Abstract
Intermolecular recombination events were monitored in Arabidopsis thaliana lines using specially designed recombination traps consisting of tandem disrupted β-glucuronidase or luciferase reporter genes in direct repeat orientation. Recombination frequencies (RFs) varied between the different lines, indicating possible position effects influencing intermolecular recombination processes. The RFs between sister chromatids and between homologous chromosomes were measured in plants either hemizygous or homozygous for a transgene locus. The RFs in homozygous plants exceeded those of hemizygous plants by a factor of >2, implying that in somatic plant cells both sister chromatid recombination and recombination between homologous chromosomes exist for recombinational DNA repair. In addition, different DNA-damaging agents stimulated recombination in homozygous and hemizygous plants to different extents in a manner dependent on the type of DNA damage and on the genomic region. The genetic and molecular analysis of recombination events showed that most of the somatic recombination events result from gene conversion, although a pop-out event has also been characterized.
INTRODUCTION
The plant genome contains large numbers of repeated DNA sequences consisting of satellite sequences, rRNA genes, transposable elements, and multigenic, highly homologous gene families (The Arabidopsis Genome Initiative, 2000; Goff et al., 2002). These sequences provide perfect targets for homologous recombination (HR), which can result in permanent alterations of the genome and/or lethal deletions. Genetic changes occurring in somatic plant cells can eventually be passed to the progeny through meiosis because plants lack a permanent germ line and instead produce gametes from somatic lineages (Puchta and Hohn, 1996). Thus, mechanisms must have evolved to control the frequency of recombination (RF), providing enough genome flexibility yet ensuring vital genome stability. HR plays an important role in somatic cells for the maintenance of genome integrity by repair of damaged DNA sequences and during meiosis for the generation of evolutionarily important allelic diversity (Lichtenstein et al., 1994; Friedberg et al., 1995; Klein, 1995; Puchta and Hohn, 1996).
Recombination between homologous sequences can occur either intramolecularly between genetically linked homologous sequences or intermolecularly between homologous sequences located on two different chromatids (Taylor, 1984; Fasullo and Davis, 1987; Bolag et al., 1989; Kadyk and Hartwell, 1992, 1993; Swoboda et al., 1994; Klein, 1995; Puchta et al., 1995). Evidence for spontaneous mitotic crossover events between homologous chromosomes derives from genetic analysis showing the existence of half-sectored colonies in Saccharomyces cerevisiae (yeast) (James, 1955). Similar molecular events have also been shown to occur in Drosophila melanogaster and in several plant species, including Nicotiana tabacum (Stern, 1936; Carlson, 1974), Arabidopsis thaliana (Jelesko et al., 1999), and Zea mays (Hu et al., 1998). Twin spots, as seen in N. tabacum, have been interpreted as reciprocal crossover events (Carlson, 1974).
Intermolecular recombination is an important DNA repair mechanism in somatic cells, essential for the elimination of damaged DNA sequences in single-copy genes. Template sequences for intermolecular recombination can be found on the sister chromatid, after DNA replication, or in diploid (or polyploid) organisms on the homologous chromosome.
In plants, intramolecular HR events and recombination events between ectopic homologous sequences have been analyzed at the molecular level (Peterhans et al., 1990; Assaad and Signer, 1992; Tovar and Lichtenstein, 1992; Swoboda et al., 1994; Puchta, 1999). By contrast, intermolecular recombination between allelic homologous sequences is poorly referenced in plants and remains difficult to detect, although some interchromosomal recombination and gene conversion events have been reported (Hu et al., 1998; Jelesko et al., 1999; Haubold et al., 2002).
Several independent transgenic A. thaliana lines were generated, carrying recombination substrates consisting of a disrupted β-glucuronidase (uidA) or luciferase (luc) gene in a special arrangement designed specifically to reveal intermolecular recombination events. Such plants were used to compare the use of sister chromatids and/or homologous chromosomes in intermolecular recombination repair. Upon recombination between the overlapping homologous sequences of the direct repeats of a marker gene, a functional gene could be restored, and its gene activity could be detected. To distinguish between sister chromatid and homolog recombination, plants either hemizygous or homozygous for the transgene locus were analyzed. Homozygous plants are expected to undergo both sister chromatid and homolog recombination, whereas hemizygous plants can only recombine between sister chromatids. RFs in homozygous and hemizygous plants were examined to demonstrate that intermolecular HR can take place between sister chromatids as well as between homologous chromosomes. Upon induction of DNA damage by treatments with UV-B radiation, UV-C radiation, bleomycin (BLM), and methyl methanesulfonate (MMS), hemizygous and homozygous plants displayed enhancements in recombination levels to different extents depending on the line tested and the type of DNA lesions introduced. Several gene conversion events and a single pop-out event have been isolated and characterized in fully recombined plants.
RESULTS
Production and Analysis of Transgenic Plants Harboring Recombination Substrates
To create recombination substrates for visualization of somatic intermolecular recombination events in whole plants, the reporter genes uidA coding for β-glucuronidase and luc coding for luciferase were used. Deletions of the 5′ and 3′ portions of either the uidA or the luc gene were made, and the resulting constructs were inserted into a binary vector plasmid. One uidA and one luciferase recombination substrate, each with sequence homology in direct orientation, were constructed: pGRU'S'G'U' with a sequence overlap of 1213 bp in the uidA gene and pCHU'C'L'U' with a sequence overlap of 1146 bp in the luc gene (Figures 1A and 1B). The recombination substrate plasmids were used to transform A. thaliana, and several generations of selfed progeny of the original transgenic plants were employed to isolate homozygous lines harboring the recombination substrates. Several independent lines containing the uidA recombination substrate (IC1, IC6, and IC9) and the luciferase recombination substrate (58F) as single-copy inserts were generated (Figure 1C).
