Abstract
In plants and other eukaryotes, long-chain acyl-CoAs are assumed to be imported into peroxisomes for β-oxidation by an ATP binding cassette (ABC) transporter. However, two genes in Arabidopsis thaliana, LACS6 and LACS7, encode peroxisomal long-chain acyl-CoA synthetase (LACS) isozymes. To investigate the biochemical and biological roles of peroxisomal LACS, we identified T-DNA knockout mutants for both genes. The single-mutant lines, lacs6-1 and lacs7-1, were indistinguishable from the wild type in germination, growth, and reproductive development. By contrast, the lacs6-1 lacs7-1 double mutant was specifically defective in seed lipid mobilization and required exogenous sucrose for seedling establishment. This phenotype is similar to the A. thaliana pxa1 mutants deficient in the peroxisomal ABC transporter and other mutants deficient in β-oxidation. Our results demonstrate that peroxisomal LACS activity and the PXA1 transporter are essential for early seedling growth. The peroxisomal LACS activity would be necessary if the PXA1 transporter delivered unesterified fatty acids into the peroxisomal matrix. Alternatively, PXA1 and LACS6/LACS7 may act in parallel pathways that are both required to ensure adequate delivery of acyl-CoA substrates for β-oxidation and successful seedling establishment.
INTRODUCTION
Plant seeds contain considerable storage reserves to supply the embryo with carbon skeletons and energy during the crucial phase of seedling establishment before the seedling becomes photoautotrophic. In oilseeds such as Arabidopsis thaliana, these reserves include large amounts of triacylglycerols (Browse, 1997). Upon germination, fatty acids are hydrolyzed from triacylglycerols stored in oilbodies and converted to CoA thioesters, which are substrates for β-oxidation in the glyoxysomes (Graham and Eastmond, 2002). A large proportion of the acetyl-CoA produced is converted via the glyoxylate cycle to produce succinate that is metabolized further to sucrose. In oil-storing seeds mobilization of fatty acids through β-oxidation in glyoxysomes is critical for germination and early seedling growth, and mutants of A. thaliana defective in β-oxidation require exogenous sucrose for seedling establishment (Hayashi et al., 1998; Zolman et al., 2001). The degradation of fatty acids requires the initial activation to acyl-CoAs and proceeds via the β-oxidation cycle with the repeated cleavage of acetate units. Four enzymatic activities are involved in this process, beginning with acyl-CoA oxidase, followed by enoyl-CoA hydratase and β-hydroxy-acyl-CoA dehydrogenase (both activities of the multifunctional protein), and finally 3-ketoacyl-CoA thiolase (KAT) catalyzing the thiolytic cleavage step. The genes and enzymes involved in β-oxidation and in the glyoxylate cycle are well characterized by enzymatic data and in A. thaliana are encoded by small gene families (Graham and Eastmond, 2002). By contrast, much less is known about the earlier steps involved in hydrolysis of fatty acids from triacylglycerol, their activation to acyl-CoAs and import into the glyoxysome. In particular, the transport mechanism of the substrate and the nature of the substrate itself are matters of debate, not only in plants but also in other organisms.
In Saccharomyces cerevisiae (yeast) as in plants, β-oxidation occurs exclusively in peroxisomes, and two independent mechanisms for fatty acid import into the organelle have been established (Hettema et al., 1996). Medium-chain fatty acids (MCFAs) are predominantly imported as free fatty acids to be activated inside the peroxisome by acyl-CoA synthetase Faa2p. By contrast, long-chain fatty acids (LCFAs) appear to be activated predominantly in the cytoplasm and transported as CoA esters across the peroxisomal membrane. This transport is mediated by an ATP binding cassette (ABC) transporter that is a heterodimer of proteins encoded by PXA1 and PXA2. Disruption of either gene resulted in a significantly decreased ability to grow on medium containing LCFAs as the sole carbon source, and β-oxidation activity was reduced to ∼30% of the wild-type level (Hettema et al., 1996; Shani and Valle, 1996). Mutation analysis indicates that a portion of the LCFAs enter the peroxisome as free fatty acid, thus demonstrating a certain extent of functional overlap between the two entry pathways (Hettema et al., 1996). The removal of the peroxisomal acyl-CoA synthetase Faa2p by gene disruption severely compromised the degradation of MCFAs but did not affect the degradation of LCFAs, and this is also consistent with formation of LCFA-CoA esters outside peroxisomes. When Faa2p was mislocalized to the cytosol (caused by blocking the peroxisomal targeting signal1, or PTS1, through addition of a Lys residue at the C terminus), MCFAs were activated outside the peroxisome, and their degradation became entirely dependent on the presence of Pxa1p/Pxa2p (Hettema et al., 1996). From these experiments it was concluded that LCFA-CoAs synthesized in the cytosol are transported by Pxa1p/Pxa2p through the peroxisomal membrane and directly serve as substrates for β-oxidation.
In mammals, the organization of β-oxidation within the cell is considerably different because most β-oxidation takes place in mitochondria, where the pathway is specific for fatty acids with a chain length of <20 carbons. Transport of fatty acids through the mitochondrion envelope uses the well-characterized carnitine shuttle (Kerner and Hoppel, 2000). Carnitine accepts fatty acids from cytoplasmic acyl-CoA and subsequently transesterifies them to CoASH in the lumen, thus maintaining separation of the cytoplasmic and mitochondrial CoA pools. Peroxisomal β-oxidation in mammals serves a special role in partial degradation of very-long-chain fatty acids (VLCFAs) of 20 to 30 carbons. In contrast with the well-characterized mitochondrial transport system, the transport of the substrate into peroxisomes involves an ABC transporter in the peroxisomal membrane. The proteins from S. cerevisiae and mammals share extensive sequence similarities. The transporter in mammals is called adrenoleukodystrophy protein (ALDP) and was originally identified as the site of mutations in patients with X-linked ALDP (X-ALDP) (Mosser et al., 1993). These patients suffer from elevated levels of VLCFAs in serum and tissue, resulting in degeneration of myelin sheaths in the central nervous system and leading to learning deficiencies, coma, and early death. The disruption of VLCFA breakdown in X-ALDP indicates that the ABC transporter is critical for acyl-CoA transport into peroxisomes as it is in S. cerevisiae. Thus, the prevailing model in S. cerevisiae and animals envisions an ABC transporter in the peroxisomal envelope accepting acyl-CoA as substrate on the cytoplasmic side and delivering this molecule (presumably using energy from ATP hydrolysis) to the lumen, where it is available for β-oxidation (Hettema et al., 1996).
In the plant kingdom, the highest flux of fatty acids through β-oxidation occurs during germination of oilseeds (Graham and Eastmond, 2002). Degradation of LCFAs is entirely dependent on the β-oxidation machinery in peroxisomes (glyoxysomes). Therefore, all the fatty acids released from the oilbodies upon germination have to be transported into the glyoxysomes to be degraded and to provide the seedling with metabolic energy as well as carbon skeletons. Recently, the transport of fatty acids across the glyoxysome membrane was shown to also involve an ABC transporter that is encoded by the A. thaliana PXA1 locus (Zolman et al., 2001), also called PED3 and CTS (Footitt et al., 2002; Hayashi et al., 2002). The PXA1 protein is homologous to the transporters described in S. cerevisiae and mammals, but in contrast with them a single gene encodes the complete transporter with two homologous half-transporters in one polypeptide. The A. thaliana pxa1 mutants require exogenous sucrose in the media for postgerminative growth and seedling establishment, indicating an inability to use reserve lipids. Based on the germination phenotype and on sequence similarities between AtPXA1 and the ABC transporter in S. cerevisiae, it was suggested that the plant PXA1 protein performs the same function as its S. cerevisiae homolog, transporting acyl-CoAs across the peroxisomal membrane (Zolman et al., 2001). Analysis of acyl-CoA levels in germinating wild-type and cts (pxa1) seedlings showed increased levels of acyl-CoAs in mutant seedlings compared with the wild-type controls (Footitt et al., 2002). This result was interpreted as indicating that the substrates for the ABC transporter are acyl-CoAs, as proposed for the S. cerevisiae and animal proteins.
We have recently identified two long-chain acyl-CoA synthetase (LACS) genes in A. thaliana, LACS6 and LACS7, that are highly expressed during germination and early seedling growth and that contain PTS1 and PTS2 peptide sequences. When fused to spectral variants of the green fluorescent protein, the PTS2 of LACS6 and the PTS2 or PTS1 present in LAC7 were sufficient to mediate targeting to peroxisomes (Fulda et al., 2002). The recent discovery of the A. thaliana PXA1 transporter would appear to obviate the need for a peroxisomal long-chain acyl-CoA synthetase (LACS) activity. Therefore, we employed a reverse-genetic approach to isolate knockout mutants deficient in LACS6 and LACS7. Surprisingly, the lacs6 lacs7 double mutant plants exhibit a phenotype similar to the phenotype of pxa1. Our results suggest that PXA1 and LACS6/LACS7 may act sequentially to provide acyl-CoA for β-oxidation in plant peroxisomes. Alternatively, PXA1 and LACS6/LACS7 may act in parallel pathways, both of which are required for successful seedling establishment. Our results also recommend a reevaluation of the proposed mechanisms of peroxisomal fatty acid import in S. cerevisiae and animals.
