Abstract
Cell polarity is essential to the function of many cell types, such as epithelial cells and neurons. The Discs large (Dlg) scaffolding protein was identified in Drosophila as a major regulator of basolateral epithelial identity. Four Dlg orthologs (Dlg1 through 4) are found in vertebrates, and mutations in the human Dlg3 gene are associated with X-linked mental retardation. We recently found that Dlg3 controls apical epithelial polarity and tight junction formation and contributes to neural induction in mouse development.1 During evolution, Dlg3 acquired specific PPxY motifs, which bind to the WW domains of the E3 ubiquitin ligases, Nedd4 and Nedd4-2. This interaction results in monoubiquitination of Dlg3, leading to directed microtubule-dependent protein trafficking, via the exocyst complex, in different polarized cell types. Directed trafficking of Dlg3 plays an important role, during both mammalian development and in adulthood, in the establishment and maintenance of specialized apical cell junctions, such as tight junctions in epithelial cells and synapses in neurons.
Keywords: Dlg3/SAP102, NEDD4, X-linked mental retardation, apical, embryo, epithelium, late-gastrula organizer, polarity, tight junctions
Cell polarity is required for morphogenesis, asymmetrical cell division and directed cell migration during embryonic development.2,3 In contrast, the loss of epithelial cell polarity leads to epithelial-mesenchymal transition and is the causal event during tumor metastasis.4-6 Polarization of cells relies on the asymmetric localization of proteins to specialized plasma membrane domains called cell junctions. The junctions maintain cell-cell adhesion, prevent free diffusion of proteins within the plasma membrane and serve as a signaling platform. Epithelial cells generate a barrier via the apical junctional complex (AJC) that includes the apical tight junction (TJ) and the more basally localized adherens junction (AJ).7 The neuro-muscular junction is another type of signaling junction that connects the axon terminal of a motor neuron to the plasma membrane of a muscle fiber. In the central nervous system (CNS), the excitatory synapses also function as signaling junctions and link the axon of one neuron to the dendrite of another neuron.
The various junctions, despite having different functions, share common structural features and molecular compositions. Cell junctions are generally composed of adhesion molecules, trans-membrane receptors, signaling molecules and cytoskeletal and scaffolding proteins. Studies in Drosophila and C. elegans led to the identification of the apical scaffolding protein complexes Crumbs and PAR-aPKC that are necessary for epithelial junction establishment and maintenance.8,9 The scaffolding complex composed of Scribble (Scrib)/Lethal giant larvae (Lgl)/Dlg defines the basolateral membrane located below the AJC. Scrib, Lgl and Dlg act as tumor suppressors that inhibit cell proliferation.10,11 Remarkably, Drosophila Dlg also localizes to neuro-muscular junctions;12 in vertebrates, Dlg1-4 are associated with both presynaptic and postsynaptic membranes, which are the most apical membrane-like domains of neurons in the brain.13
In vertebrates, deciphering Scrib/Lgl/Dlg functions in apical-basal polarity establishment is challenging due to gene duplication during evolution, as one Scribble but two Lgls (Lgl1 and Lgl2) and four Dlgs (Dlg1 through 4) orthologs have been identified.14 The Dlgs belong to the MAGUK (Membrane Associated Guanylate Kinases) family of scaffolding proteins, characterized by the presence of three different protein-protein interaction (PPI) domains, including: the Postsynaptic density 95/Discs large/Zonula occludens-1 (PDZ), the SRC Homology 3 Domain (SH3) and the Guanylate Kinase (GUK) domains. The Dlgs act as molecular scaffolds to build multi-protein complexes that establish and maintain specific membrane regions. In humans, mutations in the Dlg3/SAP102 (synaptic associated protein 102) gene go hand-in-hand with X-linked mental retardation. This genetic disorder leads to synaptic dysfunction and learning and memory impairments.15-18
We recently found that Dlg3 inactivation in mice causes midgestational lethality,1,19 the earliest phenotype during embryonic development described so far in the mammalian Dlg family. At the end of gastrulation, the Dlg3 mutant mouse embryos present with several developmental defects of variable severity, such as an absence of embryonic turning, posterior truncation and forebrain deletion. These abnormalities are a consequence of polarity defects in the embryonic late-gastrula organizer tissues; namely, the definitive endoderm and the axial mesendoderm. During development, the organizer is essential for anterior neural induction and head formation.20,21 We found that Dlg3 acts cell autonomously in the epithelial cells of the late-gastrula organizer to establish apico-basal cell polarity and maintain tissue integrity. These findings suggest that human X-linked mental retardation caused by DLG3 mutations might be due to neural induction failure during early embryonic development.