Figure 1.
Schematic Representation of the Intermolecular Recombination Substrates and Molecular Analysis of the Four Recombination Lines Used in This Study.
(A) T-DNA of the pGRU'S'G'U' plasmid integrated in IC1, IC6, or IC9 lines, carrying the partially overlapping uidA region with 1213-bp homologous sequence in direct orientation. GUS, uidA gene; Hpt, hygromycin phosphotransferase gene; LB, left border; P, 35S promoter of Cauliflower mosaic virus (CaMV); RB, right border; T, 35S terminator of CaMV.
(B) T-DNA of the pGRU'C'L'U' plasmid integrated in the 58F line, carrying the partially overlapping luc region with 1146-bp homologous sequence in direct orientation. Hpt, hygromycin phosphotransferase gene; LB, left border; LUC, luc gene; P, 2× 35S promoter of CaMV; RB, right border; T, 35S terminator of CaMV.
(C) DNA gel blot analysis of the IC1, IC6, IC9, and 58F recombination lines using the EcoRI or the HindIII restriction enzyme and hybridization with the Hpt gene.
Relative RF in Homozygous and in Hemizygous Plants
In plants carrying the luciferase recombination substrate, somatic recombination events could be visualized as light-emitting sectors using the bioluminescence detection procedure (Figure 2A). Plants harboring the β-glucuronidase recombination substrate were histochemically stained, and recombination events could be visualized as blue sectors on bleached plants (Figure 2B). These assays allow measure of unequal crossover between chromatids or homologs or conversion between near allelic sequences. In hemizygous organisms, intermolecular HR can occur only between sister chromatids after replication, whereas in homozygous organisms, intermolecular recombination can also take place between homologous chromosomes. Therefore, one would expect that in plants homozygous for the recombination marker, the RF would be more than twice that of plants hemizygous for the transgene locus. Three independent recombination lines, IC1, IC6, and IC9 containing one unit of the uidA recombination substrate each, yielded different RFs (Table 1). The average RFs in hemizygous and in homozygous plants of line IC1 were 0.07 and 0.32 events per plant, respectively, whereas line IC6 displayed RFs of 0.08 and 0.29 (Table 1). In line IC9, the average RFs were 0.07 and 0.18 (Table 1). With an average of 3.1 and 8.5 spots per plant, the RFs in the luciferase line 58F were much higher than the RFs in uidA lines. Our observations can reflect a possible position effect of the genomic locus on intermolecular HR processes.
Figure 2.
Visualization of Recombination Events in Whole Plants Using Either Luciferase Bioluminescence Detection or Histochemical GUS Assay.
(A) 58F homozygous plant revealing many independent recombination events.
(B) An IC1 homozygous plant exhibiting a single recombination event.
(C) Fully recombined 58F-derived plant exhibiting a LUC+ phenotype.
(D) Leaf of the fully recombined IC9-derived plant, AE13, exhibiting a GUS+ phenotype. Bars = 5 mm in (A) and (B), 2 mm in (C), and 1 mm in (D).
Table 1.
RFs in Homozygous and Hemizygous Plants Containing Either a uidA (IC1, IC6, and IC9) or a Luciferase (58F) Gene as Recombination Substrate
| Line | RFhemi | RFhomo | RFhomo:RFhemi |
|---|---|---|---|
| IC1 | 0.07 ± 0.01 | 0.32 ± 0.15 | 4.2 ± 1.2 |
| IC6 | 0.08 ± 0.03 | 0.29 ± 0.09 | 3.9 ± 0.3 |
| IC9 | 0.07 ± 0.04 | 0.18 ± 0.10 | 3.1 ± 0.9 |
| 58F | 3.1 ± 1.0 | 8.5 ± 2.3 | 2.8 ± 0.2 |
Hemizygous plants were generated by three independent outcrosses. Seeds homozygous and hemizygous for the recombination substrate were harvested from the same donor/recipient plants used for the corresponding crosses. The hemizygous and corresponding homozygous plants were assayed for somatic HR events at different time periods. Independent experiments were conducted three times (except for line 58F) using >80 plants per replicate. RFhemi, RF in hemizygous plants; RFhomo, RF in homozygous plants; RFhomo:RFhemi, ratio between RFs in homozygous and hemizygous plants.
Analyses of three populations obtained after three independent outcrosses of the homozygous recombination lines IC1, IC6, IC9, and 58F with the wild-type plants revealed that the average RF in homozygous plants was, in all lines tested, more than twice that of the hemizygous plants (Table 1). The average RF in homozygous plants of line IC1 was 4.2 times that of hemizygous plants. In homozygous plants of the lines IC6 and IC9, recombination was even >3 times that in hemizygous plants. Interestingly, although in line 58F recombination levels were found generally much higher than in uidA lines, recombination in homozygous plants was ∼3 times higher than in hemizygous plants. Although the RF varied to different extents during the three independent experiments (data not shown) performed at different time periods, the variation of the ratio between RFs in homozygous and hemizygous plants (RFhomo:RFhemi) was not as strong (Table 1). The finding of RFhomo:RFhemi >2 suggests that in homozygous lines, recombination between the homologs occurs. The small differences in RFhomo:RFhemi in the four lines may mean that the genomic region of the recombination target transgene somehow influences the use of either the homologous chromosome or the sister chromatid.
