen, R. (2001). A new method to measure nitrate/nitrite with a NO-sensitive electrode. J. Appl. Physiol. 90, 317–320. [DOI] [PubMed] [Google Scholar]
- Bethke, P.C., and Jones, R.L. (2001). Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. Plant J. 25, 19–29. [DOI] [PubMed] [Google Scholar]
- Binnerup, S.J., and Sorensen, J. (1992). Nitrate and nitrite microgradients in barley rhizosphere as detected by a highly sensitive denitrification bioassay. Appl. Environ. Microbiol. 58, 2375–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandok, M.R., Ytterberg, A., Van Wijk, K., and Klessig, D. (2003). The pathogen-inducible nitric oxide synthase (iNOS) in plants is a variant of the P protein of the glycine decarboxylase complex. Cell 113, 469–482. [DOI] [PubMed] [Google Scholar]
- Clarke, A., Desikan, R., Hurst, R.D., Hancock, J.T., and Neill, S.J. (2000). NO way back: Nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 24, 667–677. [DOI] [PubMed] [Google Scholar]
- Court, M.N., Stephen, R.C., and Waid, J.S. (1962). Nitrite toxicity arising from the use of urea as a fertilizer. Nature 194, 1263–1265. [Google Scholar]
- Delledonne, M., Xia, Y., Dixon, R.A., and Lamb, C. (1998). Nitric oxide functions as a signal in plant disease resistance. Nature 394, 585–588. [DOI] [PubMed] [Google Scholar]
- Drozdowicz, Y.M., and Jones, R.L. (1995). Hormonal regulation of organic and phosphoric acid release by barley aleurone layers and scutella. Plant Physiol. 108, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner, J., Wendehenne, D., and Klessig, D.F. (1998). Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95, 10328–10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano, J.M., Swanson, S.J., Blancaflor, E.B., Dowd, P.E., Kao, T.H., and Gilroy, S. (2001). Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13, 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath, A., Bethke, P.C., and Jones, R.L. (2001). Enzymes that scavenge reactive oxygen species are down-regulated prior to gibberellic acid-induced programmed cell death in barley aleurone. Plant Physiol. 126, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, T.E., and Varner, J.E. (1970). Control of nitrate reductase activity in barley aleurone layers. Proc. Natl. Acad. Sci. USA 65, 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner, I., Wendehenne, D., Langebartels, C., and Durner, J. (2000). In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 23, 817–824. [DOI] [PubMed] [Google Scholar]
- Foyer, C., Rowell, J., and Walker, D. (1983). Measurement of the ascorbate content of spinach (Spinacia-oleracea cultivar Yates) leaf protoplasts and chloroplasts during illumination. Planta 157, 239–244. [DOI] [PubMed] [Google Scholar]
- Goupy, P., Hugues, M., Boivin, P., and Amiot, M.J. (1999). Antioxidant composition and activity of barley (Hordeum vulgare) and malt extracts and of isolated phenolic compounds. J. Sci. Food Agric. 79, 1625–1634. [Google Scholar]
- Granger, D.L., Taintor, R.R., Boockvar, K.S., and Hibbs, J.B. (1996). Measurement of nitrate and nitrite in biological samples using nitrate reductase and the Griess reaction. Methods Enzymol. 268, 142–151. [DOI] [PubMed] [Google Scholar]
- Griffith, O.W. (1980). Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinlypyridine. Anal. Biochem. 106, 207–212. [DOI] [PubMed] [Google Scholar]
- Gubler, F., and Ashford, A.E. (1985). Release of ferulic acid esters from barley aleurone. I. Time course of gibberellic-acid-induced release from isolated layers. Aust. J. Plant Physiol. 12, 297–305. [Google Scholar]
- Horemans, N., Foyer, C.H., and Asard, H. (2000). Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 5, 263–267. [DOI] [PubMed] [Google Scholar]