RESULTS
Isolation of lacs6 and lacs7 Mutants by Reverse Genetics
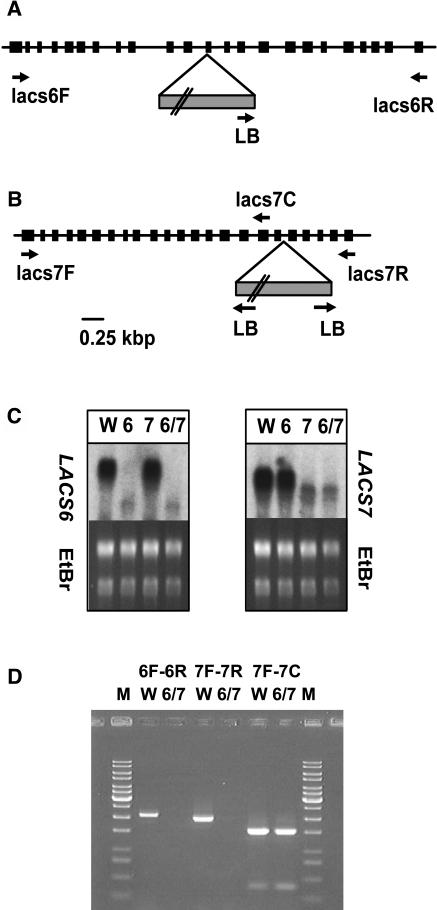
The A. thaliana LACS6 and LACS7 genes have previously been shown to encode peroxisomal LACS isozymes (Fulda et al., 2002; Shockey et al., 2002). To investigate the biological roles of these enzymes, we initiated a screen to isolate knockout mutant lines for each gene. In this process, several T-DNA knockout populations available through the ABRC and through the University of Wisconsin–Madison were subjected to a PCR-based screen. The primers for this screen were designed to the 5′ (forward [F] primers 6F and 7F) and and 3′ (reverse [R] primers 6R and 7R) portions of the target genes and used in combination with a T-DNA left border (LB) primer. Two lines containing insertions at the LACS6 locus were chosen for further analysis, and additional rounds of PCR allowed us to isolate two independent lacs6 knockout lines. Plants heterozygous for a T-DNA insert in LACS6 were used to investigate the number of T-DNA insertions by germinating the seeds under kanamycin selection. Only one line (designated lacs6-1) exhibited a segregation ratio on kanamycin that indicated the presence of a single T-DNA insertion. Of 684 seeds, 177 were kanamycin sensitive, which is a good fit to the 3:1 hypothesis for a single insertion (χ2 = 0.281, P > 0.5). DNA sequence analysis of this line revealed that the T-DNA is inserted into exon 12 having the LB oriented toward the 3′ end of the gene (Figure 1A). Homozygous progeny were identified by PCR and were not obviously distinguishable from the wild type under our normal growth conditions. Most significantly, lacs6-1 seeds germinated readily on soil or on agar plates without sucrose, and the young seedlings became established and grew as rapidly as their wild-type and heterozygous siblings. There was, thus, no indication that β-oxidation of fatty acids was significantly compromised in lacs6-1 plants.
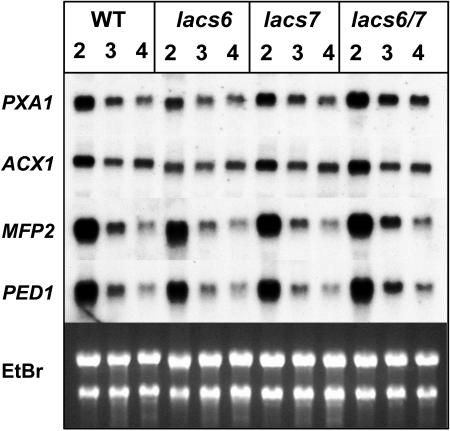
Figure 1.
Identification of lacs6 and lacs7 Mutants.
(A) and (B) Genomic structure of the lacs6-1 and lacs7-1 loci. Arrows, locations of the primers used for the PCR-based screening; closed boxes, exons.
(A) In lacs6-1, the T-DNA is inserted in exon 12 with the LB oriented toward the 3′ end of the gene.
(B) In lacs7-1, the T-DNA is located in intron 18. PCR experiments demonstrated that LB sequences are oriented toward both the 5′ and 3′ ends of the gene.
(C) Gene expression in wild-type and mutant plants. Samples of total RNA from seedlings of the wild type (W), lacs6-1 (6), lacs7-1 (7), and the lacs6 lacs7 double mutant (6/7) were subjected to gel blot analysis using a LACS6 or LACS7 cDNA as probe. Equal loading was confirmed by comparing ethidium bromide staining of rRNA bands (EtBr).
(D) RT-PCR fails to detect LACS6 or LACS7 transcript in the double mutant. Total RNA from wild-type (W) or lacs6 lacs7 double mutant (6/7) seedlings was used as template for RT-PCR reactions using 6F-6R, 7F-7R, or 7F-7C primer combinations (see [A]). The resulting products were separated by gel electrophoresis and stained using ethidium bromide. M, molecular weight markers.
A total of 140,000 lines were screened for a T-DNA insertion in LACS7, and one line of the BASTA population of the University of Wisconsin–Madison was found to contain an insertion in intron 18. The selfed progeny from a single heterozygous plant were used to investigate the number of T-DNA insertions by spraying 10-d-old seedlings with Basta solution. From 427 seedlings, 109 were found to be Basta sensitive. This result is in good agreement with the 3:1 hypothesis for a single insertion (χ2 = 0.063, P > 0.8), and the mutant line was designated lacs7-1. Interestingly, PCR products were obtained with both the 7F/LB and 7R/LB primer combinations. Sequencing of the 7F-LB product indicated a plant DNA/T-DNA junction at position 3290 of the genomic sequence (where A of the start codon is 1), whereas the sequencing of the 7R-LB product indicated a plant DNA/T-DNA junction at position 3306. These results are consistent with rearrangement of a T-DNA catena upon insertion, such that both the 5′ and 3′ junctions involve a T-DNA LB. In addition, 16 bp from intron 18 of the LACS7 gene have probably been incorporated into the T-DNA catena or lost from the mutant locus. The precise organization of the insertion was not further analyzed. The physical map of the lacs7-1 mutant is shown in Figure 1B. Basta-resistant progeny from the LACS7/lacs7-1 parent did not show segregation of a recognizable phenotype, so homozygous mutant plants were identified by PCR using 7R/LB and 7F/7R primer combinations.
Further detailed analyses of both lacs6-1 and lacs7-1, including the timing and progress of germination, in the light and in the dark, with or without sucrose, and at 22 or 6°C did not reveal any substantial difference from the wild type. There were no differences in the lipid content or fatty acid composition of germinating seedlings or in the sensitivity of seedlings to different concentrations of 2,4-dichlorophenoxybutyric acid (2,4-DB) (Hayashi et al., 1998).
Production and Characterization of a lacs6 lacs7 Double Mutant
The absence of any phenotype in the mutants was consistent with a model in which the PXA1 protein delivers acyl-CoA molecules to the glyoxysome matrix from the cytoplasm (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002). However, the LACS6 and LACS7 proteins have very similar fatty acyl substrate specificities, and the two genes are both highly expressed during germination (Fulda et al., 2002). We therefore considered the possibility that LACS6 and LACS7 perform substantially overlapping functions. To test this possibility, we crossed lacs6-1 and lacs7-1 plants and allowed the resulting F1 progeny to self. F2 seeds from the cross were plated on medium containing kanamycin (to select against individuals lacking a lacs6-1 allele) and sucrose. Sucrose supplementation has been shown as necessary and sufficient for seedling establishment in several mutants deficient in β-oxidation (Hayashi et al., 1998; Zolman et al., 2001); sucrose is not required for subsequent growth of β-oxidation–deficient plants. After 10 d on sucrose medium, we transferred F2 plants to soil and sprayed them with Basta to select against individuals lacking a lacs7-1 allele. After Basta selection, DNA was prepared from a single leaf of each of the 38 plants remaining in the experiment. One of these plants tested negative for the presence of LACS6 and LACS7 wild-type alleles when tested by PCR using 6F/6R and 7F/7R primer combinations. Subsequent PCR reactions with these primer combinations together with 6R/LB and 7R/LB combinations confirmed the isolation of a lacs6-1 lacs7-1 double mutant.