Genetic and Molecular Analysis of Recombination Events
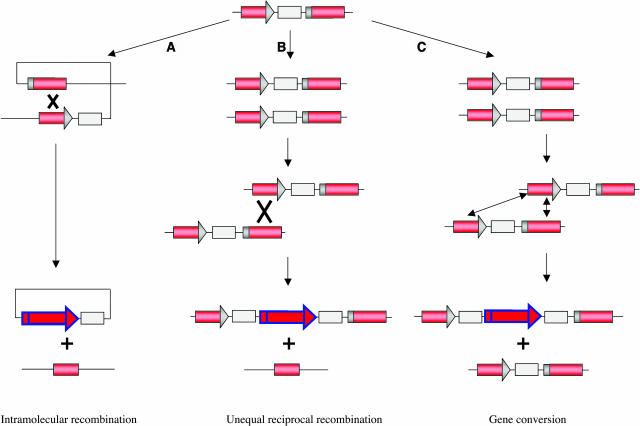
To provide molecular data on the recombination events, the progeny of homozygous 58F (∼1,000,000 plants) was screened for plants uniformly expressing the luciferase gene (LUC+, Figure 2C). Only such plants could provide enough DNA for analysis of the recombination event that had occurred. Five R1 (for generation 1 of recombined plant) 58F plants (FR1 to FR5) showing full luciferase activity were identified. Based on the design of the construct, three types of unequal recombination events between the direct repeats are expected to result in a functional reporter gene (Figure 3). Combination of genetic and molecular analysis of the fully recombined plants and their offspring should allow distinction between the three types of recombination processes.
Figure 3.
Schematic Representation of Mechanisms of Recombination between Direct Repeats (Red Rectangle), Theoretically Leading to the Restoration of a Functional Reporter Gene.
(A) An intramolecular event (pop out) can occur before or after DNA replication. This type of recombination generates an extrachromosomal circle containing the reporter gene. Such episomes are unstable and can yield a screenable sector only after reintegration.
(B) Following replication, an unequal reciprocal recombination event can occur between sister chromatids or allelic chromosomes to generate a functional reporter gene. A recombined chromatid and a chromatid containing only the region of the homologous sequence of the reporter gene are produced.
(C) A gene conversion event may occur via DSB gap repair, generating a functional recombined reporter gene on one chromatid or a chromosome maintaining the original recombination substrate locus on the other chromatid or chromosome.
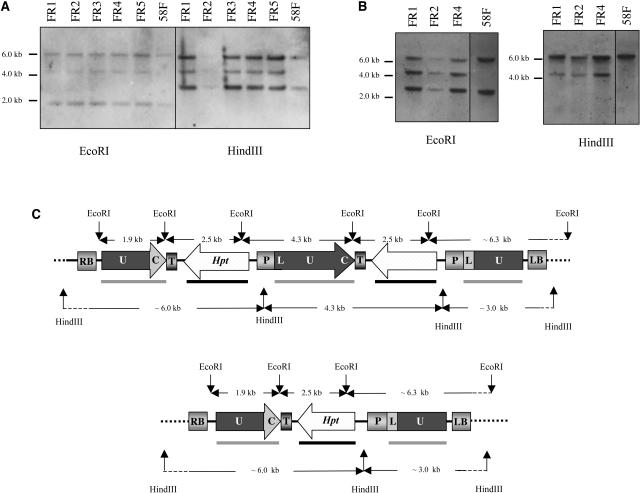
The DNA gel blot analysis of the 58F-derived plants (FR1 to FR5) revealed that in all five plants the molecular pattern of the original recombination substrate was conserved and that an additional fragment, diagnostic for the restoration of the functional luc gene (4.3-kb band), could be detected (Figures 4A to 4C). The genetic analysis of their corresponding progeny (R2 plants) showed that 100% of the plants exhibited a hygromycin-resistant (Hygr) phenotype characteristic for a gene conversion process. Molecular and genetic approaches allowed confirmation that the restoration of a functional luc gene had occurred via a gene conversion process.
Figure 4.
Molecular Analysis of Five Fully Recombined 58F-Derived Plants and Schematic Representation of the Recombination Product Having Occurred after the Gene Conversion Process.
(A) DNA gel blot analysis of the five fully recombined FR lines using the EcoRI or the HindIII restriction enzyme and hybridization with the luc probe (shaded bar, [C]).
(B) DNA gel blot analysis of three fully recombined FR lines using the EcoRI or the HindIII restriction enzyme and hybridization with the Hpt probe (closed bar, [C]).
(C) Schematic representation of the recombination product having occurred after the gene conversion process. Hpt, hygromycin phosphotransferase gene; LB, left border; LUC, luc gene; RB, right border.
Approximately 10,000 IC9 plants were screened for an individual uniformly expressing the β-glucuronidase gene (Figure 2D). Two plants, AE13 and AN32, were identified. To elucidate the mechanism of the recombination events, molecular and genetic analyses were combined. In R2 plants originating from the first plant uniformly expressing the β-glucuronidase gene (GUS+), the 3.6-kb band (EcoRI digestion), diagnostic for a reciprocal recombination or gene conversion process, corresponding to the restored uidA gene was detectable (Figures 5A and 5B). In addition, all of the examined R2 plants exhibited a Hygr phenotype fitting with the expected pattern for a gene conversion process. Both molecular and genetic analyses thus suggest that the restoration of the functional uidA gene had occurred via a gene conversion process.
Figure 5.
Molecular Analysis of the Fully Recombined IC9-Derived Plant AE13 and Schematic Representation of the Recombination Product.
(A) DNA gel blot analysis of the fully recombined R2 lines using either the EcoRI or the HindIII restriction enzyme and hybridization with the uidA overlapping part.
(B) Schematic representation of the putative recombination product having occurred after a gene conversion process. Putative missing sequences are represented in the light shaded area. GUS, uidA gene; Hpt, hygromycin phosphotransferase gene; LB, left border; RB, right border.