Furthermore, we found that some of the Dlgs functionally diverged during evolution from the common Drosophila ancestor. Dlg3 is located in the cytoplasm and along the cell membrane, with a peak in distribution at the level of the apical membrane. These observations strongly contrast with the idea that the Dlgs function exclusively at the basolateral membrane, a hypothesis that is based on studies of Dlg in Drosophila. In the brain, the Dlgs cluster at synapses in the presynaptic active zone and postsynaptic densities (PSD) in a highly dynamic manner, both spatially and temporally.22
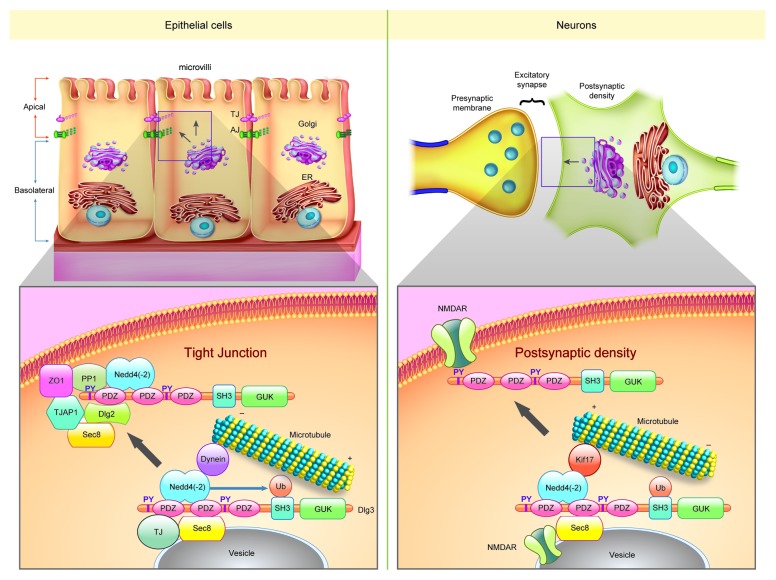
Important clues to understanding the mechanisms of Dlg3-mediated cell polarity and junction establishment came from the analysis of the protein interactome.1 We found that Dlg3 is part of an apical trafficking complex in epithelial cells,23,24 which contains the motor protein dynein25 and the exocyst proteins Sec6 and Sec8.26 Additionally, proteins important for apical polarity establishment, such as the enzymes PP1a27 and the tight junction protein TJAP-1, interact with Dlg3. Interestingly, Dlg3 is the only Dlg family member containing PPxY motifs that bind to the WW domain of Nedd4 and Nedd4-2 E3 ubiquitin ligases.28 The cytoplasmic interaction between Dlg3 and Nedd4 increases during cell polarization and results in Dlg3 monoubiquitination. The Dlg3-Nedd4 interaction is necessary for the binding of Dlg3 PDZ domains to Sec8 and subsequent entry into the secretory pathway. In our model, the Dlg3-Nedd4 multi-protein complex traffics via a microtubule-dependent dynein motor-protein driven fashion toward the TJ and apical membrane. Remarkably, a form of Dlg3 that cannot bind to Nedd4 acts as dominant negative protein and disrupts epithelial polarity establishment. In the neurons of the CNS, Dlg3 appears to interact with NMDA receptors already at the level of the ER/Golgi.26 Dlg3 would then control the NMDA receptor delivery to the membrane during synaptogenesis. In neurons, Dlg3 activity is likely also regulated by Nedd4-dependent post-translational modification. In general, Dlg3 could convey proteins or cargo and function as a docking site for the exocyst at specialized cell junctions (tight junctions, postsynaptic densities and neuro-muscular junctions).
In conclusion, we found that defects in the late-gastrula organizer tissue in the Dlg3 null embryos result in anterior region malformations with low penetrance. In less severely affected embryos, abnormal cortical development may also occur, but this was not detected due to the developmental stage studied. These findings suggest that mutations in human DLG3 could cause subtle defects in forebrain development. Moreover, these mutations could result in the interaction of the mutated DLG3 with NEDD4(-2) while also disrupting the trafficking of the complex toward the synapse26,29,30 (Fig. 1). The evolutionary expansion of the mammalian Dlg family has provided diversity in the mechanisms of cell polarity establishment and junction formation.