We isolated total RNA from single and double mutant seedlings germinated on sucrose along with wild-type controls. The RNA was subject to gel blot analysis using radiolabeled, gene-specific probes to LACS6 and LACS7. The resulting autoradiograph in Figure 1C shows high expression of LACS6 in the wild-type and lacs7-1 seedlings but only a weaker, lower molecular weight band in lacs6-1 and lacs6-1 lacs7-1 seedlings. These results are consistent with termination of lacs6-1 transcription at or near the site of T-DNA insertion. Similarly, the smaller, less abundant RNA species hybridizing to the LACS7 probe in RNA from lacs7-1 and lacs6-1 lacs7-1 seedlings likely represents termination of transcription at or near the site of T-DNA insertion in this gene. Attempts to amplify a reverse transcription (RT)–PCR product using the 6F and 6R primers were unsuccessful with RNA from lacs6 lacs7 mutant seedlings as template, even though wild-type RNA gave a PCR product of the expected molecular weight (Figure 1D). Similarly, RT-PCR using 7F and 7R primers failed to detect any full-length LACS7 transcript in the double mutant. As a positive control of the RNA prepared from lacs6 lacs7 tissue, we used the 7F primer in combination with a reverse primer designed to an exon DNA sequence immediately 5′ to the T-DNA insertion site (Figure 1A). With this combination, RT-PCR products of the expected size were produced from both wild-type and lacs6 lacs7 RNA (Figure 1D). It is very likely that lacs6-1 and lacs7-1 are null alleles because the regions of each gene 3′ to the T-DNA insertion site encode portions of the proteins that are highly conserved among the nine LACS proteins in A. thaliana and among the many LACS sequences reported from plants, animals, and microbes (Shockey et al., 2002).
The lacs6 lacs7 Double Mutant Is Defective in Postgerminative Growth
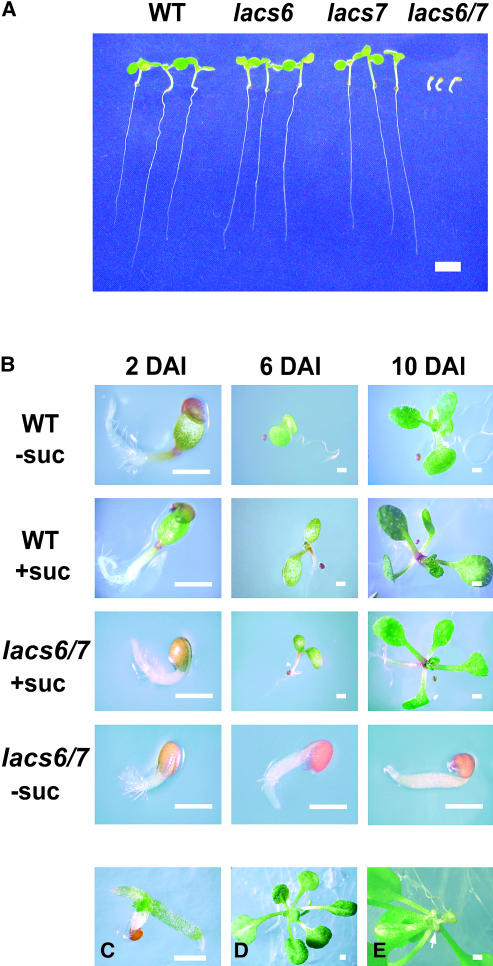
The lacs6-1 lacs7-1 mutant identified by PCR had germinated and become established normally on medium containing 1% sucrose. To discover whether sucrose is required for seedling establishment, we placed homozygous F3 seeds of the double mutant on media with or without sucrose, along with seeds of the wild-type and the lacs6 and lacs7 single mutants. After 7 d on media without sucrose, the lacs6 and lacs7 single mutants were indistinguishable from the wild type with fully expanded cotyledons, an emerging pair of leaves, and strong growth of the primary root. By contrast, the lacs6 lacs7 double mutant plants shown in Figure 2A had germinated but stalled in their postgerminative growth after emergence of the radicle. The cotyledons remained unexpanded and usually still inside the seed coat, and there was no development of true leaves. Figure 2B shows wild-type and double mutant seedlings germinated with or without sucrose compared at 2, 6, and 10 d after imbibition. Growth and development of double mutant seedlings on sucrose closely matched the wild-type controls (with or without sucrose), although we recorded a slight (∼7 to 10 h) delay in emergence of the radicle and cotyledons in the mutant (see photos at 2 d in Figure 2B). Initial germination of double mutant seeds without sucrose appeared relatively normal (see photos at 2 d), but subsequent development was arrested. This phenotype is very similar to those reported for other A. thaliana mutants deficient in β-oxidation (Hayashi et al., 1998).
Figure 2.
Phenotype of the lacs6 lac7 Double Mutant.
(A) Wild-type A. thaliana (WT), lacs6, lacs7, and lacs6 lacs7 double mutant (lacs6/7) were grown for 7 d on medium without sucrose. (Seedlings were rearranged on an agar plate before being photographed.)
(B) Seedlings of wild-type (WT) and lacs6 lacs7 (lacs6/7) were grown on media with (+suc) or without (−suc) sucrose supplementation and photographed 2, 6, and 10 d after imbibition (DAI).
(C) lacs6 lacs7 double mutant seedlings after 5-weeks growth in the absence of sucrose.
(D) A lacs6 lacs7 double mutant plant was maintained on medium without sucrose for 3 weeks and then transferred to medium containing 1% sucrose for 16 d.
(E) Same plant as in (D) from beneath. The arrow indicates a vestigial cotyledon.
Bars = 2 mm in (A) and 0.4 mm in (B) to (E).
When arrested double mutant seedlings were maintained on media without sucrose, they remained alive. Sometimes the cotyledons became exposed, but they remained white and unexpanded. This was true even after 5 weeks of incubation on medium lacking sucrose. Thus, the 5-week-old seedlings shown in Figure 2C show these relatively undeveloped cotyledons; the green tissue between the cotyledons appears to be the partially enlarged shoot meristem. The arrested seedlings retained their potential for development for >3 weeks. Figure 2D shows a typical double mutant plant that was germinated and kept on media without sucrose for 3 weeks before transfer to 1% sucrose medium. Sixteen days after transfer, the plant had developed into a robust rosette. However, the cotyledons on such plants remain vestigial, white organs (Figure 2E), in contrast with the cotyledons of double mutant plants germinated on sucrose (Figure 2B). The ability of lacs6 lacs7 seedlings to undergo further growth and development declined gradually beyond 3 weeks on sucrose-free media so that none of the 5-week-old seedlings could produce viable plants after transfer to sucrose medium.
Reduced Lipid Turnover and Persistent Oilbodies in the lacs6 lacs7 Double Mutant
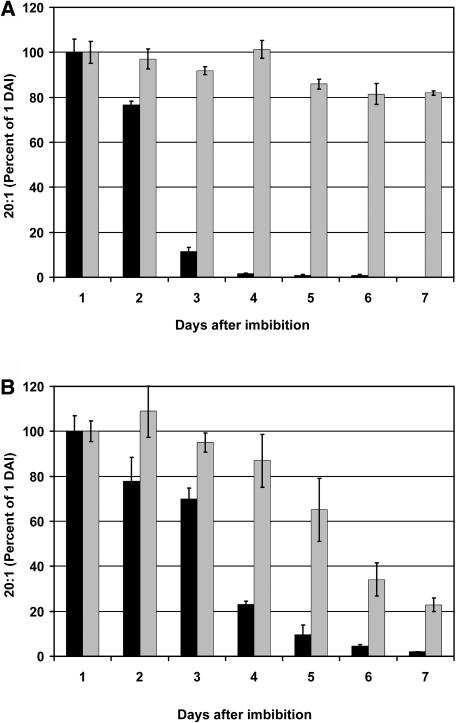
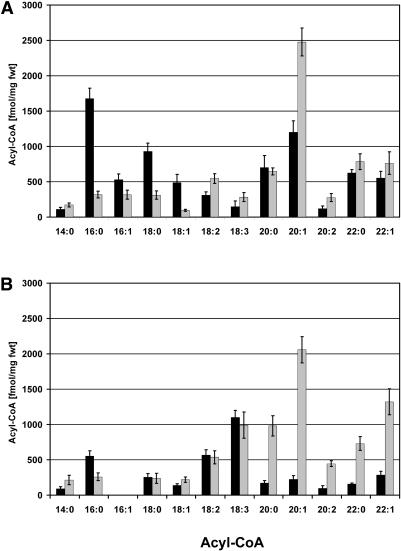
During germination of A. thaliana seeds (and other oil-storing seeds), triacylglycerol storage lipid (TAG) is broken down even as de novo fatty acid synthesis provides acyl groups for the synthesis of polar membrane lipids. Turnover of storage lipid can be followed by separating TAG from polar lipids by thin layer chromatography. However, in A. thaliana, eicosenoic acid (20:1) is a specific component of TAG that acts as a more convenient reporter of TAG breakdown (Lemieux et al., 1990; Germain et al., 2001). The results in Figure 3A show that when lacs6 lacs7 seeds were germinated on medium without sucrose, the content of 20:1 declined by <20% over 7 d. By contrast, wild-type seeds showed a rapid and complete mobilization of 20:1, so that 99% of this fatty acid had been broken down at 4 d. This result is consistent with utilization of TAG reserves during the period when cotyledons expand and become green and the seedling root becomes established (see Figure 2B). The lack of 20:1 breakdown in the double mutant is consistent with the very limited development after germination, as shown in Figure 2B.