The molecular analysis of two R2 plants originating from the AN32 plant showed the overlapping uidA region U as a single locus, corresponding to the 2-kb fragment (EcoRI digestion, Figures 6A and 6B). This fragment was only detectable in the AN32/2 plant (Figure 6A). Approximately 75% of the plants exhibited a Hygr/GUS+ phenotype, and 25% exhibited a hygromycin-sensitive (Hygs)/GUS− phenotype, as expected for the segregation of single locus. Among the R3 population of Hygr/GUS+ plants, half contained the overlapping uidA region U. This would imply that a pop-out event had occurred followed by its reintegration into a different chromosome.
Figure 6.
Molecular Analysis of the Fully Recombined IC9-Derived Plant AN32 and Schematic Representation of the Recombination Products Having Occurred after an Intramolecular Pop-Out Recombination Process.
(A) DNA gel blot analysis of the fully recombined R2 lines, AN32/2 and AN32/3, using either the EcoRI or the HindIII restriction enzyme and hybridization with the uidA overlapping part.
(B) Schematic representation of the recombination product having occurred after the intramolecular pop-out recombination process and the integration of the circular molecule in the plant genome. GUS, uidA gene; Hpt, hygromycin phosphotransferase gene; LB, left border; RB, right border.
Differential Stimulation of Recombination in Homozygous and in Hemizygous Plants by DNA-Damaging Agents
To study in detail whether the differences in the RF between homozygous and hemizygous plants reflect the different contributions of either the sister chromatid or the homologous chromosome, both hemizygous and homozygous plants were challenged with different DNA-damaging agents. In addition, such experiments would allow us to show that the contribution of either the sister chromatid or the homolog for recombinational DNA repair is influenced by the type of DNA damage and/or by the genomic position of the transgene locus.
After treatment with MMS, the RF in hemizygous and homozygous plants of lines IC1 and IC6 increased by a factor of ∼7, whereas in line IC9, an ∼18-fold induction of the RF could be observed (Table 2).
Table 2.
RFs in Homozygous and Hemizygous Plants after Treatment with MMS, UV-B Radiation, UV-C Radiation, or BLM
| RFhemi
|
RFhomo
|
RFhomo:RFhemi
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Line | Type of Treatment | Untreated | Treated | Enh | Untreated | Treated | Enh | Untreated | Treated |
| IC1 | MMS | 0.05 ± 0.01 | 0.36 ± 0.05 | 7.2 ± 0.5 | 0.20 ± 0.02 | 1.18 ± 0.10 | 5.9 ± 0.5 | 4.1 ± 0.4 | 3.3 ± 0.2 |
| UV-B | 0.05 ± 0.01 | 0.08 ± 0.02 | 1.6 ± 0.1 | 0.16 ± 0.01 | 0.35 ± 0.04 | 2.1 ± 0.1 | 3.2 ± 0.3 | 4.5 ± 0.6 | |
| UV-C | 0.02 ± 0.01 | 0.04 ± 0.01 | 2.3 ± 0.5 | 0.04 ± 0.02 | 0.17 ± 0.03 | 5.1 ± 1.4 | 2.0 ± 0.1 | 4.2 ± 0.1 | |
| BLM | 0.01 | 0.05 | 5.0 | 0.01 | 0.14 | 14.0 | 1.1 | 2.7 | |
| IC6 | MMS | 0.07 ± 0.01 | 0.44 ± 0.06 | 6.6 ± 0.4 | 0.22 ± 0.03 | 1.40 ± 0.11 | 6.8 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 |
| UV-B | 0.06 ± 0.01 | 0.11 ± 0.02 | 1.8 ± 0.1 | 0.20 ± 0.02 | 0.39 ± 0.04 | 1.9 ± 0.1 | 3.3 ± 0.2 | 3.4 ± 0.1 | |
| UV-C | 0.02 ± 0.01 | 0.08 ± 0.02 | 4.7 ± 1.3 | 0.05 ± 0.02 | 0.20 ± 0.03 | 4.5 ± 1.1 | 2.5 ± 0.0 | 2.5 ± 0.2 | |
| BLM | 0.01 | 0.03 | 3.0 | 0.009 | 0.08 | 8.9 | 0.9 | 2.4 | |
| IC9 | MMS | 0.03 ± 0.01 | 0.51 ± 0.05 | 18.5 ± 4.2 | 0.09 ± 0.01 | 1.67 ± 0.14 | 17.4 ± 0.4 | 3.2 ± 0.4 | 3.3 ± 0.0 |
| UV-B | 0.02 ± 0.01 | 0.20 ± 0.02 | 13.1 ± 6.3 | 0.09 ± 0.01 | 0.42 ± 0.04 | 4.7 ± 0.1 | 5.5 ± 1.3 | 2.1 ± 0.1 | |
| UV-C | 0.02 ± 0.01 | 0.11 ± 0.05 | 5.5 ± 0.2 | 0.05 ± 0.02 | 0.30 ± 0.04 | 6.8 ± 1.3 | 2.5 ± 0.2 | 2.8 ± 0.2 | |
| BLM | 0.008 | 0.03 | 3.7 | 0.02 | 0.08 | 4.0 | 2.5 | 2.7 | |
The determination of RFs in homozygous and hemizygous lines IC1, IC6, and IC9 was performed using 170 to 370 plants per experiment. Experiments were duplicated except for BLM treatment. Histochemical GUS assay was performed 4 d after each treatment. RFhemi, RF in hemizygous plants; RFhomo, RF in homozygous plants; Enh, average enhancement (±SD) of HRF after treatment; RFhomo:RFhemi, ratio between RFs in homozygous and hemizygous plants.