Figure 1. Common molecular mechanisms regulate the establishment and maintenance of tight junctions and post-synaptic densities. We hypothesize that Dlg3 regulates multi-protein complex trafficking toward the cell junctions in collaboration with Nedd4(-2) and the exocyst protein Sec8. Dlg3 could interact with other scaffolding proteins, signaling molecules and receptors (for instance: the NMDA receptor) at the level of the endoplasmic reticulum or the Golgi apparatus.26 In epithelial cells, Dlg3 is also found along the cilia; Dlg3 overexpression induces cilia growth (unpublished observations). These findings suggest that Dlg3 could also be involved in anterograde intraflagellar transport.
Acknowledgments
We wish to apologise to the many contributors to this important field whom we were unable to cite due to space constraints. H.L. was funded by the Helmholtz association, a ERC starting grant and the DFG. C.V.C. was a fellow of the Alexander von Humboldt Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/19792
References
- 1.Van Campenhout CA, Eitelhuber A, Gloeckner CJ, Giallonardo P, Gegg M, Oller H, et al. Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4-2 e3 ubiquitin ligases. Dev Cell. 2011;21:479–91. doi: 10.1016/j.devcel.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–35. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 3.Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb Perspect Biol. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 5.Royer C, Lu X. Epithelial cell polarity: a major gatekeeper against cancer? Cell Death Differ. 2011;18:1470–7. doi: 10.1038/cdd.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feigin ME, Muthuswamy SK. Polarity proteins regulate mammalian cell-cell junctions and cancer pathogenesis. Curr Opin Cell Biol. 2009;21:694–700. doi: 10.1016/j.ceb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23. doi: 10.1002/(SICI)1097-4644(19960616)61:4<514::AID-JCB4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 9.Etemad-Moghadam B, Guo S, Kemphues KJ. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell. 1995;83:743–52. doi: 10.1016/0092-8674(95)90187-6. [DOI] [PubMed] [Google Scholar]
- 10.Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays. 2003;25:542–53. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto M, Igaki T. Deciphering tumor-suppressor signaling in flies: genetic link between Scribble/Dlg/Lgl and the Hippo pathways. J Genet Genomics. 2011;38:461–70. doi: 10.1016/j.jgg.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Rafael JA, Hutchinson TL, Lumeng CN, Marfatia SM, Chishti AH, Chamberlain JS. Localization of Dlg at the mammalian neuromuscular junction. Neuroreport. 1998;9:2121–5. doi: 10.1097/00001756-199806220-00039. [DOI] [PubMed] [Google Scholar]
- 13.Tang VW. Proteomic and bioinformatic analysis of epithelial tight junction reveals an unexpected cluster of synaptic molecules. Biol Direct. 2006;1:37. doi: 10.1186/1745-6150-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–30. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Tarpey P, Parnau J, Blow M, Woffendin H, Bignell G, Cox C, et al. Mutations in the DLG3 gene cause nonsyndromic X-linked mental retardation. Am J Hum Genet. 2004;75:318–24. doi: 10.1086/422703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, et al. Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J Neurosci. 2007;27:2673–82. doi: 10.1523/JNEUROSCI.4457-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanni G, van Esch H, Bensalem A, Saillour Y, Poirier K, Castelnau L, et al. A novel mutation in the DLG3 gene encoding the synapse-associated protein 102 (SAP102) causes non-syndromic mental retardation. Neurogenetics. 2010;11:251–5. doi: 10.1007/s10048-009-0224-y. [DOI] [PubMed] [Google Scholar]
- 18.Gardoni F. MAGUK proteins: new targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2008;585:147–52. doi: 10.1016/j.ejphar.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 19.Cox BJ, Vollmer M, Tamplin O, Lu M, Biechele S, Gertsenstein M, et al. Phenotypic annotation of the mouse X chromosome. Genome Res. 2010;20:1154–64. doi: 10.1101/gr.105106.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–74. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 21.Kinder SJ, Tsang TE, Wakamiya M, Sasaki H, Behringer RR, Nagy A, et al. The organizer of the mouse gastrula is composed of a dynamic population of progenitor cells for the axial mesoderm. Development. 2001;128:3623–34. doi: 10.1242/dev.128.18.3623. [DOI] [PubMed] [Google Scholar]
- 22.Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–71. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009;20:2522–9. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinzel D, Boldt K, Davis EE, Burtscher I, Trümbach D, Diplas B, et al. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev Cell. 2010;19:66–77. doi: 10.1016/j.devcel.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–8. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- 26.Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, et al. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat Cell Biol. 2003;5:520–30. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 27.Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc Natl Acad Sci U S A. 2008;105:10402–7. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huibregtse JM, Scheffner M, Beaudenon S, Howley PM. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci U S A. 1995;92:2563–7. doi: 10.1073/pnas.92.7.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–50. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 30.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]