Figure 3.
Delayed Lipid Mobilization in lacs6 lacs7 Seedlings.
(A) Breakdown of storage lipid in wild-type (closed bars) and lacs6 lacs7 (shaded bars) seedlings germinated in the absence of sucrose.
(B) Breakdown of storage lipid in wild-type (closed bars) and lacs6 lacs7 (shaded bars) seedlings grown on 1% sucrose.
The weight of 20:1 was measured in three samples of 10 seedlings each at each time point and expressed as a percentage of the 20:1 content at 1 d after imbibition (1.22 and 1.01 μg/seed in wild-type and mutant seedlings, respectively). Error bars indicate standard errors (n = 3). DAI, days after imbibition.
When wild-type plants were germinated on sucrose, 20:1 declined at a somewhat slower rate (presumably because of the abundant supply of sucrose) but was nevertheless 98% metabolized by day 7 of the experiment, as shown in Figure 3B. Even though lacs6 lacs7 seedlings germinated and developed on sucrose at nearly the same rate as the wild type (Figure 2B), 20:1 was metabolized very little in the first 4 d of the experiment (Figure 3B). Somewhat surprisingly, 20:1 was turned over after day 4, although at a much slower rate than in the wild type so that the double mutant seedlings still retained 34% of their TAG at 6 d and 23% at 7 d. These results are similar to those reported for pxa1/cts seedlings germinated on sucrose (Footitt et al., 2002) but differ from the results of Germain et al. (2001) for the ped1/kat2 mutant (deficient in the major isoform of peroxisomal thiolase), which exhibited no significant breakdown of TAG up to 5 d after germination.
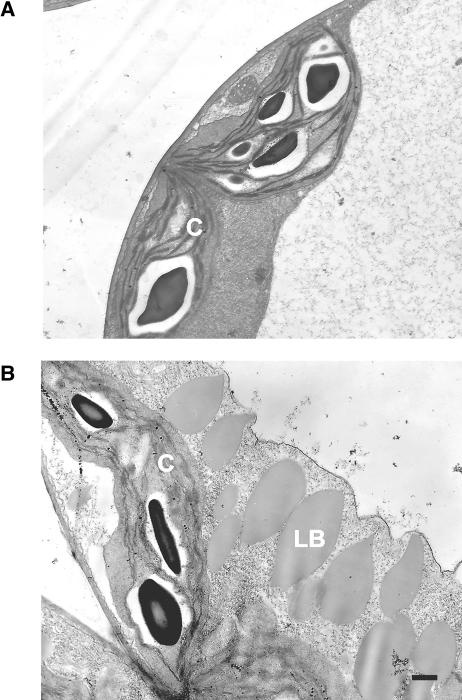
We extended our comparison of lacs6 lacs7 seedlings with seedlings of these other two classes of β-oxidation mutants by examining the ultrastructure of cotyledons from 6-d-old green seedlings that had been germinated on sucrose. The electron micrographs shown in Figure 4 show striking numbers of large lipid bodies in lacs6 lacs7 cells, but these are not seen in wild-type cells. This finding reflects the compromised degradation of stored lipid reserves, as also established by the delayed metabolism of 20:1. Persistent oilbodies are also a feature of the ped1/kat2 (Hayashi et al., 1998; Germain et al., 2001) and cts/ped3 (pxal) mutants (Footitt et al., 2002; Hayashi et al., 2002). Inspection of a number of sections by electron microscopy confirmed this result and also indicated that glyoxysomes in the lacs6 lacs7 mutant were morphologically normal, with no evidence for the enlarged, reticulate glyoxysomes seen in thiolase mutants ped1/kat2 (Hayashi et al., 1998; Germain et al., 2001). Measurements on 16 glyoxysomal sections in cells of the double mutant indicated an average diameter of 605 ± 31 nm (mean ± se) compared with 635 ± 47 nm for the wild type.
Figure 4.
Ultrastructure of Developing Cotyledons.
Transmission electron micrographs of cotyledons from 6-d-old wild-type (A) and lacs6 lacs7 (B) seedlings grown on media supplemented with 1% sucrose. C, chloroplast; LB, lipid body. Bar = 500 nm.
To investigate whether the deficiency in peroxisomal LACS and the associated reduction in TAG breakdown are correlated with changes in the pool sizes of acyl-CoAs, we used the method of Larson and Graham (2001) to analyze acyl-CoAs in wild-type and lacs6 lacs7 mutants. The results shown in Figure 5A indicate that 2-d-old wild-type and lacs6 lacs7 seedlings have a similar level of acyl-CoAs, 7.4 ± 0.11 pmol/mg fresh weight in wild-type and 7.0 ± 0.17 pmol/mg in the double mutant. However, 5-d-old seedlings of the double mutant contained dramatically increased concentrations of 20- and 22-carbon acyl-CoAs relative to the wild type (Figure 5B). The concentration of 20:1-CoA in lacs6 lacs7 seedlings is nearly 10-fold higher than in the wild type, and this mirrors the difference in the quantity of 20:1, mostly in TAG, measured in 5-d-old double mutant and wild-type seedlings shown in Figure 3B. Accumulation of 20- and 22-carbon acyl-CoAs in the double mutant results in a total acyl-CoA pool of 8.0 ± 0.10 pmol/mg fresh weight compared with 3.6 ± 0.17 pmol/mg fresh weight in the wild-type controls. Together with results from the kat2 (Germain et al., 2001) and cts mutants (Footitt et al., 2002), these results indicate that acyl-CoA accumulation occurs in germinating oilseeds whenever peroxisomal β-oxidation is blocked, even when the block is caused by a defect in acyl-CoA synthetase.
Figure 5.
Acyl-CoA Profiles of Wild-Type and lacs6 lacs7 Seedlings.
(A) Two-day-old seedlings.
(B) Five-day-old seedlings.
Seedlings were imbibed for 4 d at 0°C and then germinated at 22°C on medium containing sucrose. Values are mean ± se for four samples of 100 mg each. Closed bars, wild-type seedlings; shaded bars, lacs6 lacs7 seedlings; fwt, fresh weight.
Gene Expression in the lacs6 lacs7 Mutant
The defect in lipid mobilization observed in lacs6 lacs7 seedlings is (presumably) the result of the failure to provide acyl-CoA substrates at the site of β-oxidation in the peroxisome. It is also possible that expression of genes encoding enzymes of β-oxidation would be repressed in the absence of substrates. To test this possibility, we compared the transcript levels of PXA1, acyl-CoA oxidase1 (ACX1), multifunctional protein (MFP2), and 3-ketoacyl-CoA thiolase (PED1) in the lacs6 and lacs7 single mutants, the lacs6 lacs7 double mutant, and the wild type at 2, 3, and 4 d after imbibition. The results of RNA gel blot analysis (Figure 6) revealed no substantial differences in transcript levels for these genes between wild-type and mutant lines. These data argue against a role for acyl-CoAs as signaling molecules affecting expression of genes involved in the β-oxidation pathway.
Figure 6.
Expression of Genes Involved in Lipid Degradation.
Wild type, both single mutants (lacs6 and lacs7), and the lacs6 lacs7 double mutant (lacs6/7) were grown on medium containing sucrose. RNA was isolated at 2, 3, and 4 d after imbibition and subject to gel blot analysis. Filters were exposed to x-ray film for 5 h for the peroxisomal ABC transporter (PXA1), the multifunctional protein2 (MFP2), and the ketoacyl-CoA thiolase (PED1) and for 14 h for acyl-CoA oxidase (ACX1). Ethidium bromide staining of the gel (EtBr) was used to verify equal loading.