Upon UV-B irradiation, recombination increased by a factor of 1.6 to 13 in different lines (Table 2). However, in contrast to the MMS treatment, UV-B irradiation influenced the homologous RF (HRF) of hemizygous and homozygous plants in lines IC1, IC6, and IC9 to different extents (Table 2). In lines IC1 and IC6, the HRF in both hemizygous and homozygous plants increased approximately twofold, whereas only in hemizygous IC9 plants the HRF increased 13-fold (Table 2). By contrast, the homozygous IC9 plants exhibited a much less pronounced increase of recombination after UV-B irradiation (Table 2).
After treatment with UV-C radiation, the RF of all lines exhibited a weak to moderate increase (Table 2). The induction of DNA damage using BLM led to an enhancement of ∼3- to 14-fold in the use of the recombinational DNA repair pathway (Table 2). Unfortunately, for all the lines the HRFs in control plants were too low to allow a conclusion about a possible contribution of the sister chromatids or the homologous chromosomes to the recombinational repair process (Table 2).
These data show that the sister chromatid or the homologous chromosome could contribute to different extents to recombinational DNA repair depending on the DNA-damaging agent used. Also, the genomic loci in which the recombination targets had integrated may influence the sensitivity to specific damage and/or repair to different degrees.
DISCUSSION
A special design of sequence repeats in direct orientation was used to monitor unequal somatic intermolecular HR events in whole A. thaliana plants. The restoration of a functional β-glucuronidase or of a luciferase gene by recombination led to a gene product that could be detected by histochemical staining or by bioluminescence activity. Genomic DNA from fully recombined plants was analyzed to provide molecular evidence that GUS+ and LUC+ sectors were the result of recombination events.
Recombination Events Resulting from Homologous Interactions between Direct Repeats
The arrangement of direct repeats theoretically allows three types of recombination events generating a functional reporter gene from the recombination construct (Figure 3; Schiestl et al., 1988; Bolag et al., 1989; Haber, 1992; Belmaaza and Chartrand, 1994; Lichtenstein et al., 1994; Galli and Schiestl, 1995; Klein, 1995; Pâques and Haber, 1999).
Two types of recombination events that can generate recombination sectors are unequal reciprocal recombination and gene conversion between sister chromatids or homologous chromosomes. Unequal reciprocal recombination events can occur between sister chromatids after replication or between homologous chromosomes, thus generating a functional gene on one chromatid and a deletion on the other chromatid (Figures 3A and 3B). Interestingly, among the population of plants screened for a reciprocal recombination event, none of the analyzed plants exhibited the expected molecular and genetic characteristic. Gene conversion events may occur via double-strand break (DSB) repair producing, upon duplication, a functional gene on one chromatid and leaving behind the chromatid with the original target locus (Figure 3C). The special orientation of the direct repeats in the recombination substrate should not lead to nonconservative slippage upon replication. A similar arrangement of the recombination substrate repeats has been shown in S. cerevisiae to result exclusively in reciprocal exchanges and gene conversion events (Fasullo and Davis, 1987; Kadyk and Hartwell, 1992; 1993; Pâques and Haber, 1999). The molecular and genetic analysis of the offspring of the fully recombined luciferase plants, originating from line 58F, clearly indicated the occurrence of gene conversion events (100% Hygr plants). The exact timing of the recombination events could not be determined, although the most likely developmental stages at which they may have occurred are during meiosis or early in embryo development. Our observations are in agreement with the simulations of Haubold et al. (2002), who have estimated that up to 90% of the recombination events in a 170-kb locus of A. thaliana could be the result of gene conversion.
The gene conversion events in the 58F plants led, in addition to the restoration of the luc gene, to complete conservation of the recombination substrate (Figure 4). However, in one of the IC9-derived plants, the molecular pattern of the gene conversion event did not precisely fit to the expectation; deletions or rearrangements within both the gene conversion donor and acceptor transgene loci must have occurred before or during the recombination process (Figure 5B). Similar rearrangements have been observed between directly repeated sequences in N. tabacum (Siebert and Puchta, 2002). Because the total number of analyzed plants was too small, any influences of the transgene locus on the type of recombination cannot be discussed.
The third type of recombination event is an intramolecular reciprocal recombination event (pop out) within a single chromosome or a single chromatid, resulting in an extrachromosomal circular molecule carrying the functional gene (Figure 3A; Gal et al., 1991). Such an event was expected to remain undetected because the resulting episome lacks an origin of replication, and detection would depend on reintegration into the genome. However, such an integration event has already been described by Peterhans et al. (1990) but remained rare. One of the fully recombined plants isolated exhibited a molecular pattern fitting with such an event. The intramolecular pop-out event has led to the release of the overlapping region U and the stable integration of the extrachromosomal molecule in a different locus of the plant genome. Because the specific signatures of the recombination substrate were missing, we suggest that the intramolecular recombination and reintegration events may have occurred late in the germ line. However, an unequal reciprocal recombination process cannot be totally excluded but would have been the result of several rearrangements of the flanking regions of the recombined locus. The pop-out/reinsertion process may be of general importance for plant genome flexibility and may represent a mechanism for transposon-independent DNA translocation.
RF in Plants Hemizygous or Homozygous for the Recombination Marker Locus
The RF was found to be >2 times higher in plants homozygous for the recombination trap than in hemizygous plants (Table 1). In hemizygous lines, only a single allele containing the recombination substrate is available for recombination between sister chromatids. By contrast, in homozygous plants two alleles can be used for recombination, enabling exchange of sequences between sister chromatids and between homologous chromosomes. Assuming that the probability for recombination between sister chromatids in homozygous plants is twice the probability for sister chromatid recombination in hemizygous plants, our results strongly suggest that in homozygous plants not only recombination between sister chromatids but also an additional type of recombination, most probably recombination between homologous chromosomes, may exist. Because our assay measures unequal recombination, our data might be an underestimate of the actual recombination rates between precisely aligned sequences in which the sister chromatids are genetically identical. The somatic RFs in lines IC1, IC6, and IC9 were lower than that in line 58F. However, RFhomo:RFhemi was similar in the IC and 58F lines, suggesting that contribution of intersister and interhomolog recombination to overall HR may be balanced under our growth conditions. In S. cerevisiae, the sister chromatid and the homologous chromosome repair pathways can be modulated under some circumstances (Arbel et al., 1999). DNA DSBs can be repaired by both pathways, whereby sister chromatid repair depends on Rad54p and repair between homologous chromosome depends on Tid1p and/or Dmc1p. Only analysis of plant repair mutants will allow such distinctions.