- Jende-Strid, B., and Moller, B.L. (1981). Analysis of proanthocyanins in wild type and mutant barley Hordeum vulgare cultivar Nordal. Carlsberg Res. Commun. 46, 53–64. [Google Scholar]
- Kojima, H., Urano, Y., Kikuchi, K., Higuchi, T., Hirata, Y., and Nagano, T. (1999). Fluorescent indicators for imaging nitric oxide production. Angew. Chem. Int. Ed. Engl. 38, 3209–3212. [DOI] [PubMed] [Google Scholar]
- Macnicol, P.K., and Jacobsen, J.V. (1992). Endosperm acidification and related metabolic changes in the developing barley grain. Plant Physiol. 98, 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick, N., Rai, L.C., Mohn, F.H., and Soeder, C.J. (1999). Studies on nitric oxide (NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum. Chemosphere 39, 1601–1610. [DOI] [PubMed] [Google Scholar]
- Müller-Moulé, P., Havaux, M., and Niyogi, K.K. (2003). Zeoxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis thaliana. Plant Physiol. 133, 748–760. [DOI] [PMC free article] [PubMed]
- Neill, S.J., Desikan, R., Clarke, A., and Hancock, J.T. (2002). Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 128, 13–16. [PMC free article] [PubMed] [Google Scholar]
- Omaye, S.T., Turnbull, D., and Sauberlich, H. (1979). Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 62, 3–11. [DOI] [PubMed] [Google Scholar]
- Pagnussat, G.C., Simontacchi, M., Puntarulo, S., and Lamattina, L. (2002). Nitric oxide is required for root organogenesis. Plant Physiol. 129, 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans, C.A., Miller, N.J., and Paganga, G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20, 933–956. [DOI] [PubMed] [Google Scholar]
- Rockel, P., Strube, F., Rockel, A., Wildt, J., and Kaiser, W.M. (2002). Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 53, 103–110. [PubMed] [Google Scholar]
- Sakihama, Y., Nakamura, S., and Yamasaki, H. (2002). Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 43, 290–297. [DOI] [PubMed] [Google Scholar]
- Schuurink, R.C., Chan, P.V., and Jones, R.L. (1996). Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiol. 111, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, R.J., Laughlin, R.J., and Malone, J.P. (1998). Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol. Biochem. 30, 1119–1126. [Google Scholar]
- Stöhr, C., and Ullrich, W.R. (2002). Generation and possible roles of NO in plant roots and their apoplastic space. J. Exp. Bot. 53, 2293–2303. [DOI] [PubMed] [Google Scholar]
- Sun, B., Ricardo-da-Silva, J.M., and Spranger, I. (1998). Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 46, 4267–4274. [Google Scholar]
- Thomas, D.D., Liu, X., Kantrow, S.P., and Lancaster, J.R. (2001). The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 98, 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzberg, E., and Lundberg, J.O.N. (1998). Nonenzymatic nitric oxide production in humans. Nitric Oxide 2, 1–7. [DOI] [PubMed] [Google Scholar]
- Wink, D.A., and Mitchell, J.B. (1998). Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 25, 434–456. [DOI] [PubMed] [Google Scholar]
- Yadav, S., Luthra, Y., Sood, D., and Aggarwal, N. (2001). Malting potential of husked barley in relation to proanthocyanidins. J. Food Sci. Technol. 38, 71–74. [Google Scholar]
- Yamasaki, H. (2000). Nitrite-dependent nitric oxide production pathway: Implications for involvement of active nitrogen species in photoinhibition in vivo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355, 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, H., Shimoji, H., Ohshiro, Y., and Sakihama, Y. (2001). Inhibitory effects of nitric oxide on oxidative phosphorylation in plant mitochondria. Nitric Oxide 5, 261–270. [DOI] [PubMed] [Google Scholar]
- Yu, Q., Tang, C., and Kuo, J. (2000). A critical review on methods to measure apoplastic pH in plants. Plant Soil 219, 29–40. [Google Scholar]