The lacs6 lacs7 Double Mutant Is Not Resistant to 2,4-DB
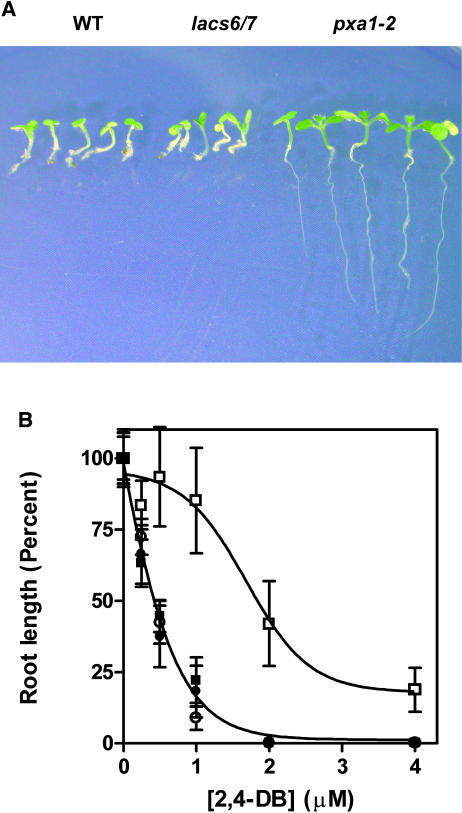
Several β-oxidation mutants in A. thaliana have been screened as lines that are resistant to the growth inhibition caused by 2,4-DB (Hayashi et al., 1998), or indolebutyric acid (IBA) (Zolman et al., 2000). 2,4-DB is converted, by one cycle of β-oxidation, to the synthetic auxin, 2,4-D, which inhibits seedling growth at micromolar concentrations (Synerholm and Zimmermann, 1947; Wain and Wightman, 1954; Epstein and Ludwig-Müller, 1993). Thus, the series of ped mutants, which include ped1 (also named kat2), deficient in seedling thiolase activity, and pxa1 (also named ped3 and cts), deficient in the peroxisomal ABC transporter PXA1, all germinate and grow on sucrose media in the presence of 0.25 to 4 μM 2,4-DB (Hayashi et al., 1998).
To find out if LACS6 and/or LACS7 are required to activate 2,4-DB to the CoA thioester—a prerequisite to β-oxidation—we germinated seeds of the wild type, lacs6, lacs7, lacs6 lacs7, and pxa1 on agar medium supplemented with 1% sucrose and 2 μM 2,4-DB. As shown in Figure 7A, the pxa1 mutant was able to grow well on this medium. However, the lacs6 lacs7 double mutant and both single mutants (data not shown) were inhibited to the same extent as wild-type controls. When the concentration of 2,4-DB was varied in the range 0.25 to 4.0 μM, the degree of growth inhibition in lacs6 lacs7 was consistently similar to the inhibition observed in the wild type (Figure 7B). The results of these experiments indicate that activation of the 2,4-DB proherbicide is not dependent on the LACS6 and LACS7 enzymes. The lacs6 lacs7 double mutant also showed wild-type sensitivity to IBA, with >90% inhibition of root growth at 5 μM IBA.
Figure 7.
lacs6 lacs7 Seedlings Are Sensitive to 2,4-DB.
(A) Wild-type A. thaliana of the Ws ecotype (WT), the lacs6 lacs7 double mutant (lacs6/7), and the 2,4-DB–resistant mutant pxa1-2 were grown for 7 d on medium containing 2 μM 2,4-DB and 1% sucrose. Before photographs were taken, seedlings were removed from the media and rearranged on agar plates.
(B) Dose–response curve for wild-type and mutant seedlings grown on different concentrations of 2,4-DB. Root lengths (10 seedlings per treatment) were measured 7 d after germination and normalized to the 0 μM 2,4-DB controls. Lengths of the roots (average ± SE) in the control treament were as follows: Ws wild type (closed square), 14.0 ± 0.3 mm; Col-0 wild type (closed circle), 14.7 ± 0.4 mm; lacs6 lacs7 (open circle), 11.4 ± 0.4 mm; and pxa1-2 (open square), 13.1 ± 0.4 mm.
DISCUSSION
We have investigated the biochemical and biological roles of peroxisomal LACS isozymes. In A. thaliana, two genes encoding peroxisomal LACS have been identified and designated LACS6 and LACS7 (Fulda et al., 2002). Further analysis of the whole family of acyl-activating enzymes containing 63 members (Shockey et al., 2003) suggested that these two genes encode the only peroxisomal LACS isozymes in A. thaliana. To elucidate the biological functions of these proteins, we used a reverse-genetics approach to isolate knockout mutants for both genes. Plants homozygous for each single mutation did not show any detectable phenotype. Earlier studies gave reasons to believe that the two proteins might be able to complement each other. Although the two LACS proteins share only 72% amino acid sequence identity, they appear to have strongly overlapping functions as indicated by a comparison of their expression profiles as well as their substrate specificities (Fulda et al., 2002). Even with knockout mutants for both genes, we are still unable to define distinguishable tasks for each of the encoded proteins.
To abolish peroxisomal LACS activity completely, we generated a lacs6 lacs7 double mutant. In contrast with both single mutants, seedlings of the double mutant arrested in postgerminative growth and were unable to elongate the primary root or to develop green leaves, unless they were provided with exogenous sucrose. The requirement for sucrose during postgerminative growth indicates an inability to retrieve metabolic energy and/or carbon skeletons from the stored lipid reserves. The requirement for sucrose in this developmental stage has been correlated with a lack of, or at least a strong reduction in, glyoxysomal fatty acid β-oxidation (Hayashi et al., 1998; Germain et al., 2001). The failure of lac6 lacs7 plants to rapidly degrade fatty acids and the persistence of oilbodies in cotyledons of the double mutants provide further evidence that the LACS6/LACS7 enzyme activity is critical for mobilizing fatty acids into β-oxidation.
The lacs6 lacs7 double mutant has the sucrose-dependent phenotype in common with the ped1/kat2 mutant deficient in thiolase activity (Hayashi et al., 1998; Germain et al., 2001), as well as with the pxa1/ped3/cts mutant deficient in a peroxisomal ABC transporter involved in the transport of fatty acids across the peroxisomal membrane (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002). Both the kat2 mutant (Germain et al., 2001) and the cts mutant (Footitt et al., 2002) have been shown to accumulate acyl-CoAs to higher levels than the wild type during germination on sucrose. These observations have been interpreted as reflecting the accumulation of acyl-CoA intermediates, either in the peroxisomes (because of a deficiency of thiolase in the kat2 mutant) or in the cytoplasm (because of a deficiency of the peroxisomal membrane acyl-CoA transporter in the cts mutant). We found that total acyl-CoA concentrations are more than twofold higher in 5-d-old lacs6 lacs7 seedlings than in wild-type controls. Most of the difference between mutant and the wild type is accounted for by 20:1-CoA and other 20- and 22-carbon acyl-CoAs (Figure 5B). These results are similar to those reported for the cts and kat2 mutants (Germain et al., 2001; Footitt et al., 2002). In each case, a block in TAG breakdown in the cotyledons is associated with very substantial increases in the accumulation of 20- and 22-carbon acyl-CoAs relative to wild-type controls. These observations are not immediately helpful in understanding the need for peroxisomal LACS activity as well as the PXA1 transporter, which is discussed below. Perhaps any block in β-oxidation results in accumulation of fatty acids as acyl-CoAs in the cytoplasm of cotyledon cells. This raises the question of whether acyl-CoAs may act to downregulate the enzymes of TAG hydrolysis.
In contrast with the ped1/kat2 and pxa1/ped3/cts mutants, the lacs6 lacs7 double mutant was found not to be resistant to the proherbicide 2,4-DB, which is converted to 2,4-D by one cycle of β-oxidation, nor to IBA, which is converted to IAA by β-oxidation. Apparently another CoA ligase is capable of activating 2,4-DB, most probably the enzyme that synthesizes IBA-CoA for conversion to IAA β-oxidation (Epstein and Ludwig-Müller, 1993). The reactions of β-oxidation also provide for the conversion of 3-oxo-2(2′[Z]-pentenyl)-cyclopentane-1-octanoic acid (OPC:8) to jasmonic acid, a plant hormone with roles in both defense signaling and development (Turner et al., 2002). The most specific phenotype of jasmonate deficiency in A. thaliana is male sterility caused by the failure of anthers and pollen to complete maturation (McConn et al., 1994; Stintzi and Browse, 2000). Because the lacs6 lacs7 double mutant is fully fertile, we conclude that activation of OPC:8 to the corresponding CoA ester is also not dependent on the LACS activity of the LACS6 and LACS7 isozymes. These observations indicate that there may be a number of distinct CoA ligase enzymes responsible for the activation of IBA, 2,4-DB, OPC:8, and other substrates for peroxisomal β-oxidation. Genomics analysis of the large family of genes encoding acyl-activating enzymes suggests that 44 of these genes encode CoA ligase activities (Shockey et al., 2003). Approximately 20 of these 44 genes have been functionally characterized to at least some extent (Fulda et al., 2002; Schnurr et al., 2002; Shockey et al., 2002, 2003; our unpublished observations). The remaining genes encode proteins that are predicted to be targeted to the peroxisome—based on the presence of identifiable PTS1 or PTS2 peroxisomal targeting sequences (Gould et al., 1989; Swinkels et al., 1991)—and other subcellular sites. We assume that the OPC:8-CoA ligase will be one of the peroxisomal isoforms because the enzyme responsible for OPC:8 synthesis, OPR3, is targeted to this organelle (Strassner et al., 2002). It is not clear whether the protein(s) catalyzing IBA/2,4-DB activation are localized inside the peroxisome or elsewhere in the cell (see below).