The surprisingly high RF in homozygous reporter lines that we found is comparable to data obtained in S. cerevisiae, in which homologs are used for recombinational repair only twofold to threefold less frequently than sister chromatids (Kadyk and Hartwell, 1992). By contrast, in mammalian cells, sister chromatids are used 100- to 1000-fold more frequently than homologs for recombinational repair (Johnson and Jasin, 2001). In N. tabacum, Gisler et al. (2002) have shown, after induction of DSB, that somatic RFs between allelic sites and ectopic sites occurred to the same extent although too rarely to be a major repair pathway compared with sister chromatid exchange. This could be because of the fact that in mammals and in N. tabacum, both having large genomes, association of homologs during interphase may occur less frequently than in A. thaliana with only five chromosome pairs.
The structure of the recombination substrate trap employed in our study was not designed to allow distinction of recombination events taking place between sister chromatids and homologous chromosomes. Only the use of molecular markers flanking the repeats of the disrupted marker gene would permit this important distinction.
DNA Damage Influences Recombination in Homozygous and Hemizygous Plants to Different Extents
Stimulation of recombination upon DNA damage occurred in homozygous and hemizygous plants to different extents and seemed to depend on the genomic position of the recombination substrate as well as on the type of DNA lesions introduced. UV-B irradiation stimulated recombination in the hemizygous IC9 line much more strongly than in the homozygous plants. By contrast, in hemizygous and homozygous IC1 and IC6 plants, the induction of HRF by UV-B/UV-C irradiation was much weaker and was not dependent on the zygosity state. Such results suggest that sister chromatids and homologous chromosomes contribute differently to recombinational repair of UV-B/UV-C radiation–induced DNA damage, depending on the genomic position of the locus to be repaired. Again, in the IC9 line MMS treatment stimulated strongly HRF in both hemizygous and homozygous plants, whereas in hemizygous and homozygous IC1 and IC6 lines treated with MMS, enhancements in the HRF were much weaker. These data suggest that DNA-damage treatments differently induce HR and that the contribution of either sister chromatids or homologs as repair template depends on the genomic position of the recombination substrate.
One explanation for the differential stimulation of recombination could be that MMS and UV radiation introduce different types of DNA damage into the genome, which subsequently involve different repair mechanisms. Whereas MMS generates repair-mediated single-strand breaks, UV-B/UV-C radiation creates mainly cyclobutyl pyrimidine dimers (CPDs) and (6-4) photoproducts [(6-4) PPs] in the plant genome (Britt, 1999). Upon elimination of CPDs and (6-4) PPs, however, other types of DNA damage, such as single-strand breaks, may also be introduced. Alkylation of DNA, the result of MMS activity, is more or less equally distributed within the genome, whereas the formation of CPDs and (6-4) PPs depends on the chromatin structure surrounding the target sequence (Mitchell et al., 1992). Therefore, some chromosomal regions might contain higher numbers of UV-induced DNA lesions than others.
Interestingly, line IC9 reacts generally much stronger toward DNA-damaging agents and involves recombinational DNA repair for different kinds of DNA damage. The sister chromatid may contribute more strongly to the repair of UV-B damage, whereas the contribution of the homologous chromosome to recombinational repair remains unchanged after MMS treatment. This indicates that in this line the regulation of recombination was more sensitive to recombinogenic damage than in the other lines tested. Sister chromatid recombination involves recognition of DNA strands after replication. In S. cerevisiae, somatic cells irradiated with x-rays in G1 were found to repair recombinogenic damage primarily by homolog recombination, whereas those irradiated in G2 repaired such damage preferentially by sister chromatid recombination (Kadyk and Hartwell, 1992). Therefore, another important factor influencing the RF between sister chromatids and that between homologs may be the cell cycle stage at which DNA damage is generated or repaired.
These unequal recombination events may be important in evolutionary terms, as they can account for amplification or deletion of repeats in the genome. The design of these special recombination substrates for monitoring interchromatid and interhomolog recombination events allows the development of a genetic approach for isolating mutant plants exhibiting high HRF. The RF between sister chromatids and those between homologous chromosomes can be differentially monitored and measured in plants either hemizygous or homozygous for the transgene locus. Analysis of individual mutants indeed allows a genetic distinction between the use of either the sister chromatid or the homologous chromosome (J. Molinier, unpublished data).
METHODS
Recombination Substrates
Nonfunctional chimeric uidA genes containing different deletions (from plasmid pGUS 23; Tinland et al., 1994) were produced. From the plasmid pUS (Tinland et al., 1994) containing the 3′ end of the uidA gene, US was cut out with XbaI and HindIII and ligated into the HincII site of pUC19. The plasmid obtained, pU'S', was excised with XbaI and HindIII and then inserted between the XbaI and HindIII sites of the plasmid pG'U' (Tinland et al., 1994). The resulting recombination substrate plasmid, pGRU'S'G'U', carried the partially overlapping uidA region with 1213-bp homologous sequence in direct orientation on its T-DNA (Figure 1A).
The recombination substrate plasmid pGRU'C'L'U' containing the partially overlapping luciferase gene with 1146-bp homologous sequence in direct orientation on the T-DNA (Figure 1B) was made from the plasmid pGN35S-luc+ (kindly provided by G. Neuhaus-Url, unpublished data). The ability of the recombination target vectors to restore a functional uidA and luciferase gene, respectively, was demonstrated by extrachromosomal recombination in leaf tissue upon delivery using a biolistic device.
Plant Transformation and Growth Conditions
The recombination substrate plasmids (pGRU'S'G'U' and pGRU'C'L'U') were mobilized into Agrobacterium tumefaciens and used to transform A. thaliana (ecotype Columbia) employing the in planta transformation method. Transgenic seedlings were selected on GM medium (MS salts [Duchefa Biochemie BV, Haarleem, The Netherlands], 1% sucrose, 0.8% agar, pH 5.8), supplemented with 20 mg/L hygromycin, and kept in a growth chamber under a 16-h/8-h photoperiod (20°C/16°C). The primary transformants (T1) were selfed, and the segregating T2 generation was analyzed for single-insert events by DNA gel blot. For all experiments, the RF in plants was determined using seeds from at least the T3 generation. Plants hemizygous for the recombination substrate were produced after crossing homozygous reporter lines with the wild-type A. thaliana (ecotype Columbia). Three independent outcrosses were performed, and seeds homozygous for the recombination substrate were harvested from the donor/recipient plants used for the corresponding crosses. The hemizygous and the corresponding homozygous plants were assayed for somatic HR events at different time periods.
Histochemical GUS Assay and Bioluminescence LUC Detection
Histochemical GUS assays were performed on 2-week-old A. thaliana seedlings (lines IC1, IC6, and IC9) as described by Jefferson et al. (1987). For the bioluminescence detection of recombination events in the luciferase gene, 2-week-old seedlings of line 58F were assayed for in vivo luciferase activity, as described by Ow et al. (1986). The approximate position of recombination events on whole plants was determined by superimposing a photon-counting image upon a reflected green light image.
Molecular Characterization of Recombination Events
Three leaves of ∼10,000 plants of the progeny of the homozygous IC9 line were screened for a full β-glucuronidase phenotype. By parallel, different batches of seeds (∼1,000,000) originating from a population of homozygous 58F line were screened for a full luciferase phenotype. Two fully recombined IC9-derived plants (AN32 and AE13) and five fully recombined 58F-derived plants (FR1 to FR5) were selfed, and their offspring (R2) were used for genetic and molecular analysis. Genomic DNA was isolated using the DNA extraction kit DNeasy (Qiagen USA, Valencia, CA). One microgram of genomic DNA was digested with the appropriate restriction enzyme. DNA gel blot analysis was performed as described by Sambrook and Russel (2001). Nonradioactive digoxigenin-labeled DNA probes were synthesized with a DIG-DNA labeling kit (Boehringer Mannheim, Mannheim, Germany) using PCR. Hybridization was done with probes specific for the hygromycin phosphotransferase (Hpt) gene or for the overlapping region of the uidA or luc gene.
Treatment of Plants with DNA Damaging Agents
For treatment with BLM and MMS, 2-week-old A. thaliana seedlings were placed in sterile liquid GM medium containing 10−6 M of BLM (Sigma, St. Louis, MO) or 100 ppm of MMS (Fluka, Milwaukee, WI). For UV-B irradiation, 2-week-old seedlings were placed in sterile solid GM medium and were irradiated for 30 min with light from TL12 F40 UV-B lamps (Philips, Eindhoven, The Netherlands) filtered through transmission cutoff filter glasses with half-maximal transmission at 295 nm. For UV-C irradiation (6000 ergs), 2-week-old seedlings were placed in sterile solid GM medium and were irradiated with light provided by a HNS 55W OFR lamp (Osram, Munich, Germany). Immediately after UV-B or UV-C irradiation, plants were moved to white light and allowed to grow for another 4 d. Histochemical GUS assay was performed 4 d after each treatment.
Calculation of RF
The average RF per plant for the different transgenic lines was calculated using mean numbers of recombination events (number of GUS+ or LUC+ spots per plant) in a population of plant siblings (at least 150), according to Sokal and Rohlf (1995).
Acknowledgments
We thank Jürg Kohli, Ingo Schubert, Moez Hanin, Jerzy Paszkowski, and Lisa Valentine for critically commenting on the manuscript and all members of our group for stimulating discussions. We also thank C. Ramos and V. Kalck for their technical support. G.R. was funded by the Swiss Chemical Industries, and J.M. was funded by the European Union PLANTREC Project QLG2-CT-2001-01397. We acknowledge the Novartis Research Foundation for financial support.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Jean Molinier (jean.molinier@fmi.ch).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.019042.
References
- Arbel, A., Zenvirth, D., and Simchen, G. (1999). Sister chromatid-based DNA repair is mediated by RAD54, not by DMC1 or TID1. EMBO J. 9, 2648–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad, F.F., and Signer, E.R. (1992). Somatic and germinal recombination of a direct repeat in Arabidopsis. Genetics 132, 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaaza, A., and Chartrand, P. (1994). One-sided invasion events in homologous recombination at double-strand breaks. Mutat. Res. 314, 199–208. [DOI] [PubMed] [Google Scholar]
- Bolag, R.J., Waldman, A.S., and Liskay, R.M. (1989). Homologous recombination in mammalian cells. Annu. Rev. Genet. 23, 199–225. [DOI] [PubMed] [Google Scholar]
- Britt, A.B. (1999). Molecular genetics of DNA repair in higher plants. Trends Plant Sci. 4, 20–24. [DOI] [PubMed] [Google Scholar]
- Carlson, P.S. (1974). Mitotic crossing-over in a higher plant. Genet. Res., Camb. 24, 109–112. [Google Scholar]
- Fasullo, M.T., and Davis, R.W. (1987). Recombinational substrate designed to study recombination between unique and repetitive sequences in vivo. Proc. Natl. Acad. Sci. USA 84, 6215–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg, E.C., Walker, G.C., and Siede, W. (1995). DNA Repair and Mutagenesis. (Washington, DC: American Society of Microbiology Press).