Our observations and those of other groups indicate that β-oxidation of fatty acids is not required for germination (Hayashi et al., 1998; Graham and Eastmond, 2002). Rather, the several processes of seedling establishment, including root elongation, expansion and greening of the cotyledons, and production of leaves from the apical meristem are blocked unless an exogenous carbon source is provided. Interestingly, the block is readily reversible for at least 3 weeks after germination in lacs6 lacs7 mutants maintained at 22°C. Presumably, carbon from nonlipid reserves is adequate to maintain the apical meristem, root meristem, and other essential organs of the seedling so that development proceeds relatively normally once an exogenous carbon source is provided.
Implications of the lacs6 lacs7 Phenotype for Fatty Acid Transport
In A. thaliana, a deficiency in fatty acid transport across the peroxisomal membrane in pxa1 and a deficiency in peroxisomal fatty acid activation in lacs6 lacs7 result in very similar phenotypes. Both mutants are compromised in the degradation of stored lipid reserves during early postgerminative growth, resulting in arrest of the seedling in this stage (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002). Our data indicate that in contrast with S. cerevisiae, peroxisomal LACS activity is essential for efficient use of LCFA in A. thaliana. Because the targeting by PTS1 and PTS2 as well as biochemical data presented by Gerbling and Gerhardt (1987) strongly suggest LACS activity to be located inside the peroxisomes, the growth phenotype of the seedlings seems to imply that, in the lacs6 lacs7 mutant, fatty acids are not in an activated state at some stage after entering the peroxisome. More detailed analysis of the fatty acids in young seedlings of the double mutant revealed that the degradation of lipid reserves in plants grown on media containing sucrose is severely delayed in the double mutant but not completely inhibited. These results establish that although peroxisomal LACS activity is a prerequisite for postgerminative growth, the double mutant is still able to slowly degrade fatty acids, which must be activated by an alternative mechanism. This resembles the situation in the pxa1 mutant provided with exogenous sucrose, in which ∼30% of TAG-derived fatty acids are degraded by 5 d after imbibition (Footitt et al., 2002) compared with ∼90% in the wild type. On the other hand, in the thiolase-deficient ped1 mutant, no appreciable decrease of triacylglycerol or fatty acids was detected up to 5 d after imbibition (Germain et al., 2001). Thus, one possibility is that blocks in the fatty acid degradation pathway set by pxa1 as well as by lacs6 lacs7 are leaky in the presence of exogenous sucrose and allow slow breakdown of fatty acids by PED1-dependent β-oxidation. However, little or no degradation of reserve lipid took place in lacs6 lacs7 seedlings incubated without sucrose.
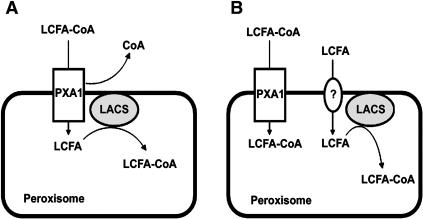
The close similarity between the phenotype of the lacs6 lacs7 double mutant and that of the pxa1 mutants suggests that these genes may encode components acting in the same pathway of fatty acid transport into the peroxisome. It may be suggested that the acyl-CoA synthetase encoded by LACS6 and LACS7 would be located on the outer surface of the peroxisomal membrane and supply acyl-CoAs to the PXA1 transporter. However, all available evidence indicates that the PTS1 and PTS2 sequences target proteins through the peroxisomal membrane into the matrix of this organelle (Gould et al., 1989; Swinkels et al., 1991; Johnson and Olsen, 2001). Furthermore, direct enzyme assays of acyl-CoA synthetase in Vigna radiata (mung bean) peroxisomes showed that the activity is protected from proteases in the intact peroxisome and is latent—that is to say the enzyme activity could only be detected after disruption of the organelles (Gerbling and Gerhardt, 1987). One model that allows the LACS6/LACS7 enzyme to act inside the peroxisome and in series with the PXA1 transporter is shown in Figure 8A. In this model, PXA1 accepts acyl-CoA as substrate on the cytoplasmic side of the peroxisomal membrane but then hydrolyzes the molecule to deliver the unesterified fatty acid to the luminal side while returning the CoA to the cytoplasm. The LACS6/LACS7 activity then synthesizes the acyl-CoA substrate required for β-oxidation. This model appears energetically wasteful, but it allows the maintenance of separate CoA pools in the cytoplasm and peroxisome. In point of fact, there is no direct evidence that the A. thaliana PXA1 transporter accepts acyl-CoA rather than unesterified fatty acid as its substrate. The observation that pxa1 seedlings accumulate long-chain acyl-CoAs during germination (Footitt et al., 2002) points to acyl-CoAs as the substrate. However, it is also possible that unesterified fatty acids accumulating in the cytoplasm of pxa1 cells are secondarily diverted into acyl-CoA synthesis. There is no direct biochemical evidence for (or against) an acyl-CoA hydrolase activity of any ABC transporter, but genetic evidence strongly suggests that acyl-CoAs are the cytoplasmic substrates and the matrix products of Pxa1p transport in S. cerevisiae (Hettema et al., 1996).
Figure 8.
Two Alternative Models for the Role of LACS6/LACS7 Acyl-CoA Synthetase in Supplying Substrates for β-Oxidation.
(A) The ABC transporter PXA1 delivers unesterified fatty acids to the peroxisome matrix, and these are activated by LACS6/LACS7.
(B) PXA1 delivers acyl-CoAs to the peroxisome matrix, while LACS6/LACS7 activate fatty acids on an independent pathway involving fatty acid entry by diffusion or by a yet unidentified transport protein (?).
In S. cerevisiae, extensive genetic analysis by Hettema et al. (1996) indicates that the peroxisomal acyl-CoA synthetase Faa2p acts in a pathway that is in parallel with the transport of acyl-CoAs by the ABC transporter encoded by Pxa1 and Pxa2. The Faa2p pathway relies on transfer of MCFAs and LCFAs through the peroxisome membrane (either passively or through the action of an unidentified carrier), followed by synthesis of acyl-CoA by Faa2p in the lumen of the organelle as shown in Figure 8B. When grown on oleic acid as sole carbon source, pxa1 mutant S. cerevisiae grow more slowly than the wild type but continue to grow because the Faa2p pathway can apparently support β-oxidation of LCFAs at 30% of the wild-type rate (Hettema et al., 1996). Interestingly, fibroblasts from human X-ALDP patients exhibit rates of VLCFA β-oxidation that are ∼20 to 30% of those measured in normal fibroblasts (Cartier et al., 1995; Braiterman et al., 1998). It is not clear whether there may be some residual transporter activity in human X-ALDP fibroblasts (Braiterman et al., 1998), but these results raise the possibility that there are parallel pathways for fatty acid transport and activation before peroxisomal β-oxidation in humans, also.
Is it possible to envisage the parallel pathway model operating in germinating A. thaliana seedlings? It seems counterintuitive that a mutational block in either one of these pathways would have the same dramatic effect in blocking seedling establishment. If one pathway were predominant, a mutation in it might block growth, but in this case a mutation in the secondary pathway should have little effect. On the other hand, if the two pathways contributed approximately equally to supplying acyl-CoAs for β-oxidation, then we would expect a mutation in either pathway to allow a reduced rate of β-oxidation — and a slower rate of seedling growth and development. One possibility to be considered is that growth and establishment of the seedlings requires a very high flux of carbon through β-oxidation followed by rapid metabolism of the resulting acetyl-CoA through gluconeogenesis and/or respiration. In this proposal, blocking either of the two pathways of acyl-CoA supply would reduce flux below a required threshold and block further development. Germination on media containing 1% sucrose obviates the need for rapid β-oxidation of lipids, and the developing cotyledons then slowly metabolize TAG through the pathway that remains functional. This could explain the gradual decline in mass of 20:1 in the lacs6 lacs7 double mutant (Figure 3), and in pxa1/cts (Footitt et al., 2002) when they are grown on sucrose. In the absence of sucrose, the reduced initial rate of β-oxidation is not adequate to support cell biological and seed development processes needed for seedling establishment. With development blocked, accumulation of fatty acids (or other metabolic intermediates) leads to feedback inhibition of TAG breakdown, thus explaining our observation that lacs6 lacs7 seeds do not break down 20:1 after germination in the absence of sucrose. Consistent with our hypothesis is the observation that reduced expression of phosphoenolpuruvate carboxykinase (a key enzyme of gluconeogenesis) mediated by RNA interference resulted in a modest decrease (25%) in 14C-acetate incorporation into sucrose but a substantial 78% reduction in seedling establishment (Rylott et al., 2003). It is well established that carbohydrate signaling influences many aspects of seedling development (Gazzarrini and McCourt, 2001; Rolland et al., 2002). It is likely that carbohydrate signaling is affected by defects in lipid mobilization and then contributes to the developmental responses observed in the lacs6 lacs7 and pxa1 mutants.