- Gal, S., Pisan, B., Hohn, T., Grimsley, N., and Hohn, B. (1991). Genomic homologous recombination in planta. EMBO J. 10, 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, A., and Schiestl, R.H. (1995). On the mechanism of UV and gamma-ray-induced intrachromosomal recombination in yeast cells synchronized in different stages of the cell cycle. Mol. Gen. Genet. 248, 301–310. [DOI] [PubMed] [Google Scholar]
- Gisler, B., Salomon, S., and Puchta, H. (2002). The role of double-strand break-induced allelic homologous recombination in somatic plant cells. Plant J. 32, 277–284. [DOI] [PubMed] [Google Scholar]
- Goff, S.A., et al. (2002). A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296, 92–100. [DOI] [PubMed] [Google Scholar]
- Haber, J.E. (1992). Exploring the pathways of homologous recombination. Curr. Opin. Cell Biol. 4, 401–412. [DOI] [PubMed] [Google Scholar]
- Haubold, B., Kroymann, J., Ratzka, A., Mitchell-Olds, T., and Wiehe, T. (2002). Recombination and gene conversion in a 170-kb genomic region of Arabidopsis thaliana. Genetics 161, 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W., Timmermans, M.C.P., and Messing, J. (1998). Interchromosomal recombination in Zea mays. Genetics 150, 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, A.P. (1955). A genetic analysis of sectoring in ultraviolet-induced variant colonies of yeast. Genetics 40, 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelesko, J.G., Harper, R., Furuya, M., and Gruissem, W. (1999). Rare germinal unequal crossing-over leading to recombinant gene formation and gene duplication in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 18, 10302–10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R.D., and Jasin, M. (2001). Double-strand-break-induced homologous recombination in mammalian cells. Biochem. Soc. Trans. 29, 196–201. [DOI] [PubMed] [Google Scholar]
- Kadyk, L.C., and Hartwell, L.H. (1992). Sister chromatids are preferred over homologs as substrate for recombinational repair in Saccharomyces cerevisiae. Genetics 132, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk, L.C., and Hartwell, L.H. (1993). Replication-dependent sister chromatid recombination in rad1 mutants of Saccharomyces cerevisiae. Genetics 133, 469–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, H.L. (1995). Genetic control of intrachromosomal recombination. Bioessays 17, 147–159. [DOI] [PubMed] [Google Scholar]
- Lichtenstein, C., Paszkowski, J., and Hohn, B. (1994). Intrachromosomal recombination between genomic repeats. In Homologous Recombination and Gene Silencing in Plants, J. Paszkowski, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 95–122.
- Mitchell, D.L., Jen, J., and Cleaver, J.E. (1992). Sequence specificity of cyclobutane pyrimidine dimers in DNA treated with solar (ultraviolet B) radiation. Nucleic Acids Res. 20, 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow, D.W., Wood, K.V., DeLuca, M., de Wet, J.R., Helsinki, D.R., and Howell, S.H. (1986). Transient and stable expression of the firefly luciferase gene in plant cells and trangenic plants. Science 234, 856–859. [DOI] [PubMed] [Google Scholar]
- Pâques, F., and Haber, J.E. (1999). Multiple pathways of recombination induced by double-strand break in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63, 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhans, A., Schlupmann, H., Basse, C., and Paszkowski, J. (1990). Intrachromosomal recombination in plants. EMBO J. 9, 3437–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta, H. (1999). Double-strand break-induced recombination between ectopic homologous sequences in somatic plant cells. Genetics 152, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta, H., and Hohn, B. (1996). From centiMorgans to base pairs: homologous recombination in plants. Trends Plant Sci. 1, 340–348. [Google Scholar]
- Puchta, H., Swoboda, P., Gal, S., Blot, M., and Hohn, B. (1995). Somatic intrachromosomal homologous recombination events in populations of plant siblings. Plant Mol. Biol. 28, 281–292. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., and Russel, I. (2001). Molecular Cloning: A Laboratory Manual, 3rd ed. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schiestl, R.H., Igarashi, S., and Hastings, P.J. (1988). Analysis of the mechanism for reversion of a disrupted gene. Genetics 119, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert, R., and Puchta, H. (2002). Efficient repair of genomic double-strand breaks by homologous recombination between directly repeated sequences in the plant genome. Plant Cell 14, 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal, R., and Rohlf, F. (1995). Biometry: The Principles and Practice of Statistics in Biological Research. (New York: W. H. Freeman and Company).
- Stern, C. (1936). Somatic crossing over and segregation in Drosophila melanogaster. Genetics 21, 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda, P., Gal, S., Hohn, B., and Puchta, H. (1994). Intrachromosomal homologous recombination in whole plants. EMBO J. 13, 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J.H. (1984). A brief history of the discovery of sister chromatid exchanges. Basic Life Sci. 29, 1–9. [DOI] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Tinland, B., Hohn, B., and Puchta, H. (1994). Agrobacterium tumefaciens transfers single-strand transferred DNA (T-DNA) into the plant cell nucleus. Proc. Natl. Acad. Sci. USA 91, 8000–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar, J., and Lichtenstein, C. (1992). Somatic and meiotic chromosomal recombination between inverted duplications in transgenic tobacco plants. Plant Cell 4, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]