Exploring the validity of the two models shown in Figure 8 (as well as considering other possibilities) will be key to understanding the metabolic/energetic requirements and the regulation of early seedling development in plants. Defining the biochemistry and cell biology of fatty acid metabolism in A. thaliana may also provide new clues to understanding peroxisomal β-oxidation of VLCFAs in humans. If there is a PXA1/ALDP-independent pathway for supplying acyl-CoAs to peroxisomal β-oxidation in higher eukaryotes, then understanding and enhancing this pathway might potentially lead to new treatments of X-ALDP.
METHODS
Plant Material and Growth Conditions
Plants (A. thaliana ecotype Ws) were grown at 22°C (70% humidity) in 16-h-light/8-h-dark cycle at 22°C in soil. For harvesting seedlings, seeds of A. thaliana were sterilized in 20% bleach/0.1% SDS for 20 min and washed repeatedly with sterile water. They were stratified for 2 d at 4°C in the dark and germinated on 0.5% agar plates containing MS media by incubation in 16-h-light/8-h-dark cycle at 22°C. When stated, 1% sucrose was included in the media. Selection was performed with Basta solution diluted to 0.25 mg/L and kanamycin at a concentration of 80 μg/mL.
To provide a positive control for experiments using 2,4-DB and IBA, we identified a T-DNA insertional line, SALK_019334 (Alonso et al., 2003), and obtained seeds from ABRC (Ohio State University). The seeds were germinated on media containing sucrose. A homozygous plant was identified by PCR and used as the basis of a mutant line designated pxa1-2 (Zolman et al., 2001). The pxa1-2 mutant requires sucrose for seedling establishment and is resistant to 2,4-DB inhibition of root growth (Figure 7). DNA sequence information at the SIGnAL database (http://signal.salk.edu) indicates that the pxa1-2 mutation is caused by T-DNA insertion 91 bp upstream of the translation start site in an intron that interrupts the 5′ noncoding sequence. The T-DNA flanking sequence also contains 18 bp of nonhomologous DNA proximal to the T-DNA LB, and this raises the possibility of additional DNA rearrangement at the insertion site. We did not confirm the exact location of the insertion or investigate the possibility that some PXA1 transcript is produced in pxa1-2 plants. However, the phenotype of this mutant is similar to those reported for other pxa1/cts/ped3 mutants (Zolman et al., 2001; Footitt et al., 2002; Hayashi et al., 2002). The pxa1-2 mutant is in the Columbia (Col-0) wild-type background. Tests showed that Col-0 and Ws plants responded similarly to the 2,4-DB.
DNA Isolation and Mutant Identification
For identification of the LACS mutants, a service at the Arabidopsis Knockout Facility at the University of Wisconsin–Madison was employed following the instructions provided at http://www.biotech.wisc.edu/Arabidopsis/. The screening was based on a PCR strategy on pooled genomic DNA from 140,000 T-DNA tagged lines. The following T-DNA–specific primers have been used: LB primers JL-202 (5′-CATTTTATAATAACGCTGCGGACATCTAC-3′) and JL-270 (5′-TTTCTCCATATTGACCATCATACTCATTG-3′). Gene-specific F and R primers were used for LACS6 and LACS7: 6F (5′-TTCGGATCATTCTCTCCTCTGATTCCGAA-3′); 6R (5′-AGGGATCAGAAGCACCAAGCTCCTTGTA-3′); 7F (5′-GAACAACGTCGTCTCGAAACCATTCGATC-3′); and 7R (5′-TTGAATGTTGGTGTGAGAAGTCCATTCTC-3′).
LACS genomic clones were labeled with digoxygenin (Roche, Indianapolis, IN) according to the manufacturer's protocol and used to detect specific PCR products by DNA gel blot hybridization. The T-DNA/LACS junctions were amplified by PCR, and PCR products were sequenced directly. For the identification of individual mutant plants, genomic DNA from subpools and finally individual plants was isolated and subjected to additional rounds of PCR.
For LACS6, a series of PCR analyses on genomic DNA pools revealed four independent T-DNA insertions in the LACS6 gene within the 60,000 lines of the alpha population of the University of Wisconsin–Madison. The PCR products were sequenced to confirm insertion of T-DNA at the LACS6 locus in all cases. To distinguish between heterozygous and homozygous mutants, two PCR reactions were performed on DNA isolated from individuals of each line. Primers 6F and 6R were used to screen for the wild-type allele, whereas LB and 6R were employed to identify the presence of the mutant allele.
RT-PCR Analysis
Total RNA (2 μg) from young leaves was treated with three units RNase-Free DNase (Fermentas, St. Leon-Rot, Germany). Subsequently, the RNA was converted to cDNA using SuperscriptII (Invitrogen, Karlsruhe, Germany). Two microliters of each RT reaction was used as template in a 50-μL PCR reaction containing gene-specific primers. The primers were the same as described above for the isolation of the T-DNA lines except for 7C (5′-GTAGTAGCCTTTGAAGATGATTGGTCCTC-3′). Amplification was performed using ExTaq (Takara, Gennevilliers, France) and the following conditions: 95°C for 3 min, 80°C for 2 min and addition of polymerase, and 35 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 1 min 30 s. A sample (8 μL) of each reaction was analyzed by Tris-acetate-EDTA-agarose gel electrophoresis and stained with ethidium bromide before visualization under UV illumination.
RNA Gel Blotting
Total RNA from seeds and seedlings was extracted following the pine tree RNA isolation method (Chang et al., 1993). Five micrograms of total RNA was separated by electrophoresis using a 1% formaldehyde gel and was transferred to nylon membrane Hybond N (Amersham Pharmacia, Buckinghamshire, UK) by using 10 × SSC. 32P-labeled probes were prepared using fragments of 154 bp of the 5′ end of the coding region from LACS6 and LACS7, respectively. These regions share only 42% of identity on DNA level to each other, with no stretch of continuous identity longer than 8 bp. The hybridization was performed at 65°C in Church buffer (1 mM EDTA, 0.25 M Na2HPO4, 7% SDS, pH 7.2). Equal gel loading was confirmed by comparing ethidium bromide–stained rRNA.
Lipid Analysis
Fatty acids from total lipids were extracted and measured following the method of Browse et al. (1986). Acyl-CoA analysis was performed as described by Larson and Graham (2001) with slight modifications: Acyl-CoAs were extracted from 100 mg of seedlings, and the resulting extract was dried under argon at 50°C. Three hundred microliters of chloroacetaldehyde reagent was then added to the samples, and the derivatization of acyl-CoAs to their ethenoderivatives was conducted at 85°C for 20 min. HPLC analysis was performed with Thermoquest HPLC equipment (Thermoquest, Egelsbach, Germany) using a LUNA 150 × 2.0 cm column with phenyl-hexyl–coated 5 μm silica particles (Phenomenex, Torrance, CA) under the same conditions as described by Larson and Graham (2001).
To provide standards for the analysis, saturated and monounsaturated acyl-CoA esters with acyl chain lengths from C12 to C18 were obtained from Sigma (St. Louis, MO). Polyunsaturated acyl-CoAs were synthesized enzymatically using an acyl-CoA synthetase from Pseudomonas sp (Sigma, St. Louis, MO). The reaction mixture (200 μL) containing 100 mM Tris-HCl, pH 8.1, 10 mM MgCl2, 5 mM ATP, 5 mM CoASH, 2 mM DTT, 25 μM free fatty acid, and 2.5 units acyl-CoA synthetase was incubated at 37°C for 2 h. The reaction was stopped with 50 μL glacial acetic acid:ethanol 1:1 (v/v), and the samples were washed with petroleum ether to extract residual free fatty acids. After purification of the acyl-CoAs on a Sep-Pak column (Strata C18-E; Phenomenex, Torrance, CA) using acetonitrile as eluting solvent, the samples were dried under argon and dissolved in 50 mM MES, pH 5.0.
Electron Microscopic Analysis
Seeds were sown on MS medium supplemented with 1% sucrose. Cotyledons from 6-d-old seedlings were removed and fixed overnight in 2% paraformaldehyde/2% glutaraldehyde/0.05 M PIPES, pH 7.2. Tissue was stained overnight with 1% OsO4. Samples were dehydrated through a graded acetone series and embedded in Spurr's epoxy resin (Sigma). Ultrathin sections were stained with uranyl acetate and lead citrate and observed with a JEM 1200EX (Tokyo, Japan).
Acknowledgments
We thank Greg Tilton and Jay Shockey for the data in Figure 3A and for many valuable discussions. We thank Ian Graham for an interesting discussion on TAG mobilization in kat2 and cts. Insertion mutant information was obtained from the SIGnAL Web site (http://signal.salk.edu). We thank the Salk Institute Genomic Analysis Laboratory for providing the sequence-indexed A. thaliana T-DNA insertion (SALK_015092.56.00), and we also thank the ABRC for providing us with the corresponding seed sample. This work was supported by Dow Chemical, Dow AgroSciences, U.S. Department of Energy Grant DE-FG06-99ER20323, and the Agricultural Research Center, Washington State University. Funding for the SIGnAL-indexed insertion mutant was provided by the National Science Foundation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: John Browse (jab@wsu.edu).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.019646.
References
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657. [DOI] [PubMed] [Google Scholar]
- Braiterman, L.T., Zheng, S., Watkins, P.A., Geraghty, M.T., Johnson, G., McGuinness, M.C., Moser, A.B., and Smith, K.D. (1998). Suppression of peroxisomal membrane protein defects by peroxisomal ATP binding cassette (ABC) proteins. Hum. Mol. Genet. 7, 239–247. [DOI] [PubMed] [Google Scholar]
- Browse, J. (1997). Synthesis and storage of fatty acids. In Advances in Cellular and Molecular Biology of Plants, I. Vasil and B. Larkins, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 407–440.
- Browse, J., McCourt, P.J., and Somerville, C.R. (1986). Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal. Biochem. 152, 141–145. [DOI] [PubMed] [Google Scholar]
- Cartier, N., Lopez, J., Moullier, P., Rocchiccioli, F., Rolland, M.-O., Jorge, P., Mosser, J., Mandel, J.-L., Bougnères, P.-F., Danos, O., and Aubourg, P. (1995). Retroviral-mediated gene transfer corrects very-long-chain fatty acid metabolism in adrenoleukodystrophy fibroblasts. Proc. Natl. Acad. Sci. USA 92, 1674–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S., Puryear, J., and Cairney, J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 11, 113–116. [Google Scholar]
- Epstein, E., and Ludwig-Müller, J. (1993). Indole-3-butyric acid in plants: Occurrence, synthesis, metabolism, and transport. Physiol. Plant 88, 382–389. [Google Scholar]
- Footitt, S., Slocombe, S.P., Larner, V., Kurup, S., Wu, Y., Larson, T., Graham, I., Baker, A., and Holdsworth, M. (2002). Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 21, 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda, M., Shockey, J., Weber, M., Wolter, F.P., and Heinz, E. (2002). Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid β-oxidation. Plant J. 32, 93–104. [DOI] [PubMed] [Google Scholar]
- Gazzarrini, S., and McCourt, P. (2001). Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr. Opin. Plant Biol. 4, 387–391. [DOI] [PubMed] [Google Scholar]
- Gerbling, H., and Gerhardt, B. (1987). Activation of fatty acids by non-glyoxysomal peroxisomes. Planta 171, 386–392. [DOI] [PubMed] [Google Scholar]
- Germain, V., Rylott, E.L., Larson, T.R., Sherson, S.M., Bechtold, N., Carde, J.P., Bryce, J.H., Graham, I.A., and Smith, S.M. (2001). Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 28, 1–12. [DOI] [PubMed] [Google Scholar]
- Gould, S.J., Keller, G.-A., Hosken, N., Wilkinson, J., and Subramani, S. (1989). A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 198, 1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, I.A., and Eastmond, P.J. (2002). Pathways of straight and branched chain fatty acid catabolism in higher plants. Prog. Lipid Res. 41, 156–181. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., Nito, K., Takei-Hoshi, R., Yagi, M., Kondo, M., Suenaga, A., Yamaya, T., and Nishimura, M. (2002). Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell Physiol. 43, 1–11. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., Toriyama, K., Kondo, M., and Nishimura, M. (1998). 2,4-dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell 10, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema, E.H., van Roermund, C.W., Distel, B., van den Berg, M., Vilela, C., Rodrigues-Pousada, C., Wanders, R.J., and Tabak, H.F. (1996). The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. EMBO J. 15, 3813–3822. [PMC free article] [PubMed] [Google Scholar]
- Johnson, T.L., and Olsen, L.J. (2001). Building new models for peroxisome biogenesis. Plant Physiol. 127, 731–739. [PMC free article] [PubMed] [Google Scholar]
- Kerner, J., and Hoppel, C. (2000). Fatty acid import into mitochondria. Biochim. Biophys. Acta 1486, 1–17. [DOI] [PubMed] [Google Scholar]
- Larson, T.R., and Graham, I.A. (2001). A novel technique for the sensitive quantificaiton of acyl CoA esters from plant tissues. Plant J. 25, 115–125. [DOI] [PubMed] [Google Scholar]
- Lemieux, B., Miquel, M., Somerville, C., and Browse, J. (1990). Mutants of Arabidopsis with alterations in seed lipid fatty acid composition. Theor. Appl. Genet. 80, 234–240. [DOI] [PubMed] [Google Scholar]
- McConn, M., Hugly, S., Somerville, C., and Browse, J. (1994). A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast ω-3 desaturase. Plant Physiol. 106, 1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser, J., Douar, A.M., Sarde, C.O., Kioschis, P., Feil, R., Moser, H., Poustka, A.M., Mandel, J.L., and Aubourg, P. (1993). Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730. [DOI] [PubMed] [Google Scholar]
- Rolland, F., Moore, B., and Sheen, J. (2002). Sugar sensing and signaling in plants. Plant Cell 14 (suppl.), S185–S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylott, E.L., Rogers, C.A., Gilday, A.D., Edgell, T., Larson, T.R., and Graham, I.A. (2003). Arabidopsis mutants in short- and medium-chain acyl-CoA oxidase activities accumulate acyl-CoAs and reveal that fatty acid beta-oxidation is essential for embryo development. J. Biol. Chem. 278, 21370–21377. [DOI] [PubMed] [Google Scholar]
- Schnurr, J.A., Shockey, J.M., de Boer, G.-J., and Browse, J. (2002). Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. Plant Physiol. 129, 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani, N., and Valle, D. (1996). A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl. Acad. Sci. USA 93, 11901–11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey, J., Fulda, M., and Browse, J.A. (2002). Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 129, 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey, J., Fulda, M., and Browse, J. (2003). Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis reveals a new class of acyl-CoA synthetases. Plant Physiol. 132, 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi, A., and Browse, J. (2000). The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 97, 10625–10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner, J., Schaller, F., Frick, U.B., Howe, G.A., Weiler, E.W., Amrhein, N., Macheroux, P., and Schaller, A. (2002). Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J. 32, 585–601. [DOI] [PubMed] [Google Scholar]
- Swinkels, B.W., Gould, S.J., Bodnar, A.G., Rachubinski, R.A., and Subramani, S. (1991). A novel, cleavable peroxisomal targeting signal at the amino-terminus of the rat 3-ketoacyl-CoA thiolase. EMBO J. 10, 3255–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synerholm, M.E., and Zimmermann, P.W. (1947). Preparation of a series of ϕ-(2,4-dichlorophenoxy)-alphatic acids and some related compounds with a consideration of their biochemical role as plant-growth regulators. Contrib. Boyce Thompson Inst. 14, 369–382. [Google Scholar]
- Turner, J.G., Ellis, C., and Devoto, A. (2002). The jasmonate signal pathway. Plant Cell 14 (suppl.), S153–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain, R.L., and Wightman, F. (1954). The growth-regulated activity of certain ω-substituted alkyl carboxylic acids in relation to their β-oxidation within the plant. Proc. R. Soc. Lond. Biol. Sci. 142, 525–536. [DOI] [PubMed] [Google Scholar]
- Zolman, B.K., Silva, I.D., and Bartel, B. (2001). The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol. 127, 1266–1278. [PMC free article] [PubMed] [Google Scholar]
- Zolman, B.K., Yoder, A., and Bartel, B. (2000). Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156, 1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]